Open Access Article
Open Access ArticleControlling hydrogen release from remaining-intact Clathrate hydrates by electromagnetic fields: molecular engineering via microsecond non-equilibrium molecular dynamics†
Yogeshwaran Krishnan,
Patricia Gomez Rosingana,
Mohammad Reza Ghaani * and
Niall J. English
* and
Niall J. English *
*
School of Chemical and Bioprocess Engineering, University College Dublin, Belfield, Dublin 4, Ireland. E-mail: mohammad.ghaani@ucd.ie; niall.english@ucd.ie; Fax: +353-1-716-1177; Tel: +353-1-716-1758 Tel: +353-1-716-1646
First published on 2nd February 2022
Abstract
In view of the recently-predicted hydrogen release from type-II (sII) clathrate hydrates in the general 140–180 K temperature range [J. Phys. Chem. C, 125, 8430–8439 (2021)], we have investigated in the present study, by means of microsecond-long non-equilibrium molecular-dynamics simulation, the effect of externally-applied electric fields (both static and alternating) on manipulating and accelerating this H2-escape process. In particular, we have found that judiciously-selected electromagnetic fields, in the microwave frequency range, serve to enhance dramatically this H2-release rate – crucially, without any breakup of the hydrate lattice itself. Of those studied, we have found that 10 GHz serves as the optimal frequency to maximise hydrogen release, owing to promotion of H2–H2 molecular collisions inside doubly-occupied 51264 cages in the sII structure and optimal field-period overlap with intra-cage tetrahedral-site hopping and opportunities for inter-cage passage via hexagonal cage faces. This study opens up the vista of “field engineering” for exquisite kinetic control of large, Grid-(terawatt hour)-scale hydrogen-storage systems.
Introduction
Clathrates hydrates are solid crystalline compounds that are constituted by hydrogen-bonded water molecules that create a three-dimensional lattice (polyhedron cages) in which guest molecules are entrapped.1 Once the solid clathrate-hydrate lattice is formed, entrapping gas molecules, there is no covalent bonding with guest molecules, and they are retained inside the cavities by physical interactions (e.g., coulombic) and stability is provided by van der Waals interactions.2 In addition, no waste or byproducts are produced as a consequence of the formation or decomposition of hydrates3 – so they are very much regarded, correctly, as a “green-storage” medium for gas, and this is especially important in the context of hydrogen storage (and particularly so for renewable hydrogen, although also more generally for any form of steam-reformed ‘blue’ H2 given hydrates' propensity to act as “filters” to allow entry only by guest molecules, and reject impurities).2Different crystalline structures can be formed depending on the size of guest molecules.4 A number-based system is used to describe each different type of polymorph structure, where the integer number makes allusion to the face type (polygon) and the power number represents the total number of faces (polyhedron).5 The three main structures that can be encountered are sI, sII and sH. Clathrates made of water that entrap molecular hydrogen form a structure-II (sII),6,7 and multiple hydrogen molecules can be found inside sII cavities.7 In the sII (type II) case, clathrates contain 136 water molecules and two different types of cavities in a unit cell: 16 pentagonal dodecahedra small cages (512) and eight large cavities (51264) formed by 12 pentagonal and 4 hexagonal faces.8
In a recent previous study, we predicted, by means of microsecond molecular-dynamics (MD), that H2 (and D2) can escape from sII hydrates in the 140–180 K range9 – importantly, without lattice break-up. This is certainly of central relevance to “green storage” of energy in an economic, safe and operationally straightforward manner for up to, and especially including, Grid-scale operations at the terawatt hour level. However, in order to probe further this intriguing “molecular-engineering” prospect, the ability to control the kinetics, and extent, of H2 release becomes of paramount importance, e.g., for planned H2 release according to an H2-demand schedule (and for consequent downstream operations, such as adding to pipeline flows or deployment in transport or fuel cells), whether on a seasonal or shorter-term (e.g., weather-, diurnal-, or emergency-dependent) basis. Clearly, from a practical, engineering perspective, any dissociation of gas hydrate's lattice cannot be countenanced in the context of cycle-based, “load-in/out”, hydrogen (or, indeed, more broadly) gas storage: aside from realising the obvious thermodynamic latent-heat penalty, there is the existential problem of a lack of (Henry's-law)10 gas (and especially hydrogen) solubility in (hydrate-melted) liquid water vis-à-vis in the enclathrated state. Therefore, it is sine qua non that any practical clathrate-based hydrogen-storage system does not allow for dissociation of the lattice, and that any H2-demand-led engineering control agent used to manipulate hydrogen release in this process does not induce lattice breakage. Low energy requirements, avoidance of chemical impurities and operationally facile implementation constitute further desiderata in any such putative and keenly-desired technology solution leveraging molecular-engineering fundamentals.
Although there have been some excellent and illuminating contributions towards practical hydrate-based hydrogen-storage systems in the wider academic literature,2,11–15 marking key progress in our understanding, there has been no practical discussion of (non-chemical/additive) hydrogen-release control agents outside of the patent literature,16 although we note that we have proposed this by means of externally-applied static electric fields regulating neon release from already-“leaky” neon hydrates.17 We note, however, that the patented invention in ref. 16 discloses a method by which external electric fields – and, more specifically, electromagnetic (e/m) ones – can be used to release H2 from hydrates without breaking up the lattice – rather akin to treating the hydrate like a “molecular sponge”, in which one can imagine one's hand's mechanical squeezing action (i.e., as an analogue of the “torque-on-dipole” mechanical action of externally-applied e/m fields16,18,19) releasing water (read H2, or other small gas molecule), from a wet (i.e., guest-saturated) sponge (hydrate). In essence, low-intensity, low-power e/m fields in a judiciously chosen (and, indeed, frequency-“tuned” or optimised) general microwave-region induce a field-period/motion-timescale “overlap” resonance, vis-à-vis lattice-vibrational modes,16,20–22 and, as we shall show in the present study, especially with respect to intra-cage interactions between guest molecules themselves, to enhance their wave-/field-resonance-enhanced, “inter-cage-hopping”-mediated,7,23,24 non-steady-state diffusional escape from the hydrate – in a similar manner to ref. 9 & 17 for H2/D2 and neon, respectively. In brief, the present work explores how electric fields (both static and e/m) may be optimised to maximise and control the H2-release rate from hydrates, as ref. 16 discloses – without inducing lattice break-up, exactly as in ref. 9's “leaky-hydrogen-hydrate” predictions – and probing underlying field-resonance mechanisms.
Methodology
H2 molecules were put inside (vide infra) a 3.3 × 3.3 × 10 nm simulation cell containing a 2 × 2 × 2-replicated sII-clathrate system of vanishingly-small dipole, as shown in Fig. 1, in a manner similar to ref. 9. Vacuum was applied in the x-direction so that Fick's-Law, chemical-potential-driven unsteady-state, outward-escaping diffusional H2-mass transfer can take place only via these (cage-truncated) crystal faces,25 following the ultra-short incipient escape of H2 molecules from the truncated cages into the vacuum in the initial picoseconds of non-equilibrium molecular-dynamics (NEMD) simulation – which do not form the focus of the present study's focus on electric fields' manipulation of the mechanisms and kinetics of inter-cage-hopping-mediated escape. In order to enforce this physical situation of Fig. 1 (similar to ref. 9), which is discussed from an analytic and elegant unsteady-state diffusional perspective in ref. 25 (albeit, naturally, under zero-field conditions), periodic boundary conditions (PBC) were applied in all Cartesian directions. As has been reported in the literature from both experimental26 and simulation standpoints,27 there is growing consensus towards respective single- and up-to-double occupancy of small (512) and large (51264) cavities by hydrogen in sII clathrates, and, for the purposes of consistency with ref. 9 and this broadly emerging consensus in the literature, this same single-double-occupation paradigm for small-large cages was adopted in the present study.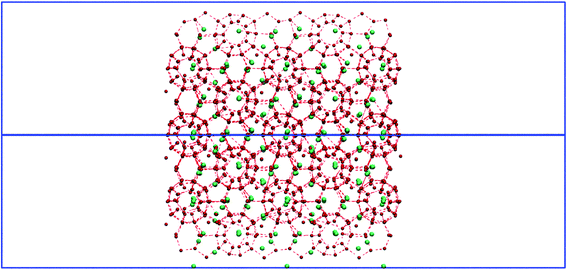 | ||
| Fig. 1 Initial snapshot of hydrogen-filled sII clathrate hydrate, similar to ref. 9. | ||
The release of hydrogen gas enclathrated in sII-type hydrates was simulated by NEMD in the absence and presence of externally-applied electric fields. Calculations were performed for all nine distinct scenarios (zero field, static field, and seven frequencies – 1, 3.162, 10, 31.62, 100, 316.2 and 1000 GHz). These frequencies were chosen to provide a “logarithmically-even” span across the microwave range of 1 GHz to 1 THz (i.e., field periods of 1 ps to 1 ns), and applied along the laboratory + x-direction (or to and fro along the +/− x-directions in the case of e/m fields). The (r.m.s.) intensity was set 0.5 V nm−1 (or 0.05 V Å−1), and this that has been selected bearing in mind previous results showing that this kept hydrate lattices intact in NEMD simulations over timescales of up to microseconds,17,20–22,28 and is still within a linear-response régime for thermodynamic and dynamical properties to external fields, e.g., importantly in the context of the present study, field effects on self-diffusivity.18,29–32 In addition, the typical range of intrinsic electric-field intensities is of the order of 10–30 V nm−1 in hydrate milieux,28 so these external-field intensities are no more than 2–3% of those intrinsically present in aqueous condensed-matter states,20,28 with experimental realisation of these field intensities now becoming much more routine with state-of-the-art instrumentation.33
In the (NE)MD simulations, the 3D Particle-Mesh Ewald (PME) method was used to handle long–range interactions, and simulations were conducted for 1 μs starting from the initial configuration detailed in Fig. 1, using the velocity-Verlet algorithm and a 2 fs time-step, which provided excellent Hamiltonian conservation.35 As in our previous work in ref. 9 & 34, we used the well-validated TIP4P/2005 (ref. 36) water and Alavi-Ripmeester–Klug H2 (ref. 37) force-field models, which have performed well for hydrogen-hydrate simulation.7,9,23,24,34,37 We note that TIP4P/2005 has been used with success in many molecular-simulation studies on gas-hydrate structures, beyond of hydrogen hydrates also.38,39 For modelling of intermolecular interactions, the Lennard-Jones potential (12–6 potential) and real-space Ewald interactions were subject to a 1 nm cut-off.35 Using the GROMACS 5.1.1. package (NE)MD was performed in conjunction with a Nosé–Hoover thermostat in the NVT ensemble at 140 K with a time constant of 0.04 ps. The temperature remained steady throughout (cf. Fig. S1, ESI†), with configuration export every 1 ps. The Báez–Clancy (BC) geometric-recognition algorithm40 was used in order to differentiate the possible structures in which water could be present. Using, inter alia, the formation of local ring structures as criteria, this approach distinguishes water molecules as forming the cages (hydrate-like), liquid and ice forms.40 The number of cage-entrapped hydrogen molecules were thus determined throughout all of the trajectories for all nine different cases under examination (zero- and static-field, and the seven e/m-field frequencies). The algorithm also allows for the tracking of the cage integrity throughout the simulation with and without applied external electric fields, although, as in ref. 16 (for electric-field effects on neon-leakage rates), the applied fields did not lead to any rupture of the hydrate lattice – given that this is not the goal of this study, which wishes, rather, to glean “molecular-engineering” insights into electric fields as a control agent for practical hydrogen storage in hydrates.
Results and discussion
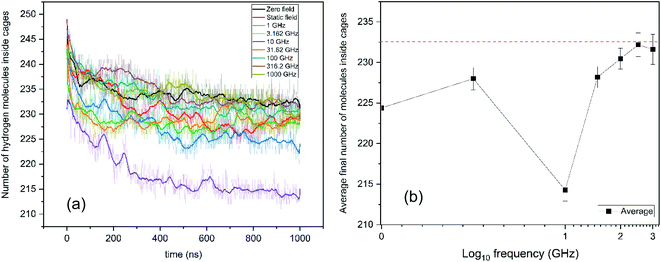
As mentioned previously, over each of the Nosé–Hoover-thermostatted 1-μs simulations, the temperature remained steady – in that the thermostat led to efficient e/m-field-imparted (especially) kinetic-energy dissipation18 (arising from partial excitation of hydrate-lattice vibrational modes20,21) – cf. Fig. S1† for representative examples. In addition, the total-system configurational energy of the systems relaxed rapidly following immediate H2-guest escape from partially-open cages at the hydrate surfaces, and rapid surface rearrangement which occurred thereat (but yet without lattice break-up), in a manner exactly analogous to the previous H2/D2- and neon-leakage studies of ref. 9 & 16; this may be seen in Fig. S2.† However, as remarked in ref. 21, which studied the application of e/m fields to bulk gas hydrates below their “dissociation threshold”, it can be seen that the lowest total-system potential energy arises for the zero-field case in the present study (cf. Fig. S2†), in that the applied e/m fields do impart a level of dynamical coupling with the underlying lattice-vibrational and local molecular-librational modes. The pressure values are calculated and shown in ESI Fig S3 and Table S1.†This observation, taken together with the findings of ref. 20 & 21 of optimal e/m-field frequencies to both excite lattice and local modes21 (and, indeed, induce hydrate dissociation per se20), prompted us in the present study to examine the effect on H2-leakage rate – sans dissociation, naturally, by counting the number of hydrate-like guest molecules entrapped in cages via the BC approach – as shown in Fig. 2 in terms of the trajectories of hydrate-like H2-guests versus time, and the associated leakage rates (beyond transient surface partial-cage escape in the early picoseconds). It can be seen in Fig. 2a – dramatically – that 10 GHz (in purple) is the most effective of those e/m-field frequencies studied at promoting the emptying rate (with ANOVA confirmation of Fig. 2b); the static electric field (in red) is intermediate relative to microwave fields in its H2-leakage-promoting efficacy vis-à-vis the zero-field (equilibrium) case (in black). A representative configuration of the system is shown in Fig. 3 for the optimal 10 GHz escape-rate case, which accelerates the already-observed (zero- and static-field) trend in ref. 9 & 16 for moves towards guest chemical-potential equalisation between the hydrate and gas phases via unsteady-state (quasi-Fickian) guest diffusion through the hydrate lattice. The plateaux reached in the guest-occupation levels after circa 0.5 μs indicate little Fick's-Law driving force for mass transfer (vide infra) and substantial progress towards H2 chemical-potential equalisation in hydrate/gas phases.
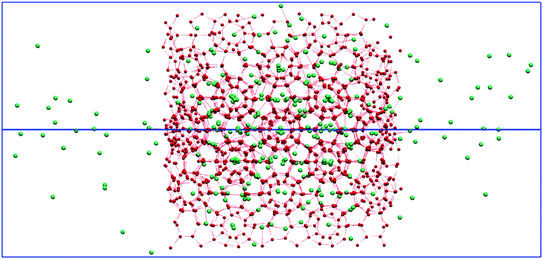 | ||
| Fig. 3 Snapshot of hydrogen escaping the cages into the vacuum under the influence of a 10 GHz e/m field (which maximises the escape rate, cf. Fig. 2). Hydrogen molecules are represented by green-coloured dots, whilst red represents hydrate-based water-based cages. | ||
As Treybal has considered for the analytic case,25 this unsteady diffusion of gas molecules in a solid (and escape therefrom) can be modelled using Fick's second law of diffusion:
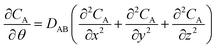 | (1) |
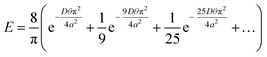 | (2) |
 | (3) |
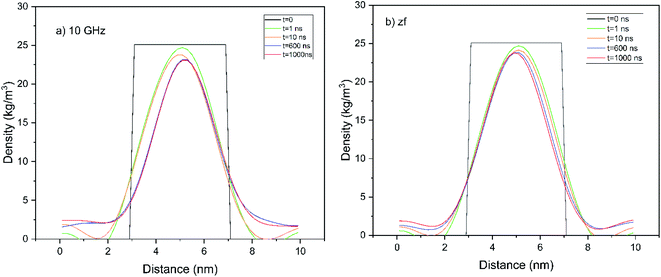 | ||
| Fig. 4 The development in time of parabolic-like spatial H2-concentration profiles along the laboratory x-direction sampled from NEMD simulation, in accord with analytic theory of unsteady-state diffusion of a gaseous guest from a solid.25 Under the influence of (a) 10 GHz electromagnetic field, and (b) zero-field conditions. | ||
Of course, the foregoing analytic theory25 was not developed for external fields, but it still provides a useful conceptual framework in the presence of electric fields. Indeed, Fig. 4a and b show semi-quantitative concentration-profile differences between 10 GHz and zero-field cases, with Fig. 4a showing a more narrow distribution within the hydrate for the 10 GHz case, owing to more rapid and extensive emptying (cf. Fig. 2). Indeed, in the context of the Fickian theory,25 it is expected that the diffusivity coefficient (D) is affected by the presence of electromagnetic fields, and evidence was shown in ref. 20 for this in the case of hydrate kinetics (albeit for hydrate dissociation). Therefore, given that we have prima facie evidence of often-accelerated clathrate emptying in Fig. 2 vis-à-vis the zero-field case (yielding the evolution of the fraction of enclathrated H2 remaining, i.e., E, versus time, θ – cf. eqn (3)), it is straightforward to see that an enhanced self-diffusivity of H2 within the bulk hydrate, D, according to the theory (cf. eqn (2)) and, indeed, physical intuition, will bear out faster hydrate leakage. Bearing this in mind, a fitting-comparison of the time evolution of E in eqn (2) with the actual NEMD-release results in Fig. 2 yields (inter-cage-hopping) H2 self-diffusivities approaching an order of magnitude higher than in bulk hydrate for the zero-field case than found previously from equilibrium MD.7,34 However, given that this system is inherently non-bulk, in that it is dominated in large part, ipso facto, by the hydrate-slab faces relatively near the centre, this is not at all unexpected – and is still broadly consistent in terms of high-level physical picture with the unsteady-state Fickian-diffusion framework (cf. eqn (1) and (2)). However, the microwave-field action in enhancing the underlying H2 self-diffusivity per se is to be noted with profit – especially with dynamical engineering control of H2 release in mind.
Naturally, the question arises as to why the 10 GHz frequency promotes hydrate leakage to the greatest extent, i.e., in boosting maximally the kinetics of inter-cage hopping. The mechanistic origins of these inter-cage phenomena lie in microwave-mediated intra-cage dynamics – more so than field coupling with lattice modes. Building on interesting experimental work of Ranieri et al.,26 English and Burnham have studied and characterised recently via molecular simulation such intra-cage hopping dynamics in doubly-occupied 51264 cavities – effectively occupying two of four tetrahedral sites at any point in time, hopping and swapping between these in a game of (thermally-activated) “musical chairs”.27 At 140 K, the relaxation time in these sites is estimated to be about 8 ps (ref. 27) (albeit using a different force-field7 to the present study). Given that there are six ways that the pair of molecules can distribute between these four sites – i.e., from simple combinatorics of 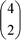 – this yields a total relaxation time of around 50 ps in terms of sampling these intra-cage sites. Bearing in mind that there are two roughly opposing hexagonal cage-faces along the general laboratory x-direction of the applied (microwave) field, this would imply a general relaxation time of the order of 100 ps for typical inter-cage jump phenomena. Given that the 10 GHz frequency has a period of 100 ps, this overlaps quite well with this more “natural”, underlying timescale – originating now from effectively “resonating” with H2–H2 collisions arising from their natural, thermally-driven intra-cage hopping dynamics. This enhances face-crossing momenta and reduces thermal activation-energy barriers for this process. For this 10 GHz case, the final averaged occupations of small cages is close to 1 (i.e., ∼0.97), as expected, whilst that of the large cages is about ∼1.30. This means that it is the large cages which have had the largest proportional decrease in occupancy, which is consistent with this hypothesis, which affects large cages only.
– this yields a total relaxation time of around 50 ps in terms of sampling these intra-cage sites. Bearing in mind that there are two roughly opposing hexagonal cage-faces along the general laboratory x-direction of the applied (microwave) field, this would imply a general relaxation time of the order of 100 ps for typical inter-cage jump phenomena. Given that the 10 GHz frequency has a period of 100 ps, this overlaps quite well with this more “natural”, underlying timescale – originating now from effectively “resonating” with H2–H2 collisions arising from their natural, thermally-driven intra-cage hopping dynamics. This enhances face-crossing momenta and reduces thermal activation-energy barriers for this process. For this 10 GHz case, the final averaged occupations of small cages is close to 1 (i.e., ∼0.97), as expected, whilst that of the large cages is about ∼1.30. This means that it is the large cages which have had the largest proportional decrease in occupancy, which is consistent with this hypothesis, which affects large cages only.
Conclusions
Microsecond-long hydrogen-release dynamics from already-loaded sII clathrate hydrate have been investigated by NEMD under the presence of lattice-preserving 0.05 V Å(r.m.s.)−1 electric fields within the range of 0 to 1 THz at 140 K, using the BC geometric-recognition algorithm to establish the population of enclathrated H2 molecules as a function of time, during this unsteady-state diffusion process. Aside from NEMD obtaining general consistency with Fickian diffusion theory for this unsteady-state process (eqn (1)–(3)), we conclude that judiciously-selected microwave fields (around 10 GHz, in the present case) can enhance H2-leakage kinetics (Fig. 2) from field-resonating analysis with general thermally-driven, intra-cage-hopping timescales – so as to enhance collisions between pairs of guest molecules in doubly-occupied large cages – across hexagonal faces, and thus promoting inter-cage hopping (and, therefore, guest self-diffusivity,7,34 and eqn. (2)25).It would be interesting for potential future study to consider a more realistic range of occupation of the large cage, given good simulation23,24,27 and experimental26 consensus about close-to-single occupation of small cages. As ref. 26 points out from experiment, and ref. 23, 24 & 27 do from molecular simulation, the possibility of triple occupation of large cages is very unlikely. Empty large cages are somewhat more likely, however, particularly during actual emptying processes. In any event, by adopting a range of different large-cage occupations, perhaps informed by Gibbs-Ensemble Monte Carlo simulation, it would be possible to take such an ensemble of different configurations that would represent a mean double occupation of large cages. By then carrying out an entire ensemble, or “swarm”, of such molecular simulations, using each such configuration as a starting point for one or more independent simulations, it would be possible to develop statistically-weighted leakage rates, and distributions thereof.
This study has shown that NEMD can be used to elucidate mechanistic effects of electric-field-manipulated guest-diffusivity control in hydrates. This demonstrates powerfully the prototyping promise of NEMD as a “molecular-design” tool for use in “field-engineering” applications, as we leverage state-of-the-art non-equilibrium simulation in crafting new control strategies for use in realising controllable, demand-led hydrogen-storage engineering systems – harnessing microwaves as powerful and versatile agents in advanced feed-back- and/or-forward control systems, as the world attempts to move towards the Hydrogen Economy urgently – at terawatt hour scale. Indeed, we note the great potential to utilise electric fields as control agents in a wide variety of technologic materials,18,33 and such judiciously-optimised fields to be a potentially near-universal method to release stored guests from host materials.
Conflicts of interest
There are no conflicts to declare.References
- E.D. Sloan Jr, E. Dendy, C. A. Koh, and C. A. Koh. Clathrate Hydrates of Natural Gases, Clathrate Hydrates of Natural Gases, September, 2007, DOI:10.1201/9781420008494.
- H. P. Veluswamy, R. Kumar, and P. Linga. Hydrogen Storage in Clathrate Hydrates: Current State of the Art and Future Directions, 2014, DOI:10.1016/j.apenergy.2014.01.063.
- Y. H. Ahn, S. Moon, D. Y. Koh, S. Hong, H. Lee, J. W. Lee and Y. Park, One-Step Formation of Hydrogen Clusters in Clathrate Hydrates Stabilized via Natural Gas Blending, Energy Storage Materials, 2020, 24, 655–661, DOI:10.1016/j.ensm.2019.06.007.
- T. A. Strobel, C. A. Koh and D. Sloan, Hydrogen Storage Properties of Clathrate Hydrate Material, Fluid Phase Equilib., 2007, 261, 382–389 CrossRef CAS . https://reader.elsevier.com/reader/sd/pii/S0378381207003998?token=8CA7534F38F05713F8E3F977ED82C8DA6A9D48BD2D83F5C53964F152C1D6754748AF9C5748DC98346963685AD48BEF6D%26originRegion=eu-west-1%26originCreation=20210707170248.
- M. Lauricella, S. Meloni, S. Liang, N. J. English, P. G. Kusalik and G. Ciccotti, Clathrate Structure-Type Recognition: Application to Hydrate Nucleation and Crystallisation, J. Chem. Phys., 2015, 142(24), 244503, DOI:10.1063/1.4922696.
- T. A. Strobel, C. A. Koh and E. Dendy Sloan, Hydrogen Storage Properties of Clathrate Hydrate Materials, Fluid Phase Equilib., 2007, 261(1–2), 382–389, DOI:10.1016/j.fluid.2007.07.028.
- H. Cao, N. J. English and J. M. D. MacElroy, Diffusive hydrogen inter-cage migration in hydrogen and hydrogen-tetrahydrofuran clathrate hydrates, J. Chem. Phys., 2013, 138, 094507 CrossRef PubMed.
- C. J. Burnham, Z. Futera and N. J. English, Study of Hydrogen-Molecule Guests in Type II Clathrate Hydrates Using a Force-Matched Potential Model Parameterised from Ab Initio Molecular Dynamics, J. Chem. Phys., 2017, 148(10), 102323, DOI:10.1063/1.4999909.
- K. Yogeshwaran, M. R. Ghaani and N. J. English, Hydrogen and Deuterium Molecular Escape from Clathrate Hydrates: “Leaky” Microsecond-Molecular-Dynamics Predictions, J. Phys. Chem. C, 2021, 125(15), 8430–8439, DOI:10.1021/acs.jpcc.1c00987.
- N. J. English and D. G. Carroll, Prediction of Henry's Law constants by a Quantitative Structure Property Relationship and neural networks, J. Chem. Inf. Comp. Sci., 2001, 41(5), 1150–1161 CrossRef CAS PubMed.
- A. Hassanpouryouzband, E. Joonaki, M. Vasheghani, S. Takeya, C. Ruppel, J. Yang, N. J. English, J. M. Schicks, K. Edlmann, H. Mehrabian, Z. M. Aman and B. Tohidi, Gas hydrates in sustainable chemistry, Chem. Soc. Rev., 2020, 49, 5225–5309 RSC.
- M. R. Ghaani, J. M. Schicks and N. J. English, A Review of Reactor Designs for Hydrogen Storage in Clathrate Hydrates, Appl. Sci., 2021, 11, 469, DOI:10.3390/app11020469.
- T. Shibata, H. Yamachi, R. Ohmura and Y. H. Mori, Engineering investigation of hydrogen storage in the form of a clathrate hydrate: Conceptual designs of underground hydrate-storage silos, Int. J. Hydrogen Energy, 2012, 37, 7612–7623 CrossRef CAS.
- M. Ozaki, S. Tomura, R. Ohmura and Y. H. Mori, Comparative study of large-scale hydrogen storage technologies: Is hydrate-based storage at advantage over existing technologies?, Int. J. Hydrogen Energy, 2014, 39, 3327–3341 CrossRef CAS.
- P. Di Profio, S. Arca, F. Rossi and M. Filipponi, Comparison of hydrogen hydrates with existing hydrogen storage technologies: Energetic and economic evaluations, Int. J. Hydrog. Energy, 2009, 34, 9173–9180 CrossRef CAS.
- Y. Krishnan, M. R. Ghaani and N. J. English, Electric-Field Control of Neon Uptake and Release to and from Clathrate Hydrates, J. Phys. Chem. C, 2019, 123, 27554–27560, DOI:10.1021/acs.jpcc.9b07257.
- N. J. English, and C. J. Burnham. Method And Apparatus For Controllable Storage Of Hydrogen, International PCT/EP2018/071626, Aug 2018 Search PubMed.
- M. R. Ghaani, P. G. Kusalki and N. J. English, Massive generation of metastable bulk nanobubbles in water by external electric fields, Sci. Adv., 2020, 6, aaz0094, DOI:10.1126/sciadv.aaz0094.
- N. J. English and J. M. D. MacElroy, Perspectives on Molecular Simulation of Clathrate Hydrates: Progress, Prospects and Challenges, Chem. Eng. Sci., 2015, 121, 133–156 CrossRef CAS.
- N. J. English and J. M. D. MacElroy, Theoretical studies of the kinetics of methane hydrate crystallization in external electromagnetic fields, J. Chem. Phys., 2004, 120(21), 10247–10256 CrossRef CAS PubMed.
- C. J. Waldron and N. J. English, Global-Density Fluctuations in Methane Clathrate Hydrates in Externally-Applied Electromagnetic Fields, J. Chem. Phys., 2017, 147(2), 024506 CrossRef PubMed.
- A. Rojey, Process and system using an electromagnetic wave to prevent the formation of hydrates, US. Pat., 5625178, 1997 Search PubMed.
- C. J. Burnham, Z. Futera and N. J. English, Quantum and classical inter-cage hopping of hydrogen molecules in clathrate hydrate: Temperature and cage-occupation effects, Phys. Chem. Chem. Phys., 2017, 19, 717–728 RSC.
- C. J. Burnham and N. J. English, Free-energy calculations of the intercage hopping barriers of hydrogen molecules in clathrate hydrates, J. Phys. Chem. C, 2016, 120, 16561–16567 CrossRef CAS.
- R. E. Treybal, Mass-Transfer Operations, 3rd edn, McGraw-Hill, New York, 1980 Search PubMed.
- U. Ranieri, M. M. Koza, W. F. Kuhs, R. Gaal, S. Klotz, A. Falenty, D. Wallacher, J. Ollivier, P. Gillet and L. E. Bove, Quantum dynamics of H2 and D2 confined in hydrate structures as a function of pressure and temperature, J. Phys. Chem. C, 2018, 123, 1888–1903 CrossRef.
- N. J. English and C. J. Burnham., 2020 Intra-Cage Structure, Vibrations and Tetrahedral-Site Hopping of H2 and D2 in Doubly-Occupied 51264 Cages in sII Clathrate Hydrates from Path-Integral and Classical Molecular Dynamics, Applied Sciences, 2021, 11(1), 54, DOI:10.3390/app11010054.
- C. J. Waldron and N. J. English, System-Density Fluctuations and Electro-Dissociation of Methane Clathrate Hydrates in Externally-Applied Static Electric Fields, J. Chem. Thermo., 2018, 117, 68–80, DOI:10.1016/j.jct.2017.08.016.
- R. Reale, N. J. English, P. Marracino, M. Liberti and F. Apollonio, Dipolar response and hydrogen-bond kinetics in liquid water in square-wave time-varying electric fields, Molec. Phys., 2014, 112(14), 1870–1878 CrossRef CAS.
- N. J. English, Molecular dynamics simulations of microwave effects on water using different long-range electrostatics methodologies, Molec. Phys., 2006, 104(2), 243–253 CrossRef CAS.
- N. J. English and J. M. D. MacElroy, Atomistic simulations of liquid water using Lekner electrostatics, Molec. Phys., 2002, 100(23), 3753–3769 CrossRef CAS.
- N. J. English and J. M. D. MacElroy, Hydrogen bonding and molecular mobility in liquid water in external electromagnetic fields, J. Chem. Phys., 2003, 119(22), 11806–11813 CrossRef CAS.
- N. J. English, Molecular Dynamics in External Electric Fields, in Electric Fields on Structure and Reactivity: New Horizons in Chemistry, ed. S. Shaik and T. Stuyver, RSC, Cambridge, 2021 Search PubMed.
- Y. Krishnan, M. R. Ghaani, A. Desmedt and N. J. English, Hydrogen Inter-Cage Hopping and Cage Occupancies inside Hydrogen Hydrate: Molecular-Dynamics Analysis, Appl. Sci., 2021, 11(1), 282, DOI:10.3390/app11010282.
- M. P. Allen and D. J. Tildesley, Computer Simulation of Liquids, 2nd edn, Oxford, 2017 Search PubMed.
- J. L. F. Abascal and C. Vega, A general purpose model for the condensed phases of water: TIP4P/2005, J. Chem. Phys., 2005, 123, 234505, DOI:10.1063/1.2121687.
- S. Alavi, J. A. Ripmeester and D. D. Klug, Molecular-dynamics study of structure II hydrogen clathrates, J. Chem. Phys., 2005, 123, 024507 CrossRef PubMed.
- S. Alavi, K. Shin and J. A. Ripmeester, Molecular dynamics simulations of hydrogen bonding in clathrate hydrates with ammonia and methanol guest molecules, J. Chem. Eng. Data, 2015, 60, 389–397 CrossRef CAS.
- S. Nath Chakraborty and L. D. Gelb, A Monte Carlo simulation study of methane clathrate hydrates confined in slit-shaped pores, J. Phys. Chem. B, 2012, 116, 2183–2197 CrossRef PubMed.
- L. A. Báez and C. Paulette, Computer Simulation of the Crystal Growth and Dissolution of Natural Gas Hydrates, Ann. N. Y. Acad. Sci., 1994, 715(1), 177–186, DOI:10.1111/j.1749-6632.1994.tb38833.x.
Footnote |
| † Electronic supplementary information (ESI) available: Temperature and total-system potential energy during NEMD simulations. See DOI: 10.1039/d1ra07471g |
| This journal is © The Royal Society of Chemistry 2022 |