Open Access Article
Open Access ArticleExtraction optimization and quality evaluation of humic acids from lignite using the cell-free filtrate of Penicillium ortum MJ51†
Shiying Li‡
ab,
Jinfang Tan‡c,
Yi Wangb,
Peipei Lib,
Desheng Hub,
Qiuzhe Shib,
Yanjun Yued,
Fang Li*b and
Yanlai Han *b
*b
aCollaborative Center Innovation of Henan Food Crops, Henan Agricultural University, Zhengzhou 450002, China
bCollege of Resources and Environmental Science, Henan Agricultural University, Zhengzhou 450002, China. E-mail: fangli0901@henau.edu.cn; hyanlai@henau.edu.cn
cSchool of Agriculture, Sun Yat-sen University, Guangzhou 510000, China
dHenan Xinlianxin Chemicals Group Co., Ltd, Xinxiang 453000, China
First published on 23rd December 2021
Abstract
Bio-solubilization of lignite is a promising technology to transform coal into humic acids (HAs) which are broadly used in agriculture. In this work, HAs were extracted from lignite using the cell-free filtrate (CFF) of Penicillium ortum MJ51. The extraction method was optimized using response surface methodology (RSM) based on the interactive effects of nitric acid concentrations, coal loading ratio, extraction temperature and time as input factors, and the absorbance of HAs at 450 nm wavelength as the output response. Under optimized conditions (lignite pretreated with 4.7 N HNO3, coal loading ratio of 4.9%, temperature of 77.3 °C and time of 8.6 hours), the absorbance at 450 nm peaked at 70.28, and the concentration and extraction yield of HAs were 31.3 g L−1 and 63.9%, respectively, which were dramatically higher than those observed for traditional biological methods (0.7 g L−1 and 14.1%, respectively). The qualities of HAs produced under optimized conditions were evaluated and compared with those extracted by the conventional chemical method. The optimized process resulted in better HA quality indices, including lower molecular mass; higher nitrogen; less aromatic carbon; more aliphatic and carboxylic carbon; and higher bioactivity for promoting plant growth. Moreover, the anti-flocculation ability was improved, thereby supporting its applicability in agriculture. Extraction of HAs from lignite using the CFF of P. ortum MJ51 provides a novel technological approach for the efficient conversion of lignite to bio-active HAs.
1. Introduction
Humic acids (HAs) are a class of natural polyelectrolyte complexes with a large number of active groups, including carboxyl groups, phenolic hydroxyl groups, carbonyl groups and methoxy groups, the pressence of these groups endows HAs with many capabilities such as complexation, ion exchange and redox properties, and makes HAs widely used in various fields, especially agriculture.1 As a fertilizer additive and soil remediation agent, HAs play an important role in stimulating plant growth, enhancing soil fertility and decreasing the toxicity of heavy metals.2–4 Accordingly, the potential biochemical activity and preparation method of HAs have aroused extensive attention.Lignite, a kind of low rank coal is an energy source as well as a HAs resource, accounts for 45% of the global low-rank coal reserves.5 Efforts had been made to extract HAs from lignite via chemical and biological methods.6–8 The amount of HAs extracted chemically depends on the extraction agent (such as NaOH or KOH), temperature, time and coal particle size.42 Although chemical methods are efficient, HAs prepared via biological methods are more environmentally safe and have better bioactivity.9 Esterase-degraded lignite HAs have a higher percentage of aliphatic carbon, but a lower percentage of aromatic carbon and ester groups than raw lignite HAs, and have been shown to promote the growth of asparagus lettuce.10 Oxidation pretreatment of lignite can also improve HA bioactivity by reducing their molecular weight and increasing their content of active functional groups.11 Among a variety of oxidants (such as hydrogen peroxide, nitric acid and sodium hydroxide), nitric acid (HNO3) was the most effective pretreatment to improve the oxidation degree of coal.12 Oxidizing pretreatment of coal with HNO3 also promotes microbial solubilization of coal, enhances the yield of HAs and the number of oxygen and nitrogen functional groups.7,13 Combining an oxidation pretreatment of coal with biological methods to obtain HAs of high quantity and quality has become a promising research area.
In the 1980s, white-rot fungi were reported to dissolve coal.14 Since then, many microorganisms, including Bacillus sp. Y7,15 Penicillium sp. P6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 and Streptomyces fulvissimus K59,18 have been reported to convert low-rank coal into HAs. Generally, microorganisms degrade coal by releasing extracellular metabolites, such as surfactants, alkaline substances and extracellular enzymes.19 Due to the diversity of microorganisms that produce various extracellular metabolites, the mechanism and efficiency of coal bio-solubilization vary. A recent study revealed that 35% of H2O2-pretreated lignite was solubilized by Trichoderma citrinoviride, which can secret oxidase, lignin peroxidase and laccase.20 Another study obtained a higher bio-solubilization rate (36.77%) using the thermostable, alkaline extracellular materials produced by Bacillus sp. Y7.15 Furthermore, it has been shown that lignite components can be solubilized using biosurfactant-containing cell-free filtrate (CFF) extracted from Bacillus licheniformis.21 Most coal-solubilizing bacteria and actinomycetes primarily rely on alkaline action and chelation, while fungi mostly rely on enzymes, but also utilize the above substances to a lesser degree.22,23 Although extracellular metabolites are widely known for their role in the lignite solubilization process, research on process optimization of HA extraction from lignite using extracellular CFF is still scarce.
16 and Streptomyces fulvissimus K59,18 have been reported to convert low-rank coal into HAs. Generally, microorganisms degrade coal by releasing extracellular metabolites, such as surfactants, alkaline substances and extracellular enzymes.19 Due to the diversity of microorganisms that produce various extracellular metabolites, the mechanism and efficiency of coal bio-solubilization vary. A recent study revealed that 35% of H2O2-pretreated lignite was solubilized by Trichoderma citrinoviride, which can secret oxidase, lignin peroxidase and laccase.20 Another study obtained a higher bio-solubilization rate (36.77%) using the thermostable, alkaline extracellular materials produced by Bacillus sp. Y7.15 Furthermore, it has been shown that lignite components can be solubilized using biosurfactant-containing cell-free filtrate (CFF) extracted from Bacillus licheniformis.21 Most coal-solubilizing bacteria and actinomycetes primarily rely on alkaline action and chelation, while fungi mostly rely on enzymes, but also utilize the above substances to a lesser degree.22,23 Although extracellular metabolites are widely known for their role in the lignite solubilization process, research on process optimization of HA extraction from lignite using extracellular CFF is still scarce.
Nowadays, efforts had been made to improve the yield and bioactivity of HAs. The amount of HAs produced via biological methods is influenced by the culture conditions, temperature, time, coal particle size, coal loading ratio and oxidation content of coal.17,24 Accordingly, an appropriate experimental design is necessary to optimize the extraction conditions. Response surface methodology (RSM) has been widely used to explore the optimal conditions related to multiple factors.25,26 RSM modeling describes the relationship between input and response variables with advantage of minimizing the number of experimental runs.27 Hence, RSM could be adopted to optimize the conditions for HA production. The biochemical activity of HAs is determined by their chemical composition, structure, molecular weight and other properties.28,29 To investigate the chemical and structural properties of HAs, numerous analytical methods have been widely used, including elemental analysis, titration analysis of acidic functional groups, ultraviolet-visible (UV-Vis) spectroscopy, fluorescence spectra, Fourier transform infrared (FTIR) spectroscopy, cross polarization magic angle spinning 13C NMR (CP/MAS 13C NMR) spectrometry, X-ray diffraction (XRD), gas chromatography-mass spectrometry (GC-MS), high-performance size-exclusion chromatography (HPSEC) and scanning electron microscopy (SEM).30–33
Here, Penicillium ortum MJ51 was isolated from lignite, which has strong ability to solubilize lignite. Some strains of Penicillium spp., such as P. simplicissimum, P. citrinum and P. decumbens P6 are reportedly able to solubilize and degrade lignite.22,34,35 However, the extraction of HAs from lignite using the CFF of the P. ortum strain has not been conducted yet. In view of the important role of extracellular metabolites secreted by microorganisms in lignite solubilization, it can be hypothesized that extracellular CFF of P. ortum MJ51 could be used to extract HAs from lignite and that HAs have better bioactivity than that extracted by traditional chemical methods. To test this hypothesis, HAs were extracted from lignite using the CFF of P. ortum MJ51, the extraction conditions were optimized through RSM based on the interactive effects of the input factors (specifically, HNO3 concentration, coal loading ratio, extraction temperature and time) and the output response (specifically, the absorbance of HAs at 450 nm wavelength). Meanwhile, the quality of HAs extracted under optimized conditions was evaluated and compared with that extracted by conventional chemical methods via elemental analysis, flocculation, HPSEC, FTIR spectroscopy and CP/MAS 13C NMR spectroscopy.
2. Materials and methods
2.1. Coal samples
The coal samples used in this experiment were collected from the Mile Coal Mine in Yunnan Province, China, and then transported to the laboratory and stored at 4 °C. The coal samples were ground to a diameter of 0.18–0.25 mm and dried to a constant weight in an oven at 80 °C. Chemical characterization of the coal samples, which included proximate analysis, calorific value and vitrinite reflectance, was performed according to Sabar et al. (2019).17 The gross calorific value and vitrinite reflectance of the coal were measured using a 5E-C5508 Kaiyuan Calorimeter (Changsha, China) and a Leitz Orthoplan microscope (Solms, Germany), respectively. The ash and volatile contents of the coal samples were 29.5% and 44.04%, respectively, and the gross calorific value and vitrinite reflectance index were 18.63 MJ kg−1 and 0.39%, respectively. These results indicate that the coal was meta-lignite.2.2. Fungus and cell-free filtrate (CFF)
Penicillium ortum MJ51, which was isolated from fresh lignite samples, has 99% sequence homology of the internal transcribed spacer (ITS) region with the P. ortum strain MK450708.1. This strain has been demonstrated to effectively degrade coal (unpublished data).The fungal strain MJ51 was inoculated on potato dextrose agar (PDA; 200 g potato, 20 g glucose, 20 g agar and 1000 mL distilled water) and cultivated for 6 d at 28 °C. After cultivation, 0.1% sterilized NaCl was added to form a spore suspension. The number of spores was observed under a microscope using a hemocytometer. Next, 2.5 mL of spore suspension (1 × 108 spores per mL) was inoculated into a 250 mL flask containing 50 mL of culture fluid (20.0 g L−1 sucrose, 3 g L−1 KNO3, 1 g L−1 KH2PO4, 0.5 g L−1 Na2HPO4, 0.5 g L−1 MgSO4·7H2O; pH 6.0). The inoculated medium was cultured at 160 rpm and 30 °C. The CFF obtained from the different fermentation times (12 hours intervals) was prepared by centrifuging 50 mL of the culture at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min. The pH of the CFF was measured and the mycelium was dried to a constant weight at 75 °C.
000g for 10 min. The pH of the CFF was measured and the mycelium was dried to a constant weight at 75 °C.
2.3. Optimization of the HA production process
The CFF (20 mL) was incubated with lignite in a 50 mL flask in a SHA-CA reciprocating water bath oscillator (Changzhou, China). Different parametric effects for HA production were studied, including the bio-solution type, fungal growth phase, HNO3 concentration for coal pretreatment, coal loading ratio, temperature and time; see Table 1 for the specific conditions. Pretreated lignite samples were prepared by placing raw lignite into HNO3 at a ratio of 1 g coal per 25 mL solution. Pretreatments were performed using 0, 0.5, 1, 2, 4, 6 N HNO3. Chemical pretreatment was conducted in 50 mL glass vials at 30 °C for 48 hours. After pretreatment, the samples were centrifuged at 7000g for 10 min and then washed with deionized water until the filtrate was colorless and the pH was greater than 5. Finally, the treated coal was dried in an oven at 80 °C.| Group | Variables | Ranges | Conditions |
|---|---|---|---|
| 1 | Bio-solution type | A: culture solution inoculation with P. ortum MJ51 for 0 hour | 0.5% raw lignite, 30 °C, 160 rpm |
| B: CFF of P. ortum MJ51 cultured to the 60th hour | |||
| 2 | Incubation time | 0 h, 12 h, 24 h, 36 h, 48 h, 60 h, 72 h, 84 h, 96 h, 108 h, 120 h, 132 h, 144 h, 156 h, 168 h | 0.5% raw lignite, 30 °C, 160 rpm |
| 3 | Temperature | 30 °C, 45 °C, 60 °C, 75 °C, 90 °C | 0.5% raw lignite, 160 rpm |
| 4 | HNO3 concentration | 0 N, 0.5 N, 1 N, 2 N, 4 N, 6 N | 0.5% raw lignite, 75 °C, 160 rpm |
| 5 | Coal loading ratio | 0.5%, 1%, 2%, 3%, 4%, 5%, 6% | 4 N HNO3, 75 °C, 160 rpm |
For the solubilization study, three groups of parallel experiments were performed. After solubilization, the samples were centrifuged at 8000g for 12 min, and the content of coal solubilization products in the supernatant was determined using a Thermo Scientific UV1510 Spectrophotometer (Waltham, USA) at a wavelength of 450 nm, which is related to the HA content.18
The optimal condition for HA extraction from lignite was determined by the Box–Behnken design of RSM. Based on the univariate analysis, ranges for variable value design of the RSM were as follows: HNO3 concentration: 2, 4, 6 N; coal loading ratio: 4, 5, 6%; time: 6, 8, 10 h; and temperature: 60, 75, 90 °C. Variables and experimental design of the RSM are shown in Tables 2 and 3. The RSM design was analyzed by Design-Expert 8.0 software.
| Factor | Name | Units | Minimum | Maximum | Mean |
|---|---|---|---|---|---|
| A | HNO3 concentration | N | 2 | 6 | 4 |
| B | Temperature | oC | 60 | 90 | 75 |
| C | Coal loading ratio | % | 4 | 6 | 5 |
| D | Time | hours | 6 | 10 | 8 |
| Run | Factor 1 A: HNO3 concentration (N) | Factor 2 B: temperature (oC) | Factor 3 C: coal loading ratio (%) | Factor 4 D: time (h) | A450 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Coded | Actual | Coded | Actual | Coded | Actual | Coded | Actual | ||
| a Note: results are presented as the mean ± standard deviation. | |||||||||
| 1 | 0 | 4 | −1 | 60 | 0 | 5 | −1 | 6 | 50.36 ± 0.56 |
| 2 | −1 | 2 | 0 | 75 | 1 | 6 | 0 | 8 | 26.34 ± 0.39 |
| 3 | −1 | 2 | −1 | 60 | 0 | 5 | 0 | 8 | 29.79 ± 0.40 |
| 4 | 0 | 4 | 0 | 75 | 0 | 5 | 0 | 8 | 68.61 ± 0.63 |
| 5 | 0 | 4 | 1 | 90 | −1 | 4 | 0 | 8 | 59.87 ± 1.31 |
| 6 | 0 | 4 | 0 | 75 | −1 | 4 | −1 | 6 | 52.16 ± 0.84 |
| 7 | 0 | 4 | −1 | 60 | 0 | 5 | 1 | 10 | 55.65 ± 1.09 |
| 8 | −1 | 2 | 1 | 90 | 0 | 5 | 0 | 8 | 31.71 ± 0.32 |
| 9 | 1 | 6 | 0 | 75 | −1 | 4 | 0 | 8 | 54.90 ± 1.51 |
| 10 | −1 | 2 | 0 | 75 | 0 | 5 | 1 | 10 | 29.96 ± 0.72 |
| 11 | 0 | 4 | 0 | 75 | 1 | 6 | −1 | 6 | 49.77 ± 0.34 |
| 12 | 0 | 4 | 0 | 75 | −1 | 4 | 1 | 10 | 61.73 ± 1.18 |
| 13 | 0 | 4 | 0 | 75 | 0 | 5 | 0 | 8 | 68.02 ± 1.31 |
| 14 | 0 | 4 | 0 | 75 | 0 | 5 | 0 | 8 | 67.49 ± 1.24 |
| 15 | 0 | 4 | 0 | 75 | 0 | 5 | 0 | 8 | 65.45 ± 1.67 |
| 16 | 1 | 6 | 0 | 75 | 0 | 5 | 1 | 10 | 57.11 ± 0.74 |
| 17 | 0 | 4 | 1 | 90 | 1 | 6 | 0 | 8 | 51.95 ± 0.70 |
| 18 | 1 | 6 | 0 | 75 | 1 | 6 | 0 | 8 | 50.92 ± 1.49 |
| 19 | −1 | 2 | 0 | 75 | −1 | 4 | 0 | 8 | 26.89 ± 0.68 |
| 20 | 0 | 4 | −1 | 60 | 1 | 6 | 0 | 8 | 45.96 ± 1.20 |
| 21 | 0 | 4 | 1 | 90 | 0 | 5 | −1 | 6 | 56.40 ± 1.38 |
| 22 | 0 | 4 | 0 | 75 | 1 | 6 | 1 | 10 | 54.94 ± 0.94 |
| 23 | 1 | 6 | −1 | 60 | 0 | 5 | 0 | 8 | 45.02 ± 0.71 |
| 24 | 0 | 4 | −1 | 60 | −1 | 4 | 0 | 8 | 51.26 ± 1.20 |
| 25 | 1 | 6 | 0 | 75 | 0 | 5 | −1 | 6 | 52.20 ± 1.08 |
| 26 | −1 | 2 | 0 | 75 | 0 | 5 | −1 | 6 | 28.60 ± 0.48 |
| 27 | 1 | 6 | 1 | 90 | 0 | 5 | 0 | 8 | 55.92 ± 1.25 |
| 28 | 0 | 4 | 0 | 75 | 0 | 5 | 0 | 8 | 66.49 ± 1.49 |
| 29 | 0 | 4 | 1 | 90 | 0 | 5 | 1 | 10 | 64.68 ± 1.31 |
2.4. Concentration and extraction yield of HAs
HAs were extracted from raw lignite, HNO3-pretreated lignite with 60 h CFF (HA-CFF), and 0.1 M NaOH solution as the control (HA-control). The volume of extractant was 20 mL. The method for HA extraction was modified from Sabar et al. (2020).9 The specific HNO3 concentration used for pretreating lignite, coal loading ratio, extraction time and temperature referred to the optimum conditions of RSM. The HA concentration and yield were determined gravimetrically as per eqn (1) and (2), respectively.
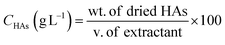 | (1) |
 | (2) |
2.5. Quality evaluation of HAs
| Flocculation limit (mmol L−1) = M·V × 1000/5 = 200MV | (3) |
3. Results and discussion
3.1. Optimization of HA extraction conditions
To enhance the release of water-soluble HAs from coal, crucial parameters that could potentially affect the concentration of dissolved coal products via coal solubilization were studied, including the fungal incubation time, HNO3 concentration for coal pretreatment, coal loading ratio, reaction temperature and time.17,18 The HA content was evaluated by measuring the absorbance value of bio-solubilized products at a wavelength of 450 nm (A450), which is the characteristic UV/Vis absorbance wavelength of HAs.Coal solubilization depends on the concentration of extracellular secondary metabolites of fungi, which are associated with the fungal growth period.38 Thus, we studied the growth curve of P. ortum MJ51 and further explored the relationship between the pH of the CFF and the A450 value of the bio-solubilized products. The growth of P. ortum MJ51 changed over time and was divided into four stages: the lag phase (0–12 hours), exponential phase (12–60 hours), stationary phase (60–96 hours) and death phase (96–168 hours) (Fig. 1a). As fermentation proceeded, the pH of the CFF increased from 6.05 to 8.17 during the exponential phase, then further increased slowly during the stationary and death phases (Fig. 1c). The trend in A450 was similar to that of the CFF pH, which indicates that the alkaline substances secreted by P. ortum MJ51 promote the release of HAs in coal. This result was supported by regression analysis (Fig. 1d), which showed a significant positive correlation between pH and A450 (R2 = 0.9098, p < 0.001). Our results were consistent with those of previous studies.15,39,40 Some microorganisms have been shown to secrete alkaline materials that increased the medium pH to more than 8 after 6 days;15,22,41 however, the pH of the CFF from P. ortum MJ51 surpassed 8.0 after only 60 hours. This indicates that P. ortum MJ51 is more efficient than the previously used microorganisms for increasing the pH of the CFF, which is helpful for shortening the coal solubilization process.
 | ||
| Fig. 2 Effect of different factors on coal solubilization. (a) Temperature, (b) HNO3 concentration, and (c) coal loading ratio. Note: error bars indicate standard deviation. | ||
Generally, alkaline substances, surfactants and extracellular enzymes in CFF participate during lignite solubilization. Previous research demonstrated that the degradation capacity was dramatically decreased when the CFF of P. decumbens P6 was heated.22 In this work, the CFF still had strong activity when heated to 90 °C, indicating that the solubilization mechanism of P. ortum MJ51 is different from that of P. decumbens P6, and that the main active substances of the CFFs were resistant to high temperature. Due to the fact that few enzymes can resist such high temperatures and gradually increase in pH of CFF, we speculated that the main CFF substances contributing to coal solubilization were alkaline substances or surfactants; similar results have been found in the CFF from bacteria.15,43 Currently, the reported bacterial metabolites with coal-dissolving function include glycerophosphocholine, proveratrol A, proveratrol B and surfactin, etc.44 To evaluate the composition of alkaline substances in the CFF of this study, whole genome-sequencing of P. ortum MJ51 was conducted. A large quantity of genes involved in aromatic compound degradation and alkaloid (isoquinoline, tropane, piperidine and pyridine alkaloid, pyridine and indole diterpene alkaloid etc.) biosynthesis were annotated (Fig. S1†). Living cells of P. ortum MJ51 depolymerize coal molecules by expressing genes involved in aromatic substance degradation, while CFF degrades coal mainly through alkaloids. Therefore, the coal solubilization mechanism by the CFF from P. ortum MJ51 was clarified.
The same trend has been found during the coal dissolution process by living organisms. Under such conditions, previous studies have shown that the best coal loading ratio is 1%, which greatly limits the acquisition of fermentation products with a high HA concentration.38,46 The reason for this is that the presence of too much pulverized coal will cause cell damage and inhibit the activity of cells and enzymes related to coal degradation.17 Conversely, CFF has a stronger tolerance to coal than living cells during the coal degradation process; thus, CFF can withstand greater coal addition, which provides greater potential for an improved HA concentration in fermentation products.
3.2. HA concentration optimization by RSM
In the present study, optimization of process parameters was carried out via Box–Behnken design to maximize the HA concentration. The following regression equations for coded values (eqn (4)) and actual experimental values (eqn (5)) were set up by collecting the data from actual experimental conditions (Table 3) according to the Box–Behnken model, which expresses the relationship between the dependent variable Y (the A450 value) and the independent variables A (HNO3 concentration), B (temperature), C (coal loading ratio) and D (time).| Y = 67.21 + 11.90A + 3.54B − 2.24C + 2.88D + 2.24AB − 0.86AC + 0.89AD − 0.66BC + 0.75BD + 1.10CD − 20.11A2 − 6.46B2 − 7.94C2 − 4.58D2 | (4) |
| Y = −490.66 + 40.92A + 4.26B + 86.55C + 19.76D + 0.07AB − 0.43AC + 0.22AD − 0.04BC + 0.02BD − 0.55CD − 5.03A2 − 0.03B2 − 7.94C2 − 1.15D2 | (5) |
Analysis of variance (ANOVA) has been suggested as a way to examine the significance and accuracy of the quadratic model by RSM. The ANOVA results for the current optimization case are presented in Table 4. The F-value of the model was 83.35 and the p-value was less than 0.0001, which indicates that the regression model was extremely significant since the term is considered significant when the “P-value” is less than 0.05.27 Furthermore, A, B, C, D, AB, A2, B2, C2 and D2 were also seen as significant model terms. The value of the “lack of fit” denotes the fitting degree of the current model and actual results.26 In this study, the lack of fit value was 0.1332, which was larger than 0.05, so the current model can be considered to have a good confidence level. The coefficient of determination (R2) of a good statistical model should be greater than 0.75, and explains a best fitting degree of regression equation to data.27 In this case, the R2 value of 0.9881 indicates that the model showed a good fit in the test range (Table 4). In addition, the normal probability and residual plots for the A450 value (Fig. 3a) further analyzed the adequacy of the fitted model. Meanwhile, predicted and measured values were virtually identical (Fig. 3b), which shows that the experiment design was appropriate.
| Source | Sum of squares | df | Mean square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 4809.38 | 14 | 343.53 | 83.35 | <0.0001 |
| A – HNO3 concentration | 1698.84 | 1 | 1698.84 | 412.20 | <0.0001 |
| B – temperature | 150.45 | 1 | 150.45 | 36.50 | <0.0001 |
| C – coal loading ratio | 60.44 | 1 | 60.44 | 14.66 | 0.0018 |
| D – time | 99.65 | 1 | 99.65 | 24.18 | 0.0002 |
| AB | 20.16 | 1 | 20.16 | 4.89 | 0.0441 |
| AC | 2.94 | 1 | 2.94 | 0.71 | 0.4124 |
| AD | 3.15 | 1 | 3.15 | 0.76 | 0.3967 |
| BC | 1.72 | 1 | 1.72 | 0.42 | 0.5292 |
| BD | 2.24 | 1 | 2.24 | 0.54 | 0.4736 |
| CD | 4.84 | 1 | 4.84 | 1.17 | 0.2968 |
| A2 | 2622.28 | 1 | 2622.28 | 636.26 | <0.0001 |
| B2 | 270.29 | 1 | 270.29 | 65.58 | <0.0001 |
| C2 | 408.95 | 1 | 408.95 | 99.23 | <0.0001 |
| D2 | 136.15 | 1 | 136.15 | 33.03 | <0.0001 |
| Residual | 57.70 | 14 | 4.12 | ||
| Lack of fit | 51.39 | 10 | 5.14 | 3.26 | 0.1332 |
| Pure error | 6.31 | 4 | 1.58 | ||
| Cor total | 4867.08 | 28 | |||
| Coefficient of determination (R2) = 0.9881 | |||||
 | ||
| Fig. 3 Diagnostics of response surface quadratic model. (a) Normal plot of residuals, and (b) actual and predicted plot. | ||
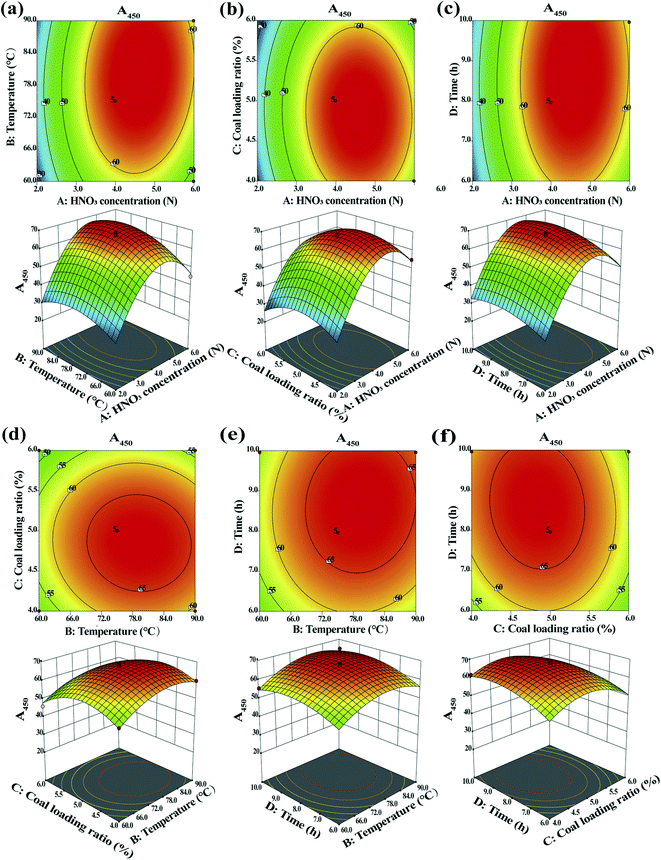
Fig. 4 shows the contour plot and 3-D surface for the HA concentration (quantified with the A450 value) as a function of the HNO3 concentration, temperature, coal loading ratio and time, as imitated by eqn (5). Color changes from blue to red indicate increasing HA concentrations. The shape of the contour plot can determine the intensity of the interaction effects, with an ellipse indicating significant interaction effects and a circle indicating the opposite.47 With the increasing HNO3 concentration and temperature, the A450 value continuously increased until peaking, and then decreased (Fig. 4a). The maximum A450 value was calculated at an HNO3 concentration of 4.7 N and temperature of 77.3 °C. Moreover, a strong interaction was found between the HNO3 concentration and temperature, as the two-dimensional contour plot was elliptical with a large eccentricity (Fig. 4a). This observation was also validated via ANOVA (p-value of 0.0441 for AB) (Table 4). The A450 value followed a parabolic shape with an increase in the HNO3 concentration and coal loading ratio (Fig. 4b). The maximum A450 value was calculated at an HNO3 concentration of 4.7 N and a coal loading ratio of 4.9%. However, no significant interaction between the HNO3 concentration and coal loading ratio was observed. This result was further confirmed via ANOVA (p-value of 0.4124 for AC) (Table 4). The contour plots and response surface plots for the effects of time and HNO3 concentration, coal loading ratio and temperature, time and temperature, and time and coal loading ratio on the HA concentration (Fig. 4c–f, respectively) shared the same trend as that in Fig. 4b.
Through RSM, the optimum response value (A450 = 70.23) was achieved at an HNO3 concentration of 4.7 N, coal loading ratio of 4.9%, time of 8.6 hours and temperature of 77.3 °C. In order to compare this with the predicted value, the experiment was repeated under the determined optimum conditions. A very similar A450 value of 70.28 was observed, thereby demonstrating that these optimization conditions had good repeatability.
3.3. Concentration and extraction yield of HAs
After optimization of HA extraction conditions, the concentration of HAs increased considerably from 0.71 g L−1 to 31.3 g L−1, and the HA extraction yield increased from 14.1% to 63.9% (Table 5). Under the same extraction conditions, the yield of HAs extracted from raw lignite and HNO3-pretreated lignite with NaOH solution was 58.7% and 66.4%, respectively. In our study, the extraction yield of HAs from lignite by the newly optimized method was comparable to that by the chemical method. While in previous study the yield of HAs extracted by the biological method (36.4%) from HNO3-treated coal was lower than that by the chemical method (54.2%).9 This indicates that the CFFs of P. ortum MJ51 could directly replace sodium hydroxide to extract HAs.| Extraction method | HNO3 concentration (N) | Temperature (°C) | Coal loading ratio (%) | Time (h) | CHAs (g L−1) | HA yield (%) |
|---|---|---|---|---|---|---|
| a Note: results are presented as the mean ± standard deviation. | ||||||
| Fermentation broth | 0 | 30 | 0.5 | 240 | 0.7 ± 0.1 | 14.1 ± 0.9 |
| CFF | 4.7 | 77.3 | 4.9 | 8.6 | 31.3 ± 1.9 | 63.9 ± 2.8 |
| NaOH (0.1 M) | 0 | 77.3 | 4.9 | 8.6 | 28.8 ± 1.3 | 58.7 ± 2.4 |
| NaOH (0.1 M) | 4.7 | 77.3 | 4.9 | 8.6 | 32.5 ± 2.1 | 66.4 ± 3.1 |
3.4. Quality evaluation of HAs
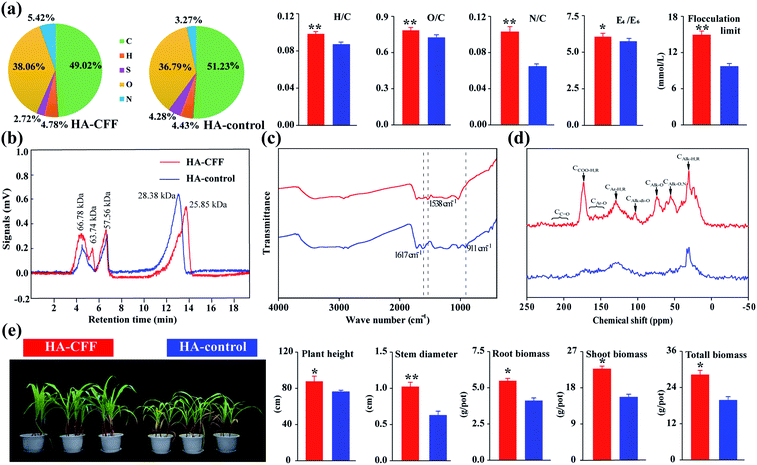
Multiple chemical and spectral analyses were used for quality evaluation of HAs extracted from lignite using CFF (HA-CFF), with HAs extracted using NaOH solution as the control (HA-control). The elemental composition of HA-CFF was obviously different from that of the HA-control, with lower carbon (C) and sulfur (S) contents, and higher nitrogen (N), hydrogen (H) and oxygen (O) contents (Fig. 5a). This result is consistent with previous reports.7,48 The increased O content may be directly related to the addition of oxygen-containing functional groups in the macromolecule. The new N in the HAs produced microbiologically was in the form of free or ionized NH2-groups of amino acids, and its amount was related to the N content initially present in the medium.49The H/C ratio represents the degree of unsaturation in the HAs, with a high H/C ratio often representing small molecular and low aromaticity compounds. The O/C ratio reflects the proportion of oxygen-containing groups in organic matter. Previous research has revealed that fungal-transformed HAs have higher O/C and H/C ratios than raw coal-derived HAs,48,50 which is consistent with our results (Fig. 5a). The N/C ratio reflects the N content in the organic material, the HA-CFF showed a higher N/C atomic ratio of 0.11 than the HA-control, while previous studies reported that the N/C value of lignite-derived HAs was usually less than 0.05.51,52 The E4/E6 ratio is known as the humification index. Generally, the higher the E4/E6 ratio, the lower molecular mass and content of condensed aromatic rings.7 In the spectroscopic estimations, the E4/E6 ratio was also higher for HA-CFF when compared to the HA-control, suggesting that the CFF may attack the aromatic structure of coal and decrease the HA molecular mass. In addition, the HPSEC results confirmed that the molecular weight of the HAs was reduced by the CFF. The lowest molecular weight elution peak (25.85 kDa) of HA-CFF was lower than that of HA-control (28.38 kDa), while the highest molecular weight elution peak in HA-control was divided into two elution peaks (66.78 and 63.74 kDa) in HA-CFF (Fig. 5b). Low molecular HAs can enter root cells and directly elicit intracellular signals;53 thus, HA-CFF may be more active in stimulating plant metabolism than HA-control.
When an HA product is applied to agriculture as a water-soluble fertilizer synergist, anions in the HAs easily form insoluble humic acid salts with calcium and magnesium ions, which will limit its application as a water-soluble fertilizer in drip irrigation or as a foliar fertilizer on leaf surface.54 Accordingly, the flocculation limit was used to evaluate the anti-flocculation ability of the HAs. The flocculation limit value was significantly higher in HA-CFF when compared to HA-control (Fig. 5a), suggesting higher hydrophilicity of HA-CFF; that is, the larger the hydrophilic components in humic samples, the higher the activity of HAs on plant physiology.55 Moreover, HA-CFF exhibiting high hydrophilicity and anti-flocculation is very suitable for the preparation of liquid fertilizer, particularly when mixed with various trace elements and applied to drip irrigation and sprinkler irrigation systems.
Both the spectra of HA-CFF and HA-control had similar primary absorption bands (Fig. 5c). The common peaks were: 3500–3300 cm−1 (O–H stretching in alcohols and phenols), 1720 cm−1 (carboxylic and carbonyl groups), 1380–1480 cm−1 (deformation vibrations of methylene and methyl groups) and 1035 cm−1 (C–O stretch of polysaccharide-like components).52,56 Compared with HA-control, the spectra of HA-CFF did not contain strong absorption bands in wavenumbers 1617 cm−1 and 911 cm−1, which are assigned to stretching C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups in aromatic rings and aromatic C–H bending, respectively,51 which suggests the collapse of aromatic rings. Conversely, HA-CFF had an absorption band at 1538 cm−1, which was caused by C
C groups in aromatic rings and aromatic C–H bending, respectively,51 which suggests the collapse of aromatic rings. Conversely, HA-CFF had an absorption band at 1538 cm−1, which was caused by C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching and N–H deformation, implying that more N was introduced in HA-CFF.57
N stretching and N–H deformation, implying that more N was introduced in HA-CFF.57
More detailed information on the structure of the HAs was obtained using solid-state 13C CP/MAS NMR spectroscopy, which provides a non-destructive appraisal of the relative amounts of different structures in a sample. The spectra were divided into three regions: carboxyl/carbonyl carbons (160–220 ppm), aromatic carbons (110–160 ppm) and aliphatic carbons (0–110 ppm) (Table 6). The HA-CFF spectra were obviously different from those of HA-control (Fig. 5d), which contained more aliphatic carbon (CAlk–O and CAlk–O,N), carboxyl and ketone carbon (CCOO–H,R and CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), but less aromatic carbon (CAr–O, CAr–H,R and CAlk–di-O). These results were consistent with the results of the elemental analysis and FTIR. O-Alkyl groups play a positive role on root and coleoptile elongation.29 The carboxyl (R–COOH) and hydroxyl (R–OH) groups of HAs play an important role in growth-related biological functions.58 The physiological experiment also confirmed the stronger bioactivity of HA-CFF. The stem diameter, plant height, root biomass, shoot biomass and total plant biomass of maize were significantly higher for the HA-CFF treatment (Fig. 5e). These results reveal that HAs extracted using CFF have excellent chemical and physiological properties.
O), but less aromatic carbon (CAr–O, CAr–H,R and CAlk–di-O). These results were consistent with the results of the elemental analysis and FTIR. O-Alkyl groups play a positive role on root and coleoptile elongation.29 The carboxyl (R–COOH) and hydroxyl (R–OH) groups of HAs play an important role in growth-related biological functions.58 The physiological experiment also confirmed the stronger bioactivity of HA-CFF. The stem diameter, plant height, root biomass, shoot biomass and total plant biomass of maize were significantly higher for the HA-CFF treatment (Fig. 5e). These results reveal that HAs extracted using CFF have excellent chemical and physiological properties.
| ppm | 190–220 | 160–190 | 140–160 | 110–140 | 90–110 | 60–90 | 45–60 | 0–45 |
|---|---|---|---|---|---|---|---|---|
| C% | Carboxyl/carbonyl carbons | Aromatic carbons | Aliphatic carbons | |||||
CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
CCOO–H,R | CAr–O | CAr–H,R | CAlk–di-O | CAlk–O | CAlk–O,N | CAlk–H,R | |
a Note: CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O represents carbonyl C; CCOO–H,R represents carboxyl C; CAr–O represents O-aromatic C; CAr–H,R represents aromatic C; CAlk–di-O represents di-O-alkyl C (anomeric); CAlk–O represents O-alkyl C; CAlk–O,N represents methoxyl and N-alkyl C; CAlk–H,R represents alkyl C. O represents carbonyl C; CCOO–H,R represents carboxyl C; CAr–O represents O-aromatic C; CAr–H,R represents aromatic C; CAlk–di-O represents di-O-alkyl C (anomeric); CAlk–O represents O-alkyl C; CAlk–O,N represents methoxyl and N-alkyl C; CAlk–H,R represents alkyl C. |
||||||||
| HA-control | 2.0 | 8.8 | 10.0 | 26.9 | 4.0 | 8.0 | 7.2 | 32.9 |
| HA-CFF | 2.4 | 12.2 | 5.1 | 15.6 | 3.1 | 16.3 | 12.2 | 33.2 |
4. Conclusion
A new method to efficiently and cleanly convert lignite into HAs using fungal CFF was proposed. The HA production process was optimized by RSM, and the results showed that the maximum HA concentration of 31.3 g L−1 was achieved with 4.7 N HNO3-pretreated lignite at a coal loading ratio of 4.9% and reaction temperature of 77.3 °C for 8.6 hours. The optimized method improved the HA extraction yield, shortened the reaction time and increased the coal loading ratio relative to the previous biological method. Quality evaluation showed that the HAs extracted from lignite using the CFF of P. ortum had a lower molecular weight, higher nitrogen and flocculation limit, and stronger biological activity than the chemically-extracted HAs. In conclusion, the findings of this work could be a promising technique for biologically active HA production, as well as for clean utilization of lignite.Author contributions
Shiying Li: experimental, data generation, writing – original draft. Jinfang Tan: conceptualization, review & editing. Yi Wang: investigation. Peipei Li: project administration. Desheng Hu: methodology. Qiuzhe Shi: draw the graphical abstract. Yanjun Yue: resources. Fang Li: methodology, writing – review & editing. Yanlai Han: conceptualization, review & editing, funding acquisition.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
We thank the editors and reviewers for their efforts in the publication of this article, we also thank Professor Fengqin Wang and Mr Haifeng Shao for their technical support and recommendations for this study. This work was supported by the National Key R&D Program of China (2018YFD0200600).References
- B. A. G. De Melo, F. L. Motta and M. H. A. Santana, Mater. Sci. Eng., C, 2016, 62, 967–974 CrossRef CAS PubMed
.
- T. A. van Tol De Castro, R. L. L. Berbara, O. C. H. Tavares, D. F. D. G. Mello, E. G. Pereira, C. D. C. B. Souza, L. M. Espinosa and A. C. García, Plant Physiol. Biotechnol., 2021, 162, 171–184 CrossRef CAS PubMed
.
- Y. Li, F. Fang, J. Wei, X. Wu, R. Cui, G. Li, F. Zheng and D. Tan, Sci. Rep., 2019, 9, 12014 CrossRef PubMed
.
- M. Bahemmat, M. Farahbakhsh and M. Kianirad, J. Hazard. Mater., 2016, 312, 307–318 CrossRef CAS PubMed
.
- E. Sarlaki, A. Sharif Paghaleh, M. H. Kianmehr and K. Asefpour Vakilian, Renewable Energy, 2021, 163, 105–122 CrossRef CAS
.
- N. Fatima, A. Jamal, Z. Huang, R. Liaquat, B. Ahmad, R. Haider, M. I. Ali, T. Shoukat, Z. A. ALOthman, M. Ouladsmane, T. Ali, S. Ali, N. Akhtar and M. Sillanpää, Sustainability, 2021, 13, 8969 CrossRef CAS
.
- M. J. Ghani, K. Akhtar, S. Khaliq, N. Akhtar and M. A. Ghauri, Process Biochem., 2021, 107, 1–12 CrossRef CAS
.
- E. Sarlaki, A. Sharif Paghaleh, M. H. Kianmehr and K. Asefpour Vakilian, J. Cleaner Prod., 2019, 235, 712–723 CrossRef CAS
.
- M. A. Sabar, M. I. Ali, N. Fatima, A. Y. Malik, A. Jamal, R. Liaquat, H. He, F. Liu, H. Guo, M. Urynowicz and Z. Huang, Fuel, 2020, 278, 118301 CrossRef CAS
.
- Y. Yang, J. Yang, B. Li, E. Wang and H. Yuan, Fuel, 2018, 214, 416–422 CrossRef CAS
.
- S. I. Zherebtsov, N. V. Malyshenko, L. V. Bryukhovetskaya and Z. R. Ismagilov, Coke Chem., 2016, 58, 400–403 CrossRef
.
- Z. Huang, C. Liers, R. Ullrich, M. Hofrichter and M. A. Urynowicz, Fuel, 2013, 112, 295–301 CrossRef CAS
.
- H. Machnikowska, K. Pawelec and A. Podgórska, Fuel Process. Technol., 2002, 77, 17–23 CrossRef
.
- M. S. Cohen and P. D. Gabriele, Appl. Environ. Microbiol., 1982, 44, 23–27 CrossRef CAS PubMed
.
- F. Jiang, Z. Li, Z. Lv, T. Gao, J. Yang, Z. Qin and H. Yuan, Fuel, 2013, 103, 639–645 CrossRef CAS
.
- H. L. Yuan, J. S. Yang, F. Q. Wang and W. X, Prikl. Biokhim. Mikrobiol., 2006, 42, 59–62 CAS
.
- M. A. Sabar, M. I. Ali, N. Fatima, A. Y. Malik, A. Jamal, M. Farman, Z. Huang and M. Urynowicz, Fuel, 2019, 253, 257–265 CrossRef CAS
.
- J. Sobolczyk-Bednarek, A. Choińska-Pulit and W. Łaba, Fuel, 2021, 301, 121082 CrossRef CAS
.
- L. M. Sekhohola, E. E. Igbinigie and A. K. Cowan, Biodegradation, 2013, 24, 305–318 CrossRef CAS PubMed
.
- X. Feng, J. Sun and Y. Xie, Fuel, 2021, 291, 120204 CrossRef CAS
.
- J. K. Polman, K. S. Miller, D. L. Stoner and C. R. Breckenridge, J. Chem. Technol. Biotechnol., 1994, 61, 11–17 CrossRef CAS
.
- H. Yuan, J. Yang and W. Chen, Fuel, 2006, 85, 1378–1382 CrossRef CAS
.
- G. Willmann and R. Fakoussa, Fuel Process. Technol., 1997, 52, 27–41 CrossRef CAS
.
- A. Wali, I. Ben Salah, M. Zerrouki, A. Choukchou-Braham, Y. Kamoun and M. Ksibi, Euro-Mediterranean Journal for Environmental Integration, 2019, 4, 1–9 CrossRef
.
- X. Song, J. Li, L. Chen, Z. Cai, C. Liao, H. Peng and H. Xiong, J. Braz. Chem. Soc., 2012, 23, 132–141 CrossRef CAS
.
- C. Lu, Z. Zhang, X. Ge, Y. Wang, X. Zhou, X. You, H. Liu and Q. Zhang, Int. J. Hydrogen Energy, 2016, 41, 13399–13407 CrossRef CAS
.
- T. Lian, W. Zhang, Q. Cao, S. Wang, F. Yin, Y. Chen, T. Zhou and H. Dong, Bioresour. Technol., 2021, 336, 125307 CrossRef CAS PubMed
.
- H. Monda, A. M. McKenna, R. Fountain and R. T. Lamar, Front. Plant Sci., 2021, 12, 958 Search PubMed
.
- D. Savy, Y. Brostaux, V. Cozzolino, P. Delaplace, P. du Jardin and A. Piccolo, Front. Plant Sci., 2020, 11, 581 CrossRef PubMed
.
- L. Zhou, L. Yuan, B. Zhao, Y. Li and Z. Lin, PLoS One, 2019, 14, e217469 Search PubMed
.
- M. Arabi, A. Ostovan, A. R. Bagheri, X. Guo, J. Li, J. Ma and L. Chen, Talanta, 2020, 215, 120933 CrossRef CAS PubMed
.
- X. Song, J. Li, S. Xu, R. Ying, J. Ma, C. Liao, D. Liu, J. Yu and L. Chen, Talanta, 2012, 99, 75–82 CrossRef CAS PubMed
.
- P. Liu, Y. H. Liao, H. B. Zheng and Y. Tang, Anal. Methods, 2020, 12, 2308–2316 RSC
.
- O. K. Achi, Bioresour. Technol., 1994, 48, 53–57 CrossRef CAS
.
- J. K. Polman, D. L. Stoner and K. M. Delezene-Briggs, J. Ind. Microbiol. Biotechnol., 1994, 13, 292–299 CrossRef CAS
.
- G. Zhou, J. Zhang, C. Zhang, Y. Feng, L. Chen, Z. Yu, X. Xin and B. Zhao, Sci. Rep., 2016, 6, 1–12 CrossRef PubMed
.
- M. S. Cohen, W. C. Bowers, H. Aronson and E. T. Gray, Appl. Environ. Microbiol., 1987, 53, 2840–2843 CrossRef CAS PubMed
.
- R. Haider, M. A. Ghauri, E. J. Jones, W. H. Orem and J. R. SanFilipo, Int. Biodeterior. Biodegrad., 2015, 100, 149–154 CrossRef CAS
.
- M. G. Baylon, Y. David, S. D. V. N. Pamidimarri, K. Baritugo, C. G. Chae, Y. J. Kim, T. W. Kim, M. Kim, J. G. Na and S. J. Park, Korean J. Chem. Eng., 2017, 34, 105–109 CrossRef CAS
.
- D. R. Quigley, B. Ward, D. L. Crawford, H. J. Hatcher and P. R. Dugan, Appl. Biochem. Biotechnol., 1989, 20–21, 753–763 CrossRef
.
- N. Akimbekov, I. Digel, G. Abdieva, P. Ualieva and K. Tastambek, Biofuels, 2021, 12, 247–258 CrossRef CAS
.
- G. Cheng, Z. Niu, C. Zhang, X. Zhang and X. Li, Appl. Sci., 2019, 9, 1356 CrossRef CAS
.
- I. Romanowska, B. Strzelecki and S. Bielecki, Fuel Process. Technol., 2015, 131, 430–436 CrossRef CAS
.
- B. Wang, F. Ndayisenga, G. Zhang and Z. Yu, GCB Bioenergy, 2021, 13, 967–978 CrossRef CAS
.
- Y. Başaran, A. Denizli, B. Sakintuna, A. Taralp and Y. Yürüm, Energy Fuels, 2003, 17, 1068–1074 CrossRef
.
- H. Kang, X. Liu, Y. Zhang, S. Zhao, Z. Yang, Z. Du and A. Zhou, Energy Sources, Part A, 2021, 43, 1162–1180 CrossRef CAS
.
- C. Lu, Y. Jing, H. Zhang, D. Lee, N. Tahir, Q. Zhang, W. Li, Y. Wang, X. Liang, J. Wang, P. Jin and X. Zhang, Bioresour. Technol., 2020, 304, 123007 CrossRef CAS PubMed
.
- L. Dong, Q. Yuan and H. Yuan, Fuel, 2006, 85, 2402–2407 CrossRef CAS
.
- L. Dong and H. Yuan, Geomicrobiol. J., 2009, 26, 484–490 CrossRef CAS
.
- R. Haider, M. A. Ghauri and K. Akhtar, Geomicrobiol. J., 2015, 32, 944–953 CrossRef CAS
.
- Y. Zhang, Y. Li, L. Chang, C. Zi, G. Liang, D. Zhang and Y. Su, RSC Adv., 2020, 10, 22002–22009 RSC
.
- L. Doskočil, J. Burdíková-Szewieczková, V. Enev, L. Kalina and J. Wasserbauer, Fuel, 2018, 213, 123–132 CrossRef
.
- S. Nardi, M. Schiavon and O. Francioso, Molecules, 2021, 26, 2256 CrossRef CAS PubMed
.
- T. Ahmad, R. Khan and T. Nawaz Khattak, J. Plant Nutr., 2018, 41, 2438–2445 CrossRef CAS
.
- S. Nardi, A. Muscolo, S. Vaccaro, S. Baiano, R. Spaccini and A. Piccolo, Soil Biol. Biochem., 2007, 39, 3138–3146 CrossRef CAS
.
- Z. Wang, T. Shen, Y. Yang, B. Gao, Y. Wan, Y. C. Li, Y. Yao, L. Liu, Y. Tang, J. Xie, F. Ding and J. Chen, J. Cleaner Prod., 2020, 243, 118585 CrossRef CAS
.
- G. Ait Baddi, M. Hafidi, J. Cegarra, J. A. Alburquerque, J. Gonzálvez, V. Gilard and J. Revel, Bioresour. Technol., 2004, 93, 285–290 CrossRef CAS PubMed
.
- M. Y. Byun, D. Kim, U. J. Youn, S. Lee and H. Lee, Plant Physiol. Biochem., 2021, 159, 37–42 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra08019a |
| ‡ The first two authors contributed equally to this study. |
| This journal is © The Royal Society of Chemistry 2022 |