Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enhancing the photocatalytic hydrogen production activity of BiVO4 [110] facets using oxygen vacancies†
Jing Pan‡
 ,
Xiaoxue Ma‡,
Wannian Zhang and
Jingguo Hu
,
Xiaoxue Ma‡,
Wannian Zhang and
Jingguo Hu *
*
College of Physics Science and Technology, Yangzhou University, Yangzhou, 225002, China. E-mail: jghu@yzu.edu.cn
First published on 22nd December 2021
Abstract
The activity of the hydrogen evolution reaction (HER) during photoelectrochemical (PEC) water-splitting is limited when using BiVO4 with an exposed [110] facet because the conduction band minimum is below the H+/H2O potential. Here, we enhance the photocatalytic hydrogen production activity through introducing an oxygen vacancy. Our first-principles calculations show that the oxygen vacancy can tune the band edge positions of the [110] facet, originating from an induced internal electric field related to geometry distortion and charge rearrangement. Furthermore, the induced electric field favors photogenerated electron–hole separation and the enhancement of atomic activity. More importantly, oxygen-vacancy-induced electronic states can increase the probability of photogenerated electron transitions, thus improving optical absorption. This study indicates that oxygen-defect engineering is an effective method for improving the photocatalytic activity when using PEC technology.
1. Introduction
Photoelectrochemical (PEC) water-splitting using solar energy to generate hydrogen is considered to be one of the more promising approaches for renewable energy production.1,2 Since the initial report of a TiO2-based photocatalyst, many semiconductors have been investigated, but the photocatalytic efficiencies are still low and far from being practically applicable because of the following problems: (1) weak visible-light adsorption due to wide band gaps; (2) fast electron–hole pair recombination; (3) low carrier mobility; and (4) band edge positions that do not match the water redox potentials.3–5 For an ideal photocatalyst, its valence band maximum (VBM) should be energetically lower than the O2/H2O potential and its conduction band minimum (CBM) should be higher than the H+/H2O potential; in addition, it should be active towards the H2 evolution reaction (HER) and O2 evolution reaction (OER).6,7 Recently, monoclinic clinobisvanite bismuth scheelite (ms-BiVO4) has attracted extensive attention due to its abundance, strong visible-light adsorption (with a direct band gap of 2.4 eV), and high activity for O2 evolution. For this photocatalyst, the VBM of BiVO4 is located at ca. 2.4 V vs. RHE, providing a sufficient overpotential for holes to photo-oxidize water. However, the CBM is below the H+/H2O potential, and the excited electrons cannot photo-reduce water.8,9 Additionally, poor carrier transport properties and rapid electron–hole recombination also limit the PEC performance.10,11 As a consequence, many measures have been taken, such as doping, morphology control, regulating different exposed facets, heterojunction construction, and surface decoration, to enhance its PEC activity.12–15 As is known, many examples of faceted BiVO4 polyhedra have been synthesized, and each exposed facet exhibits different thermodynamic and photocatalytic behavior; photogenerated electrons and holes can be preferentially separated and accumulated on [010] and [110] facets, where the [010] facet favors proton reduction and the [110] facet favors water oxidation.16,17 Zhao et al. realized a so-called hydrogen farm project via precisely tuning the (110)/(010) facets, achieving an overall solar-to-chemical efficiency of over 1.9% and a solar-to-hydrogen efficiency exceeding 1.8%. If the photocatalytic activity of a single (110) or (010) facet can be enhanced, the efficiency of photocatalytic hydrogen generation at a (110)/(010) facet heterojunction can also be enhanced. In this case, the oxygen reaction on the (110) facet is a complex reaction, thus, it is important to improve the photocatalytic activity of the (110) facet.18 Vacancy-defect engineering is a feasible method, utilizing electron redistribution and special chemical properties to enhance the photocatalytic activity.19–21Recently, experimental studies have shown that oxygen vacancies (Ovac) in a crystal structure could greatly improve the photocatalytic activity. For example, Zhao et al. reported the Ovac-boosted photocatalytic nitrogen fixation of TiO2 via providing more active sites with electron redistribution and enhanced electron transport.22 Our group have shown that surface Ovac supported charge separation and transfer, thus improving the OER performance of 3D nanoporous BiVO4.23 Although the use of Ovac, which widely exist in metal oxides and are important for the PEC performance, is a promising strategy for enhancing the photocatalytic activity, the mechanism explaining the effects of Ovac on photocatalytic water-splitting remains contentious and poorly understood. In this work, we adopt the use of an Ovac to improve the photocatalytic hydrogen production activity of the BiVO4 [110] facet and investigate the mechanism via first-principles calculations. The calculated results show that the Ovac not only upshifts the band edge positions to satisfy the requirements of PEC water-splitting but it also assists photogenerated electron–hole separation and optical absorption, as a result of the Ovac-induced electric field.
2. Computational model and methods
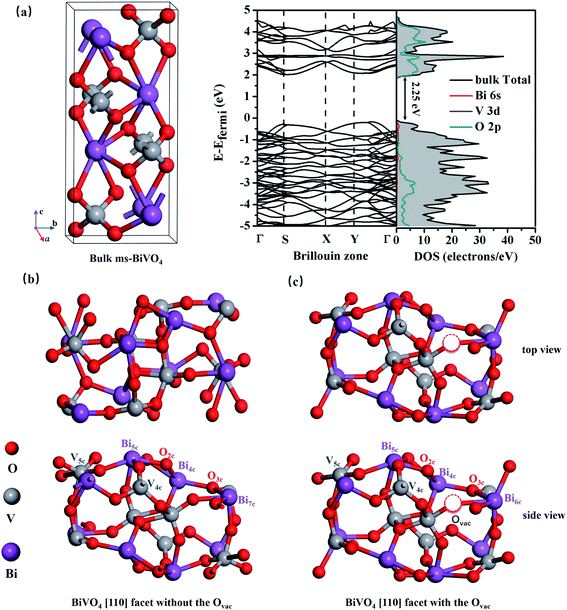
All calculations were based on the Vienna ab initio simulation package (VASP) with density functional theory (DFT),24,25 and the generalized gradient approximation (GGA) of the Perdew–Burke–Ernzerhof (PBE) functional was adopted.26,27 As shown in Fig. 1(b) and (c), supercells with a size of 11.9 × 7.3 × 23.5 Å3 containing 48 atoms were used to model bilayer BiVO4 with and without oxygen vacancies, and the models were constructed from the [110] surface of optimized bulk monoclinic BiVO4 with a 12 Å vacuum slab along the z-direction (see Fig. 1(a)). A plane wave cutoff energy of 400 eV and a total change in energy of 1.0 × 10−5 eV for geometrical optimization were employed, and the maximum stress was less than 0.01 eV Å−1. Monkhorst–Pack k-point grids of 5 × 5 × 1 for geometric optimization and 7 × 7 × 1 for electronic structure calculations were sampled as the Brillouin zones.3. Results and discussion
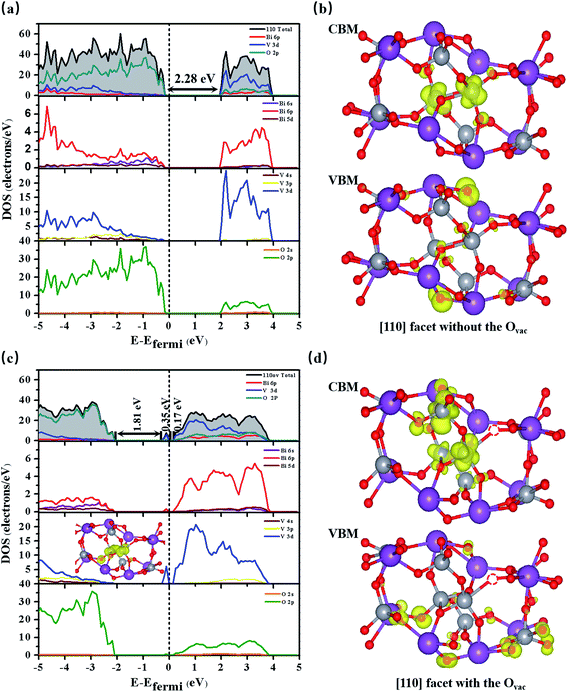
As shown in Fig. 1(a), bulk BiVO4 is a layered monoclinic scheelite-phase structure containing BiO8 dodecahedra and VO4 tetrahedra, which are linked via Bi3+–O2−–V5+ connections and stacked along the main [001] axis direction with an interplanar distance of 2.97 Å (close to the experimental value of 2.89 Å).28 Bulk BiVO4 is a semiconductor, and the calculated band gap is 2.25 eV, basically in accordance with the experimental band gap of 2.40 eV. Additionally, the PBE-calculated lattice constants are a = 5.04 Å, b = 5.27 Å, and c = 11.89 Å, which are consistent with the experimental values of a = 5.09 Å, b = 5.20 Å, and c = 11.70 Å,29 confirming the reliability of the PBE method.As shown in Fig. 1(b), the BiVO4 [110] facet is made up of 7-, 5-, and 4-coordinated Bi, 5- and 4-coordinated V, and 2- and 3-coordinated O; it retains semiconductor behavior with a band gap of 2.28 eV, and the VBM mainly consists of O 2p while the CBM is primarily composed of Bi 6p, O 2p, and V 3d (see Fig. 2(a)).
We create an Ovac near Bi7c, which is considered as an active site, as can be judged from the adsorption energy of H2O molecules given in the ESI.†30 As shown in Fig. 1(c), near the Ovac, 7-coordinated Bi changes to 6-coordinated Bi, and Bi and V atoms bond to neighboring O atoms with smaller bond lengths and angles compared with the pure [110] facet. For example, the Bi1–O1 bond length varies from 2.33 Å to 2.27 Å, the V1–O1 bond length varies from 1.78 Å to 1.72 Å, and the O1–Bi1–O2 angle changes from 134.96° to 122.06° (see Table 1). This change in structure will result in a change in the electronic structure of the [110] facet. As shown in Fig. 2(c), the energy of the VBM in the [110] facet with the Ovac is lower than that in pure [110], indicating that holes are easily excited and easier to separate from the bulk to the surface.31 When the Fermi level is set to zero, the CBM is at 1.93 eV for the pure [110] facet, while it moves to 0.16 eV for the [110] facet with the Ovac; the Fermi level is nearer the CBM for the [110] facet with the Ovac, indicating an increase in the electron concentration and the generation of n-type semiconductor behavior. Furthermore, localized states exist in the band gap, coming from hybridization between V 3d and O 2p states neighbouring the Ovac, which can also be seen in the partial density analysis in the inset of Fig. 2(c). These Ovac-induced electronic states are conducive to electron transition from the VBM to these states, with a gap of 1.81 eV, and subsequently to the CBM, with a gap of 0.17 eV, benefiting the optical absorption.
Comparing the partial charge densities of [110] facets with and without an Ovac, as shown in Fig. 2(b) and (d), we find that charge in the VBM region is mainly concentrated on O atoms and charge in the CBM region is located mainly on V atoms for the pure [110] facet (see Fig. 2(b)). There are obvious changes in the [110] facet with the Ovac: the charge in the VBM and CBM regions greatly increases because unmatched electrons and dangling bonds are present, favoring the catalytic behavior. Specially, the charge in the CBM region is distributed more on O atoms but still in the sublayer, the charge in the VBM moves to the surface, and the increased distance between the VBM and CBM is beneficial for electron–hole separation. Furthermore, we quantitatively analyse the electric dipole moments of BiVO4; an internal electric field is present with a magnitude of 13.01 D for the [110] facet with the Ovac, which is associated with the greater geometry distortion and charge rearrangement and is stronger than that of the pure [110] facet (7.59 D). As is known, an induced electric field can effectively improve surface charge separation and change the photoelectrochemical impedance spectroscopy and transient absorption spectroscopy responses. For example, Zhang et al. have shown that tantalum doping induced an electric field in hematite homojunction nanorods, providing additional driving force to significantly improve charge separation both in the bulk and at the surface.32 Hussain et al. have shown that an oxygen-vacancy-induced internal electric field between [BiO]+ and [Br]− had the remarkable capacity to assist effective charge separation and move charge to the surface from the bulk.33
Fig. 3(a) shows the average electrostatic potentials along the z-direction of the BiVO4 systems. The work function, defined as the difference between the vacuum level and the Fermi level, is 6.04 eV for the pure [110] facet, which is larger than that of the [110] facet with the Ovac (5.87 eV), indicating that charge is more easily transferred to the surface due to the existence of the Ovac. Based on the electrostatic potential, the band edge energies (e.g., the VBM and CBM) can be obtained via aligning the eigenvalues to the vacuum level.34 For the pure [110] facet, the VBM is −6.318 eV and the CBM is −4.038 eV, straddling the oxidation potential but not the H+/H2O potential; this means there is a lack of driving force for the HER, limiting the photocatalytic hydrogen generation abilities of BiVO4, which is in accordance with the experimental results.35 Compared to the pure [110] facet, the band edge positions of the [110] facet with the Ovac are upshifted by 0.161 eV; the VBM position is −6.216 eV and the CBM position is −3.877 eV, straddling the water redox region. The upshift mainly comes from the Ovac-induced internal electric field caused by geometry distortion and charge rearrangement. As we know, the total dipole perpendicular to the surface component (μ⊥) causes the work function change, that is, ΔW⊥ = μ⊥/Aε0,34,36 where A and ε0 refer to the surface area of the unit cell and dielectric constant, respectively. Here, the dipole density is −0.428 D nm−2 and, therefore, the resultant work function change is −0.161 eV; based on ΔV⊥ = −ΔW⊥, the band edge upshifts by 0.161 eV, matching with the calculated energy shift based on the mean electrostatic potential.
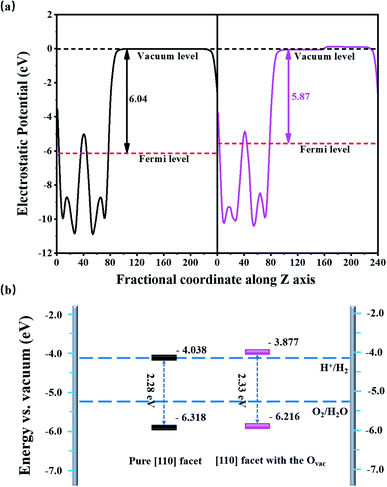 | ||
| Fig. 3 (a) The average electrostatic potentials along the z axis and (b) the band edge positions of BiVO4 [010] facets relative to the water redox potential with (pink) and without (black) the Ovac. | ||
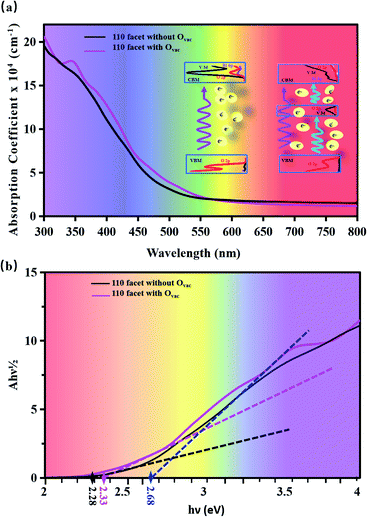
Fig. 4 displays the optical absorption of the BiVO4 [110] facets. We find that the absorption peak is at 196.4 nm for the pure [110] facet and at 197.7 nm for the [110] facet with the Ovac. To clearly describe the change in band gap, we display (Ahν)1/2 as a function of hν in Fig. 4(b). The intercept of a tangent line to the first peak with the x-axis relates to the band gap. The intercept is 2.28 eV for the pure [110] facet, relating to the band gap. Compared to the pure [110] facet, two peaks appear for the [110] facet with the Ovac: the intercept of the first peak relates to a band gap of 2.33 eV and the intercept of the second peak gives a band gap of 2.68 eV. The difference between the intercept of the first peak and the second small peak is 0.35 eV, relating to the energy range of local states due to the introduction of the Ovac [see Fig. 2(c)]. An enhancement of the optical absorption can be observed between 300 and 700 nm in the case of the [110] facet with the Ovac. In fact, the absorption spectrum is closely connected with the electron transitions between the conduction bands and the valence bands. For the pure [110] facet, optical adsorption is derived from electronic inter-band transitions from O 2p at the VBM to O 2p, Bi 6p, and V 3d at the CBM (see the PDOS in Fig. 2(a)). For the [110] facet with the Ovac, a peak appears at 350 nm, relating to an energy of 3.54 eV; the corresponding energy in the PDOS is 1.48 eV, as seen in Fig. 2(c), and, compared to the pure [110] facet, some electronic states appear at 1.48 eV due to the effects of the introduction of the Ovac. Due to the presence of local states, the electron transitions include transitions not only from the host VBM but also from local states (i.e., V 3d and O 2p) to the CBM. This indicates that the local electronic states not only favor electron transitions but they also enhance the transition probability.
Furthermore, the effects of the Ovac on the [110] facet have been explored via calculating its formation energy (Eform = EOvac − Esurf + ½EO2, where EOvac, Esurf, and EO2 are the total energies of BiVO4 [110] facets with and without the Ovac and molecular O2, respectively). The formation energy of the [110] facet with the Ovac is 3.86 eV, and this calculated result is comparable to what is reported in ref. 37, indicating that oxygen vacancies can easily be formed in the BiVO4 [110] facet. To further investigate the effects of the Ovac site and the Ovac concentration in the [110] facet, we create an Ovac neighbouring V5c (see Fig. S2†), and create two and three Ovac, forming Ovac concentrations of 6.25% and 9.38%, as shown in Fig. S3 in the ESI; †30 the calculated results show that the Ovac site and concentration have a great influence on the electronic structure and optical adsorption, thus affecting the photocatalytic properties.
4. Conclusions
Based on electronic structure calculations and band edge alignment analysis, we demonstrate that vacancy-defect engineering is a feasible strategy for improving the photocatalytic water-splitting activity of BiVO4. To this end, the Ovac plays an important role: (1) the Ovac excites the activity of neighbouring atoms due to unmatched electrons and dangling bonds; (2) the Ovac-induced internal electric field is conducive to photogenerated electron–hole separation and can tune the band edges; and (3) the Ovac-induced local electronic states favor electron transitions and enhance the optical absorption. As a result, the BiVO4 [110] facet can become a promising photocatalyst for water-splitting owing to the ideal band gap for enhanced optical absorption, the reduced electron–hole recombination, and the suitable band edges for water redox.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the NSFC (11774302, 12074332, and 21903014). The authors are grateful for access to the computational resources at YZU.References
- Z. Liu, Z. Xiao, G. Luo, R. Chen, C.-L. Dong, X. Chen, J. Cen, H. Yang, Y. Wang, D. Su, Y. Li and S. Wang, Small, 2019, 15, 1904903 CrossRef CAS PubMed.
- J. Cen, Q. Wu, M. Liu and A. Orlov, Green Energy Environ., 2017, 2, 100–111 CrossRef.
- K. Edalati, K. Fujiwara, S. Takechi, Q. Wang, M. Arita, M. Watanabe, X. Sauvage, T. Ishihara and Z. Horita, ACS Appl. Energy Mater., 2020, 3, 1710–1718 CrossRef CAS.
- X. Liu, J. Li and W. Yao, ACS Omega, 2020, 5, 27463–27469 CrossRef CAS PubMed.
- Y. Huang, J. Liu, C. Zhao, X. Jia, M. Ma, Y. Qian, C. Yang, K. Liu, F. Tan, Z. Wang, X. Li, S. Qu and Z. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 52603–52614 CrossRef CAS PubMed.
- K. Maeda, J. Photochem. Photobiol., C, 2011, 12, 237–268 CrossRef CAS.
- B. Huang and J. N. Hart, Phys. Chem. Chem. Phys., 2020, 22, 1727–1737 RSC.
- W. Qiu, S. Xiao, J. Ke, Z. Wang, S. Tang, K. Zhang, W. Qian, Y. Huang, D. Huang, Y. Tong and S. Yang, Angew. Chem., Int. Ed. Engl., 2019, 58, 19087–19095 CrossRef CAS PubMed.
- Y. Hermans, S. Murcia-López, A. Klein, R. van de Krol, T. Andreu, J. R. Morante, T. Toupance and W. Jaegermann, Phys. Chem. Chem. Phys., 2019, 21, 5086–5096 RSC.
- Y. Yang, X. Zhong, K. Liu, J. Du, Y. Yang, H. He, Y. Zhou, F. Dong, C. Fu and J. Wang, J. Electrochem. Soc., 2019, 166, H513–H520 CrossRef CAS.
- R. Irani, I. Y. Ahmet, J.-W. Jang, S. P. Berglund, P. Plate, C. Höhn, R. Böttger, S. W. Schmitt, C. Dubourdieu, S. Lardhi, L. Cavallo, M. Harb, P. Bogdanoff, R. van de Krol and F. F. Abdi, Sol. RRL, 2020, 4, 1900290 CrossRef CAS.
- K. Zhu, F. Shi, X. Zhu and W. Yang, Nano Energy, 2020, 73, 104761 CrossRef CAS.
- B. He, Z. Li, D. Zhao, H. Liu, Y. Zhong, J. Ning, Z. Zhang, Y. Wang and Y. Hu, ACS Appl. Nano Mater., 2018, 1, 2589–2599 CrossRef CAS.
- D. K. Lee and K.-S. Choi, Nat. Energy, 2018, 3, 53–60 CrossRef CAS.
- W. Kim Tae and K.-S. Choi, Science, 2014, 343, 990–994 CrossRef PubMed.
- H. Li, H. Yu, X. Quan, S. Chen and H. Zhao, Adv. Funct. Mater., 2015, 25, 3074–3080 CrossRef CAS.
- R. Li, F. Zhang, D. Wang, J. Yang, M. Li, J. Zhu, X. Zhou, H. Han and C. Li, Nat. Commun., 2013, 4, 1432 CrossRef PubMed.
- Y. Zhao, C. Ding, J. Zhu, W. Qin, X. Tao, F. Fan, R. Li and C. Li, Angew. Chem., Int. Ed., 2020, 59, 9653–9658 CrossRef CAS PubMed.
- W. Zhang, L. Song, J. Cen and M. Liu, J. Phys. Chem. C, 2019, 123, 20730–20736 CrossRef CAS.
- S. Wang, P. Chen, Y. Bai, J.-H. Yun, G. Liu and L. Wang, Adv. Mater., 2018, 30, 1800486 CrossRef PubMed.
- N. Daelman, F. S. Hegner, M. Rellán-Piñeiro, M. Capdevila-Cortada, R. García-Muelas and N. López, J. Chem. Phys., 2020, 152, 050901 CrossRef CAS PubMed.
- Y. Zhao, Y. Zhao, R. Shi, B. Wang, G. I. N. Waterhouse, L.-Z. Wu, C.-H. Tung and T. Zhang, Adv. Mater., 2019, 31, 1806482 CrossRef PubMed.
- S. Jin, X. Ma, J. Pan, C. Zhu, S. E. Saji, J. Hu, X. Xu, L. Sun and Z. Yin, Appl. Catal., B, 2021, 281, 119477 CrossRef CAS.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 14251–14269 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef PubMed.
- F. S. Hegner, D. Forrer, J. R. Galán-Mascarós, N. López and A. Selloni, J. Phys. Chem. Lett., 2019, 10, 6672–6678 CrossRef CAS PubMed.
- A. W. Sleight, H. y. Chen, A. Ferretti and D. E. Cox, Mater. Res. Bull., 1979, 14, 1571–1581 CrossRef CAS.
- ESI.†.
- J. Hu, X. Zhao, W. Chen, H. Su and Z. Chen, J. Phys. Chem. C, 2017, 121, 18702–18709 CrossRef CAS.
- H. Zhang, D. Li, W. J. Byun, X. Wang, T. J. Shin, H. Y. Jeong, H. Han, C. Li and J. S. Lee, Nat. Commun., 2020, 11, 4622 CrossRef PubMed.
- A. Hussain, J. Hou, M. Tahir, X. Wang, M. U. Qadri, T. jiang, X. Tu, T. Zhang, Q. Dou and J.-j. Zou, J. Environ. Chem. Eng., 2021, 9, 104766 CrossRef CAS.
- J. Pan, X. Shao, X. Xu, J. Zhong, J. Hu and L. Ma, J. Phys. Chem. C, 2020, 124, 6580–6587 CrossRef CAS.
- A. Kudo, K. Omori and H. Kato, J. Am. Chem. Soc., 1999, 121, 11459–11467 CrossRef CAS.
- J. Pan, W. Zhang, X. Xu and J. Hu, Phys. Chem. Chem. Phys., 2020, 22, 9415–9423 RSC.
- G.-L. Li, RSC Adv., 2017, 7, 9130–9140 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra07121a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |