 Open Access Article
Open Access ArticleProfiling of co-metabolic degradation of tetracycline by the bio-cathode in microbial fuel cells†
Luxiang Wang *ab,
Dongmin Liangab and
Yunqi Shiab
*ab,
Dongmin Liangab and
Yunqi Shiab
aSchool of Environment and Energy, South China University of Technology, Guangzhou Higher Education Mega Centre, Guangzhou 510006, P. R China. E-mail: luxiangscut@163.com
bThe Key Lab of Pollution Control and Ecosystem Restoration in Industry Clusters, Ministry of Education, Guangzhou Higher Education Mega Centre, Guangzhou 510006, P. R China
First published on 21st December 2021
Abstract
In this paper, a system of tetracycline (TEC) degradation by the bio-cathode in a microbial fuel cell (MFC) was constructed. Overall, the co-metabolic degradation performance of TEC was studied through single factor experiments and the ecological risk was evaluated using the E. coli growth inhibition rate and resistance genes. High throughput sequencing (HTS) was utilized to profile the biofilm community structure of the bio-cathode. Results showed that the degradation rate of TEC reached greater than 90% under optimal conditions, which was 10 mg L−1 initial TEC concentration, 0.2–0.7 g L−1 sodium acetate concentration and 12–18 L h−1 aeration. Furthermore, compared with the aerobic biodegradation of TEC, the degradation efficiency of the MFC bio-cathode for TEC was significantly increased by 50% and the eco-toxicity of TEC after 36 hour degradation was reduced by 60.9%, and TEC ARGs in effluent were cut down. HTS results showed that electrochemically active bacteria Acetobacter and TEC-resistant degradation bacteria Hyphomicrobium, Clostridium and Rhodopseudomonas were the main dominant bacteria in the cathode biofilm. Besides, based on 5 intermediates, degradation pathways involving deamidation, denitro dimethylation, dedimethylation and dehydroxylation of TEC were proposed. The degradation of TEC on the bio-cathode was mainly caused by microbial co-metabolism action. This study would enrich the study of MFC bio-cathodic degradation of antibiotics in water.
1. Introduction
In recent years, tetracycline (TEC) has been highly detected in water environments, with a detection range between μg and ng.1–4 For example, in the Pearl River Basin of China, TEC is always detected and the highest concentration has been up to 385.5 ng L−1.5 Moreover, a great deal of studies have implied that TEC enriched in the aquatic environment can inhibit the growth of microbes, plants and aquatic organisms, reduce biodiversity, spread or diffuse the resistance genes, and even affect the survival and development of human beings,6 which has attracted widespread attention. Therefore, it is imperative to reduce TEC in water.Conventional water treatment processes cannot meet the requirements of removing TEC from municipal sewage, pharmaceutical wastewater and aquaculture wastewater.7 Therefore, researchers have been committed to questing efficient and low-cost treatment methods to dispose wastewater containing TEC. For the past few years, many investigations have verified that microbial fuel cells (MFC) can degrade antibiotics and other refractory contaminants, among which some have explored the means of MFC to degrade TEC. Yan et al.8 started MFC via inoculating pig manure supernatant and then replaced sodium acetate in the anolyte with TEC gradually. After a long-term operation for 330 d, the degradation rate of TEC with an initial concentration of 10 mg L−1 on MFC anode reached 99% within 72 h. Furthermore, compared with control groups containing microbes, the higher ATP concentration and continuous electrical stimulation in MFC process may be the reasons for the higher degradation efficiency of TEC. The results of high throughput sequencing (HTS) implied that the total resistance genes in the effluent of MFC were significantly reduced compared with that of the microbial control group.8 Peng et al.9 added glucose into the anode chamber of MFC for co-metabolism of TEC. Their results showed that MFC displayed good removal efficiency for TEC in a concentration range of 1–20 mg L−1. When the concentration of TEC was 20 mg L−1, the degradation rate of TEC could reach 87% within 80 h and the maximum power density was 660 mW m−3. It was also proved that the toxicity of degradation products was weakened. Therefore, the anode of MFC has advantages of high efficiency and no harm to degrade TEC, but it still has disadvantages of long-running time and slow degradation rate.
With the further investigations, it is found that degradation of antibiotics by anode of MFC has some problems, such as the low degradation efficiency, the slow microbial growth, the long start-up and running time, and so on. On the contrary, the bio-cathode of MFC usually don't need to add catalyst to improve the electron transfer efficiency to improve the degradation rate, indicating a higher degradation efficiency. Moreover, the cathode chamber is an aerobiotic environment, so the MFC start-up and running time can be accelerated. For example, Liang et al.10 reported that the degradation rate of chloramphenicol (CAP) on bio-cathode was 96% within 24 h under an applied voltage of 0.5 V, which was much higher than that of non-bio-cathode (73%). They also found that the degradation process of CAP on bio-cathode would not generate highly toxic degradation intermediates. Sun et al.11 also proved that the degradation rate of CAP on bio-cathode outclassed that on non-bio-cathode and was about 3.2 times. The electrochemical performance of bio-cathode was superior to non-bio-cathode as well. What is said above showed that degradation of antibiotics on bio-cathode possesses merits of high degradation efficiency/rate and non-toxic degradation products. Additionally, Chen et al.12 also explored the degradation of oxytetracycline (OTC) by bio-cathode of MFC and confirmed the feasibility of bio-cathode in degrading TEC. Nonetheless, no further researches were conducted to investigate the degradation conditions, mechanism and efficiency of bio-cathode. In general, investigations involving the performance and mechanism of degrading TEC on bio-cathode are still scarce at present.
Therefore, this study took TEC as the target pollutant and planned to construct a system to degrade TEC via bio-cathode of MFC. The influences involving initial concentration of TEC, type of carbon sources, concentration of carbon sources and aeration strength on the degradation of TEC via bio-cathode were investigated through single-factor experiments. From this, the suitable conditions for degradation of TEC on bio-cathode in MFC were obtained, and then, the pseudo-first order kinetics model was used to fit degradation kinetics. Moreover, the ecological risk of TEC degradation on bio-cathode in MFC was evaluated through analyzing the toxicity of degradation intermediates and the variations of antibiotics resistance genes (ARGs) in order to comprehensively assess the performance of TEC degradation by bio-cathode in MFC. Finally, the probable degradation pathway of TEC was speculated via authenticating degradation intermediates, while the mechanism of co-metabolic degradation for TEC on bio-cathode was elucidated via analyzing HTS of cathode biofilm communities. This study provided a theoretical basis for the decontamination of wastewater containing TEC by bio-cathode in MFC.
2. Materials and methods
2.1 Materials and reagents
The inoculated sludge was taken from the sludge concentration tank in the aerobic stage of a sewage treatment plant in Guangzhou, Guangdong province.Tetracycline (C22H24N2O8) was purchased from Macklin Company. Sodium chloride (NaCl), ammonium chloride (NH4Cl), sodium dihydrogen phosphate (NaH2PO4), disodium hydrogen phosphate (Na2HPO4) and glucose (C6H12O6) were all purchased from Guangzhou Chemical Reagent Works.
2.2 Construction, inoculation and operation of MFC bio-cathode
The configuration of the two-chamber MFC reactor followed the previous literature published by our research group.13 The two-chamber MFCs used were cubic in appearance with two cylinder reactors (2.2 cm in depth and 5 cm in diameter) in both sides, composed of carbon felt cathode and anode. The carbon felts used were cut into circles (3 cm in diameter) and cleaned by acetone before use. The main components of the anolyte were glucose (1000 mg L−1), PBS buffer solution (50 mmol L−1), vitamins and microelements (12.5 mL L−1).14 The catholyte mainly included sodium bicarbonate (500 mg L−1), sodium acetate (500 mg L−1), PBS buffer solution (50 mmol L−1), vitamins and microelements (12.5 mL L−1).Due to the slow start-up speed of anaerobic anode, single-chamber MFC was used to make the anode operate in advance, and the anolyte was renewed every 7 d. After the output voltage stabilized, the anode was taken down and assembled into a new two-chamber MFC. Then, a concentration array including 5, 10, 15, and 20 mg L−1 TEC was added into the medium, with one concentration used for each cycle (3d), as described in previous reports.15,16 The overall time for a successful operation was about half a month. The aeration rate in cathode chamber was about 12 L h−1. The concentration of TEC was measured every 4 h. When the degradation rate of TEC (C = 20 mg L−1) on bio-cathode reached more than 70%, the operation of this MFC reactor was considered to be successful.
2.3 Analysis and measurement methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 69, v(acetonitrile)
69, v(acetonitrile)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v(oxalic acid)); detection wavelength was 355 nm; column temperature was 30 °C; flow rate was 1 mL min−1; injection volume was 20 μL.
v(oxalic acid)); detection wavelength was 355 nm; column temperature was 30 °C; flow rate was 1 mL min−1; injection volume was 20 μL.The degradation intermediates were detected by liquid chromatograph-mass spectrometer/mass spectrometer (LC-MS/MS). The samples were collected at the time of 12 h, 24 h and 36 h, and then mixed and centrifuged. Then, the supernatant was concentrated with nitrogen bubbling, with a concentration factor of 50 times. The concentrated solution was then filtered by 0.22 μm cellulose acetate membrane and injected into the LC-MS/MS instrument.
In order to investigate the effects of TEC on the succession of microbial communities in bio-cathode, HTS analysis was adopted. The samples of original activated sludge, sludge containing TEC and operated for 60 d, sludge containing no TEC and operated for 60 d, and finally, the samples of open circuit MFC containing TEC and operated for 60 d were collected respectively. After extracting the DNA with the kit, the DNA was amplified by PCR and then sent to BioMaker Company (Beijing) for sequencing.
3. Results and discussion
3.1 TEC degradation performances and kinetics on MFC bio-cathode
By carrying out the singlet-factor degradation experiment, it was helpful to explore the impacts of main factors on the biological degradation of TEC in bio-cathode system, as well as inquiry into the degradation kinetics according to the pseudo-first order kinetic fitting. The results are shown in Fig. 1.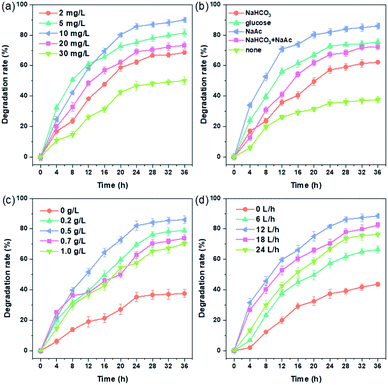 | ||
| Fig. 1 Influence factors on TEC degradation: (a) initial concentration of TEC, (b) type of carbon sources, (c) concentration of carbon sources (sodium acetate), (d) aeration intensity. | ||
3.2 Mechanism of TEC degradation on MFC bio-cathode
 | ||
| Fig. 2 TEC degradation processes in different units (degradation condition: CTEC = 10 mg L−1, NaAC as carbon source, Ccarbon source = 0.5 g L−1, aeration strength = 12 L h−1). | ||
The degradation efficiency of TEC in MFC bio-cathode system was 88.86%, which was about 50% higher than that in bio-degradation system, and the reaction rate was about twice as high as that in bio-degradation system. The reason might be that the weakly electric stimulation of MFC can promote the growth and metabolism of microbes, thus improved the degradation efficiency and rate of TEC. However, further verifications were needed from the perspective of microbial communities.
Compared with the TEC-free sample A2 and open circuited cathode sample A4, the MFC bio-cathode sample A1 produced different microbial communities, indicating that different external conditions had a great influence on the evolution of microbial communities. At gate level in Fig. 3(a), Proteobacteria had the highest content in all the four systems, which were 60.58% (A1), 86.08% (A2), 40.43% (A3) and 64.21% (A4), respectively. Other dominant bacteria were Firmicutes, Bacteroides and Actinobacteria. By comparison with the unacclimated sludge, the content of Proteobacteria in A1, A2 and A4 raised by 20.15%, 45.65% and 23.78%, respectively. Especially, in the bio-cathode sample without TEC (A2), Proteobacteria was the dominant group plenarily, because Proteobacteria is a common dominant bacteria phylum in wastewater treatment systems and it contains a large number of electric-producing bacteria.24 It is worth mentioning that in sample A1 with TEC, the relative abundance of Firmicutes was 21.93%, which was 18.68% higher than the original sample (3.25%), while the relative abundance of Firmicutes in another sample A4 containing TEC also increased by 5.98%. However, in sample A2 without TEC, the relative abundance of Firmicutes did not change significantly. It implied that Firmicutes had a strong resistance to TEC, which might be the vital bacteria in cathode biofilm to degrade TEC, and the result was consistent with the research results of Wang et al.25
The heat map of bacterial community distribution at genus level for different biological samples was shown in Fig. 3(b). Under the stress of TEC and electron transport, the abundance and species of dominant microflorae in samples A1, A2 and A4 evolved greatly, compared with that in sludge sample A3. However, A1 and A2 had relatively similar bacterial community, might because that both of them were microbial electrochemical systems, leading to the enrichment of a large number of electrochemically active bacteria on the cathode. In detail, the dominant microbe on cathode biofilm included Rhodopseudomonas, Hyphomicrobium, Acetobacter, Clostridium, etc. There into, Rhodopseudomonas was enriched in all the three samples A1, A2 and A4. Wu et al.26 proved that Rhodopseudomonas has a good degradation performance for TEC. Besides, Hyphomicrobium was a common facultative anaerobe in bioelectrochemical systems with the function of promoting hydrolysis and reducing refractory organic contaminants.27 The existence of Hyphomicrobium in the bio-cathode was likely to promote the degradation of TEC. Acetobacter was also an electrochemically active bacterium. When the carbon source was sodium acetate, Acetobacter can easily accomplish electron transfer process in MFC.28 It could be inferred that Acetobacter is primarily a medium for electron transport to the bio-cathode, thus provided continuous electrical stimulation for microbes. In addition, Clostridium was a TEC-resistant bacterial genus commonly found in animal intestines.29 In sample A4, the TEC degrading bacterium Rhodopseudomonas was the dominant specie in the process of aerobic microbial degradation and the relative abundance was 18.5%, which was much higher than other microbes. However, the relative abundance of electrochemically active bacterium Acetobacter in A4 was much lower than in sample A1, indicating that aerobic microbial degradation system could enrich TEC degrading bacteria, but could not enrich electrochemically active bacteria.30
In conclusion, in the process of degrading TEC by MFC bio-cathode, a large number of electroactive bacteria and TEC degrading bacteria were enriched on the bio-cathode. As a result, TEC could be rapidly degraded under the interaction of electrochemically active bacteria and TEC degrading bacteria. Therefore, the efficient performance of MFC bio-cathode to degrade TEC was further proved from the perspective of microbial communities.
The overall degradation pathways of TEC degraded on MFC bio-cathode was shown in Fig. S3.† The substance with the molecular fragment of 444 m/z was judged as TEC parent. Among the degradation intermediates of TEC by MFC bio-cathode, the substances represented by the fragments of 430 m/z and 427 m/z could be inferred as the demethylation and deamination products of TEC respectively, based on the structural formula of TEC molecular. The molecular fragment of 417 m/z might be the further demethylation product of B430, while the molecular E404 was the further demethylation product of D417. Besides, F384 was the deamination product by removing two methyl groups of C427. It could be seen from these intermediates that the degradation process of TEC by MFC bio-cathode mainly includes demethylation, deamination, dedimethylation and other processes. In the process of TEC degradation, the active sites of amides and dimethyl groups on TEC were gradually destroyed and the antibacterial activity of tetracycline decreased, which explained why the eco-toxicity of effluent from MFC bio-cathode was eliminated to some extent.
3.3 Ecological risk assessment of TEC degradation on MFC bio-cathode
4. Conclusions
To sum up, the method of gradient domestication was utilized to successfully construct and operate the MFC containing bio-cathode, as well as to realize the high-efficiency degradation of TEC. The optimal degradation conditions were determined as followed: 10 mg L−1 of initial concentration of TEC, sodium acetate as the carbon source, 0.2–0.7 g L−1 of concentration of carbon source, 12–18 L h−1 of aeration strength, and the highest degradation rate of TEC reached more than 90% after 36 hour degradation. The degradation efficiency was significantly enhanced compared with aerobic biodegradation or MFC bio-anodic degradation of TEC. The growth inhibition rate of E. coli indicated that the final degraded sample showed less toxicity compared with TEC parent, reducing by 60.9%, leading to the very low ecological toxicity of the effluent. Moreover, the results of ARGs determination showed that the concentrations of tetA, tetC, tetM and tetX were all enriched in MFC bio-cathode system except for tetO, but the magnitude of ascent was much lower than in the aerobic biodegradation system, implying that MFC bio-cathode degradation was beneficial to cutting down ARGs in the effluent. The analysis of HTS showed that dominant microflorae on bio-cathode were electrochemically active bacteria such as Acetobacter, Rhodopseudomonas and TEC-resistant degradation bacteria such as Hyphomicrobium, Clostridium, Stenotrophomonas and Mycobacterium. Five kinds of intermediates were determined. Meanwhile, two degradation pathways involving demethylation, deamination, dehydroxyl and dedimethylation of TEC were speculated. The degradation of TEC by MFC bio-cathode was owing to the action of microbes and the improvement of microbial metabolic activity under electrical stimulation, and that the degradation of TEC was improved by leaps and bounds consequently.Author contributions
Conceptualization, Luxaing Wang; methodology, Luxaing Wang and Dongmin Liang; software, Luxaing Wang; investigation, Luxaing Wang, Dongmin Liang and Yunqi Shi; data curation, Dongmin Liang and Yunqi Shi; writing – original draft preparation, Luxaing Wang. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is funded by the National Natural Science Foundation of China and Foundation of Guangdong Province of China (No. 21477039, No. U1401235, No. 2016B020240005).References
- E. T. Furlong, D. W. Kolpin, M. T. Meyer, E. Michael Thurman, S. D. Zaugg, L. B. Barber and H. T. Buxton, Environ. Sci. Technol., 2002, 36, 1202–1211 CrossRef PubMed.
- Y. Zhang, C. Zhang, D. B. Parker, D. D. Snow, Z. Zhou and X. Li, Sci. Total Environ., 2013, 463–464, 631–638 CrossRef CAS PubMed.
- W.-J. Deng, N. Li and G.-G. Ying, Environ. Geochem. Health, 2018, 40, 2191–2203 CrossRef CAS PubMed.
- C. Yan, Y. Yang, J. Zhou, M. Liu, M. Nie, H. Shi and L. Gu, Environ. Pollut., 2013, 175, 22–29 CrossRef CAS PubMed.
- L. Xu, H. Zhang, P. Xiong, Q. Zhu, C. Liao and G. Jiang, Sci. Total Environ., 2021, 753, 141975 CrossRef CAS PubMed.
- J. Scaria, K. V. Anupama and P. V. Nidheesh, Sci. Total Environ., 2021, 771, 145291 CrossRef CAS PubMed.
- Q. Liao, H. Rong, M. Zhao, H. Luo, Z. Chu and R. Wang, Sci. Total Environ., 2021, 757, 143981 CrossRef CAS PubMed.
- W. Yan, Y. Guo, Y. Xiao, S. Wang, R. Ding, J. Jiang, H. Gang, H. Wang, J. Yang and F. Zhao, Water Res., 2018, 142, 105–114 CrossRef CAS PubMed.
- X. Peng, J. Cao, B. Xie, M. Duan and J. Zhao, Ecotoxicol. Environ. Saf., 2020, 188, 109869 CrossRef CAS PubMed.
- B. Liang, H. Y. Cheng, D. Y. Kong, S. H. Gao, F. Sun, D. Cui, F. Y. Kong, A. J. Zhou, W. Z. Liu, N. Q. Ren, W. M. Wu, A. J. Wang and D. J. Lee, Environ. Sci. Technol., 2013, 47, 5353–5361 CrossRef CAS PubMed.
- F. Sun, H. Liu, B. Liang, R. Song, Q. Yan and A. Wang, Bioresour. Technol., 2013, 143, 699–702 CrossRef CAS PubMed.
- J. Chen, Y. Hu, W. Huang, Y. Liu, M. Tang, L. Zhang and J. Sun, Bioresour. Technol., 2018, 270, 482–488 CrossRef CAS PubMed.
- L. Wang, Y. Wu, Y. Zheng, L. Liu and F. Zhao, RSC Adv., 2015, 5, 56430–56437 RSC.
- B. Qiu, Y. Hu, C. Liang, L. Wang, Y. Shu, Y. Chen and J. Cheng, Bioresour. Technol., 2020, 300, 122703 CrossRef CAS PubMed.
- X. Li, X. Zhang, X. Zhao, B. Yu, L. Weng and Y. Li, Appl. Biochem. Biotechnol., 2019, 189, 384–395 CrossRef CAS PubMed.
- X. Li, Y. Li, L. Zhao, Y. Sun, X. Zhang, X. Chen, L. Weng and Y. Li, Int. J. Environ. Res. Public Health, 2019, 16, 3897 CrossRef CAS PubMed.
- B. Hou, Y. Hu and J. Sun, Bioresour. Technol., 2012, 111, 105–110 CrossRef CAS PubMed.
- N. Guo, Y. Wang, L. Yan, X. Wang, M. Wang, H. Xu and S. Wang, Water Res., 2017, 117, 95–101 CrossRef CAS PubMed.
- S. K. Butti, G. Velvizhi, M. L. K. Sulonen, J. M. Haavisto, E. Oguz Koroglu, A. Yusuf Cetinkaya, S. Singh, D. Arya, J. Annie Modestra, K. Vamsi Krishna, A. Verma, B. Ozkaya, A.-M. Lakaniemi, J. A. Puhakka and S. Venkata Mohan, Renewable Sustainable Energy Rev., 2016, 53, 462–476 CrossRef CAS.
- S. Shao, Y. Hu, J. Cheng and Y. Chen, Bioresour. Technol., 2019, 288, 121567 CrossRef CAS PubMed.
- S. Long, L. Zhao, J. Chen, J. Kim, C. H. Huang and S. G. Pavlostathis, Bioresour. Technol., 2021, 322, 124534 CrossRef CAS PubMed.
- W. Yan, Y. Xiao, W. Yan, R. Ding, S. Wang and F. Zhao, Chem. Eng. J., 2019, 358, 1421–1437 CrossRef CAS.
- D. Wu, F. Sun and Y. Zhou, Electrochim. Acta, 2017, 240, 136–145 CrossRef CAS.
- J. W. Park, V. Krumins, B. V. Kjellerup, D. E. Fennell, L. A. Rodenburg, K. R. Sowers, L. J. Kerkhof and M. M. Haggblom, Appl. Microbiol. Biotechnol., 2011, 89, 2005–2017 CrossRef CAS PubMed.
- Q. Wang, X. Li, Q. Yang, Y. Chen and B. Du, Ecotoxicol. Environ. Saf., 2019, 171, 746–752 CrossRef CAS PubMed.
- D. Wu, G. Chen, X. Zhang, K. Yang and B. Xie, Sci. Rep., 2017, 7, 41230 CrossRef CAS PubMed.
- Z. Kong, L. Li, H. Kato, T. Zhang, Y. Xue and Y. Y. Li, Bioresour. Technol., 2019, 282, 482–493 CrossRef CAS PubMed.
- H. Toh, V. K. Sharma, K. Oshima, S. Kondo, M. Hattori, F. B. Ward, A. Free and T. D. Taylor, J. Bacteriol., 2011, 193, 6411–6412 CrossRef CAS PubMed.
- M. Park and F. Rafii, Anaerobe, 2019, 56, 124–129 CrossRef CAS PubMed.
- B. Halling-Sorensen, G. Sengelov and J. Tjornelund, Arch. Environ. Contam. Toxicol., 2002, 42, 263–271 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra07600k |
| This journal is © The Royal Society of Chemistry 2022 |



