Open Access Article
Open Access ArticleIndividual iron(III) glycerolate: synthesis and characterisation†
Tat'yana G. Khonina *a,
Elena Yu. Nikitinaa,
Alexander Yu. Germov
*a,
Elena Yu. Nikitinaa,
Alexander Yu. Germov b,
Boris Yu. Goloborodskyb,
Konstantin N. Mikhalevb,
Ekaterina A. Bogdanovac,
Denis S. Tishina,
Alexander M. Demin
b,
Boris Yu. Goloborodskyb,
Konstantin N. Mikhalevb,
Ekaterina A. Bogdanovac,
Denis S. Tishina,
Alexander M. Demin a,
Victor P. Krasnov
a,
Victor P. Krasnov a,
Oleg N. Chupakhin
a,
Oleg N. Chupakhin ad and
Valery N. Charushinad
ad and
Valery N. Charushinad
aPostovsky Institute of Organic Synthesis, Russian Academy of Sciences, Ural Branch, Yekaterinburg 620108, Russia. E-mail: khonina@ios.uran.ru
bMiheev Institute of Metal Physics, Russian Academy of Sciences, Ural Branch, Yekaterinburg 620108, Russia
cInstitute of Solid State Chemistry, Russian Academy of Sciences, Ural Branch, Yekaterinburg 620108, Russia
dInstitute of Chemical Technology, Ural Federal University, Yekaterinburg 620002, Russia
First published on 2nd February 2022
Abstract
Iron(II) and iron(III) salts of strong acids form iron glycerolates on heating at 180 °C with glycerol in the presence of an equivalent amount of alkali. Individual iron(III) glycerolate was obtained for the first time. When Fe3O4 magnetic nanoparticles were heated with glycerol, an iron(III) glycerolate shell was formed on their surface.
Currently, glycerolates of various metals (Ti, Co, Fe, Zn, etc.) are used as catalytic systems1,2 or as precursors to obtain nanoparticles, including iron oxide magnetic nanoparticles (MNPs),3 and nanostructure materials for technical and biomedical applications.4–8
Glycerolates of biogenic elements (Si, Zn, B and Ti) are of particular interest because of their biological activity. They are used as biocompatible precursors in the sol–gel synthesis of pharmacologically active hydrogels with reparative, regenerative, antioxidant, immunotropic and antimicrobial effects.8–10 In this regard, glycerolate of the biogenic iron element can be considered as an innovative biocompatible precursor in the sol–gel synthesis of composite bioactive hydrogels possessing a haemostatic effect characteristic of various iron compounds.11
A promising trend in biomedicine is the core–shell modification of Fe3O4 MNPs for MRI diagnostics or magnetic hyperthermia of tumors.12–14 So, the development of an iron glycerolate shell on the surface of Fe3O4 MNPs15 and studying an opportunity of using modified nanoparticles in magnetic hyperthermia is of particular interest. In addition, the antibacterial activity of Fe3O4 MNPs with glycerol adsorbed on the surface was also demonstrated.16,17
In the literature, individual iron(II) and iron(III) glycerolates have not so far been described. At the same time, the synthesis of individual forms is extremely important for biomedical purposes in order to determine bioavailability parameters. The available literature data concern only mixed iron(II,III) glycerolate that is usually formed as a result of the interaction of di- or trivalent iron oxides, hydroxides or salts (mainly oxalates) with glycerol at elevated temperatures (up to 245 °C).15,18–20 It is worth noting that all attempts to synthesize iron glycerolates from chlorides and sulfates of ferrous or ferric irons proved to be unsuccessful.20 At the same time, iron glycerolate was obtained from iron(III) nitrate in boiling glycerol under reflux (280 °C);3 however, contents of iron(III) and iron(II) were not determined in that product.
Regardless of the iron valence state in the starting compound, Fe(II) and Fe(III) are present in the resulting glycerolate in all cases. It should be noted that the possible pathways of the redox process for obtaining mixed iron(II,III) glycerolate are not discussed in the literature. The quantitative Fe(II)/Fe(III) ratio is usually determined by the Mössbauer spectroscopy21 or the colorimetric method.19 The composition of iron(II,III) glycerolate is mainly described by the following formulas: Fe2C6H11O6 (powder diffraction file JCPDSD-ICDD PDF 2, card [23-1731])18 and Fe3+2Fe2+3(C3H5O3)4.19–21 It was not possible to obtain single crystals of iron glycerolate and calculate the unit cell parameters.4,18
We have found that the reactions of iron(II) or iron(III) chlorides and sulfates with glycerol proved to proceed only in the presence of an equivalent amount of alkali to give glycerolates of various chemical compositions. Thus, for the first time, individual iron(III) glycerolate FeC3H5O3 (1) was obtained in 91% yield on heating iron(III) chloride hexahydrate FeCl3·6H2O with sodium hydroxide in an excess of glycerol C3H8O3 at 180 °C for 18 h (Scheme 1) (see ESI†).
The resulting product 1 is a light green powder insoluble in water and organic solvents, thus indicating a probable polymeric structure. It should be noted that the reaction temperature (180 °C) and duration (18 h) appear to be optimal taking into account a high yield of the product and its purity.
Heating iron(II) sulfate heptahydrate FeSO4·7H2O in glycerol in the presence of an equivalent amount of NaOH under the same conditions (180 °C, 18 h) resulted in mixed iron(II,III) glycerolate Fe3+2Fe5+3(C3H5O3)7 (2) in 83% yield (see ESI†). The resulting product is a dark green powder that is poorly soluble in water and organic solvents.
Iron glycerolates 1 and 2 were formed as colored powders; they are storage stable with no change in structure and no noticeable change in color; they do not melt to decomposition temperature. Dilute acids or hot water caused decomposition with the production of glycerol and iron (hydroxy)oxides or salts, as it was noted earlier.18 Plausible pathways for the formation of iron glycerolate 1, as well as iron glycerolate 2 (Scheme 2) and the features of the process are discussed below.
Magnetic materials based on Fe3O4 nanoparticles with a biologically compatible coating are of great interest for biology and medicine.12–14 Previously, we were the first to demonstrate the possibility of forming a shell of iron glycerolate on the surface of Fe3O4 MNPs by a simple and reproducible method, namely, by interacting Fe3O4 MNPs with glycerol at 220 °C for 40 h.15 In this work, we optimized the synthetic procedure and chose the optimum conditions (180 °C, 18 h) (see ESI†). The composition of the resulting shell was found to correspond to iron glycerolate 1.
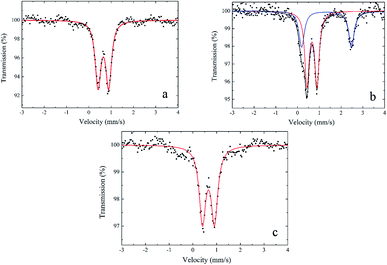
To determine the Fe(II)/Fe(III) ratio in the obtained products, we used the Mössbauer spectroscopy. Fig. 1 shows the Mössbauer spectra of iron glycerolates 1 (a and c) and 2 (b). The samples were prepared by deposition of the powder onto aluminum foil with a diameter of 22 mm (see ESI†).
The Mössbauer spectrum of iron glycerolate 1 (Fig. 1a) contains only one doublet (red line) with quadrupole splitting value of 0.48 mm s−1 (Table 1) which is typical for Fe(III) positions,21 at the same time there are no signals typical for Fe(II). The Mössbauer spectrum of iron glycerolate 2 (Fig. 1b) contains two doublets (red and blue lines) with quadrupole splitting Qs values of 0.46 and 2.29 mm s−1 (Table 1) corresponding to Fe(III) and Fe(II) positions, respectively.21 In this case, the content of Fe(II) was 38%; Fe(III), 62%.
| Sample | Starting material | Spectral lines | Isomer shift, δiso (mm s−1) | QS (mm s−1) | Relative content (%) | Line width (mm s−1) |
|---|---|---|---|---|---|---|
| Iron(III) glycerolate 1 (a) | FeCl3·6H2O | Fe3+ | 0.66 | 0.48 | 100 | 0.31 |
| Iron(III) glycerolate 1 (c) | Fe3O4 MNPs | Fe3+ | 0.66 | 0.51 | 100 | 0.33 |
| Iron(II,III) glycerolate 2 (b) | FeSO4·7H2O | Fe3+ | 0.66 | 0.46 | 62 | 0.24 |
| Fe2+ | 1.33 | 2.29 | 38 | 0.30 |
The Mössbauer spectrum of a sample obtained from Fe3O4 MNPs (Fig. 1c) also contains a doublet (red line) with Qs = 0.51 mm s−1 (Table 1) characteristic of Fe(III). Any signals typical for Fe(II) are absent, which confirms the presence of a shell of iron glycerolate 1. It should be noted that the signals of Fe(II) contained in the core of the Fe3O4 MNPs were not recorded under these conditions of spectrum registration.
The results of the quantitative determination of Fe(II) and Fe(III) by the Mössbauer spectroscopy in the studied products, as well as the data of their elemental analyses (Table 2), allowed us to propose the molecular formulas for iron glycerolates 1 and 2. Elemental compositions of the synthesized samples were determined on a CHN analyzer, the Fe content was calculated from the amount of incombustible residue (Fe2O3). The elemental composition of glycerolate 1 is in good agreement with the molecular formula FeC3H5O3 and practically does not differ from the elemental composition calculated for iron glycerolate Fe2C6H11O6 (card [23-1731]).
| Iron glycerolate | Composition (%) | |||||
|---|---|---|---|---|---|---|
| Experimental | Calculated | |||||
| C | H | Fe | C | H | Fe | |
| a Iron(II,III) glycerolate synthesized from goethite in boiling glycerole.18b Iron(II,III) glycerolate synthesized from goethite, lepidocrocite, hematite at 245 °C.19 | ||||||
| FeC3H5O3 (1) | 24.75 | 3.44 | 38.40 | 24.86 | 3.48 | 38.54 |
| Fe2C6H11O6a | 24.70 | 3.25 | 41.00 | 24.78 | 3.81 | 38.40 |
| Fe3+2Fe2+3(C3H5O3)4b | 22.47 | 3.31 | 43.69 | 22.68 | 3.17 | 43.94 |
| Fe3+2Fe5+3(C3H5O3)7 (2) | 23.12 | 3.20 | 41.89 | 23.66 | 3.48 | 41.22 |
It should be noted that the experimentally determined Fe content (%) in iron(II,III) glycerolate18 proved to differ considerably from the calculated composition for the formula Fe2C6H11O6. At the same time, the experimental results for iron(II,III) glycerolate19 are in good agreement with the formula Fe3+2Fe2+3(C3H5O3)4. The data of elemental analyses for the mixed iron glycerolate 2 obtained in this study correspond to the formula Fe3+2Fe5+3(C3H5O3)7. However, both mixed iron glycerolates differ significantly in their compositions. Thus, the chemical compositions of iron glycerolates differs significantly depending on the nature of the starting compounds and reaction conditions.
Fig. 2 shows the X-ray diffraction (XRD) spectra of the powders of (a) iron glycerolate 1, (b) iron glycerolate 2, and (c) Fe3O4 MNPs with a shell of iron(III) glycerolate 1. All spectra contain peak at 12.7 deg. 2θ (8.1 Å), which is the main diffraction line of iron glycerolate.18
 | ||
| Fig. 2 X-ray diffraction spectra of (a) iron(III) glycerolate 1, (b) iron(II,III) glycerolate 2, (c) Fe3O4 MNPs coated with glycerolate 1. In the insets: photos of the analysed powders. | ||
Fig. 3 shows HRTEM images of (a) starting Fe3O4 MNPs and (b) Fe3O4 MNPs coated with iron glycerolate 1. The modified Fe3O4 MNPs had a core–shell structure with an average magnetite core size of 10 nm coated with an iron glycerolate shell 2–4 nm thick.
 | ||
| Fig. 3 HRTEM images of (a) starting Fe3O4 MNPs Fe3O4 and (b) MNPs coated with iron glycerolate 1; in inset, an electron diffraction pattern. | ||
Fig. 4 shows the IR spectra of (a) iron glycerolate 1, (b) iron glycerolate 2, (c) Fe3O4 MNPs, and (d) MNPS coated with glycerolate 1. The most intensive bands at 2850–2840 cm−1 correspond to the stretching vibration of C–H bonds. The bands in the range 1480–1200 cm−1 are attributed to the deformation vibrations of C–H bonds. The high intensity bands in the ranges 1150–880 and 780–690 cm−1 can be assigned to deformation vibrations of C–O–Fe groups in glycerolate fragments. Band at 537 cm−1 is characteristic of the initial Fe3O4 MNPs. In modified nanoparticles, it is likely to be shifted to 582 cm−1 and superimpose on the bands in the range 600–610 cm−1 corresponding to C–O–Fe vibrations. It should be noted that the IR spectrum of modified Fe3O4 MNPs is similar in position and shape of absorption bands to the spectrum of glycerolate 1. Our measurements are in agreement with the previous IR studies of iron glycerolates.3,15,21
 | ||
| Fig. 4 (a) IR spectra of (a) iron(III) glycerolate 1, (b) iron(II,III) glycerolate 2, (c) Fe3O4 MNPs and (d) MNPs coated with glycerolate 1. | ||
Thus, the IR and XRD spectra of iron glycerolates 1 and 2 turned out to be similar. Therefore, the quantitative determination of Fe(II) and Fe(III) contents in iron glycerolates 1 and 2 proved to be possible only by using the Mössbauer spectroscopy. The results obtained have allowed us to refine the information on the composition of iron glycerolate available in the XRD database [card 23-1731].18
Considering the possible pathways for the formation of iron glycerolates 1 and 2, it can be assumed that the process includes the ion exchange reaction between iron salts and alkali with the formation of amorphous iron(III) and iron(II) hydroxides, respectively. In this case, iron(II) hydroxide is partially oxidized by atmospheric oxygen to iron(III) hydroxide; however, the reduction of iron(III) to iron(II) with glycerol does not occur under the reaction conditions. Then iron(II) and iron(III) hydroxides enter the reversible condensation reaction with glycerol to form iron glycerolates. As noted above, the resulting products are hydrolysed in hot water, which corresponds to a shift in the equilibrium in the condensation reaction towards the starting materials. However, an excess of glycerol and removal of water when the reaction mixture is heated up to 180 °C leads to a shift in the equilibrium towards the reaction products, as evidenced by their high yields.
Thus, iron glycerolate 1 is formed from iron(III) hydroxide when the latter is reacted with glycerole (Scheme 1); iron glycerolate 2 is formed from iron(II) and iron(III) hydroxides (Scheme 2). The graphical formula of iron glycerolate 2 is represented as a conditional notation corresponding to the molecular formula Fe3+2Fe5+3(C3H5O3)7. When m = n = 1, the graphical formula does correspond to the molecular formula Fe3+2Fe2+3(C3H5O3)4 (ref. 21) in iron glycerolate 2, Fe3+2Fe2+3(C3H5O3)4·3FeC3H5O3. It should be noted that all our attempts to synthesize individual iron(II) glycerolate have failed, even in an inert gas atmosphere.
It should be noted that the formation of iron hydroxides and, consequently, iron glycerolates does not occur without alkali, since the hydrolysis of iron salts of strong acids proceeds stepwise and results mainly in the formation of basic salts as the first step of the hydrolysis process. In addition, we cannot exclude that sodium monoglycerolate derived from equilibrium interaction of NaOH with an excess of glycerol is also involved in the formation of iron glycerolates 1 and 2. Sodium monoglycerolate, like NaOH, can enter the ion exchange reaction with iron salts with the formation of glyceroxy iron derivatives.
The proposed chemistry of the process, in our opinion, is characteristic of all iron(II) and iron(III) salts of strong acids.
It is known that iron salts of weak acids, for example, iron(II) oxalate (as dihydrate FeC2O4·2H2O) or iron(III) oxalate (as pentahydrate Fe2(C2O4)3·5H2O) are capable of reacting with glycerol without alkali at 240 °C to form mixed iron(II,III) glycerolate Fe3+2Fe2+3(C3H5O3)4.21 It is interesting to note that the chemical composition of iron(II,III) glycerolate is the same in both cases. It can be assumed that complete hydrolysis of these salts takes place, which is facilitated by an increased temperature, thus resulting in the formation of iron(II) or iron(III) hydroxides and oxalic acid. At the same time, in cases of FeC2O4 (as well as FeSO4), the formed iron(II) hydroxide is partially oxidized by atmospheric oxygen to give iron(III) hydroxide. Iron(II) and iron(III) hydroxides are supposed to react further with glycerol to form a mixed iron(II,III) glycerolate. We believe that in the case of Fe2(C2O4)3, the formed iron(III) hydroxide is reduced by oxalic acid, which is also the product of hydrolysis, to iron(II) hydroxide (Scheme 3, reaction 1). Further, when reacting with glycerol, a mixture of iron(II) and iron(III) hydroxides is expected to form a mixed iron(II,III) glycerolate. In our opinion, the reduction with glycerol (Scheme 3, reaction 2), according to the work,19 cannot be considered as the determining process.
 | ||
| Scheme 3 Reduction of iron(III) hydroxide with oxalic acid (1) or glycerol (2).19 | ||
At the same time, to the best of our knowledge there are scarcely available data on oxidation of glycerol on reacting with iron(III) salts. It has been established,19 that a carbonyl compound is present in the reaction mixture, as indicated by a low intensity CO stretching vibrational band. However, qualitative tests carried out to determine the character of the CO function proved to be negative for aldehydes and ketones.
Thus, the key difference in the pathways for obtaining iron glycerolates from iron(II) or (III) salts of strong or weak acids is associated with the step of the formation of iron hydroxides: in case of iron salts of strong acids, iron hydroxides are formed due to the ion exchange reaction of iron salts with alkali, while iron salts of weak acids undergo their complete hydrolysis.
In summary, it has first been shown that iron(II) and iron(III) salts of strong acids (FeCl3, FeSO4) are able to form iron glycerolates. The previously undescribed individual iron(III) glycerolate FeC3H5O3 was obtained from FeCl3 as an example, glycerol and NaOH at 180 °C in one-pot synthesis. It has been found that due to the direct interaction of Fe3O4 MNPs with glycerol at 180 °C, an iron(III) glycerolate shell is formed on the nanoparticle surface. The obtained iron glycerolates were characterized by Mössbauer and IR spectroscopy, XRD and elemental analysis.
Individual iron(III) glycerolate can be considered as a novel biocompatible precursor in the sol–gel synthesis of pharmacologically active nanocomposite materials and for further preparation of advanced composite magnetic nanomaterials with glycerolate shell to be used in magnetic hyperthermia of tumors. In addition, it can be used as a precursor for in the preparation of MNPs and as a catalyst for various chemical processes.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was carried out with financial support from the Ministry of Science and Higher Education of the Russian Federation (grant 075-15-2020-777) using the equipment of the Centre for Joint Use “Spectroscopy and Analysis of Organic Compounds” at the Postovsky Institute of Organic Synthesis of UB RAS. Mössbauer spectroscopy, XRD, and HRTEM studies were carried out within the state programs of Miheev Institute of Metal Physics of UB RAS («Function»), Institute of Solid State Chemistry of UB RAS, and Ural Federal University, correspondingly. Authors are grateful to Dr Maksim S. Karabanov (Ural Center for Shared Use “Modern Nanotechnology” at the Ural Federal University) for HRTEM study.References
- M. Wang, J. Jiang and L. Ai, ACS Sustainable Chem. Eng., 2018, 6, 6117–6125 CrossRef CAS.
- P. C. Lau, T. L. Kwong and K. F. Yung, Sci. Rep., 2016, 6, 23822 CrossRef CAS PubMed.
- V. Bartůněk, D. Průcha, M. Švecová, P. Ulbrich, S. Huber, D. Sedmidubský and O. Jankovský, Mater. Chem. Phys., 2016, 180, 272–278 CrossRef.
- J. Zhao, Y. Liu, M. Fan, L. Yuan and X. Zou, Inorg. Chem. Front., 2015, 2, 198–212 RSC.
- H. Dong and C. Feldmann, J. Alloys Compd., 2012, 513, 125–129 CrossRef CAS.
- O. Jankovský, V. Rach, D. Sedmidubský, S. Huber, P. Ulbrich, M. Svecova and V. Bartunek, J. Alloys Compd., 2017, 723, 58–63 CrossRef.
- M. Y. Cheong, A. H. Hazimah, A. A. H. Zafarizal and I. Rosnah, J. Oil Palm Res., 2012, 24, 1287–1295 Search PubMed.
- T. G. Khonina, A. P. Safronov, E. V. Shadrina, M. V. Ivanenko, A. I. Suvorova and O. N. Chupakhin, J. Colloid Interface Sci., 2012, 365, 81–89 CrossRef CAS PubMed.
- T. G. Khonina, M. V. Ivanenko, O. N. Chupakhin, A. P. Safronov, E. A. Bogdanova, M. S. Karabanalov, V. V. Permikin, L. P. Larionov and L. I. Drozdova, Eur. J. Pharm. Sci., 2017, 107, 197–202 CrossRef CAS PubMed.
- T. G. Khonina, N. V. Kungurov, N. V. Zilberberg, N. P. Evstigneeva, M. M. Kokhan, A. I. Polishchuk, E. V. Shadrina, E. Yu. Nikitina, V. V. Permikin and O. N. Chupakhin, J. Sol–Gel Sci. Technol., 2020, 95, 682–692 CrossRef CAS.
- M. T. Matter, F. Starsich, M. Galli, M. Hilber, A. A. Schlegel, S. Bertazzo, S. E. Pratsinis and I. K. Herrmann, Nanoscale, 2017, 9, 8418–8426 RSC.
- N. D. Thorat, H. E. Townley, R. M. Patil, S. A. M. Tofail and J. Bauer, Drug Discovery Today, 2020, 25, 1245–1252 CrossRef CAS PubMed.
- A. M. Demin, A. G. Pershina, A. S. Minin, O. Y. Brikunova, A. M. Murzakaev, N. A. Perekucha, A. V. Romashchenko, O. B. Shevelev, M. A. Uimin, I. V. Byzov, D. Malkeyeva, E. Kiseleva, L. V. Efimova, S. V. Vtorushin, L. M. Ogorodova and V. P. Krasnov, ACS Appl. Mater. Interfaces, 2021, 13, 36800–36815 CrossRef CAS PubMed.
- A. M. Demin, A. V. Maksimovskikh, A. V. Mekhaev, D. K. Kuznetsov, A. S. Minin, A. G. Pershina, M. A. Uimin, V. Y. Shur and V. P. Krasnov, Ceram. Int., 2021, 47, 23078–23087 CrossRef CAS.
- A. M. Demin, T. G. Khonina, E. V. Shadrina, E. A. Bogdanova, D. K. Kuznetsov, A. V. Mekhaev, V. Ya Shur and V. P. Krasnov, Russ. Chem. Bull., 2019, 68, 1178–1182 CrossRef.
- C. L. Popa, A. M. Prodan, P. Chapon, C. Turculet and D. Predoi, J. Nanomater., 2015, 465034 Search PubMed.
- A. M. Prodan, M. Beuran, C. S. Turculet, M. Popa, E. Andronescu, C. Bleotu, S. M. Raita, M. Soare and O. Lupescu, Rom. Biotechnol. Lett., 2018, 23, 13901–13908 CAS.
- E. W. Radoslovich, M. Raupach, P. G. Slade and R. M. Taylor, Aust. J. Chem., 1970, 23, 1963–1970 CrossRef CAS.
- P. F. Fuls, L. Rodrique and J. J. Fripiat, Clays Clay Miner., 1970, 18, 53–62 CrossRef CAS.
- L. Rodrique, G. Delvaux and A. Dardenne, Powder Technol., 1978, 19, 93–101 CrossRef CAS.
- P. Bruylants, A. Munaut, G. Poncelet, J. Ladriere, J. Meyers and J. Fripiat, J. Inorg. Nucl. Chem., 1980, 42, 1603–1611 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra08485b |
| This journal is © The Royal Society of Chemistry 2022 |