Open Access Article
Open Access ArticleTannic acid induces dentin biomineralization by crosslinking and surface modification†
Weijing Kong‡
a,
Qiaolin Du‡b,
Yinan Quc,
Changyu Shaod,
Chaoqun Chenb,
Jian Sunb,
Caiyun Maob,
Ruikang Tang d and
Xinhua Gu
d and
Xinhua Gu *b
*b
aStomatology Hospital, School of Stomatology, Zhejiang University School of Medicine, Clinical Research Center for Oral Diseases of Zhejiang Province, Key Laboratory of Oral Biomedical Research of Zhejiang Province, Cancer Center of Zhejiang University, Hangzhou, P. R. China
bDepartment of Stomatology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, P. R. China. E-mail: guxh@zju.edu.cn
cReal Dental, Guangzhou, P. R. China
dCenter for Biomaterials and Biopathways, Department of Chemistry, Zhejiang University, Hangzhou, P. R. China
First published on 26th January 2022
Abstract
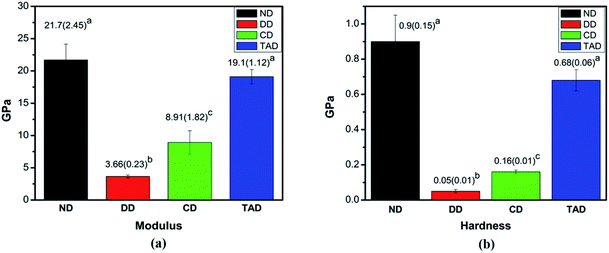
It is currently known that crosslinking agents can effectively improve the mechanical properties of dentin by crosslinking type I collagen. However, few scholars have focused on the influence of crosslinking agents on the collagen-mineral interface after crosslinking. Analysis of the Fourier transform infrared spectroscopy (FTIR) results showed that hydrogen bonding occurs between the tannic acid (TA) molecule and the collagen. The crosslinking degree of TA to collagen reached a maximum 41.28 ± 1.52. This study used TA crosslinked collagen fibers to successfully induce dentin biomineralization, and the complete remineralization was achieved within 4 days. The crosslinking effect of TA can improve the mechanical properties and anti-enzyme properties of dentin. The elastic modulus (mean and standard deviation) and hardness values of the remineralized dentin pretreated with TA reached 19.1 ± 1.12 GPa and 0.68 ± 0.06 GPa, respectively, which were close to those of healthy dentin measurements, but significantly higher than those of dentin without crosslinking (8.91 ± 1.82 GPa and 0.16 ± 0.01 GPa). The interface energy between the surface of collagen fibers and minerals decreased from 10.59 mJ m−2 to 4.19 mJ m−2 with the influence of TA. The current work reveals the importance of tannic acid crosslinking for dentin remineralization while providing profound insights into the interfacial control of biomolecules in collagen mineralization.
1. Introduction
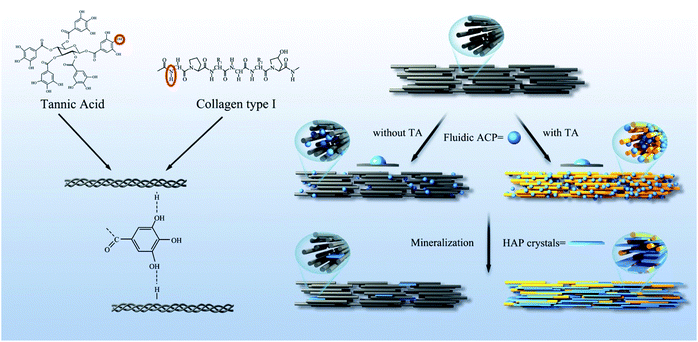
As calcified hard tissues, dentin presents an intricate composition comprised of 70% (by weight) mineral, 20% organic matrix, and 10% water.1,2 Type I collagen fibers account for 90% of the organic matrix and are arranged into highly organized hierarchical structures. As a template for hydroxyapatite (HAP),3 type I collagen can have a number of structural roles, such as forming a strong and elastic mineralized scaffold to direct the nucleation and growth of crystals.4 The HAP mineral is embedded in the collagen and oriented along its longitudinal axis, which endows the hard tissue with excellent mechanical properties.5,6 Generally, due to the lack of self-repair ability, in the environment where cariogenic bacteria appear, hard tissues are easily attacked by bacteria-derived enzymes, and mineral loss leads to irreversible dentin destruction.7,8 Biomineralization is an approach that imitates natural biomineralization. Current research shows that through intrafibrillar mineralization, bare collagen fibers can be better protected, and excellent mechanical properties can be achieved.9,10 Novel remineralization methods are accepted for clinical use and show great promise for the future.11Dentin biomineralization is a process completed under the regulation and induction of noncollagenous proteins (NCPs), known as nonclassical particle-mediated crystallization pathways.12,13 The pathways and mechanisms by which the amorphous phase mediates crystallization are the basis for dentin biomineralization.14 Amorphous calcium phosphate (ACP) was found to be the precursor phase that mediated crystallization pathways; precursor mineral phases exist in the liquid-like state and remain well hydrated.15,16 Charged polymers mimicking NCPs may make ACP precursors liquid-like and highly charged, which ultimately enables ACP precursors to enter collagen fibrils.17,18 In particular, recent studies have found that adjusting the collagen–mineral interface can accelerate the accumulation of minerals and reduce the duration of collagen mineralization. Shao and coworkers have reported that citrate molecules promote the binding of ACP to collagen by increasing wetting, thereby changing the properties of the mineralized interface.19 In addition, we found that as a surface modifier, polydopamine can make it easier for ACP to enter collagen fibrils and simultaneously reduce local heterogeneous nucleation barriers.20
However, so far, the process of remineralization remains too time-consuming for applications. It has been shown that host matrix metalloproteinases (MMPs) derived from dentin or saliva are readily capable of degrading the dentin matrix, which has been previously demineralized.21 Therefore, it is extremely important to provide a more efficient demineralized dentin collagen. The use of exogenous collagen crosslinkers induces further crosslinking of collagen and inhibits collagen degradation.22 Meanwhile, a more stable and dense collagen fiber network can effectively maintain the original minerals and promote collagen remineralization.23,24
Tannic acid (TA) is a plant-derived polyphenol composed of a complex mixture of polygalloyl glucose esters that has biological stability and high crosslinking potential for collagen.25 Bedran et al. found that the formation of hydrogen bonds between the amide NH group of collagen and hydroxyl group of TA enhanced the mechanical properties of dentin.26 In addition, as a novel desensitizer, the pyrogallol groups of TA combine with calcium ions to promote the mineral nucleation of dentin collagen.27
In this paper, we report that TA induces biomineralization of dentin by dual effects. The first objective of this study was to demonstrate that the TA modification of demineralized dentin collagen matrix is resistant to enzymatic degradation of collagenase and restores the biomechanical properties of the tissue. The second objective was to investigate the mechanism of promoting mineralization by inducing the intrafibrillar mineralization of dentin collagen crosslinked by TA.
2. Experimental methods
2.1 Material and reagents
Thymol, hexamethyldisilazane, phosphoric acid, CaCl2, Na2HPO4, poly-acrylic acid (PAA average Mw = 1800), NaCl, Tris, NaN3 and bacterial collagenase from Clostridium histolyticum (type I, ≥125 CDU per mg solid) were purchased form Sigma-Aldrich, USA. Fifty wt% of glutaraldehyde, TA, HCl, NaOH, ninhydrin were obtained from Aladdin, Shanghai, China. A hydroxyproline (HYP) detection kit was obtained from Jiancheng, Nanjing, China. Hank's Balanced Salt Solution (HBSS) was purchased from Solarbio, Beijing, China.2.2 Specimen preparation
All experiments were performed in accordance with“international Ethical Guideline and Helsinki Declaration” and “national Ethics Censorship of Biomedical Research Involving Human Subject”, and were approved by the ethics committee of the First Affiliated Hospital of Zhejiang University School of Medicine. Informed consents were obtained from human participants of this study. We collected freshly extracted human non-caries third molars. An ultramicrotome (Boeckeler Instruments, Tucson, USA) was used to remove the crown enamel, and then the dentin was cut into discs approximately 1 mm thick below the enamel-dentinal Junction (EDJ). During the experiment, the prepared dentin was made into dentin slices with dimensions of 5 mm × 5 mm × 1 mm (n = 60) and dentin strips with dimensions of 10 mm × 0.8 mm × 0.8 mm (n = 168), and the dentin was polished using 600-, 1200- and 2000-grit silicon carbide papers, respectively. The dentin sample was immersed in 37% phosphoric acid for 15 s and rinsed with deionized water for 1 min to produce a 3–5 μm thick artificial demineralized dentin layer.
The prepared solution was equally divided into two mineralized solutions for the experimental group and the control group. TEM-grid-loaded collagen fibrils were floated over the mineralization solution (partially demineralized dentin slices and collagen membranes were immersed in the mineralization solution) at 37 °C for a designated time. Remineralization was carried out at a constant temperature of 37 °C in an incubator. During the experiment of TEM-grid-loaded collagen fibrils and dentin, the remineralization solution was maintained, while the mineralization solution of the collagen membrane was changed after 5 days of mineralization. At designated time intervals (0 days, 2 days, and 4 days), 10 nickel meshes were removed from the mineralization solution of the control group and the TA group and sequentially washed with deionized water, 50% ethanol, and 100% ethanol. At the same time, 10 dentin slices were removed, washed with deionized water, dehydrated in 35%, 50%, 70%, 90% and 100% alcohol, and immersed in hexamethyldisilazane (HMDS) for 1 hour. Subsequently, each dentin slice was divided equally for TEM and scanning electron microscopy (SEM) observation. After 10 days of mineralization, the collagen membranes were taken out and washed three times with deionized water, and then freeze-dried for 24 hours.
2.3 Experimental part
| Crosslinking degree (%) = 100(Mo − Mt)/Mo |
| Group (name, sample size) | TA crosslinking | Remineralization |
|---|---|---|
| A (demineralized dentin, n = 42) | — | — |
| B (dentin with TA crosslinking, n = 42) | TA crosslinking for 2 hours | — |
| C (dentin collagen without TA crosslinking and 7 days of remineralization, n = 42) | — | 7 days of remineralization |
| D (dentin collagen with TA crosslinking and 7 days of remineralization, n = 42) | TA crosslinking for 2 hours | 7 days of remineralization |
In addition, for monitoring the effect of TA on minerals, the remineralized dentin strips were demineralized with 1% ethylenediaminetetraacetic acid (EDTA) at pH 7.4 and room temperature.
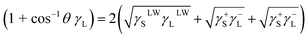 | (1) |
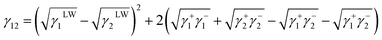 | (2) |
3. Results and discussion
3.1 Analysis of TA crosslinking
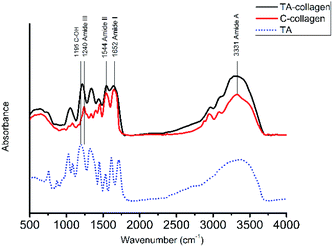 | ||
| Fig. 1 FTIR for natural C-collagen (red line) and TA-collagen (black line) as well as for TA (blue dashed line). | ||
| Band | Band frequency (cm−1) | |
|---|---|---|
| C-collagen | TA-collagen | |
| Amide A | 3331 | 3317 |
| Amide I | 1652 | 1641 |
| Amide III | 1240 | 1217 |
| TA: C–OH | — | 1195 |
TA is a specific class of hydrolysable tannins, containing a penta galloyl glucose core, which has a five-arm polyphenol structure.34,35 The type I collagen which contains three repeating sequences of polypeptide α-chains, each consisting of more than 1000 amino acids. The interactions between proteins and polyphenols can involve hydrogen bonds, covalent bonds, ions, and hydrophobic bonds.36 A large number of phenolic hydroxyls in the polyphenol derivative crosslinker have a good modification effect on the collagen.37 TA crosslinked collagen mainly by noncovalent bonding under nonoxidized conditions.38 The H bond between amide NH and OH of the TA galloyl group was shown in Fig. 10.39
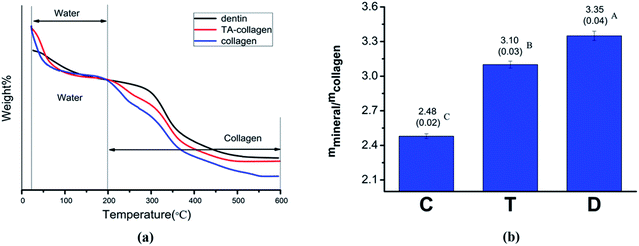
Analysis of the FTIR results confirmed that a slight redshift was shown in TA crosslinked collagen, indicating that hydrogen bond occurred between the TA molecule and the collagen. The absorption peak of phenolic hydroxyl vibration due to TA was observed. The crosslinking degree of the collagen membrane pretreated with TA reached a maximum 41.28 ± 1.52, which was also supported by the ultrastructure of type I collagen fibrils. The anti-enzymatic hydrolysis effect was tested on the crosslinked type I collagen of dentin. Therefore, TA can be used as a cross-linker in demineralized dentin collagen.
3.2 Influence of TA on mineralization
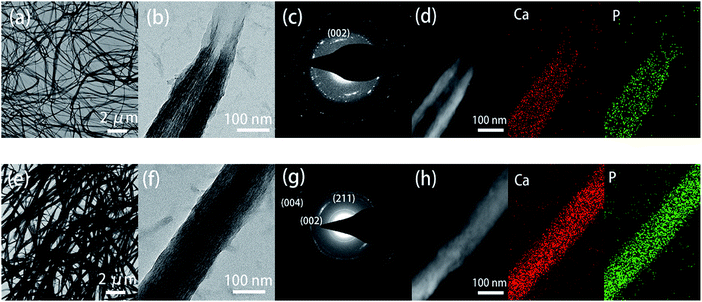
As the in vivo mineralization of deposited crystals cannot be directly monitored, self-assembly collagen can be used as a model system to study the mineralization of dentin collagen fibers. The surface structure of the unmineralized collagen membrane was relatively smooth, showing a thin strip-like appearance. After mineralization, the collagen diameter was significantly thickened, and the rope-like structure was more pronounced after TA crosslinking. Energy dispersive X-ray spectrometer (EDS) analysis showed that obvious Ca and P signals appeared in the experimental group and control group after mineralization, and the Ca/P ratio was approximately 1.62, which proved that a large amount of HAP was compounded on the collagen after mineralization (Fig. S2†).
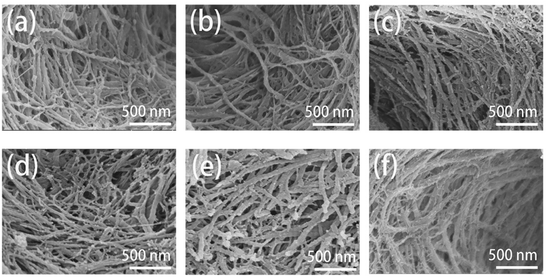 | ||
| Fig. 5 SEM images of the surface morphologies of remineralized dentin without (a)–(c) and with TA-pretreatment (d)–(f) for 0 day, 2 days, and 4 days. | ||
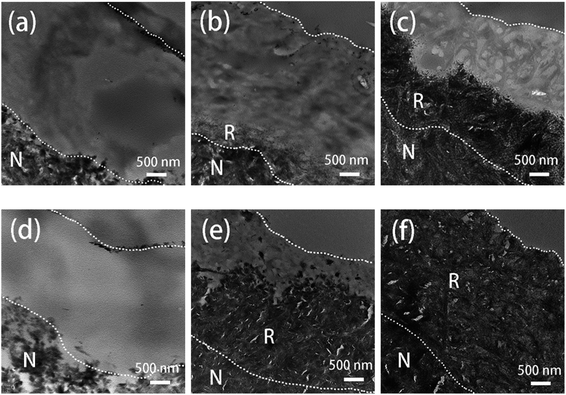 | ||
| Fig. 6 TEM images of remineralized dentin without (a–c) and with TA-pretreatment (d–f) for 0 day, 2 days, and 4 days. N: natural dentin. R: remineralized dentin. | ||
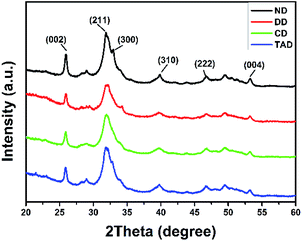
Fig. 7 showed the XRD results of the surface of the dentin disks. After demineralization, the characteristic HAP peaks decreased, and the low crystallinity comes from the incomplete demineralization of the dentin. After repair, significant characteristic HAP diffraction peaks were observed on surface of both remineralization dentin (C-dentin) and TA crosslinked remineralization dentin (TA-dentin) indicating that the precipitates were HAP. Especially, the XRD pattern of TA-dentin exhibited the characteristic HAP diffraction peaks (300) at 2θ = 33, which was not found in C-dentin.
| γ | γLw | γ+ | γ− | γij | |
|---|---|---|---|---|---|
| C-collagen | 22.06 | 21.40 | 0.6 | 0.18 | 10.59 |
| TA-collagen | 28.53 | 21.62 | 1.04 | 11.49 | 4.19 |
According to the results, the mechanism of TA promoting remineralization in this study can be described as follows. The experiments demonstrated that the crosslinking of the collagen fibrils during the TA pretreatment, and the triple helix conformation of collagen was not destroyed. Collagen was considered as a template for the growth of mineral crystals. TA binds to the endogenous protease cleavage site and prevents enzyme substrate interactions, protecting the exposed collagen from solubilization by proteases. In addition, we confirmed that the interface energy between ACP and collagen was decreased after TA treatment, which facilitated the heterogeneous formation of ACP precursors on collagen. The lower interface promoted more ACP to penetrate into collagen fibers and subsequent intrafibrillar mineralization by a wetting effect. TA provided additional nucleation sites for calcium phosphate crystals. TA facilitated the mineralization of dentin by binding to calcium ions and accumulating them at the interface due to the presence of abundant phenolic hydroxyls, thereby accelerating the formation of HAP (Fig. 10).44
4. Conclusions
The biomimetic mineralization of dentin is rarely transformed into clinical application based on the slow restoration of the polymer-induced liquid-precursor (PILP) phase. The demineralized dentin collagen fiber network degrades when attacked by MMPs.45 This research demonstrates that the mechanical properties and enzymatic resistance of collagen improved after the dentin was treated with TA. In addition, the interfacial energy can be reduced, which makes it easier for the liquid nanoprecursor to enter the collagen fiber and promotes the mineralization process. Therefore, TA adjusts the mineralization interface through the wetting effect, this finding may provide unique insight for the crosslinking agent in promoting mineralization.Author contributions
Weijing Kong and Qiaolin Du: methodology, investigation, writing – original draft,writing – review & editing. Yinan Qu: investigation, formal analysis. Changyu Shao: validation. Chaoqun Chen: software. Jian Sun: project administration. Caiyun Mao: funding acquisition. Ruikang Tang: resources. Xinhua Gu: conceptualization, supervision, funding acquisition, methodology, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge project funding provided by the Public Welfare Technology Application Research Project of Zhejiang Province (LGF18H140001), Natural Science Foundation of Zhejiang Province (LY20H140002), Medical and Health Science and Technology Project of Zhejiang Province (2018KY073, 2019KY383).References
- A. Linde, Anat. Rec., 1989, 224, 154–166 CrossRef CAS PubMed.
- J. Ma, J. Wang, X. Ai and S. Zhang, Biotechnol. Adv., 2014, 32, 744–760 CrossRef CAS PubMed.
- K. Gelse, E. Poschl and T. Aigner, Adv. Drug Delivery Rev., 2003, 55, 1531–1546 CrossRef CAS PubMed.
- F. Nudelman, A. J. Lausch, N. A. J. M. Sommerdijk and E. D. Sone, J. Struct. Biol., 2013, 183, 258–269 CrossRef CAS PubMed.
- T. A. Ulrich, A. Jain, K. Tanner, J. L. MacKay and S. Kumar, Biomaterials, 2010, 31, 1875–1884 CrossRef CAS PubMed.
- J. B. Forien, C. Fleck, P. Cloetens, G. Duda, P. Fratzl, E. Zolotoyabko and P. Zaslansky, Nano Lett., 2015, 15, 3729–3734 CrossRef CAS PubMed.
- V. Hass, I. V. Luque-Martinez, M. F. Gutierrez, C. G. Moreira, V. B. Gotti, V. P. Feitosa, G. Koller, M. F. Otuki, A. D. Loguercio and A. Reis, Dent. Mater., 2016, 32, 732–741 CrossRef CAS PubMed.
- L. B. He, Y. Hao, L. Zhen, H. L. Liu, M. Y. Shao, X. Xu, K. N. Liang, Y. Gao, H. Yuan, J. S. Li, J. Y. Li, L. Cheng and C. van Loveren, J. Struct. Biol., 2019, 207, 115–122 CrossRef CAS PubMed.
- Y. Liu, L. Tjaderhane, L. Breschi, A. Mazzoni, N. Li, J. Mao, D. H. Pashley and F. R. Tay, J. Dent. Res., 2011, 90, 953–968 CrossRef CAS PubMed.
- L. F. Barbosa-Martins, J. P. Sousa, L. A. Alves, R. P. W. Davies and R. M. Puppin-Rontanti, Materials, 2018, 11 Search PubMed.
- J. D. Featherstone, M. Fontana and M. Wolff, J. Dent. Res., 2018, 97, 125–127 CrossRef CAS PubMed.
- A. George and A. Veis, Chem. Rev., 2008, 108, 4670–4693 CrossRef CAS PubMed.
- A. Rao, J. K. Berg, M. Kellermeier and D. Gebauer, Eur. J. Mineral., 2014, 26, 537–552 CrossRef CAS.
- W. J. Jin, S. Q. Jiang, H. H. Pan and R. K. Tang, Crystals, 2018, 8, 48 CrossRef.
- M. J. Olszta, D. J. Odom, E. P. Douglas and L. B. Gower, Connect. Tissue Res., 2003, 44(1), 326–334 CrossRef CAS PubMed.
- M. A. S. Melo, S. F. F. Guedes, H. H. K. Xu and L. K. A. Rodrigues, Trends Biotechnol., 2013, 31, 459–467 CrossRef CAS PubMed.
- M. J. Olszta, X. G. Cheng, S. S. Jee, R. Kumar, Y. Y. Kim, M. J. Kaufman, E. P. Douglas and L. B. Gower, Mater. Sci. Eng., 2007, 58, 77–116 CrossRef.
- F. Nudelman, K. Pieterse, A. George, P. H. H. Bomans, H. Friedrich, L. J. Brylka, P. A. J. Hilbers, G. de With and N. A. J. M. Sommerdijk, Nat. Mater., 2010, 9, 1004–1009 CrossRef CAS PubMed.
- C. Y. Shao, R. B. Zhao, S. Q. Jiang, S. S. Yao, Z. F. Wu, B. Jin, Y. L. Yang, H. H. Pan and R. K. Tang, Adv. Mater., 2018, 30 Search PubMed.
- Y. N. Qu, T. Y. Gu, Q. L. Du, C. Y. Shao, J. Wang, B. Jin, W. J. Kong, J. Sun, C. Q. Chen, H. H. Pan, R. K. Tang and X. H. Gu, ACS Biomater. Sci. Eng., 2020, 6, 3327–3334 CrossRef CAS PubMed.
- C. Chaussain-Miller, F. Fioretti, M. Goldberg and S. Menashi, J. Dent. Res., 2006, 85, 22–32 CrossRef CAS PubMed.
- T. R. Aguiar, C. M. P. Vidal, R. S. Phansalkar, I. Todorova, J. G. Napolitano, J. B. McAlpine, S. N. Chen, G. F. Pauli and A. K. Bedran-Russo, J. Dent. Res., 2014, 93, 417–422 CrossRef CAS PubMed.
- T. M. Du, X. F. Niu, Z. W. Li, P. Li, Q. L. Feng and Y. B. Fan, Int. J. Biol. Macromol., 2018, 113, 450–457 CrossRef CAS PubMed.
- C. M. P. Vidal, T. R. Aguiar, R. Phansalkar, J. B. McAlpine, J. G. Napolitano, S. N. Chen, L. S. N. Araujo, G. F. Pauli and A. Bedran-Russo, Acta Biomater., 2014, 10, 3288–3294 CrossRef CAS PubMed.
- A. C. Machado, E. Dezan Junior, J. E. Gomes-Filho, L. T. Cintra, D. B. Ruviere, R. Zoccal, C. A. Damante and E. G. Jardim Junior, J. Appl. Oral Sci., 2012, 20, 414–418 CrossRef PubMed.
- A. K. B. Bedran-Russo, K. J. Yoo, K. C. Ema and D. H. Pashley, J. Dent. Res., 2009, 88, 807–811 CrossRef CAS PubMed.
- D. X. Oh, E. Prajatelistia, S. W. Ju, H. J. Kim, S. J. Baek, H. J. Cha, S. H. Jun, J. S. Ahn and D. S. Hwang, Sci. Rep., 2015, 5, 10884 CrossRef CAS PubMed.
- J. Wang, Y. N. Qu, C. Q. Chen, J. Sun, H. H. Pan, C. Y. Shao, R. K. Tang and X. H. Gu, Mater. Sci. Eng., C, 2019, 104, 109959 CrossRef CAS PubMed.
- P. Velmurugan, E. R. A. Singam, J. R. Rao and V. Subramanian, Biopolymers, 2014, 101, 471–483 CrossRef CAS PubMed.
- M. Jastrzebska, J. Zalewska-Rejdak, R. Wrzalik, A. Kocot, I. Mroz, B. Barwinski, A. Turek and B. Cwalina, J. Biomed. Mater. Res., Part A, 2006, 78, 148–156 CrossRef CAS PubMed.
- O. I. Ulusoy and G. Gorgul, Aust Endod J., 2013, 39, 66–72 CrossRef PubMed.
- M. P. Gashti, M. Bourquin, M. Stir and J. Hulliger, J. Mater. Chem. B, 2013, 1, 1501–1508 RSC.
- M. G. Gandolfi, P. Taddei, A. Pondrelli, F. Zamparini, C. Prati and G. Spagnuolo, Materials, 2018, 12, 25 CrossRef PubMed.
- X. Zhang, M. D. Do, P. Casey, A. Sulistio, G. G. Qiao, L. Lundin, P. Lillford and S. Kosaraju, J. Agric. Food Chem., 2010, 58, 6809–6815 CrossRef CAS PubMed.
- J. Guo, W. Sun, J. P. Kim, X. Lu, Q. Li, M. Lin, O. Mrowczynski, E. B. Rizk, J. Cheng, G. Qian and J. Yang, Acta Biomater., 2018, 72, 35–44 CrossRef CAS PubMed.
- C. Simon, K. Barathieu, M. Laguerre, J. M. Schmitter, E. Fouquet, I. Pianet and E. J. Dufourc, Biochemistry, 2003, 42, 10385–10395 CrossRef CAS PubMed.
- S. Varma, J. P. Orgel and J. D. Schieber, Biophys. J., 2016, 111, 50–56 CrossRef CAS PubMed.
- L. Wu, H. Shao, Z. Fang, Y. Zhao, C. Y. Cao and Q. Li, ACS Biomater. Sci. Eng., 2019, 5, 4272–4284 CrossRef CAS PubMed.
- F. H. Heijmen, J. S. du Pont, E. Middelkoop, R. W. Kreis and M. J. Hoekstra, Biomaterials, 1997, 18, 749–754 CrossRef CAS PubMed.
- P. J. M. Smeets, A. R. Finney, W. Habraken, F. Nudelman, H. Friedrich, J. Laven, J. J. De Yoreo, P. M. Rodger and N. Sommerdijk, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, E7882–E7890 CrossRef CAS PubMed.
- W. Traub, T. Arad and S. Weiner, Proc. Natl. Acad. Sci. U. S. A., 1989, 86, 9822–9826 CrossRef CAS PubMed.
- S. S. Yao, B. A. Jin, Z. M. Liu, C. Y. Shao, R. B. Zhao, X. Y. Wang and R. K. Tang, Adv. Mater., 2017, 29 Search PubMed.
- Y. N. Wang, S. Q. Jiang, H. H. Pan and R. K. Tang, CrystEngComm, 2016, 18, 379–383 RSC.
- J. Guo, Y. Ping, H. Ejima, K. Alt, M. Meissner, J. J. Richardson, Y. Yan, K. Peter, D. von Elverfeldt, C. E. Hagemeyer and F. Caruso, Angew. Chem., Int. Ed., 2014, 53, 5546–5551 CrossRef CAS PubMed.
- J. Sun, C. Q. Chen, H. H. Pan, Y. Chen, C. Y. Mao, W. Wang, R. K. Tang and X. H. Gu, J. Mater. Chem. B, 2014, 2, 4544–4553 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra07887a |
| ‡ Weijing Kong and Qiaolin Du contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) <
<