Open Access Article
Open Access ArticleCatalytic activity, thermal stability and structural evolution of PdCu single-atom alloy catalysts: the effects of size and morphology
Qing Liuab,
Xiaoxu Wangc,
Lu Liab,
Keke Song*ab,
Yanzhou Wangab and
Ping Qian *ab
*ab
aBeijing Advanced Innovation Center for Materials Genome Engineering, University of Science and Technology Beijing, Beijing, 100083, China. E-mail: qianping@ustb.edu.cn; KeKSong@163.com
bDepartment of Physics, University of Science and Technology Beijing, Beijing, 100083, China
cDP Technology, Beijing, 100080, China
First published on 20th December 2021
Abstract
Single-atom alloys (SAAs) have been emerging as an important field of research in electrocatalysis owing to extremely high atom utilization, unique structure and high catalytic activity. In this work, the catalytic properties and thermal stability of PdCu SAAs with a crown-jewel (CJ) structure are studied by density functional theory (DFT) calculations and the molecular dynamics (MD) simulation method. The DFT results reveal that CJ-structured PdCu SAAs show excellent HER and ORR catalytic performance, and can be regarded as a promising alternative to Pt catalysts towards the ORR or HER. Additionally, we attempt to explain the high catalytic activity of PdCu SAAs by electronic structure analysis. In addition, MD simulation results confirm the thermal stability of CJ-structured PdCu. More importantly, we found that CJ-structured PdCu clusters undergo a structural transformation from cuboctahedral (Cubo) to icosahedral (Ico) structure by heating or after the adsorption of reaction intermediate, which indicates that Cubo is less stable than the Ico structure. Besides, Cubo–Ico transformation is size-dependent and only found in small clusters. Furthermore, the effects of size and morphology on melting properties are discussed. The melting point increases as cluster size increases, which agrees well with Pawlow's law.
1. Introduction
Metallic nanoclusters (NCs) have received great attention in materials science and engineering for their excellent properties owing to their small size and large surface-volume ratio.1,2 Bimetallic nanoclusters exhibit enhanced and more complex properties than the corresponding monometallic components attributed to the synergistic effect of the different elements.3,4 By tuning their size, structures, and chemical compositions as well as the surface state properties, the unique properties of bimetallic NCs can be utilized in a large range of fields, especially in heterogeneous catalysis.5,6 With regard to electrocatalysis, much effort has been dedicated to improving the catalytic performance of nanoalloys by designing different kinds of structures. Some special structural models of NCs, such as core–shell,7,8 onion-like,9 quasi-janus structures10 and crown-jewel (CJ) structures11,12 have been found to have structure-dependent properties and are an important focus of nanostructure research. Specifically, CJ-structured bimetallic NCs, where the one metal clusters serve as the crowns and another metal atoms serve as jewels decorating the special positions of the crowns, which is regarded as one kind of single-atom-alloy (SAA). SAA contains isolated metal atoms singly dispersed on the surface of a host metal, which turns out to a research frontier in heterogeneous catalysis.13–15 SAAs show extremely high atom utilization efficiency, which is especially beneficial to precious metal catalysts. It is reported that when the size of cluster is about 9.03 nm, the cluster can efficiently represent the properties of bulk metal surface.5 However, not only the metal surface, but metal clusters can also be the supports. Comparable clusters with the dispersions of isolated noble metal atoms have been prepared and applied in catalysis experimentally. It is reported that SAAs with CJ structure exhibit excellent catalytic property, compared to conventional nanocluster catalysts.11,12,16 For example, Zhang et al. have prepared crown-jewel-structured AuPd nanocluster by a galvanic replacement reaction method, which located Au single atoms at the top position of Pd host clusters. This single atom structure showed markedly high catalytic activity for aerobic glucose oxidation.12 Besides, AuPd single atom structures were also prepared toward oxygen reduction reaction. It was showed that discrete Pd single atoms dispersed in Au nanocluster surfaces will significantly improve reactivity of the surface toward H2O2 production.16 In addition, Zhang et al. prepared the colloidal SAAs of “crown jewel” structured (IrPd)/Au nanoclusters and found that this SAAs were the most effective catalysts for aerobic glucose oxidation tested to date.11 The unique electronic structure and coordinative unsaturation of the active sites for CJ structure modify the adsorption properties of intermediates, and thus significantly enhances the catalytic performance of CJ. Additionally, the strength of metal bonds between single atoms and host metal results in the high stability of CJ systems.17 Thus, SAAs catalysts with CJ structure are worth studying deeply as an attractive candidate for the catalytic applications.Pt-based materials have been long known as the most efficient metal catalysts for many conventional chemical reactions, especially for oxygen reduction reaction (ORR) and hydrogen evolution reaction (HER). However, deficiencies such as high cost and scarcity hamper their large-scale industrial application. Thus, searching Pt-free alternatives has been an important focus on the design of advanced electrocatalysts. Especially, Pd-based nanoalloy is an attractive alternative to Pt in electrocatalytic materials since Pd has similar physicochemical characteristics with Pt. In recent years, it has become common to alloy Pd with non-noble transition metals (e.g. Fe, Co, Ni, Cu, W) to design advanced catalysts.18–21 In this regard, compared with other transition metals, Cu is cheap and highly abundant, which will significantly reduce the high cost of precious metal catalysts. Besides, it has reported that Pd alloyed with Cu achieved high catalytic activity for both ORR and HER owing to strong synergistic effect.22–24 For example, Tang et al. investigated the effect of alloy composition on the oxygen reduction activity of PdCu nanoparticles. They found that the average oxygen binding reduced when Cu is added to Pd, indicating an increase in catalytic activity up to a peak at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Pd/Cu ratio.25 Besides, Zhang et al. prepared monodispersed bimetallic PdCu nanoparticles. They found that both the HER activity and stability of PdCu/C are highly dependent on the Pd/Cu atomic ratios.22 Zhang et al. synthesized CuPd nanocrystals with a variety of nonspherical shapes and found that the ORR activity is remarkably shape-dependent.26
1 Pd/Cu ratio.25 Besides, Zhang et al. prepared monodispersed bimetallic PdCu nanoparticles. They found that both the HER activity and stability of PdCu/C are highly dependent on the Pd/Cu atomic ratios.22 Zhang et al. synthesized CuPd nanocrystals with a variety of nonspherical shapes and found that the ORR activity is remarkably shape-dependent.26
As the CJ-structured SAA is emerging as an important field of research in catalysis, it seems interesting and challengeable to investigate PdCu nanoalloys with the CJ structure, which may possess excellent catalytic activity and could be a promising alternative to Pt catalysts. However, the detailed investigations about structural properties and catalytic properties towards ORR and HER of CJ-structured PdCu nanoclusters are still scarce, which are important for their potential application in catalysis.
In recent years, density functional theory (DFT) calculations and molecular dynamics (MD) simulation method, are playing an increasingly important role in studying nanostructure and predicting material properties.27–31 Herein, our work is mainly consisted of two parts: the study of catalytic properties towards ORR and HER and thermal stability of CJ-structured PdCu SAAs. The thermal stability is an important property for CJ-structured nanoclusters to function as a catalyst. The catalyst will not be able to work if the thermal stability is poor. Besides, the melting point is strongly linked with the thermal stability of the clusters. Generally, the more stable of the cluster, the higher the melting point. Thus, the study of melting behaviors is important for CJ-structured nanocluster for the catalytic applications. By DFT calculations, we explore the catalytic activity of PdCu SAAs toward ORR and HER, respectively. On the other hand, MD simulations based on the EAM potential are used to investigate the melting properties and structural evolution of PdCu nanoclusters during heating process. Moreover, a structural transformation from cuboctahedral (Cubo) to icosahedral (Ico) structure is observed by heating or after the adsorption of intermediates. The Pawlow's law is also observed in CJ structure. Besides, the effects of the morphology and size on these properties are also discussed.
2. Computational methods and models
2.1. DFT calculations
The DFT calculations are performed using the Vienna Ab initio Simulation Package (VASP).32 The generalized gradient approximation (GGA) of Perdew–Burke–Ernzerhof (PBE) is applied to describe the electron exchange-correlation energy.33 The ion–electron interactions are described by the projector augmented wave (PAW) method.34 Plane-wave expansions are set with a kinetic energy cutoff of 500 eV. The Brillouin zone is sampled with a gamma (1 × 1 × 1) point. A 25.0 Å cubic supercell is composed of a PdCu nanocluster with an enough large vacuum space to separates the NC from its periodic image. The atomic structure is optimized with the energy converged to 1.0 × 10−4 eV and the force converged to 0.01 eV Å−1.We calculate the change in Gibbs free energy (ΔG) for ORR and HER pathways on PdCu systems, which is defined as ΔG = ΔE + ΔEZPE − TΔS, where ΔE is the total energy change of the reaction obtained from DFT calculations, ΔEZPE and ΔS are the changes in zero-point energy and the entropy between the adsorbed state and the gas phase, respectively. T is temperature (298.15 K, in this work). Zero-point energies and S corrections are calculated from the vibrational frequencies in the harmonic oscillator approximation as done by Peterson et al.35 Besides, the adsorption energy of an adsorbate (ΔEads) on nanoclusters are defined as: Eads = Ecluster + adsorbate − Ecluster − Eadsorbate, where Ecluster + adsorbate is the total energy of metal nanoclusters with an adsorbate, Ecluster is the energy of the nanocluster, and Eadsorbate is the energy of an isolated adsorbate species, respectively.
The free energies of the ORR elementary reactions are calculated based on a computational standard hydrogen electrode (SHE) model proposed by Nørskov et al.36 This model defines that the free energy of a proton/electron pair (H+ + e−) in solution is equal to half of the free energy of H2 molecule. Besides, the effect of external bias on the reaction can be applied by shifting ΔG by −neU, where e is the transferred charge and U is the applied electrode potential, and n is the number of proton–electron transfer pairs. The effects of pH value can be treated as an energy shift to free energy change: ΔGpH = −kBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 × pH and pH is assumed to be zero for acidic medium in this work.
10 × pH and pH is assumed to be zero for acidic medium in this work.
2.2. MD simulation
Molecular dynamics simulations using the Large-scale Atomic/Molecular Massively Parallel Simulator (LAMMPS) software37 are performed to study the heating process at the atomistic level of PdCu CJ nanoalloys. The interaction between metal atoms is described based on the embedded atom method (EAM). The EAM, developed by Zhou et al., is described with the identical analytic form and has been extended to enable calculations of alloys with any combination of 16 metals.38 The detailed description of this potential function used in this study is given in previous papers.39 Besides, this EAM potential has been successfully used in describing thermodynamic properties of metal nanoclusters and nanoalloys.40,41 In MD simulation, the temperature is controlled by the Nosé–Hoover thermostat. The equations of motion are integrated using Verlet leapfrog algorithm and the time step is set to 1 fs. The heating processes of alloy clusters are simulated as follows: firstly, the nanoclusters are fully relaxed at 0 K to reach a minimum energy configuration. Then, the clusters are heated up from 0 K to 1600 K (which is above the melting pointing of the alloy nanoclusters) with a temperature increment of 20 K, each with 240 ps equilibrated simulation time. In this work, the melting properties and structural evolution of PdCu SAA nanoclusters are studied by MD simulations.2.3. Models of CJ-structured PdCu nanoclusters
In this work, we conduct an investigation into the catalytic property and thermal stability of CJ-structured PdCu SAAs with different morphologies and cluster sizes. It is known that clusters with certain special numbers of atoms are much more abundant than others when generated in typical cluster experiments, and these numbers are called geometric “magic numbers”.42 For metal nanoclusters, the relative stable geometries are normally those consisting of a magic number N of atoms (N = 55, 147, 309, 561, 923, etc.),43,44 which normally corresponds to highly symmetric structures, such as icosahedral and cuboctahedral models. The highly symmetric structures Cubo and Ico are the most commonly used structural models for the nanocluster research, experimentally and theoretically.45–47 Therefore, closed atomic shell structures of Ico and Cubo models including the magic number of atoms are used as host cluster in this work. For PdCu nanoclusters, all vertices of Cu clusters are replaced by Pd atoms, where the Cu clusters act as the host cluster (crowns) and Pd atoms serve as jewels decorating the vertex positions of the crowns, corresponding to the typical CJ structure. To study the size effect, PdCu nanoclusters with sizes of 55, 147, 309, 561 and 923 atoms are used. Fig. 1a and b displays our model system of CJ-structured PdCu nanoclusters with Cubo and Ico morphologies. In this work, the catalytic properties are studied based on DFT calculations. Besides, thermal stability and structural evolution of PdCu SAA structures during heating are investigated by MD simulation.3. Results and discussion
3.1. Catalytic properties
In this section, we investigate the HER and ORR catalytic properties on PdCu SAAs, respectively, by the DFT calculations. For the catalytic property researches, two morphologies are considered, that is the highly symmetric Cubo, and Ico structures. Besides, size effects are also discussed and the PdCu nanoclusters with sizes of 55 and 147 atoms are selected.To investigate the effect of the addition of single atom Pd on HER or ORR catalytic activity of CJ-structured PdCu nanoclusters, three equivalent adsorption sites according to the Pd-centered symmetry for Ico configuration (T1, B1, H1 sites) and four adsorption sites for Cubo configuration (T1, B1, H1 and H2 sites) are considered, as shown in Fig. 1c and d. The adsorption energy of H atom (EH*) on PdCu clusters with Ico and Cubo structures at different adsorption sites are calculated. For comparison, the hydrogen adsorption of Pt and Pd clusters are also calculated. The most favorable adsorption energies and adsorption sites for hydrogen atom are listed in Table 1. It is seen that the hydrogen adsorption energies for these systems are all negative, indicating that hydrogen adsorption is energetically favorable. Besides, the calculated Gibbs free energy diagram of hydrogen evolution of PdCu, Pt and Pd nanoclusters are displayed in Fig. 2c. Theoretically, the overall HER reaction can be described by a three-state diagram consisting of an initial H+ state, an intermediate H* state and the final product 1/2H2, as seen in the figure.
| Cluster | Configuration | Adsorption site | EH* (eV) | ΔGH (eV) |
|---|---|---|---|---|
| Pd12Cu43 | Ico | H1 | −0.34 | −0.10 |
| Pd12Cu43 | Cubo | — | — | — |
| Pd12Cu135 | Ico | H1 | −0.26 | −0.02 |
| Pd12Cu135 | Cubo | — | — | — |
| Pd55 | Ico | H1 | −0.66 | −0.42 |
| Pd55 | Cubo | H1 | −0.56 | −0.32 |
| Pt55 | Ico | B1 | −0.82 | −0.58 |
| Pt55 | Cubo | B1 | −0.75 | −0.51 |
| Pt147 | Ico | B1 | −0.63 | −0.39 |
From the figure we can draw the following conclusions: firstly, when Pd single atoms embedded in Cu nanocluster surface, the |ΔGH*| of this SAAs is smaller than Pd and even Pt catalysts, indicating that CJ-structured PdCu shows better HER activity. Thus, CJ-structured PdCu catalyst is promising to realize highly efficient and low-cost electrocatalytic H2 production. Secondly, we found that the HER activity of Pd clusters with Ico and Cubo configurations are both higher than Pt clusters. Thirdly, catalysts with Cubo configuration for both Pd and Pt clusters have better HER catalytic performance than that with Ico configuration.
In addition, we are surprised to find a ligand-induced structural evolution for Pd12Cu43 cluster with Cubo. Pd12Cu43 cluster with Cubo structure transforms into Ico structure after adsorbed H atom on cluster surface, as seen in Fig. 2a. Besides, the total energy of the adsorption system which transformed into icosahedral is almost the same with that of icosahedral adsorption system. This structure transformation indicates that Cubo structure is metastable structure and less stable than Ico. For example, Pd12Cu43–Cubo structure is less stable than that icosahedral structure by −4.07 eV. The similar ligand-induced structural transformation is also found in Pt55 cluster. It is reported that Pt55 cluster undergo a structural evolution from Cubo to an Ico structure by the Mackay transformation with the help of the adsorption of methylamine.51 Cubo–Ico transformation is driven by a combination of both the external forces resulting from the adsorption of the ligand, which pull out the Pt atoms on the surface of NCs in a radial direction, and the contraction forces in a tangential direction.
To elucidate the excellent catalytic property of PdCu SAA structure, we take Pd12Cu43 SAA as a representative and we calculate the electronic structure. It is known that the high catalytic activity of SAA is attributed not only to the unique structure, but also to the electronic structure of single atom. Bader analysis is used to quantify the charge changes around each atom in PdCu SAA structure.52 The Bader charges change of some atoms for Pd12Cu43 SAA and Pd55 cluster are shown in the Fig. 2d and e. For Pd55 cluster, we see that vertex Pd atoms are weakly negatively charged. However, when Pd single atoms embedded in Cu nanocluster, the negative charge density of single Pd atoms significantly increases and Cu atoms are all positively charged. This is attributed to electron transfer from the surrounding Cu atoms to single Pd atoms. This similar results are also found in AuPd12 and (IrPd)/Au.11 The anionic charge on vertex Pd atoms may act as catalytically active sites for HER. Thus, the negatively charged Pd single atoms maybe the cause for the high catalytic performance of PdCu SAA catalyst.
| Structures | EO* (eV) | Adsorption site | RDS | Overpotential (V) |
|---|---|---|---|---|
| Pd12Cu43-Ico | −4.73 | H1 | R4 | 0.87 |
| Pd55-Ico | −4.55 | H1 | R3 | 0.81 |
| Pt55-Ico | −4.50 | H1 | R4 | 0.78 |
| Pt55-Cubo | −4.55 | B1 | R4 | 0.88 |
| Pd12Cu135-Ico | −4.38 | H1 | R3 | 0.67 |
| Pd147-Ico | −4.41 | H1 | R3 | 0.74 |
| Pt147-Ico | −4.38 | H1 | R4 | 0.65 |
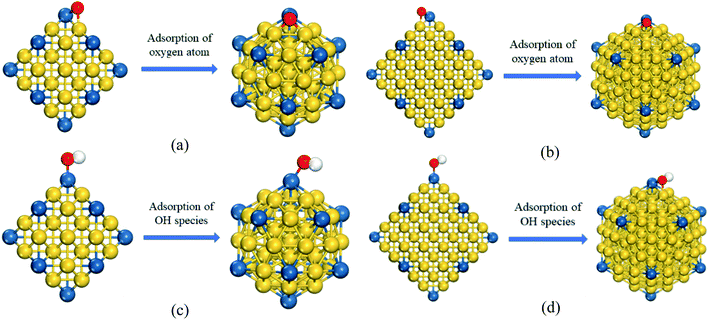
As shown in the Table 2, we can see that the O adsorption energy of Pd12Cu43 SAAs with Ico is slightly stronger than Pt55 and Pd55 clusters, regretfully, which means that ORR activity of Pd12Cu43 may be lower than Pd and Pt clusters. However, when PdCu cluster size increases to 147 atoms, the EO* of Pd12Cu135-ico is weaker than Pd147-ico cluster and close to Pt147-ico cluster, indicating that PdCu with 147 atoms may show better ORR activity than Pd cluster and is even comparable to Pt cluster. Thus, CJ-structured PdCu can be considered as a promising ORR catalyst with low cost. Besides, we also see that the O adsorption energy of Pt55 with Cubo is stronger than that with Ico structure. This indicates that the Ico morphology may have higher ORR catalytic activity than Cubo. Furthermore, a ligand-induced structural transformation is also found. As shown in Fig. 3a and c, Pd12Cu43 cluster with Cubo structure transforms into Ico structure with the help of the adsorption of ORR intermediates: O and OH species on cluster surface. This further confirms that Cubo is less stable than Ico structure.
To further study the ORR catalytic performance of CJ-structured PdCu nanoclusters, we calculate the Gibbs free energy diagram, which are used to explore the energetics of ORR elementary steps. In general, for alloy nanoclusters and SAA structures,54–56 ORR prefer to proceed through the associative mechanism in acid media. The associative pathway of ORR at the PEMFC cathode is described by the following four elementary steps:
| O2(g) + H+ + e− + * → *OOH |
| *OOH + H+ + e− → *O + H2O(l) |
| *O + H+ + e− → *OH |
| *OH + H+ + e− → H2O(l) + * |
 | ||
| Fig. 4 The Gibbs free energy diagrams of the ORR pathway occurring on CJ-structured PdCu, Pt and Pd clusters at 0 V, at 1.23 V and at the limiting potential. | ||
However, the overpotentials of PdCu SAAs are slightly larger than state-of-the-art ORR catalyst, for example, Pt(111) (∼0.45 V)36 and 2D-supported SACs (∼0.55 V).60 Although the overpotentials of PdCu clusters are not comparable to state-of-the-art ORR catalyst, CJ-structured cluster catalysts still show several major advantages over those ORR catalysts. Firstly, Cu cluster as host cluster reduces the usage of precious metals and will significantly reduce production costs. Secondly, the strength of metal bonds between single atoms and host metal ensure that CJ systems are often more stable than oxide-supported or 2D-supported SACs. Thirdly, the nanoclusters with more uniform particle sizes can be obtained in the dispersed systems compared with those in the supported cases, especially under conditions of a high metal concentration, because the dispersed metal nanoclusters can be easily concentrated by evaporating the solvent without changing the structures.61 Thus, CJ-structured PdCu cluster can be considered as a promising ORR catalyst with low cost for fuel cells cathode applications.
Moreover, the structural transformations from the Cubo structure to Ico structure are also found for CJ-structured PdCu clusters with 147 atoms when O (seen Fig. 3b), OH species (Fig. 3d) or H atom (Fig. 2b) are adsorbed on the cluster surface. This indicates that CJ-structured Pd12Cu135 SAA with Cubo is also a metastable structure.
3.2. Melting properties
In this section, the heating process for PdCu nanoclusters is studied by MD simulation with the embedded atom method, in hope to explore the effects of morphology and cluster size on the thermodynamic stability and structural evolution during heating. Fig. 6a–c shows the temperature dependence of total potential energy (PE) during heating for various sizes (147, 309, 561, 923 atoms) and morphologies (cuboctahedral and icosahedral structures) of CJ-structured PdCu SAAs. We can see that there is a sharp jump at about 800 K for Pd12Cu135 clusters both with Ico and Cubo configurations, which corresponds to the melting point. Besides, we found that Pd12Cu135 with Cubo structure undergo a solid–solid structural transformation from Cubo to Ico structure at 0 K (the snapshot images near the structural transformation point are shown in the inset), which indicates that Cubo is less stable than Ico. This conclusion is consistent with the ligand-induced structural transformation results mentioned earlier. From the potential energy curve of Pd12Cu297-Cubo, we see that apart from the melting point with 846 K, Pd12Cu297 with Cubo structure also shows a cuboctahedral to icosahedral (Cubo to Ico) structure transformation at 320 K with a sharp drop in potential energy. However, when the cluster size increases to 561 and 923 atoms, the Cubo–Ico structural transformation phenomenon disappears, which indicates that Cubo–Ico transformation is size-dependent and occurs only for small nanoclusters.The radial distribution function (RDF) is commonly used to study the structural evolution and the degree of disordering of the nanostructure. The RDF is defined as the probability of finding a pair of atoms with a distance r, relative to the probability expected for a completely random distribution, compared with a homogeneous distribution.62,63 We take Pd12Cu297 SAA with Cubo as an example, total RDFs at 100 K (before Cubo–Ico structural transformation), 320 K (at Cubo–Ico transformation point), 500 K, 700 K, 800 K and 850 K (near the melting point) are shown in Fig. 5. The corresponding structural evolution snapshots at different temperatures during heating are depicted in the inset. We see that the RDFs show sharp peaks at 100 K, which corresponds to a regular cuboctahedral structure. When it is heated up to 320 K, the RDF peaks suddenly change and exhibit the main structural characteristics of an icosahedral structure, which is attributed to the Cubo–Ico structural transformation. When it comes to 500 K, 700 K and 800 K, the RDF peaks gradually become smaller and broader, indicating that the icosahedral structure becomes more disordered. The peak intensity is getting weaker as the temperature increases. When the temperature reaches melting point 850 K, the long-range order characteristic lost in RDF, suggesting the characteristic of amorphous liquid structure. Besides, we observe that Pd single atoms still stay at the original vertex sites until the melting point and the CJ structure is well maintained, which proves the thermal stability of PdCu SAA.
 | ||
| Fig. 5 Total RDFs for CJ-structured Pd12Cu297 SAA with Cubo at different temperatures during heating. Inset: the corresponding structural evolution snapshot at different temperatures. | ||
In addition, the Cubo–Ico transformation temperature and melting points of CJ-structured PdCu SAAs changing with the different cluster sizes and morphologies are listed in Table 3. We found that the melting point of PdCu with Ico structures is higher than that with Cubo structure for each cluster size, which further confirms that Ico structure of PdCu is more stable than Cubo. For different sizes, the melting point of CJ-structured PdCu nanoclusters increases as the cluster size increases and the melting points of Ico configurations against N−1/3 (N is total number of atoms in PdCu icosahedral cluster) are plotted in Fig. 6d. The melting point of Ico correlates linearly with N−1/3, which agrees well with Pawlow's law.64
| Structure | Ttran (K) | Tmelt (K) | Structure | Tmelt (K) |
|---|---|---|---|---|
| Cubo-147 | 0 | 812 | Ico-147 | 788 |
| Cubo-309 | 320 | 846 | Ico-309 | 858 |
| Cubo-561 | — | 918 | Ico-561 | 938 |
| Cubo-923 | — | 947 | Ico-923 | 974 |
4. Conclusions
In this work, the HER and ORR catalytic activity and thermal stability of CJ-structured PdCu SAAs are studied by using DFT calculations and MD simulation, respectively. The effects of the morphology and size on these properties are discussed. For the catalytic property, we found that CJ-PdCu show higher HER catalytic activity than the state-of-the-art Pt catalysts, which can be considered as low cost and highly efficient HER electrocatalysts for H2 production. On the other hand, by calculating the adsorption energy of oxygen atom and Gibbs free energy diagram, we found that PdCu SAA also exhibits a higher ORR activity than Pd cluster and comparable activity to Pt cluster. Thus, CJ-structured PdCu SAA can also be used as ORR catalysts for fuel cells cathode applications. Besides, size effects are also discussed and HER and ORR catalytic performance both improve as cluster size increases. In addition, MD simulation proved the thermal stability of CJ-structured PdCu and PdCu SAA structure can still be stable during heating until melting. Furthermore, a structural transformation from Cubo to Ico structure is found by heating or the adsorption of reaction intermediates. For example, DFT results show that CJ-structured PdCu clusters of 55 and 147 atoms both undergo structural evolution from Cubo to Ico structure after the adsorption of reaction intermediates. On the other hand, MD simulations show that PdCu SAA of Cubo structure transform into Ico during heating process and this structural transformation occurs before melting point. Besides, the effects of the morphology and size on melting properties are also discussed. Our results demonstrate the potential applications of SAAs with CJ structure in catalysis. The catalytic activity and thermal stability of CJ-structured PdCu nanoalloys can be tuned by the size and morphology, which provides a guidance for the rational design of SAA for catalytic applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (Grant No. 2018YFB0704300). The first author thanks the China Scholarship Council (CSC) for financial support (202006460065).References
- R. Ferrando, J. Jellinek and R. L. Johnston, Chem. Rev., 2008, 108, 845–910 CrossRef CAS PubMed.
- F. Baletto and R. Ferrando, Rev. Mod. Phys., 2005, 77, 371 CrossRef CAS.
- I. Mosleh and A. Abbaspourrad, RSC Adv., 2021, 11, 32615–32621 RSC.
- X. Cheng, X. Sui, J. Xu, X. Liu, M. Chen and Y. Zhu, RSC Adv., 2021, 11, 32526–32532 RSC.
- L. Li, Y.-Z. Wang, X.-X. Wang, K.-K. Song, X.-D. Jian, P. Qian, Y. Bai and Y.-J. Su, J. Phys. Chem. C, 2020, 124, 8706–8715 CrossRef CAS.
- X. Zhao, Y. Chang, J. Ji, J. Jia and M. Jia, RSC Adv., 2021, 11, 33179–33185 RSC.
- Y. Tuo, Q. Lu, C. Chen, T. Liu, Y. Pan, Y. Zhou and J. Zhang, RSC Adv., 2021, 11, 26326–26335 RSC.
- L. Li, H. Xin Ma, X. Dong Jian, P. Qian and Y. Jing Su, Phys. Chem. Chem. Phys., 2020, 22, 9467–9476 RSC.
- D. Cheng, W. Wang, S. Huang and D. Cao, J. Phys. Chem. C, 2008, 112, 4855–4860 CrossRef CAS.
- I. Parsina and F. Baletto, J. Phys. Chem. C, 2010, 114, 1504–1511 CrossRef CAS.
- H. Zhang, L. Lu, K. Kawashima, M. Okumura, M. Haruta and N. Toshima, Adv. Mater., 2015, 27, 1383–1388 CrossRef CAS PubMed.
- H. Zhang, T. Watanabe, M. Okumura, M. Haruta and N. Toshima, Nat. Mater., 2011, 11, 49–52 CrossRef PubMed.
- M. K. Samantaray and V. D'Elia, et al., Chem. Rev., 2020, 120, 734–813 CrossRef CAS PubMed.
- L. Liu and A. Corma, Chem. Rev., 2018, 118, 4981–5079 CrossRef CAS PubMed.
- Y. Ren, X. Liu, Z. Zhang and X. Shen, Phys. Chem. Chem. Phys., 2021, 23, 15564–15573 RSC.
- J. S. Jirkovsky, I. Panas, E. Ahlberg, M. Halasa, S. Romani and D. J. Schiffrin, J. Am. Chem. Soc., 2011, 133, 19432–19441 CrossRef CAS PubMed.
- J. Mao, J. Yin, J. Pei, D. Wang and Y. Li, Nano Today, 2020, 34, 100917 CrossRef CAS.
- K. R. Reddy, K. P. Lee, A. I. Gopalan, M. S. Kim, A. M. Showkat and Y. C. Nho, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 3355–3364 CrossRef CAS.
- J. Chen, G. Xia, P. Jiang, Y. Yang, R. Li, R. Shi, J. Su and Q. Chen, ACS Appl. Mater. Interfaces, 2016, 8, 13378–13383 CrossRef CAS PubMed.
- S. Shen, T. Zhao, J. Xu and Y. Li, J. Power Sources, 2010, 195, 1001–1006 CrossRef CAS.
- G. Hu, F. Nitze, E. Gracia-Espino, J. Ma, H. R. Barzegar, T. Sharifi, X. Jia, A. Shchukarev, L. Lu, C. Ma, G. Yang and T. Wågberg, Nat. Commun., 2014, 5, 5253 CrossRef CAS PubMed.
- X. Zhang, D. Wu and D. Cheng, Electrochim. Acta, 2017, 246, 572–579 CrossRef CAS.
- Z.-P. Wu, S. Shan, Z.-H. Xie, N. Kang, K. Park, E. Hopkins, S. Yan, A. Sharma, J. Luo and J. Wang, ACS Catal., 2018, 8, 11302–11313 CrossRef CAS.
- J. Wu, S. Shan, J. Luo, P. Joseph, V. Petkov and C.-J. Zhong, ACS Appl. Mater. Interfaces, 2015, 7, 25906–25913 CrossRef CAS PubMed.
- W. Tang, L. Zhang and G. Henkelman, J. Phys. Chem. Lett., 2011, 2, 1328–1331 CrossRef CAS PubMed.
- L. Zhang, F. Hou and Y. Tan, Chem. Commun., 2012, 48, 7152–7154 RSC.
- L.-B. Shi, Y.-Y. Zhang, X.-M. Xiu and H.-K. Dong, Carbon, 2018, 134, 103–111 CrossRef CAS.
- L.-B. Shi, M. Yang, S. Cao, Q. You, Y.-Y. Niu and Y.-Z. Wang, Appl. Surf. Sci., 2019, 492, 435–448 CrossRef CAS.
- X. Wang, C. Wang, S. Ci, Y. Ma, T. Liu, L. Gao, P. Qian, C. Ji and Y. Su, J. Mater. Chem. A, 2020, 8, 23488–23497 RSC.
- Q. Liu, H. Zhao, X. Wang, J. Huo, L. Li, P. Gao, P. Qian, Y. Su and N. Chen, Prog. Nat. Sci.: Mater. Int., 2019, 29, 525–532 CrossRef CAS.
- K. H. Pham and T. T. T. Giap, RSC Adv., 2021, 11, 32435–32445 RSC.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef PubMed.
- A. A. Peterson, F. Abild-Pedersen, F. Studt, J. Rossmeisl and J. K. Nørskov, Energy Environ. Sci., 2010, 3, 1311–1315 RSC.
- J. K. Nørskov, J. Rossmeisl, A. Logadottir, L. Lindqvist, J. R. Kitchin, T. Bligaard and H. Jónsson, J. Phys. Chem. B, 2004, 108, 17886–17892 CrossRef.
- S. Plimpton, J. Comput. Phys., 1995, 117, 1–19 CrossRef CAS.
- X. Zhou, R. Johnson and H. Wadley, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 144113 CrossRef.
- X. W. Zhou, H. N. G. Wadley, R. A. Johnson, D. J. Larson, N. Tabat, A. Cerezo, A. K. Petford-Long, G. D. W. Smith, P. H. Clifton, R. L. Martens and T. F. Kelly, Acta Mater., 2001, 49, 4005–4015 CrossRef CAS.
- Q. Li, M. Wang, Y. Liang, L. Lin, T. Fu, P. Wei and T. Peng, Phys. E: Low-Dimens. Syst. Nanostructures, 2017, 90, 137–142 CrossRef CAS.
- D. Schebarchov, S. Hendy and W. Polak, J. Phys.: Condens. Matter, 2009, 21, 144204 CrossRef CAS PubMed.
- B. K. Teo and N. J. A. Sloane, Inorg. Chem., 1985, 24, 4545–4558 CrossRef CAS.
- I. A. Solov'yov, A. V. Solov'yov, W. Greiner, A. Koshelev and A. Shutovich, Phys. Rev. Lett., 2003, 90, 053401 CrossRef PubMed.
- E. Pahl, F. Calvo, L. Koči and P. Schwerdtfeger, Angew. Chem., Int. Ed., 2008, 47, 8207–8210 CrossRef CAS PubMed.
- A. P. Tsai, Sci. Technol. Adv. Mater., 2008, 9, 013008 CrossRef PubMed.
- Y. Shichibu, K. Suzuki and K. Konishi, Nanoscale, 2012, 4, 4125–4129 RSC.
- J. K. Seo, A. Khetan, M. H. Seo, H. Kim and B. Han, J. Power Sources, 2013, 238, 137–143 CrossRef CAS.
- J. K. Nørskov, T. Bligaard, A. Logadottir, J. Kitchin, J. G. Chen, S. Pandelov and U. Stimming, J. Electrochem. Soc., 2005, 152, J23 CrossRef.
- B. Hinnemann, P. G. Moses, J. Bonde, K. P. Jørgensen, J. H. Nielsen, S. Horch, I. Chorkendorff and J. K. Nørskov, J. Am. Chem. Soc., 2005, 127, 5308–5309 CrossRef CAS PubMed.
- T. Yang, Y. Bao, W. Xiao, J. Zhou, J. Ding, Y. P. Feng, K. P. Loh, M. Yang and S. J. Wang, ACS Appl. Mater. Interfaces, 2018, 10, 22042–22049 CrossRef CAS PubMed.
- J. H. Ryu, S. S. Han, D. H. Kim, G. Henkelman and H. M. Lee, ACS Nano, 2011, 5, 8515–8522 CrossRef CAS PubMed.
- W. Tang, E. Sanville and G. Henkelman, J. Phys.: Condens. Matter, 2009, 21, 084204 CrossRef CAS PubMed.
- M. C. S. Escaño, Nano Res., 2015, 8, 1689–1697 CrossRef.
- H. Xu, D. Cheng and Y. Gao, ACS Catal., 2017, 7, 2164–2170 CrossRef CAS.
- M. T. Darby and M. Stamatakis, ChemPhysChem, 2021, 22, 499 CrossRef CAS PubMed.
- A. S. Nair and B. Pathak, J. Phys. Chem. C, 2019, 123, 3634–3644 CrossRef CAS.
- J. Shin, J.-H. Choi, P.-R. Cha and S. K. Kim, et al., Nanoscale, 2015, 7, 15830–15839 RSC.
- A. S. Nair and B. Pathak, J. Phys. Chem. C, 2019, 123, 3634–3644 CrossRef CAS.
- D.-H. Lim and J. Wilcox, J. Phys. Chem. C, 2012, 116, 3653–3660 CrossRef CAS.
- Y. Wang, H. Yuan, Y. Li and Z. Chen, Nanoscale, 2015, 7, 11633 RSC.
- L. Wang, L. Huang, F. Liang, S. Liu, Y. Wang and H. Zhang, Chin. J. Catal., 2017, 38, 1528–1539 CrossRef CAS.
- R. Subbaraman and S. K. Sankaranarayanan, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 075434 CrossRef.
- Q. Liu, Y. Su, K. Song, X. Wang, P. Gao, C. Wang, Y. Xiao, X. Jian and P. Qian, Prog. Nat. Sci.: Mater. Int., 2020, 30, 477–484 CrossRef CAS.
- P. Pawlow, Z. fur Phys. Chem, 1909, 65, 1–35 CAS.
| This journal is © The Royal Society of Chemistry 2022 |