Open Access Article
Open Access ArticleRetracted Article: Preparation of NiFe2O4@MIL-101(Fe)/GO as a novel nanocarrier and investigation of its antimicrobial properties
Fatemeh Shaterana,
Mohammad Ali Ghasemzadeh *a and
Seyyed Soheil Aghaeib
*a and
Seyyed Soheil Aghaeib
aDepartment of Chemistry, Qom Branch, Islamic Azad University, Qom, I. R. Iran. E-mail: Ghasemzadeh@qom-iau.ac.ir
bDepartment of Microbiology, Qom Branch, Islamic Azad University, Qom, I. R. Iran
First published on 2nd March 2022
Abstract
In this research, we have investigated a novel magnetic nanocomposite including NiFe2O4@MIL-101(Fe)/GO for the delivery of the antibiotic tetracycline (TC). Moreover, the antibacterial activity of NiFe2O4@MIL-101(Fe)/GO, NiFe2O4@MIL-101(Fe)/GO/TC and pure TC was evaluated by agar well diffusion and minimum inhibitory concentration (MIC) methods on both Gram-negative (Escherichia coli) and Gram-positive (Staphylococcus aureus) bacteria. In addition, the cytotoxicity of NiFe2O4@MIL-101(Fe)/GO/TC on HeLa cells was determined by an MTT assay which showed good results. The structure of the prepared nanocarrier was investigated by various spectroscopic techniques such as Fourier-transform infrared spectroscopy (FT-IR), energy-dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD), field emission scanning electron microscopy (FE-SEM), Brunauer–Emmett–Teller (BET), and thermal gravimetric analysis (TGA). The results of this study showed that 98% of the TC was loaded on the synthesized nanocomposite. Drug release occurred at pH: 7.4 (phosphate buffer saline) and pH: 5.0 (acetate buffer) within 3 days, resulting in 77% and 85% release of the drug, respectively.
1. Introduction
The design of drug delivery systems is very useful in the diagnosis and treatment of diseases and the controlled and targeted release of drugs. Today, great effort has been made to develop new methods for targeted drug delivery and controlled drug release.1 The aim of the new drug delivery is to optimize the drug's effects while reducing side effects in the body. Therefore, the use of drug nanocarriers for targeted drug delivery can provide these optimal conditions.2Recently, numerous compounds have been investigated, such as solid lipid nanoparticles,3 polymer nanoparticles,4 micelles,5 hydrogel nanoparticles,6 liposomes,7 and metal–organic frameworks (MOFs)8 as drug carriers.
MOFs are a new class of porous materials made from metal ions and organic ligands. They have significant properties such as: low density, high porosity, adjustable pore size, easy synthesis, high stability and high loading capacity, which have made them a promising drug carrier in recent years.9–11 MIL-100 and MIL-101 are the first group of metal–organic frameworks to be considered as a nanocarrier in the release of ibuprofen.1 In recent years, different MOFs including ZIF-8, MIL-101(Fe)–NH2, and UiO-66 have been used for drug delivery of some drugs such as doxorubicin, naproxen, and ciprofloxacin.12–14
Magnetic nanoparticles (MNPs) with highlighted properties including high stability, easy separation, low toxicity, ease of surface modification and magnetic attributes15 have been used as highly potential materials in diverse fields like biomedical,16 biochemical,17 magnetic imaging,18 catalysis19 and industrial applications.20 However, their role in biomedicine, particularly in the field of drug delivery, is important because their inherent magnetism aids many tasks, including targeting, which is very important and necessary in drug delivery.21
Among the various ferrites, NiFe2O4 with the general formula (AB2O4) has attracted significant attention due to its excellent magnetic and catalytic properties, high saturation magnetization, strong chemical stability, and low toxicity.22 The ferromagnetic properties of nickel ferrite (NiFe2O4) resulting from the magnetic moments of anti-parallel spins between Fe3+ ions at tetrahedral sites and Ni2+ ions at octahedral sites make them one of the most widely used spinel ferrites in various biomedical applications.23
The combination of MNPs and MOFs results in the formation of magnetic frameworks composites (MFCs). Combinations of MOFs with NPs have been made due to their high adsorption capacity, easy functionality, and easy isolation with an external magnetic field.24 There are many ways to improve and modify nanocarrier structures by adding a functional group and surface modification using different materials like PEG,25 chitosan,26 alginate,27 carboxymethyl cellulose28 and graphene oxide.29
Graphene oxide (GO) with properties including special surfaces, pore size, non-toxicity and excellent biological compatibility which has oxygen-containing agents, epoxy and hydroxyl, carboxyl, carbonyl, and phenol functional groups, is a suitable substrate for drug loading through hydrogen bonding and physical absorption.30
One of the advantages of this nanomaterial is that graphene oxide can be well-dispersed in water and physiological environments due to its abundant hydrophilic groups, such as hydroxyl, epoxide and carboxylic groups on its large surfaces. In addition, its good biocompatibility and lack of obvious toxicity make it a promising material for drug carrier substances.31,32
The hydroxyl and epoxy functional groups of GO permit the metal ions in MOFs to be formed as a composite hence, the idea of MOF-GO nanocomposites is developed. So, by merging the complementary features of the two materials, GO becomes dense arrays of layers and nonporous.
Recently, some MOF-GO structures like Fe3O4@MOFs/GO and CMC/MOF-5/GO have been used as an efficient drug carrier for the drug release of ibuprofen and doxorubicin, respectively.33,34
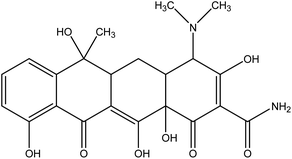
Tetracyclines (TC) are a family of bacteriostatic antibiotics that are active against various Gram-negative and Gram-positive bacteria and are used to treat acne and skin infections.35
Tetracyclines inhibit protein production, they are able to inhibit bacterial protein synthesis by binding reversibly to ribosomal units (Fig. 1).
Considering the significance of the previous research related to the need of new drug delivery systems to find a suitable and practical nanocarrier,36–38 herein, we were able to report a novel magnetic nanocomposite including NiFe2O4@MIL-101(Fe)/GO, as a carrier for the drug delivery of TC (Scheme 1). Moreover, the antibacterial properties of NiFe2O4@MIL-101(Fe)/GO, pure TC and the drug-loaded nanocarrier were compared against Escherichia coli and Staphylococcus aureus bacteria by agar well diffusion and MIC methods which showed significant results. An MTT assay was performed to evaluate the toxicity of NiFe2O4@MIL-101(Fe)/GO/TC.
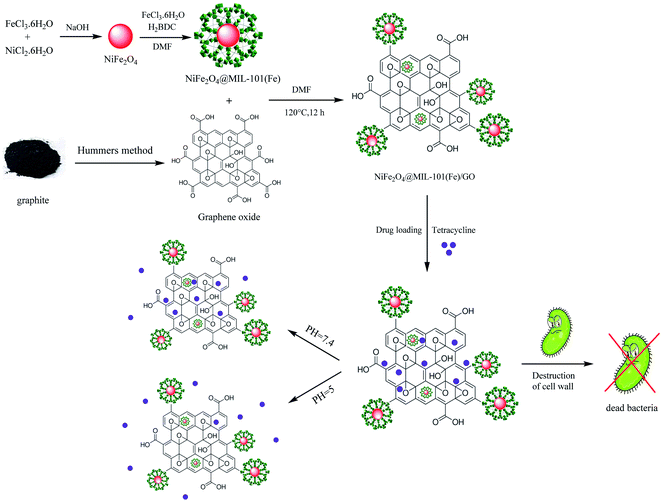 | ||
| Scheme 1 A schematic exhibition of the synthetic procedure of NiFe2O4@MIL-101(Fe)/GO as a TC carrier with antibacterial activities. | ||
2. Results and discussion
2.1. Characterization of NiFe2O4@MIL-101(Fe)/GO and NiFe2O4@MIL-101(Fe)/GO@TC
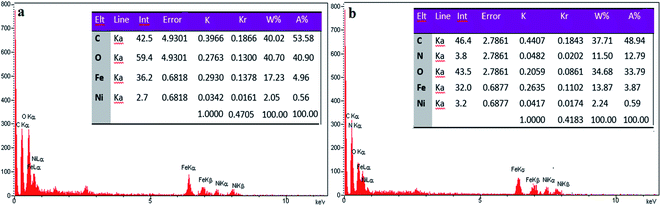
In this research, the structures of NiFe2O4@MIL-101(Fe)/GO and NiFe2O4@MIL-101(Fe)/GO@TC were fully characterized by different spectroscopy techniques.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of BDC, respectively.
C of BDC, respectively.
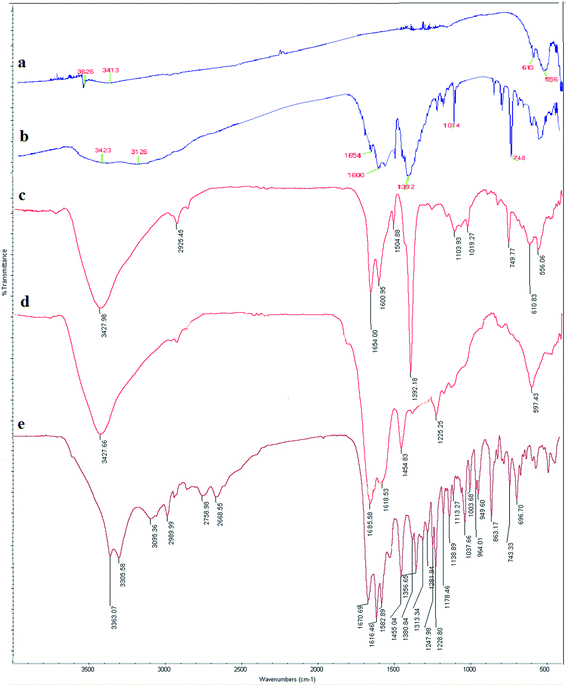 | ||
| Fig. 2 FT-IR spectra of (a) NiFe2O4, (b) MIL-101(Fe), (c) NiFe2O4@MIL-101(Fe)/GO, (d) NiFe2O4@MIL-101(Fe)/GO/TC, and (e) TC. | ||
In the FT-IR spectrum of NiFe2O4@MIL-101(Fe)/GO (Fig. 2c), the presence of Ni–O and Fe–O bonds in the magnetic particles was confirmed by the peaks appearing at 556 cm−1 and 610 cm−1, respectively. The peaks observed at 1654 cm−1 and 1600 cm−1 are related to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of BDC, respectively, and the band at 1392 cm−1 was from the aromatic carbon.39 The peak at 1019 cm−1 was from C–O, the absorbed water in GO is shown by a broad peak at 3427 cm−1, contributed to by the O–H stretching of the H2O molecules. This supports the fact that GO is a highly absorptive material.
C of BDC, respectively, and the band at 1392 cm−1 was from the aromatic carbon.39 The peak at 1019 cm−1 was from C–O, the absorbed water in GO is shown by a broad peak at 3427 cm−1, contributed to by the O–H stretching of the H2O molecules. This supports the fact that GO is a highly absorptive material.
The FT-IR spectra of NiFe2O4@MIL-101(Fe)/GO/TC confirms the presence of TC in the MOF (Fig. 2d). The peak at 3427 cm−1 is associated with the OH group. The absorption band at 3427 cm−1 belongs to the NH2 group of TC, representing the loading of TC on the nanocomposite. The bands at 1454 cm−1 are related to the stretching vibrations of C–N. Moreover, the peak at 1685 cm−1 refers to the carbonyl group of the drug.
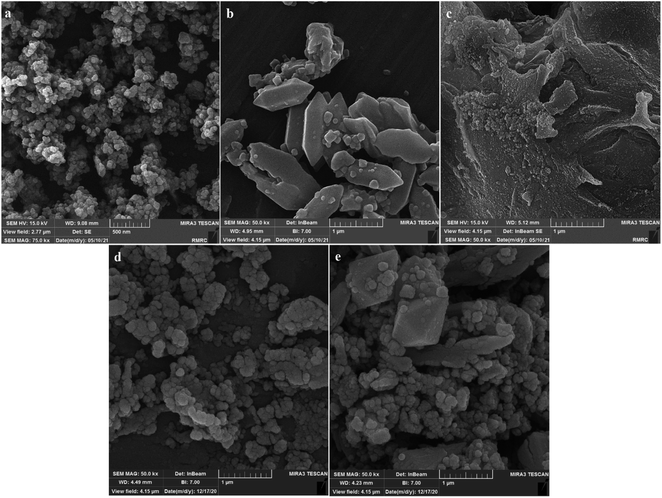 | ||
| Fig. 4 SEM analysis of (a) NiFe2O4, (b) MIL-101(Fe), (c) GO, (d) NiFe2O4@MIL101(Fe)/GO, and (e) NiFe2O4@MIL-101(Fe)/GO/TC. | ||
SEM images for the nanocarrier before and after drug loading are shown in Fig. 4d and e. The image of the NiFe2O4@MIL-101(Fe)/GO shows that the particles have a regular and spherical shape (Fig. 4d). After loading, it is shown that the particle size has become larger and the shape has become irregular (Fig. 4e).
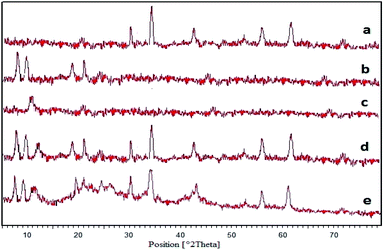 | ||
| Fig. 5 XRD patterns of (a) pure NiFe2O4, (b) MIL-101(Fe), (c) GO, (d) NiFe2O4@MIL-101(Fe)/GO, and (e) NiFe2O4@MIL-101(Fe)/GO/TC. | ||
Fig. 5d shows the XRD pattern of NiFe2O4@MIL-101(Fe)/GO. The peaks observed at 2θ = 30.29°, 35.70°, 43.36°, 57.36° and 62.92° are related to the NiFe2O4 NPs. Also the peaks at 8.9°, 9.7°, 19.6° and 21.7° (2θ°) show the presence of the MIL-101(Fe) frameworks as well as the peak at 2θ = 11.1° indicated the construction of the GO, which confirms the formation of the nanocomposite.
From this information, it can be concluded that after loading TC, the crystallite structure of NiFe2O4@MIL-101(Fe)/GO is preserved although the peak intensities declined (Fig. 5e).
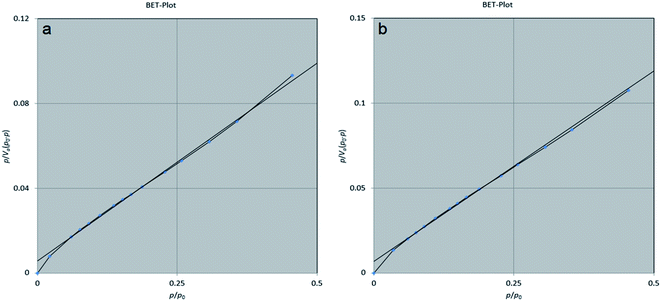
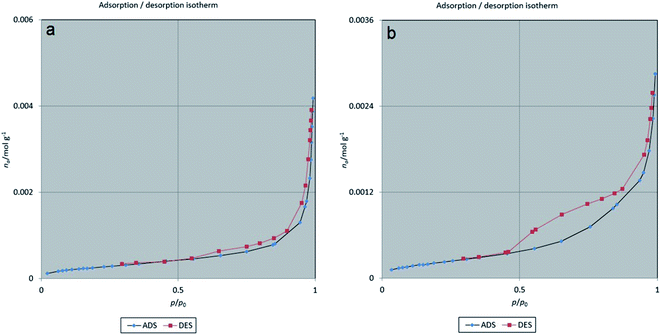
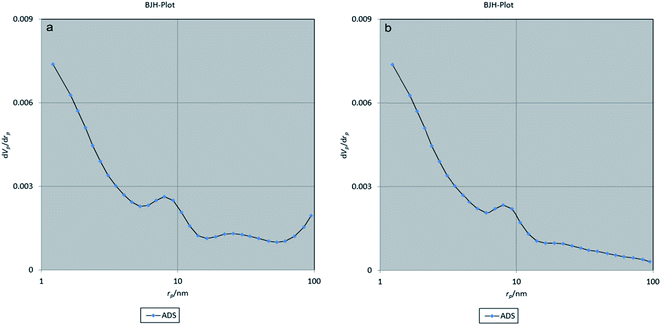
In addition, the BET method was used to evaluate the porous structure of NiFe2O4@MIL-101(Fe)/GO before and after encapsulating TC based on nitrogen adsorption. As shown in Fig. 7, the adsorption–desorption isotherm for NiFe2O4@MIL-101(Fe)/GO is a type I sorption isotherm. Furthermore, the result of the BJH (Barrett–Joyner–Halenda) analysis indicates that the average pore diameter of the nanocarrier is 1.21 nm, which indicates the porosity of the composite (Fig. 8).
2.2. Study on drug release
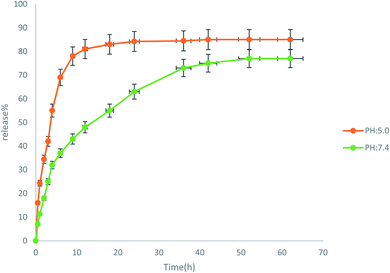
Before the release, different concentrations of the drug were prepared in two buffer (phosphate buffer and acetate buffer) solutions. Their absorptions were then obtained by a UV-Vis device at 360 nm. Then, their calibration curves were plotted. The drug release diagram at pH: 7.4 and pH: 5 is shown in Fig. 10. As indicated, the structure of NiFe2O4@MIL-101(Fe)/GO is unstable in acidic buffer (pH: 5) and degraded very rapidly. For this reason, drug release at pH: 5 is much faster than pH: 7.4 which means it is unsuitable for a desirable drug delivery system. The obtained results show that the rate of drug release at pH: 5 increased to 70% during the first 4 h. Whereas, the release of the drug at the physiological pH of the body (pH: 7.4) was about 77% over 72 h and then fixed. Based on these observations, the TC release of NiFe2O4@MIL-101(Fe)/GO is controlled at pH 7.4.2.3. Results of the antimicrobial tests
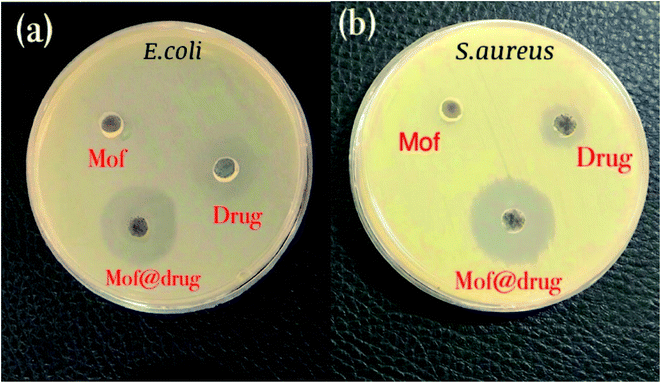 | ||
| Fig. 11 Results of the antibacterial test: (a) the disk containing E. coli bacteria and (b) the disk containing S. aureus bacteria. | ||
| Bacteria | TC | NiFe2O4@MIL-101(Fe)/GO | NiFe2O4@MIL-101(Fe)/GO/TC |
|---|---|---|---|
| E. coli | 15 | 0 | 21 |
| S. aureus | 11 | 0 | 23 |
We provided Table 2 to show a comparison between our research with similar studies.37,45–48 As can be seen, the newly prepared nanocomposite including NiFe2O4@MIL-101(Fe)/GO had a satisfactory drug loading percentage, drug release, and also antibacterial activities in comparison with previous structures.
| Entry | Nanocomposite | Drug | Drug release (pH: 7.4) | Drug loading | Bacterial strain | Inhibited zone | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Fe3O4@PAA@ZIF-8 | Ciprofloxacin | 74% | 93% | E. coli | 32 mm | 37 |
| S. aureus | 15 mm | ||||||
| 2 | ZIF-8/GO/MgFe2O4 | Tetracycline | 92% | 90% | E. coli | 22 mm | 45 |
| S. aureus | 25 mm | ||||||
| 3 | ZnO@ZIF-8 | Ampicillin | 60% | E. coli | 12 mm | 46 | |
| S. aureus | 48 mm | ||||||
| 4 | GO-PEG | Nigella sativa | 88% | E. coli | 30 mm | 47 | |
| S. aureus | 36 mm | ||||||
| 5 | Zn2(bdc)2(dabco) | Gentamicin | 65% | E. coli | 16 mm | 48 | |
| S. aureus | 9 mm | ||||||
| 6 | NiFe2O4@MIL-101(Fe)/GO | Tetracycline | 77% | 98% | E. coli | 21 mm | This research |
| S. aureus | 23 mm |
| Bacteria | TC | NiFe2O4@MIL-101(Fe)/GO | NiFe2O4@MIL-101(Fe)/GO/TC |
|---|---|---|---|
| E. coli | 3.125 | >100 | 0.19 |
| S. aureus | 25 | >100 | 6.25 |
2.4. Cytotoxicity of NiFe2O4@MIL-101(Fe)/GO/TC
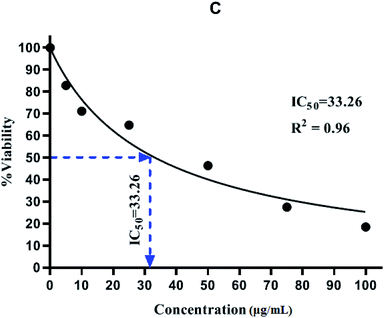
In this research, an MTT assay was used to determine the cytotoxicity of NiFe2O4@MIL-101(Fe)/GO/TC. In the MTT assay, only cells which are viable after 24 hours exposure to the sample were capable of metabolizing a dye (3-(4,5-dimethylthiozol-2-yl)-2,5-diphenyl tetrazolium bromide) efficiently and the purple-colored precipitate which is dissolved in a detergent was analyzed spectrophotometrically. After 24 hours post treatment, HeLa cells showed excellent viability even up to the concentration of 100 μg mL−1 of NiFe2O4@MIL-101(Fe)/GO/TC. As shown in Table 4, The IC50 value of the NiFe2O4@MIL-101(Fe)/GO/TC was 33.26 (Fig. 12).| Concentration (μg mL−1) | Absorbance at 570 nm | Average | Average-blank | % viability | IC50 (μg mL−1) | ||
|---|---|---|---|---|---|---|---|
| 100 | 0.211 | 0.241 | 0.238 | 0.23 | 0.222 | 18.5 | 33.26 |
| 75 | 0.355 | 0.312 | 0.347 | 0.338 | 0.33 | 27.5 | |
| 50 | 0.577 | 0.561 | 0.558 | 0.565333 | 0.557333 | 46.44 | |
| 25 | 0.798 | 0.778 | 0.781 | 0.785667 | 0.777667 | 64.801 | |
| 10 | 0.877 | 0.845 | 0.863 | 0.861667 | 0.853667 | 71.14 | |
| 5 | 1.008 | 1.001 | 0.998 | 1.002333 | 0.994333 | 82.86 | |
| Control (untreated) | 1.214 | 1.197 | 1.202 | 1.208 | 1.2 | 100 | |
| Blank | 0.008 | 0.009 | 0.007 | 0.008 | 0 | ||
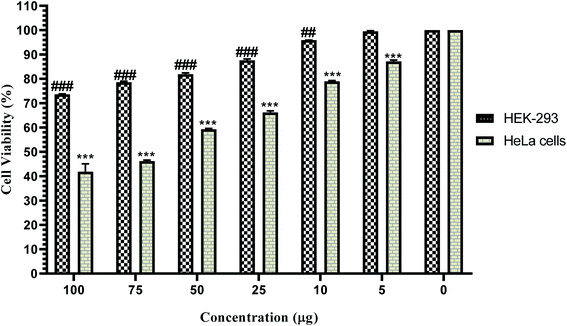
The results of the MTT assay showed that the TC extract in a concentration-dependent manner could inhibit cell proliferation. In this way, by increasing its concentration, the proliferation of HELA cancer cells is inhibited and makes a significant difference compared to the control group. By determining the inhibitory concentration of IC50, it was found that the TC concentration was 33.26 μg mL−1 in 24 hours, which resulted in the death of 50% of HELA cancer cells. Also, the results of the MTT assay to evaluate the effect of TC on the normal HEK-293 cell line showed that the TC extract has a less toxic effect on the HEK-293 cell line than the HELA cancer cell line (Fig. 13).
3. Experimental section
3.1. Materials
Chemicals were purchased from Sigma-Aldrich and Merck in high purity. All of the materials were of commercial reagent grade and were used without further purification. FT-IR spectra were recorded on the Magna-IR spectrometer 550. Powder X-ray diffraction (XRD) was carried out on a Philips diffractometer of X'pert company with mono chromatized Cu Kα radiation (λ = 1.5406 Å). The compositional analysis was done by X-ray energy dispersive analysis (EDX, Kevex (Newark, DE) Delta Class I). The microscopic morphology of products was visualized by SEM (LEO 1455VP). Thermogravimetric analysis (TGA) was performed on a Mettler Toledo TGA under argon and heated from room temperature to 825 °C. The approximate sample weight was 10 mg in the TG experiment with a 10 °C min−1 heating rate. Nitrogen adsorption–desorption isotherms were measured at 196 °C using a Belsorp mini automatic adsorption instrument after degassing the samples at 150 °C for 5 h. Absorption spectra were recorded in the range 200–800 nm on a Shimadzu model 1601 PC UV-visible spectrophotometer (Shimadzu, Tokyo, Japan).3.2. Preparation of NiFe2O4 nanoparticles
NiFe2O4 was prepared using a method described by Zhang et al.40 via a coprecipitation method. Briefly, FeCl3·6H2O (1.35 g, 5 mmol) and NiCl2·6H2O (0.595 g, 2.5 mmol) were dissolved in distilled water (10 mL) and then sonicated to form a clear solution. Then, 20 mL of 5 mol L−1 NaOH solution was added into the mixture and stirred for 15 min. The resulting mixture was transferred into a 50 mL Teflon-lined autoclave and heated at 180 °C for 12 h. After cooling slowly to room temperature, the black product was washed several times with distilled water and ethanol and then dried at 50 °C for 24 h.3.3. Synthesis of NiFe2O4@MIL-101(Fe)
Synthesis of NiFe2O4@MIl-101(Fe) was performed by a solvothermal method. In this way, terephthalic acid (0.415 g, 2.5 mmol) and FeCl3·6H2O (1.35 g, 5 mmol) were added into 30 mL of DMF. Then, 0.12 g of the prepared NiFe2O4 was added to it. The mixture was suspended under ultrasonic irradiation for 15 min. Next, the mixture was transferred to a Teflon autoclave at 120 °C for 20 h. When the reaction was completed, the obtained residue was washed with DMF and EtOH to eliminate unreacted materials. The purified product was dried in an oven for 30 min at 70 °C and then activated at 150 °C after10 h. The obtained NiFe2O4@MIL-101(Fe) was 0.51 g which proved that the content of the MOF in the composite is about 0.39 g.3.4. Synthesis of GO
GO was synthesized from graphite powder according to the Hummers' method.49 In this regard, graphite (2 g) and NaNO3 (2 g) were mixed in 50 mL of H2SO4 (98%) and kept in an ice bath (0–5 °C) with continuous stirring. The mixture was stirred for 2 hours at this temperature and KMnO4 (6 g) was added to the suspension very slowly. The rate of addition was carefully controlled to keep the reaction temperature lower than 15 °C. The ice bath was then removed, and the mixture was stirred at 35 °C until it became pasty brownish and kept under stirring for 2 days. It is then diluted with a slow addition of 100 mL of water. The reaction temperature was rapidly increased to 98 °C. Further, this solution was diluted by adding additional 200 mL of water under continuous stirring, followed by a slow addition of 10 mL of H2O2 to terminate the reaction by the appearance of a yellow color. Finally, the mixture was washed with HCl (10%) and deionized water several times. The product was dried at 50 °C for 24 h to obtain GO.3.5. Synthesis of NiFe2O4@MIL-101(Fe)/GO
A mixture of GO (0.05 g) and NiFe2O4@MIL-101(Fe) (0.1 g) was dispersed in DMF (30 mL) under ultrasonic treatment for 20 min. Then, the mixture was transferred to a Teflon autoclave at 120 °C for 12 h. When the reaction was completed, the product was washed with DMF, deionized water and EtOH. Finally, it was dried at 70 °C for 24 h.3.6. Loading TC on the NiFe2O4@MIL-101(Fe)/GO
Drug loading of NiFe2O4@MIL-101(Fe)/GO was as follows: 0.1 g of TC was dissolved in 5 mL of deionized water which continued by the addition of NiFe2O4@MIL-101(Fe)/GO (0.05 g). This solution was stirred for the duration of five days. After completion of the loading, the prepared NiFe2O4@MIl-101(Fe)/GO/TC was washed three times with deionized water to remove unloaded drug, then dried in an oven at 60 °C for 24 h. The amount of drug loading was 98% based on eqn (1):| Drug loading (%) = (TC weight in sample/total weight of sample) × 100% | (1) |
3.7. Drug release from NiFe2O4@MIL-101(Fe)/GO
The release of the drug was performed in two buffers, phosphate buffer saline (pH: 7.4) and buffer acetate (pH: 5). In this procedure, 0.02 g of NiFe2O4@MIL-101(Fe)/GO/TC was added to 50 mL of each buffer separately. Then, the solution was stirred at 37 °C for 3 days. Each time, 5 mL of the solution was removed and quickly replaced with the same amounts of fresh buffer. Further, measurement of the TC released from NiFe2O4@MIL-101(Fe)/GO was done by an instrument of spectroscopy UV/Vis at 360 nm. Finally, the release percentage was expressed by eqn (2):| Release percentage (%) = mr (amount of released TC)/ml (total amount of loaded TC) | (2) |
3.8. Assays for antibacterial activity
3.9. MTT assay
The cytotoxicity of NiFe2O4@MIL-101(Fe)/GO/TC on HeLa cells was determined by MTT assay. Approximately 1 × 105 mL−1 HeLa cells in their exponential growth phase were seeded in a flat-bottomed 96-well polystyrene coated plate and were incubated for 24 h at 37 °C in a 5% CO2 incubator. A series of dilutions (5, 10, 25, 50, 75 and 100 μg mL−1) of NiFe2O4@MIL-101(Fe)/GO/TC in the medium was added to the plate in hexaplets. After 24 hours of incubation, 10 μL of the MTT reagent was added to each well and further incubation was carried out for 4 hours. Formazan crystals formed after 4 hours in each well, which were dissolved in 150 μL of detergent and the plates were read immediately in a microplate reader (BIO-RAD microplate reader-550) at 570 nm. Wells with complete medium, NiFe2O4@MIL-101(Fe)/GO/TC and MTT reagent, without cells were used as blanks. Untreated HeLa cells as well as the cell treated with (5, 10, 25, 50, 75 and 100 μg mL−1) concentrations of NiFe2O4@MIL-101(Fe)/GO/TC for 24 h were subjected to the MTT assay for cell viability determination.524. Conclusions
In this study, a novel nanocomposite including NiFe2O4@MIL-101(Fe)/GO was successfully produced and its structure was confirmed by FT-IR, EDX, XRD, SEM, BET and TGA techniques. In addition, tetracycline as an antibiotic drug was encapsulated into the NiFe2O4@MIL-101(Fe)/GO with a high loading of 98% due to the structure, high surface area and cavities in the structure of the nanocarrier. Furthermore, the drug release from the nanocomposite was tested at pH: 7.4 and pH: 5.0. The results of this release indicated that pH: 7.4 is more appropriate because of the controlled release and withdrawal of the drug from the nanocarrier. Furthermore, E. coli and S. aureus were used for antibacterial testing, and the results showed that the TC drug loaded on the NiFe2O4@MIL-101(Fe)/GO better inhibited the growth of the bacterium. As a result, NiFe2O4@MIL-101(Fe)/GO/TC has a stronger antibacterial property than pure TC. At the end, MTT studies exhibited that NiFe2O4@MIL-101(Fe)/GO/TC was of low toxicity.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The author gratefully acknowledges the financial support of this work by the Research Affairs Office of the Islamic Azad University, Qom Branch, Qom, I. R. Iran [grant number 2019-2898].References
- S. Khan, M. Imran, T. T. Butt, S. Ali Shah, M. Sohail and A. Malik, Trends Food Sci. Technol., 2018, 80, 8–22 CrossRef CAS.
- R. C. Alves, Z. M. Schulte, M. T. Luiz, P. Da Silva, R. C. G. Frem and N. L. Rois, Inorg. Chem., 2021, 60, 11739–11744 CrossRef CAS PubMed.
- Y. Duan, A. Dhar, C. Patel, M. Khimani, S. Neogi, P. Sharma and N. Siva Kumar, RSC Adv., 2020, 10, 26777–26791 RSC.
- Y. Liu, G. Yang, Th. Baby, T. Dong Chen and D. A. Weitz, Angew. Chem., Int. Ed., 2020, 132, 4750–4758 CrossRef.
- M. Ghezzi, S. Pescina, C. Padula, P. Santi, E. Del Favero, L. Cantù and S. Nicoli, J. Control. Release, 2021, 332, 312–336 CrossRef CAS PubMed.
- S. Jacob, A. B. Nair, J. Shah, N. Sreeharsha, S. Gupta and P. Shinu, Pharmaceutics, 2021, 13, 357 CrossRef CAS PubMed.
- D. E. Large, R. G. Abdelmessih, E. A. Fink and D. T. Auguste, Adv. Drug Deliv. Rev., 2021, 176, 113851 CrossRef CAS PubMed.
- M. Safaei, M. H. Foroughi, N. Ebrahimpoor, S. Jahani, A. Omidi and M. Khatami, Trends Analyt Chem., 2019, 118, 401–425 CrossRef CAS.
- A. Lajevardi, M. Hossaini Sadr, A. Badiei and M. Armaghan, J. Mol. Liq., 2020, 307, 112996 CrossRef CAS.
- H. D. Lawson, S. Patrick Walton and Ch. Chan, ACS Appl. Mater. Interfaces, 2021, 13, 7004–7020 CrossRef CAS PubMed.
- Y. Han, W. Liu, J. Huang, S. Qiu, H. Zhong, D. Liu and J. Liu, Pharmaceutics, 2018, 10, 271 CrossRef CAS PubMed.
- I. Vasconcelos, T. da Silva, G. Militão, T. Soares, N. Rodrigues, M. Rodrigues, N. Costa, R. Freire and S. Junior, RSC Adv., 2012, 2, 9437–9442 RSC.
- M. Almáši, V. Zeleňák, P. Palotai, E. Beňová and A. Zeleňákov, Inorg. Chem. Commun., 2018, 93, 115–120 CrossRef.
- M. Nasrabadi, M. A. Ghasemzadeh and M. R. Zand Monfared, New J. Chem., 2019, 43, 16033–16040 RSC.
- G. Simonsen and M. Strand, J. Pet. Sci. Eng., 2018, 165, 488–495 CrossRef CAS.
- X. Gao, L. P. Xu, Sh. F. Zhou, G. Liu and X. Zhang, Am. J. Biomed. Life Sci., 2014, 6, 41–57 CrossRef.
- A. K. Gupta and M. Gupta, Biomaterial, 2005, 26, 3995–4021 CrossRef CAS PubMed.
- D. D. Stueber, J. Villanova, I. Aponte, Zh. Xiao and V. L. Colvin, Pharmaceutics, 2021, 13, 943–954 CrossRef CAS PubMed.
- A. Lazzarini, R. Colaiezzi, M. Passacantando, F. D'Orazio, L. Arrizza, F. Ferella and M. Crucianelli, J. Phys. Chem. Solids, 2021, 153, 110003 CrossRef CAS.
- Y. Zhu, L. P. Stubbs, F. Ho, R. Liu, Ch. Peng Ship, J. Maguire and N. Hosmane, ChemCatChem, 2010, 2, 365–374 CrossRef CAS.
- J. K. patra, G. Das, L. F. Fraceto, E. V. Ramos Campos, M. Rodriguez-Torres, L. S. Acosta-Torres, L. A. Diaz-Torres, M. K. Swamy, Sh. Sharma, S. Habtemariam and H. Shin, J. Nanobiotechnol., 2018, 16, 71 CrossRef PubMed.
- M. Dhiman, A. Goyal, V. Kumar and S. Singhal, New J. Chem., 2016, 40, 10418–10431 RSC.
- M. R. Phadatare, V. M. Khot, A. B. Salunkhe, N. D. Thorat and S. H. Pawar, J. Magn. Magn. Mater., 2012, 324, 770–772 CrossRef CAS.
- F. Canfarotta and S. A. Piletsky, Adv. Healthc. Mater., 2014, 3, 160–175 CrossRef CAS PubMed.
- P. Zhang, Y. Huang, H. Liu, R. Marquez, J. Lu, W. Zhao, X. Zhang, X. Gao, J. Li, R. Venkataramanan, L. Xu and S. Li, Biomaterials, 2014, 35, 7146–7156 CrossRef CAS PubMed.
- Q. Hu and Y. Luo, Int. J. Biol. Macromol., 2021, 179, 125–135 CrossRef CAS PubMed.
- T. Azizi Vahed, M. R. Naimi-Jamal and L. Panahi, J. Drug Deliv. Sci. Technol., 2019, 49, 570–576 CrossRef CAS.
- S. Darvishi, S. Javanbakht, A. Heydari, F. Kazeminava, P. Gholizadeh, M. Mahdipour and A. Shaabani, Int. J. Biol. Macromol., 2021, 181, 937–944 CrossRef CAS PubMed.
- T. Zhou, X. Zhou and D. Xing, Biomaterials, 2014, 35, 4185–4194 CrossRef CAS PubMed.
- H. Zhao, R. Ding, X. Zhao, Y. Li, L. Qu, H. Pei, L. Yildirimer, Zh. Wu and W. Zhang, Drug Discov. Today, 2017, 22, 1302–1317 CrossRef CAS PubMed.
- X. Yang, Y. Wang, X. Huang, Y. Ma, Y. Huang, R. Yang, H. Duan and Y. Chen, J. Mater. Chem., 2011, 21, 3448–3454 RSC.
- S. Goenka, V. Sant and Sh. Sant, J. Controlled Release, 2014, 173, 75–88 CrossRef CAS PubMed.
- L. Li, Y. Q. Wu, K. K. Sun, R. Zhang, L. Fan, K. K. Liang and L. B. Mao, Mater. Lett., 2015, 162, 207–210 CrossRef.
- S. Javanbakht, M. Pooresmaeil and H. Namazi, Carbohydr. Polym., 2018, 208, 294–301 CrossRef PubMed.
- I. Chopra and M. Roberts, Microbiol. Mol. Biol. Rev., 2001, 65, 232–260 CrossRef CAS PubMed.
- M. Akbari, M. A. Ghasemzadeh and M. Fadaeian, ChemistrySelect, 2020, 5, 14564–14571 CrossRef CAS.
- M. Esfahanian, M. A. Ghasemzadeh and S. M. H. Razavian, Artif. Cells, Nanomed., Biotechnol., 2019, 47, 2024–2030 CrossRef CAS PubMed.
- S. Farhadi, M. A. Ghasemzadeh and S. S. Aghaei, ChemistrySelect, 2019, 4, 729–736 CrossRef CAS.
- B. Paulchamy, G. Arthi and B. D. Lignesh, J. Nanomed. Nanotechnol., 2015, 6, 253–256 Search PubMed.
- H. Zhang, X. Hao, L. Mo, S. Liu, W. Zhang and Z. Zhang, New J. Chem., 2017, 41, 7108–7115 RSC.
- Y. Wang, W. Guo and X. Li, RSC Adv., 2018, 8, 36477–36483 RSC.
- C. Petit and T. J. Bandosz, J. Mater. Chem., 2009, 19, 6521–6528 RSC.
- J. Lin, H. Hu, N. Gao, J. Ye, Y. Chen and H. Ou, J. Water Proc. Eng., 2020, 33, 101010 CrossRef.
- N. Uregen, K. Pehlivanoglu, Y. Ozdemir and Y. Devrim, Int. J. Hydrog. Energy, 2017, 42, 2636–2647 CrossRef CAS.
- S. Sanaei-Rad, M. A. Ghasemzadeh and S. M. H. Razavian, Sci. Rep., 2021, 11, 18734 CrossRef CAS PubMed.
- G. C. Mohanta, S. K. Pandey, I. K. Maurya, T. S. Sahota, S. K. Mondal and A. Deep, ChemistrySelect, 2019, 4, 12002–12009 CrossRef CAS.
- M. A. Jihad, F. T. M. Noori, M. S. Jabir, S. Albukhaty, F. A. AlMalki and A. A. Alyamani, Molecules, 2021, 26, 3067 CrossRef CAS PubMed.
- H. Nabipour, B. Soltani and N. Ahmadi Nasab, J. Am. Chem. Soc., 2018, 28, 1206–1213 CAS.
- S. William, J. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef.
- S. Aguado, J. Quiros, J. Canivet, D. Farrusseng, K. Boltes and R. Rosal, Chemosphere, 2014, 113, 188–192 CrossRef CAS PubMed.
- Y. Fan, A. C Pauer, A. A. Gonzales and H. Fenniri, Int. J. Hydrogen Energy, 2019, 14, 7281–7289 CAS.
- F. Nuzhath, M. Mohd and A. Imran, Saudi J. Biol. Sci., 2019, 26, 1146–1153 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2022 |