Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fine-tuning of the pharmacological potential of novel thiazolium ionic liquids by anion alteration†
Mohammad Y. Alfaifia,
Ali A. Shatia,
Serag Eldin I. Elbehairiab,
Reda F. M. Elshaarawy *cd and
Emad M. Gade
*cd and
Emad M. Gade
aBiology Department, Faculty of Science, King Khalid University, 9004 Abha, Saudi Arabia
bCell Culture Lab, Egyptian Organization for Biological Products and Vaccines (VACSERA Holding Company), Giza 12311, Egypt
cChemistry Department, Faculty of Science, Suez University, 43533 Suez, Egypt
dInstitut für Anorganische Chemie und Strukturchemie, Heinrich-Heine Universität Düsseldorf, Düsseldorf, Germany. E-mail: reda.elshaarawy@suezuniv.edu.eg; reel-001@hhu.de
eChemistry Department, Faculty of Science, Suez Canal University, Ismalia, Egypt
First published on 22nd December 2021
Abstract
A novel series of thiazolium ionic liquids (TILs) bound to chloride (2a–c), tetrafluoroborate (BF4) (3a–c), and bis-(trifluoromethanesulfonimide) (Tf2N) anions (4a–c) was synthesized and their physicochemical characteristics were investigated using various microanalytical techniques. The pharmacological potential of the new TILs was assessed as chemotherapeutic agents for bacterial infections and ovarian cancer (SKOV-3). Notably, ILs with the same cations become more bactericidal upon their binding with the strongest chaotropic anion (TN2f). The in vitro toxicity of the TILs toward ovarian carcinoma cell lines (SKOV-3) and normal human skin fibroblast cells (HSF) revealed that all tested TILs have the capacity to induce a dose- and time-dependent decline in SKOV-3 cell viability, with Tf2N-linked TILs (4a–c) having a preferable efficacy. In addition, the new compounds showed excellent selectivity for cancer cells (SKOV-3) over healthy cells (HSF). [iPBzTh][Tf2N] (4b) is the most cytotoxic and specific one and may act as a promising anti-ovarian cancer agent.
1. Introduction
Ovarian cancer (OC) is one of the main death causes among females and the 3rd most prevalent gynecological cancer in developing countries.1 Cytoreductive surgery followed by adjuvant chemotherapy (platinum-paclitaxel therapy cocktail) is the most common protocol used in OC therapy.2 Chemotherapy was used prior to surgery to reduce tumor size in order to facilitate surgery. However, after surgery, the role of chemotherapy is to eliminate any remaining tumor cells.2 As many as 75% of cancer patients initially respond to chemotherapeutic treatment, but most of them relapse due to intrinsic drug resistance and die.3 Besides, traditional chemotherapy exhibits a number of disadvantages, including the adverse effects observed when using large dosages, the inability to be absorbed into aqueous media, and a short circulation half-life in the bloodstream.4,5Meanwhile, antimicrobial resistance (AMR) is of great concern nowadays. It is expected that some of the currently utilized drugs will no longer be useful in the future due to their limited efficiencies and side impacts. As a matter of fact, there are even lists established for some AMR microorganisms.6 Despite the variety of therapeutic techniques used to prevent this resistance, such as rational medication usage and decreased antibiotic abuse, it is vital to tackle resistance through a variety of strategies, including the production of new bioactive compounds.6,7 This is why it is important to continuously seek new, highly effective chemotherapeutic treatments with minimal side effects.
The amazing physical and chemical properties of ionic liquids (ILs), such as excellent thermal and chemical stability, non-flammability, non-volatile, amphiphilic nature, reusability, and electric conductivity,8 make them promising candidates for many applications.9 In this scenario, they have been efficiently used as solvents and/or catalysts for organic reactions,10,11 fabrication of nanomaterials,12 electrochemical sensors and biosensors,13 optical chemosensors,14,15 pH nano-fluorosensing,16 scavenging of pollutants from aqueous effluents,17,18 etc.9 Furthermore, ILs have been implicated in a variety of biological and pharmaceutical applications due to their low toxicity toward many biological targets and their exceptional pharmacological capabilities.19 In addition, the physicochemical and biological characteristics of ILs could be fine-tuned using the numerous reported potential protocols for modifying cations and anions, the main components of ILs.9 Noteworthy, tagging of active pharmaceutical ingredients (APIs) by ionic liquids, helps in improving their pharmaceutical properties.9 Several reports have documented the effective application of ILs as chemotherapeutic agents.20 Despite the many studies that have been reported on the synthesis and applications of ILs, however, most of these reports have been focused only on the common cations (ammonium, imidazolium, pyridinium, or phosphonium and their derivatives).9,20 Therefore, exploring other cations is imperative to get more diversified smart ILs for specific-task applications.
The broad-spectrum biochemical and pharmacological properties of the thiazole ring, have made it a popular choice as an active nucleus to design novel chemotherapeutic agents. As a result, its derivatives have been effectively applied in many pharmacological fields, such as antimicrobial, antitumor, antioxidant, antitubercular, anticonvulsant, anti-inflammatory, diuretic, and neuroprotective agents.21 In addition, the thiazole ring acts as the main active ingredient in many promising biomolecules, pro-drugs, and clinical drugs.14 This motivated us to fabricate novel room-temperature thiazolium ILs for chemotherapeutic applications. To the best of our knowledge, very few efforts have been made by researchers to date to design and fabricate thiazolium ionic liquids. Davis and Forrester have reported the first series of thiazolium ILs for application as catalysts in the benzoin condensation reaction.22 Subsequently, a few studies have been performed to explore the potential of other new thiazolium ILs as catalysts,23–25 anion exchangers,26 and gas fractionators.27 Therefore, the current work will be the first study to fine-tune the pharmacological activity of thiazolium ILs by modification of thiazolium cation and its bound anion.
In continuation of our exploring trip for searching of novel multifunctional chemotherapeutic agents,17,28 the aforesaid facts motivated us to synthesize a new series of thiazolium ionic liquids (TILs) bound to various N-aralkyl side chains and different anions. The newly synthesized TILs were structurally characterized based on their spectral characteristics. The pharmacological potentials of new thiazolium derivatives were in vitro investigated against two pathogenic bacterial strains as well as ovarian cancer cell lines.
2. Experimental part
2.1. Materials and instrumentation
Solvents and chemicals utilized in this study, as well as their suppliers, are listed in the ESI.† Also, the chloromethylation protocol that was used in the preparation of benzyl derivatives was shown in detail in the ESI.† In addition, the structural characterization methods used for the obtained starting materials and ionic liquids have been described in the ESI.†2.2. Synthesis of thiazolium ionic liquids (TILs): 3-aralkyl-4-methylthiazol-3-ium chloride
The following procedure was followed for the preparation of quaternary thiazolium chlorides: a solution of benzyl chloride derivative (1.2 eq.) in 10 mL of dry toluene was gradually added to a solution of 4-methylthiazole (4-MeTh) (1 eq.) in the same solvent under vigorous stirring at room temperature. After the addition was completed, the temperature of the reaction mixture was raised to 80 °C and kept overnight under stirring and reflux at this temperature. After allowing the reaction to cool to ambient temperature and settling the desired products, the soluble unreacted materials are removed by filtration or decantation from the solid or semi-solid products. The obtained products were thoroughly washed with dry toluene (3 × 10 mL), a mixture of diethyl ether-ethyl acetate (3 × 10 mL), and eventually diethyl ether (3 × 10 mL) for the complete removal of any impurities. The solid and semi-solid thiazolium chloride products (2a–c) were dried overnight under a vacuum and have been used for the following reactions without any more purification.![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.85 (d, J = 7.4 Hz, 1H, Ph–
), 7.85 (d, J = 7.4 Hz, 1H, Ph–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.73 (t, J = 7.4 Hz, 1H, Ph–
), 7.73 (t, J = 7.4 Hz, 1H, Ph–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.43 (d, J = 7.3 Hz, 1H, Ph–
), 7.43 (d, J = 7.3 Hz, 1H, Ph–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.31–7.15 (m, 2H, Th–
), 7.31–7.15 (m, 2H, Th–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) and Ph–
and Ph–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 5.39 (s, 2H, Ph–C
), 5.39 (s, 2H, Ph–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2), 3.79 (s, 3H, Th–C
2), 3.79 (s, 3H, Th–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 2.67 (s, 3H, Ph–C
3), 2.67 (s, 3H, Ph–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3). 13C NMR (126 MHz, DMSO-d6) δ (ppm): 157.54 (Th–
3). 13C NMR (126 MHz, DMSO-d6) δ (ppm): 157.54 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 2), 144.86 (Th–
2), 144.86 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 4), 137.71 (Ph–
4), 137.71 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 129.27 (Ph–
), 129.27 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 128.57 (Ph–
), 128.57 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 125.68 (Ph–
), 125.68 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 117.83 (Th–
), 117.83 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 5), 56.75 (Ph–
5), 56.75 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2), 21.41 (Ph–
H2), 21.41 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3), and 14.51 (Th–
H3), and 14.51 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3). ESI-MS (+ve): 204.09 m/z [M − Cl−, C11H12NS]+; ESI-MS (−ve): 34.97 m/z [Cl]−.
H3). ESI-MS (+ve): 204.09 m/z [M − Cl−, C11H12NS]+; ESI-MS (−ve): 34.97 m/z [Cl]−.![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.72 (s, br, 3H, Ph–
), 7.72 (s, br, 3H, Ph–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.48 (s, br, 1H, Ph–
), 7.48 (s, br, 1H, Ph–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.30–7.14 (m, 1H, Th–
), 7.30–7.14 (m, 1H, Th–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 5.57 (s, 2H, Ph–C
), 5.57 (s, 2H, Ph–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2), 3.31 (p, J = 1.8 Hz, 1H, C
2), 3.31 (p, J = 1.8 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) (CH3)2), 2.86 (s, 3H, Th–C
(CH3)2), 2.86 (s, 3H, Th–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.21 (d, J = 6.9 Hz, 6H, CH(C
3), 1.21 (d, J = 6.9 Hz, 6H, CH(C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3)2). 13C NMR (125 MHz, CDCl3) δ (ppm): 160.06 (Th–
3)2). 13C NMR (125 MHz, CDCl3) δ (ppm): 160.06 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 2), 148.84 (Th–
2), 148.84 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 4), 144.71 (Ph–
4), 144.71 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 129.43 (Ph–
), 129.43 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 128.62 (Ph–
), 128.62 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 123.44 (Ph–
), 123.44 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 120.71 (Th–
), 120.71 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 5), 55.52 (Ph–
5), 55.52 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2), 52.04 (
H2), 52.04 (![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H(CH3)2), 22.54 (CH(
H(CH3)2), 22.54 (CH(![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3)2), and 14.42 (Th–
H3)2), and 14.42 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3). ESI-MS (+ve): 232.22 m/z [M − Cl−, C14H18NS]+; ESI-MS (−ve): 34.97 m/z [Cl]−.
H3). ESI-MS (+ve): 232.22 m/z [M − Cl−, C14H18NS]+; ESI-MS (−ve): 34.97 m/z [Cl]−.![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.68–7.59 (m, 3H, Ph–
), 7.68–7.59 (m, 3H, Ph–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.42 (d, J = 7.1 Hz, 1H, Ph–
), 7.42 (d, J = 7.1 Hz, 1H, Ph–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.22 (s, 1H, Th–
), 7.22 (s, 1H, Th–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 5.49 (s, 2H, Ph–C
), 5.49 (s, 2H, Ph–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2), 2.79 (s, 3H, Th–C
2), 2.79 (s, 3H, Th–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.32 (s, 9H,
3), 1.32 (s, 9H, ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) (C
(C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3)3). 13C NMR (126 MHz, DMSO-d6) δ (ppm): 161.46 (Th–
3)3). 13C NMR (126 MHz, DMSO-d6) δ (ppm): 161.46 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 2), 148.67 (Th–
2), 148.67 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 4), 140.07 (Ph–
4), 140.07 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 129.24 (Ph–
), 129.24 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 126.02 (Ph–
), 126.02 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 123.14 (Ph–
), 123.14 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 118.44 (Th–
), 118.44 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 5), 55.24 (Ph–
5), 55.24 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2), 35.98 (
H2), 35.98 (![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) (CH3)3), 31.60 (C(
(CH3)3), 31.60 (C(![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3)3), and 14.47 (Th–
H3)3), and 14.47 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3). ESI-MS (+ve): 246.24 m/z [M − Cl−, C15H20NS]+; ESI-MS (−ve): 34.97 m/z [Cl]−.
H3). ESI-MS (+ve): 246.24 m/z [M − Cl−, C15H20NS]+; ESI-MS (−ve): 34.97 m/z [Cl]−.![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.71 (s, 1H, Ph–
), 7.71 (s, 1H, Ph–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.64 (s, 2H, Ph–
), 7.64 (s, 2H, Ph–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.32 (s, 1H, Ph–
), 7.32 (s, 1H, Ph–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.24 (s, 1H, Th–
), 7.24 (s, 1H, Th–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 5.32 (s, 2H, Ph–C
), 5.32 (s, 2H, Ph–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2), 3.88 (s, 3H, Th–C
2), 3.88 (s, 3H, Th–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 2.63 (s, 3H, Ph–C
3), 2.63 (s, 3H, Ph–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3). 13C NMR (126 MHz, DMSO-d6) δ (ppm): 157.52 (Th–
3). 13C NMR (126 MHz, DMSO-d6) δ (ppm): 157.52 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 2), 144.88 (Th–
2), 144.88 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 4), 136.86 (Ph–
4), 136.86 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 129.49 (Ph–
), 129.49 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 128.09 (Ph–
), 128.09 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 125.69 (Ph–
), 125.69 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 117.60 (Th–
), 117.60 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 5), 56.68 (Ph–
5), 56.68 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2), 21.86 (Ph–
H2), 21.86 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3), and 14.59 (Th–
H3), and 14.59 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3). 11B NMR (97 MHz, DMSO-d6) δ (ppm): −1.31 (singlet). 19F NMR (471 MHz, DMSO-d6) δ (ppm): −148.69 (singlet) (B
H3). 11B NMR (97 MHz, DMSO-d6) δ (ppm): −1.31 (singlet). 19F NMR (471 MHz, DMSO-d6) δ (ppm): −148.69 (singlet) (B![[F with combining low line]](https://www.rsc.org/images/entities/char_0046_0332.gif) 4). ESI-MS (+ve): 204.08 m/z [M − BF4−, C11H12NS]+; ESI-MS (−ve): 86.81 m/z [BF4]−.
4). ESI-MS (+ve): 204.08 m/z [M − BF4−, C11H12NS]+; ESI-MS (−ve): 86.81 m/z [BF4]−.![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.74 (d, J = 7.1 Hz, 2H, Ph–
), 7.74 (d, J = 7.1 Hz, 2H, Ph–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.67 (d, J = 7.1 Hz, 1H, Ph–
), 7.67 (d, J = 7.1 Hz, 1H, Ph–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.60 (d, J = 7.2 Hz, 1H, Ph–
), 7.60 (d, J = 7.2 Hz, 1H, Ph–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.48–7.33 (m, 1H, Th–
), 7.48–7.33 (m, 1H, Th–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 5.40 (s, 2H, Ph–C
), 5.40 (s, 2H, Ph–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2), 3.78 (s, 3H, Th–C
2), 3.78 (s, 3H, Th–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 3.33 (p, J = 1.8 Hz, 1H, C
3), 3.33 (p, J = 1.8 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) (CH3)2), 1.23 (d, J = 6.8 Hz, 6H, CH(C
(CH3)2), 1.23 (d, J = 6.8 Hz, 6H, CH(C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3)2). 13C NMR (125 MHz, CDCl3) δ (ppm): 160.06 (Th–
3)2). 13C NMR (125 MHz, CDCl3) δ (ppm): 160.06 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 2), 146.03 (Th–
2), 146.03 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 4), 144.71 (Ph–
4), 144.71 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 132.05 (Ph–
), 132.05 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 129.94 (Ph–
), 129.94 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 128.62 (Ph–
), 128.62 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 120.71 (Th–
), 120.71 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 5), 55.77 (Ph–
5), 55.77 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2), 36.41 (
H2), 36.41 (![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H(CH3)2), 26.99 (CH(
H(CH3)2), 26.99 (CH(![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3)2), and 14.40 (Th–
H3)2), and 14.40 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3). 11B NMR (96 MHz, DMSO-d6) δ (ppm): −1.29 (singlet). 19F NMR (471 MHz, DMSO-d6) δ (ppm): −148.28 (singlet) (B
H3). 11B NMR (96 MHz, DMSO-d6) δ (ppm): −1.29 (singlet). 19F NMR (471 MHz, DMSO-d6) δ (ppm): −148.28 (singlet) (B![[F with combining low line]](https://www.rsc.org/images/entities/char_0046_0332.gif) 4). ESI-MS (+ve): 232.22 m/z [M − BF4−, C14H18NS]+; ESI-MS (−ve): 86.81 m/z [BF4]−.
4). ESI-MS (+ve): 232.22 m/z [M − BF4−, C14H18NS]+; ESI-MS (−ve): 86.81 m/z [BF4]−.![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.73 (dd, J = 7.2, 1H, Ph–
), 7.73 (dd, J = 7.2, 1H, Ph–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.71–7.62 (m, 3H, Ph–
), 7.71–7.62 (m, 3H, Ph–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.61 (d, J = 7.3 Hz, 1H, Th–
), 7.61 (d, J = 7.3 Hz, 1H, Th–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 5.39 (s, 2H, Ph–C
), 5.39 (s, 2H, Ph–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2), 3.77 (s, 3H, Th–C
2), 3.77 (s, 3H, Th–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.39 (s, 9H,
3), 1.39 (s, 9H, ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) (C
(C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3)3). 13C NMR (126 MHz, DMSO-d6) δ (ppm): 160.29 (Th–
3)3). 13C NMR (126 MHz, DMSO-d6) δ (ppm): 160.29 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 2), 148.88 (Th–
2), 148.88 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 4), 144.94 (Ph–
4), 144.94 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 131.97 (Ph–
), 131.97 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 125.94 (Ph–
), 125.94 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 123.08 (Ph–
), 123.08 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 121.33 (Th–
), 121.33 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 5), 55.48 (Ph–
5), 55.48 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2), 34.96 (
H2), 34.96 (![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) (CH3)3), 29.34 (C(
(CH3)3), 29.34 (C(![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3)3), and 14.53 (Th–
H3)3), and 14.53 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3). 11B NMR (97 MHz, DMSO-d6) δ (ppm): −1.30 (singlet). 19F NMR (471 MHz, DMSO-d6) δ (ppm): −148.69 (singlet) (B
H3). 11B NMR (97 MHz, DMSO-d6) δ (ppm): −1.30 (singlet). 19F NMR (471 MHz, DMSO-d6) δ (ppm): −148.69 (singlet) (B![[F with combining low line]](https://www.rsc.org/images/entities/char_0046_0332.gif) 4). ESI-MS (+ve): 246.26 m/z [M − BF4−, C15H20NS]+; ESI-MS (−ve): 86.81 m/z [BF4]−.
4). ESI-MS (+ve): 246.26 m/z [M − BF4−, C15H20NS]+; ESI-MS (−ve): 86.81 m/z [BF4]−.![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.72 (d, J = 7.1 Hz, 1H, Ph–
), 7.72 (d, J = 7.1 Hz, 1H, Ph–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.65 (d, J = 7.2 Hz, 3H, Ph–
), 7.65 (d, J = 7.2 Hz, 3H, Ph–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.59 (d, J = 7.3 Hz, 1H, Th–
), 7.59 (d, J = 7.3 Hz, 1H, Th–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 5.38 (s, 2H, Ph–C
), 5.38 (s, 2H, Ph–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2), 3.77 (s, 3H, Th–C
2), 3.77 (s, 3H, Th–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 2.64 (s, 3H, Ph–C
3), 2.64 (s, 3H, Ph–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3). 13C NMR (75 MHz, DMSO-d6) δ (ppm): 158.30 (Th–
3). 13C NMR (75 MHz, DMSO-d6) δ (ppm): 158.30 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 2), 149.97 (Tf2N–
2), 149.97 (Tf2N–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) F3), 144.92 (Th–
F3), 144.92 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 4), 137.59 (Ph–
4), 137.59 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 130.70 (Ph–
), 130.70 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 128.82 (Ph–
), 128.82 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 126.32 (Ph–
), 126.32 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 117.49 (Th–
), 117.49 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 5), 56.44 (Ph–
5), 56.44 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2), 22.47 (Ph–
H2), 22.47 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3), and 14.68 (Th–
H3), and 14.68 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3). 19F NMR (565 MHz, DMSO-d6) δ (ppm): −81.66 (singlet) (Tf2N–C
H3). 19F NMR (565 MHz, DMSO-d6) δ (ppm): −81.66 (singlet) (Tf2N–C![[F with combining low line]](https://www.rsc.org/images/entities/char_0046_0332.gif) 3). ESI-MS (+ve): 204.09 m/z [M − Tf2N−, C11H12NS]+; ESI-MS (−ve): 279.93 m/z [C2F6NO4S2]−.
3). ESI-MS (+ve): 204.09 m/z [M − Tf2N−, C11H12NS]+; ESI-MS (−ve): 279.93 m/z [C2F6NO4S2]−.![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.77 (d, J = 7.1 Hz, 1H, Ph–
), 7.77 (d, J = 7.1 Hz, 1H, Ph–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.70 (d, J = 7.1 Hz, 3H, Ph–
), 7.70 (d, J = 7.1 Hz, 3H, Ph–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.63 (d, J = 7.3 Hz, 1H, Th–
), 7.63 (d, J = 7.3 Hz, 1H, Th–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 5.43 (s, 2H, Ph–C
), 5.43 (s, 2H, Ph–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2), 3.82 (s, 3H, Th–C
2), 3.82 (s, 3H, Th–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 3.37–3.29 (m, 1H, C
3), 3.37–3.29 (m, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) (CH3)2), 1.26 (d, J = 6.9 Hz, 6H, CH(C
(CH3)2), 1.26 (d, J = 6.9 Hz, 6H, CH(C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3)2). 13C NMR (126 MHz, DMSO-d6) δ (ppm): 158.30 (Th–
3)2). 13C NMR (126 MHz, DMSO-d6) δ (ppm): 158.30 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 2), 149.48 (Tf2N–
2), 149.48 (Tf2N–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) F3), 148.58 (Th–
F3), 148.58 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 4), 144.93 (Ph–
4), 144.93 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 130.70 (Ph–
), 130.70 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 126.34 (Ph–
), 126.34 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 123.06 (Ph–
), 123.06 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 121.09 (Th–
), 121.09 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 5), 55.50 (Ph–
5), 55.50 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2), 33.80 (
H2), 33.80 (![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H(CH3)2), 22.47 (CH(
H(CH3)2), 22.47 (CH(![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3)2), and 14.41 (Th–
H3)2), and 14.41 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3). 19F NMR (565 MHz, DMSO-d6) δ (ppm): −81.68 (singlet) (Tf2N–C
H3). 19F NMR (565 MHz, DMSO-d6) δ (ppm): −81.68 (singlet) (Tf2N–C![[F with combining low line]](https://www.rsc.org/images/entities/char_0046_0332.gif) 3). ESI-MS (+ve): 232.22 m/z [M − Tf2N−, C15H20NS]+; ESI-MS (−ve): 279.93 m/z [C2F6NO4S2]−.
3). ESI-MS (+ve): 232.22 m/z [M − Tf2N−, C15H20NS]+; ESI-MS (−ve): 279.93 m/z [C2F6NO4S2]−.![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.73 (d, J = 7.2 Hz, 1H, Ph–
), 7.73 (d, J = 7.2 Hz, 1H, Ph–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.65 (dd, J = 7.3, 3H, Ph–
), 7.65 (dd, J = 7.3, 3H, Ph–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.61 (d, J = 7.3 Hz, 1H, Th–
), 7.61 (d, J = 7.3 Hz, 1H, Th–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 5.39 (s, 2H, Ph–C
), 5.39 (s, 2H, Ph–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2), 3.77 (s, 3H, Th–C
2), 3.77 (s, 3H, Th–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.39 (s, 9H,
3), 1.39 (s, 9H, ![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) (C
(C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3)3). 13C NMR (126 MHz, DMSO-d6) δ (ppm): 160.28 (Th–
3)3). 13C NMR (126 MHz, DMSO-d6) δ (ppm): 160.28 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 2), 149.43 (Tf2N–
2), 149.43 (Tf2N–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) F3), 148.76 (Th–
F3), 148.76 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 4), 144.94 (Ph–
4), 144.94 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 131.97 (Ph–
), 131.97 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 125.95 (Ph–
), 125.95 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 123.08 (Ph–
), 123.08 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 121.08 (Th–
), 121.08 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) 5), 55.58 (Ph–
5), 55.58 (Ph–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2), 34.96 (
H2), 34.96 (![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) (CH3)3), 29.34 (C(
(CH3)3), 29.34 (C(![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3)3), and 14.57 (Th–
H3)3), and 14.57 (Th–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3). 19F NMR (565 MHz, DMSO-d6) δ (ppm): −81.34 (singlet) (Tf2N–C
H3). 19F NMR (565 MHz, DMSO-d6) δ (ppm): −81.34 (singlet) (Tf2N–C![[F with combining low line]](https://www.rsc.org/images/entities/char_0046_0332.gif) 3). ESI-MS (+ve): 246.24 m/z [M − Tf2N−, C15H20NS]+; ESI-MS (−ve): 279.93 m/z [C2F6NO4S2]−.
3). ESI-MS (+ve): 246.24 m/z [M − Tf2N−, C15H20NS]+; ESI-MS (−ve): 279.93 m/z [C2F6NO4S2]−.2.3. Biological studies
3. Results and discussion
3.1. Design and synthesis of target benzyl thiazolium ionic liquids (BTILs)
As part of our continued interest in exploring and synthesizing novel heterocyclic derivatives for many uses, particularly in biological implementations,31,32 we present here a set of novel thiazolium ionic liquid derivatives for biological applications. The protocol adopted for the preparation of N-alkyl thiazolium ionic liquids from 1,3-thiazole involves three consecutive steps (see Scheme 1). In the first step, a chloromethylation reaction was performed on some alkylbenzenes (toluene, cumene, tert-butylbenzene) using a mixture of dimethoxymethane-chlorosulfonic acid-zinc iodide as a chloromethylating agent to yield the corresponding chlorobenzyl derivatives [1a–c]. Subsequently, the quaternization of 1,3-thiazole utilizing chlorobenzyl derivatives was conducted under reflux to produce the corresponding N-benzyl thiazolium chlorides [2a–c]. Eventually, these compounds were subjected to anion metathesis with tetrafluoroborate (BF4−) and bis-(trifluoromethanesulfonimide) (Tf2N−) at room temperature to yield the corresponding thiazolium ionic liquids [3a–c] and [4a–c] (Scheme 1).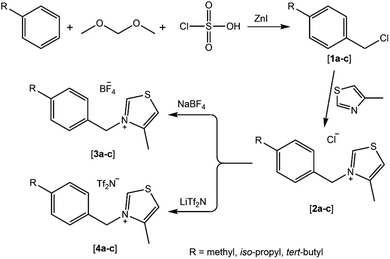 | ||
| Scheme 1 The stepwise pathway for the preparation of the targeted N-benzyl thiazolium ionic liquids. | ||
All the synthesized thiazolium ionic liquids were obtained in good to excellent yields (58–79%) and their structural formulas were elucidated based on the spectral analyses (NMR (1H, 13C, 11B, 19F) and ESI-MS).
3.2. Physical properties of new thiazolium ionic liquids
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = −3.383), whereas, the thiazolium cation that bearing the most hydrophobic side chain (tert-butyl) and bound to a hydrophobic anion (Tf2N−) (4c) was the most insoluble one (log
S = −3.383), whereas, the thiazolium cation that bearing the most hydrophobic side chain (tert-butyl) and bound to a hydrophobic anion (Tf2N−) (4c) was the most insoluble one (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = −7.86).
S = −7.86).
| Compd | MW (g mol−1) | Nature | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sb Sb |
C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pc Pc |
Dd (g cm−3) | ηe (cP) | Tdecf (°C) |
|---|---|---|---|---|---|---|---|
| a MW, molecular weight.b Calculated using ChemDraw 16.c Calculated using ChemDraw 16.d Measured density using COSMOtherm.e Viscosity was measured at 50 °C and concentrations 0.5 mg mL−1 using a capillary viscometer.34f Decomposition temperature taken from DTG curves. | |||||||
| 2a | 239.76 | Solid | −3.383 | −1.182 | NA | NA | NA |
| 2b | 267.82 | Solid | −4.142 | −0.254 | NA | NA | NA |
| 2c | 281.84 | Solid | −4.48 | 0.145 | NA | NA | NA |
| 3a | 291.11 | Oil | −4.569 | −1.181 | 1.543 | 439.25 ± 5.3 | 340.3 |
| 3b | 319.17 | Semi-solid | −5.319 | −0.250 | NA | NA | NA |
| 3c | 333.20 | Semi-solid | −5.653 | 0.147 | NA | NA | NA |
| 4a | 484.45 | Oil | −6.789 | −0.352 | 1.536 | 428.75 ± 4.8 | 323.6 |
| 4b | 512.50 | Oil | −7.53 | 0.074 | 1.526 | 561.41 ± 6.1 | 298.7 |
| 4c | 526.53 | Oil | −7.86 | 0.205 | 1.531 | 587.35 ± 5.9 | 277.3 |
As shown in Table 1, the C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values revealed that the methylbenzyl-methylthiazolium cation is the least lipophilic cation with C
P values revealed that the methylbenzyl-methylthiazolium cation is the least lipophilic cation with C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values in the range of (−1.182)–(−0.352), depending on the type of counter anion. In contrast, replacing the methyl group with a tert-butyl group has remarkably increased the C
P values in the range of (−1.182)–(−0.352), depending on the type of counter anion. In contrast, replacing the methyl group with a tert-butyl group has remarkably increased the C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values to be in the range of 0.145–0.205, depending on the bound anion, confirming their more lipophilic nature. Interestingly, the low C
P values to be in the range of 0.145–0.205, depending on the bound anion, confirming their more lipophilic nature. Interestingly, the low C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P and high aqueous solubility values of thiazolium ionic liquids containing methylbenzyl-methylthiazolium and iso-propylbenzyl-methylthiazolium cations result in better bioavailability and reduced dosages required to obtain a good clinical response during treatment.33 Further, increasing the lipophilicity of the cation results in strong interactions between the ionic liquids and the outer lipophilic layer of the microbial cell surface.19
P and high aqueous solubility values of thiazolium ionic liquids containing methylbenzyl-methylthiazolium and iso-propylbenzyl-methylthiazolium cations result in better bioavailability and reduced dosages required to obtain a good clinical response during treatment.33 Further, increasing the lipophilicity of the cation results in strong interactions between the ionic liquids and the outer lipophilic layer of the microbial cell surface.19
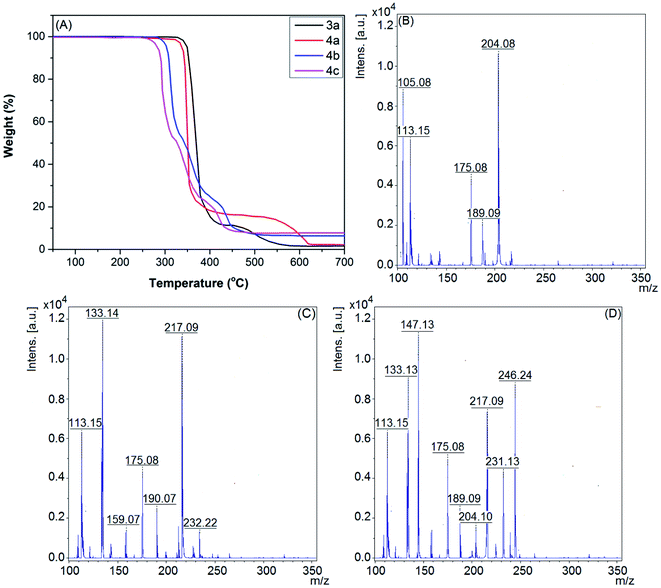
The thermogravimetric (TG) curves of the room temperature thiazolium ionic liquids (RTTILs) (3a, 4a–c) (Fig. 1A) were employed for the investigation of their thermal stabilities. It is evident that they are thermally stable up to 270 °C and then undergo a sharp degradation phase in the temperature range of 270–350 °C. As shown in Fig. 1A, RTTILs with long side chains linked to the N-atom of the thiazolium ring (4b,c) exhibited more complicated thermal decomposition styles and lower initial decomposition temperatures as compared to those with methyl groups (3a and 4a). For instance, the TGA diagram of compound 4a shows two distinct thermal degradation phases. The first main decomposition phase (∼81% weight loss) starts at 323 °C, followed by a modest decomposition stage between 496 and 618 °C. Conversely, IL 4b exhibits three characteristic decomposition steps. The first thermal degradation step begins at 298 °C (∼52% weight loss) and is followed by two smaller degradation steps at 353 and 418 °C, respectively.
3.3. Structural characterization
In addition, common fragmentation peaks were observed in the mass spectra of the thiazolium ionic liquids as a result of the consecutive liberation of alkyl side chains, methylthiazole and/or methylthiazolium fragments from the parent molecule, [M − Tf2N− − R˙]+, [M − Tf2N− − MeTh]+, and [M − Tf2N− − MeThH˙]+.
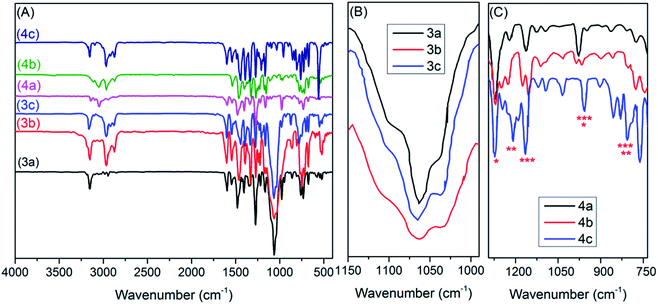
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N/C–S of thiazolium cation,38 and benzyl moiety,33 respectively. On the other hand, the characteristic bands for anions of TILs 3a–c are readily observable in a 1062 ± 2 cm−1 region (see Fig. 2B) that can be assigned to the vibrational modes of [BF4−], as the thiazolium cations do not exhibit strong absorption bands in this region.39 Conversely, the inspection of FTIR spectra for TILs 4a–c (Fig. 2C) revealed remarkable changes in the characteristic bands for anion (TN2f) such as the emergence of several peaks in the regions: 1272 ± 2 cm−1 assigned to νas(CF3) + νas(SO2); 1223 ± 3 cm−1 due to νs(CF3) + νs(SO2); 1155 ± 5 cm−1 characteristic for νas(CF3) + ν(C–S); 1017 ± 2; 905 ± 5 cm−1 distinctive for ν(N–S); 796 ± 3 cm−1 due to δ(CF3); and 747 ± 3 cm−1 assigned to ν(C–S), of the TN2f anion.40,41 In general, the new thiazolium ILs exhibited FTIR signatures comparable to those previously reported in the literature.33
N/C–S of thiazolium cation,38 and benzyl moiety,33 respectively. On the other hand, the characteristic bands for anions of TILs 3a–c are readily observable in a 1062 ± 2 cm−1 region (see Fig. 2B) that can be assigned to the vibrational modes of [BF4−], as the thiazolium cations do not exhibit strong absorption bands in this region.39 Conversely, the inspection of FTIR spectra for TILs 4a–c (Fig. 2C) revealed remarkable changes in the characteristic bands for anion (TN2f) such as the emergence of several peaks in the regions: 1272 ± 2 cm−1 assigned to νas(CF3) + νas(SO2); 1223 ± 3 cm−1 due to νs(CF3) + νs(SO2); 1155 ± 5 cm−1 characteristic for νas(CF3) + ν(C–S); 1017 ± 2; 905 ± 5 cm−1 distinctive for ν(N–S); 796 ± 3 cm−1 due to δ(CF3); and 747 ± 3 cm−1 assigned to ν(C–S), of the TN2f anion.40,41 In general, the new thiazolium ILs exhibited FTIR signatures comparable to those previously reported in the literature.33
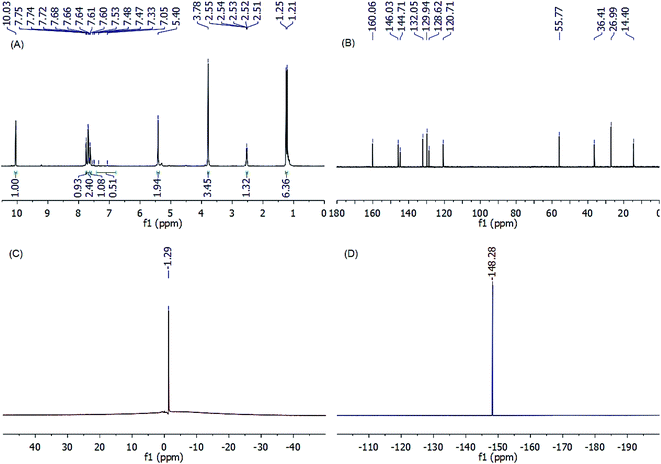 | ||
| Fig. 3 (A) 1H NMR (200 MHz, CDCl3), (B) 13C NMR (125 MHz, CDCl3), (C) 11B NMR (96 MHz, DMSO-d6), and (D) 19F NMR (471 MHz, DMSO-d6) of TIL (3b). | ||
On the other hand, the emergence of eleven distinct carbon peaks in the 13C NMR spectrum of TIL 3b validates the molecular structure of the synthesized ionic liquid. As shown in Fig. 3B, the carbon signal at 14.4 ppm was assigned to the CH3 group of methylthiazole moiety, whereas, the three peaks at 26.99, 55.77, and 36.41 ppm were ascribed to the resonance of methyl and methine carbons of the isopropyl group and benzylic carbon atom, respectively. The emergence of four characteristic carbon signals at 144.71, 132.05, 129.94, and 128.62 confirms the presence of a benzyl ring on the thiazole ring. Another three peaks were observed in the low-field region at δ 120.71, 160.06, and 146.03 ppm corresponding to N–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) –H, N–
–H, N–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) –Me, and S–
–Me, and S–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) –H of thiazolium ring, respectively.
–H of thiazolium ring, respectively.
Notable, the structural identities of the counter-anions for the synthesized thiazolium ionic liquids were identified using negative-mode electrospray mass spectrometry (ESI-MS (−ve)), 11B NMR, and 19F NMR analyses. Where, the ESI-MS (−ve) of the TILs showed major common peaks at m/z 34.97 for Cl−, 86.81 for BF4−, and 279.93 for Tf2N−. Additionally, the emergence of a singlet (δ −1.29 ppm) in 11B NMR spectra, as well as a singlet (δ −148.28 ppm) in the 19F NMR spectra of TILs 3a–c (ESI†), confirms the presence of BF4− as a counter anion for these ILs. Conversely, the emergence of a singlet at δ −81.66 ppm in the 19F NMR spectra of TILs 4a–c (ESI†) is indicative of the binding of these ILs to Tf2N− as a counter anion.
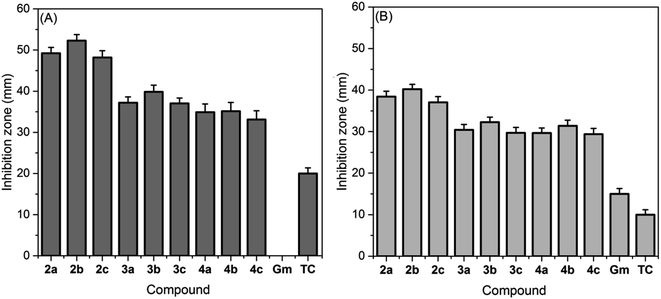
3.4. Antimicrobial study
The amazing antimicrobial characteristics of ILs42 have unlocked new vistas in tackling the current challenges related to fighting antibiotic-resistant pathogens. Motivated by these facts, the antibacterial activities of the newly synthesized thiazolium ionic liquids were investigated against the most common foodborne bacteria, S. aureus and E. coli.| TIL | S. aureus | E. coli | ||
|---|---|---|---|---|
| MIC ± SD | MBC ± SD | MIC ± SD | MBC ± SD | |
| a NA = not assigned. | ||||
| 2a | 4.05 ± 0.29 | 4.25 ± 0.31 | 6.75 ± 0.35 | 12.25 ± 0.36 |
| 2b | 2.35 ± 0.27 | 2.35 ± 0.28 | 5.25 ± 0.31 | 5.25 ± 0.29 |
| 2c | 3.75 ± 0.19 | 3.75 ± 0.14 | 6.25 ± 0.27 | 6.25 ± 0.29 |
| 3a | 3.25 ± 0.19 | 3.25 ± 0.21 | 5.75 ± 0.41 | 5.75 ± 0.45 |
| 3b | 2.56 ± 0.13 | 2.56 ± 0.15 | 5.25 ± 0.29 | 6.25 ± 0.23 |
| 3c | 3.12 ± 0.19 | 3.12 ± 0.14 | 6.50 ± 0.39 | 6.50 ± 0.39 |
| 4a | 1.05 ± 0.19 | 1.05 ± 0.15 | 3.37 ± 0.31 | 6.25 ± 0.29 |
| 4b | 0.75 ± 0.11 | 0.75 ± 0.09 | 2.55 ± 0.42 | 3.25 ± 0.51 |
| 4c | 1.25 ± 0.30 | 1.25 ± 0.33 | 3.50 ± 0.49 | 3.46 ± 0.47 |
| Gentamycin | NA | NA | 6.85 ± 0.63 | NA |
| Tetracycline | 7.20 ± 0.66 | NA | NA | NA |
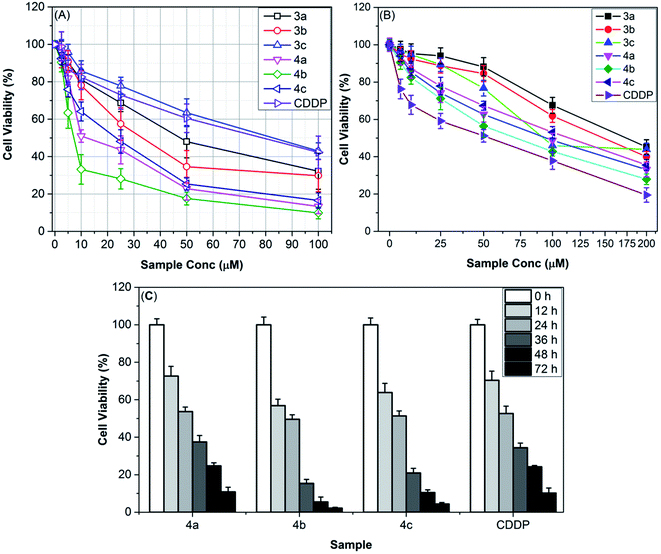
3.5. Cytotoxicity study
As shown in Table 3, the IC50 values of the new TILs against both cell lines are in accordance with the aforementioned hypotheses. It can be seen that the IC50 values for these TILs against ovarian cancer cells were in the range of 7.37–77.95 μM (IC50 for CDDP 79.56 μM), whereas, the IC50 values against HSF cells were 75.42–191.26 μM (IC50 for CDDP 58.28 μM), indicating that SKOV-3 cells are more sensitive than HSF toward treatments with tested thiazolium ILs. Besides, again, Tf2N-tethered thiazolium ILs (4a–c) are more toxic for SKOV-3 cells (IC50 7.37–21.31 μM) as compared to the corresponding tetrafluoroborate salts (3a–c) (IC50 35.73–77.95 μM).
| Cell line | IC50 (μM) ± SD | ||||||
|---|---|---|---|---|---|---|---|
| 3a | 3b | 3c | 4a | 4b | 4c | CDDP | |
| SKOV-3 | 77.95 ± 1.35 | 35.73 ± 1.03 | 51.07 ± 2.33 | 21.31 ± 1.05 | 7.37 ± 1.11 | 15.22 ± 1.23 | 79.48 ± 1.18 |
| HSF | 177.52 ± 8.79 | 152.24 ± 9.15 | 191.26 ± 9.85 | 113.19 ± 7.39 | 75.42 ± 8.09 | 91.81 ± 7.35 | 58.28 ± 6.67 |
| SI | 2.28 | 4.26 | 3.76 | 5.87 | 10.23 | 6.03 | 0.73 |
Furthermore, the selectivity levels of the tested thiazolium ILs for the cancer cells (SKOV-3) over normal ones (HSF) can be investigated by calculating the selectivity index (SI) for the tested TILs using the following equation;51
It is evident in Table 3 that [iPBzTh][Tf2N] (4b) (SI = 10.23) exhibited the highest level of selectivity for attacking SKOV-3 cells over normal cells (HSF). Thus, [iPBzTh][Tf2N] could act as a promising anti-ovarian cancer agent without inducing harm to healthy human skin fibroblasts. Consequently, this study offers the first ionic liquid that can serve as an anti-ovarian cancer agent which may open a wide window for exploring a new generation of TILs-based anti-ovarian cancer agents.
To study the effect of the duration of remediation using TILs on their anti-ovarian cancer activity, the SKOV-3 cell lines were injected with aliquot IC50 doses of the most potent thiazolium ILs (4a–c) and cisplatin and incubated for 72 h. At definite time intervals, the SKOV-3 cell viability has been measured. As shown in Fig. 5C, the viability of SKOV-3 cells was dramatically reduced after incubation for 24 h with the most potent anti-ovarian cancer candidate [iPBzTh][Tf2N] (4b) and virtually totally suppressed after 72 h, reaching a minimum viability (2.2 ± 0.43%). CDDP also caused a time-dependent decrease in SKOV-3 cell viability, albeit with less efficiency than IL 4b, which caused limited SKOV-3 survival at 72 h (10.2 ± 2.7%).
4. Conclusion
A novel series of thiazolium ionic liquids (TILs) bound to chloride (2a–c), tetrafluoroborate (BF4) (3a–c), and bis-(trifluoromethanesulfonimide) (Tf2N) anions (4a–c) has been successfully synthesized. The new TILs were obtained in good yields and their structural formulas were investigated based on microanalytical, spectral (FTIR, UV-Vis, NMR (1H, 13C, 19F, 11B) and ESI-MS), and thermal analyses. The in vitro antibacterial study revealed that the respective chaotropic activity of the IL anion significantly influences their respective antibacterial performance. According to the Hofmeister series and its related impacts, ILs with identical cations become more bactericidal upon binding with a strong chaotropic anion (eg, TN2f). The in vitro toxicity of the newly synthesized TILs toward the ovarian carcinoma cell line (SKOV-3) was assessed using the MTT assay, in comparison to cisplatin (CDDP). The selectivity of new TILs for attacking cancer cells over healthy ones (e.g. human skin fibroblast cells (HSF)) was also investigated. The findings revealed that all the TILs have the capacity to induce a dose- and time-dependent decline in the cell viability of SKOV-3, with a preferable efficacy for Tf2N-linked TILs (4a–c). In addition, the new compounds showed excellent selectivity for cancer cells (SKOV-3) over healthy cells (HSF), as revealed by their selectivity index values. [iPBzTh][Tf2N] (4b) is the most cytotoxic one and can reduce the SKOV-3 cellular viability to a minimum value (10.5 ± 1.7%) at 100 μM dose. Besides, it exhibited the highest level of selectivity (SI = 10.23) for attacking SKOV-3 cells over normal cells (HSF). Therefore, our findings demonstrated the potential application of [iPBzTh][Tf2N] as a novel anti-ovarian cancer agent, in the future. However, further work on remaining issues for these thiazolium ILs is still needed, which will be the aim of our future studies.Conflicts of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through General Group Research Project under grant number (RGP. 2/66/42).References
- B. M. Reid, J. B. Permuth and T. A. Sellers, Cancer Biol. Med., 2017, 14, 9, DOI:10.20892/j.issn.2095-3941.2016.0084.
- C. Katherine, M. P. H. Kurnit, F. Gini, M. D. Fleming and E. Lengyel, Obstet. Gynecol., 2021, 137, 108, DOI:10.1097/aog.0000000000004173.
- S.-S. Li, J. Ma and A. S. T. Wong, J. Gynecol. Oncol., 2018, 29, e32, DOI:10.3802/jgo.2018.29.e32.
- M. Chidambaram, R. Manavalan and K. Kathiresan, J. Pharm. Pharm. Sci., 2011, 14, 67, DOI:10.18433/j30c7d.
- M. E. Davis, Z. Chen and D. M. Shin, Nanosci. Nanotechnol., 2009, 239, DOI:10.1142/9789814287005_0025.
- M. Gajdács, E. Urbán, A. Stájer and Z. Baráth, Eur. J. Investig. Health Psychol. Educ., 2021, 11, 71, DOI:10.3390/ejihpe11010006.
- S. Ghanem, C. J. Kim, D. Dutta, M. Salifu and S. H. Lim, J. Intern. Med., 2021, 290, 40, DOI:10.1111/joim.13238.
- P. Nancarrow and H. Mohammed, ChemBioEng Rev., 2017, 4, 106, DOI:10.1002/cben.201600021.
- S. K. Singh and A. W. Savoy, J. Mol. Liq., 2020, 297, 112038 CrossRef CAS.
- S. Sowmiah, C. I. Cheng and Y.-H. Chu, Curr. Org. Synth., 2012, 9, 74, DOI:10.2174/157017912798889116.
- H. Hisahiro, Heterocycles, 2012, 85, 281, DOI:10.3987/contents-12-85-02.
- X. Duan, J. Ma, J. Lian and W. Zheng, CrystEngComm, 2014, 16, 2550, 10.1039/c3ce41203b.
- V. V. Singh, A. K. Nigam, A. Batra, M. Boopathi, B. Singh and R. Vijayaraghavan, Int. J. Electrochem., 2012, 165683, DOI:10.1155/2012/165683.
- S. M. Saleh, R. Ali and R. F. M. Elshaarawy, RSC Adv., 2016, 6, 68709, 10.1039/c6ra12750a.
- R. F. M. Elshaarawy, R. Ali, S. M. Saleh and C. Janiak, J. Mol. Liq., 2017, 241, 308, DOI:10.1016/j.molliq.2017.06.016.
- R. Ali, S. M. Saleh and R. F. M. Elshaarawy, RSC Adv., 2016, 6, 86965, 10.1039/c6ra18097c.
- R. F. M. Elshaarawy, W. A. Mokbel and E. A. El-Sawi, J. Mol. Liq., 2016, 223, 1123, DOI:10.1016/j.molliq.2016.09.042.
- W. N. El-Sayed, J. Alkabli, K. Althumayri, R. F. M. Elshaarawy and L. A. Ismail, J. Mol. Liq., 2020, 114525, DOI:10.1016/j.molliq.2020.114525.
- K. S. Egorova, E. G. Gordeev and V. P. Ananikov, Chem. Rev., 2017, 117, 7132, DOI:10.1021/acs.chemrev.6b00562.
- A. R. Dias, J. Costa-Rodrigues, M. H. Fernandes, R. Ferraz and C. Prudêncio, ChemMedChem, 2017, 12, 11, DOI:10.1002/cmdc.201600480.
- A. Petrou, M. Fesatidou and A. Geronikaki, Molecules, 2021, 26, 3166, DOI:10.3390/molecules26113166.
- J. H. Davis and K. J. Forrester, Tetrahedron Lett., 1999, 40, 1621 CrossRef CAS.
- F. L. Yu, R. L. Zhang, C. X. Xie and S. T. Yu, Green Chem., 2010, 12, 1196, DOI:10.1016/s0040-4039(99)00025-8.
- D. M. Wolfe and P. R. Schreiner, Eur. J. Org. Chem., 2007, 2825, DOI:10.1002/ejoc.200700114.
- Q. Yan, H. Zang, C. Wu, J. Feng, M. Li, M. Zhang, L. Wang and B. Cheng, J. Mol. Liq., 2015, 204, 156, DOI:10.1016/j.molliq.2015.01.016.
- L. Liu, S. He, S. Zhang, M. Zhang, M. D. Guiver and N. Li, ACS Appl. Mater. Interfaces, 2016, 8, 465–466, DOI:10.1021/acsami.5b11519.
- P. C. Hillesheim, S. M. Mahurin, P. F. Fulvio, J. S. Yeary, Y. Oyola, D. Jiang and S. Dai, Ind. Eng. Chem. Res., 2012, 51, 11530, DOI:10.1021/ie3015632.
- A. R. Sofy, A. A. Hmed, A. E. M. Alnaggar, R. A. Dawoud, R. F. M. Elshaarawy and M. R. Sofy, Int. J. Biol. Macromol., 2020, 163, 1261–1275, DOI:10.1016/j.ijbiomac.2020.07.066.
- R. F. M. Elshaarawy, L. A. Ismail, M. Y. Alfaifi, M. A. Rizk, E. E. Eltamany and C. Janiak, Int. J. Biol. Macromol., 2020, 152, 709, DOI:10.1016/j.ijbiomac.2020.02.284.
- M. Y. Alfaifi, S. E. I. Elbehairi, H. S. Hafez and R. F. M. Elshaarawy, J. Mol. Struct., 2019, 1191, 118, DOI:10.1016/j.molstruc.2019.04.119.
- H. K. Ibrahim, S. H. El-Tamany, R. F. El-Shaarawy and I. M. El-Deen, Maced. J. Chem. Chem. Eng., 2008, 27, 22, DOI:10.20450/mjcce.2008.248.
- R. F. M. Elshaarawy and C. Janiak, Arab. J. Chem., 2016, 9, 825, DOI:10.1016/j.arabjc.2015.04.010.
- L. He, D. Li, C. Zhang, P. Bai and L. Chen, Bioorg. Med. Chem. Lett., 2017, 27, 4171, DOI:10.1016/j.bmcl.2017.07.009.
- S. M. Tawfik, J. Mol. Liq., 2015, 209, 320–326, DOI:10.1016/j.molliq.2015.05.054.
- M. Niemczak, T. Rzemieniecki, Ł. Sobiech, G. Skrzypczak, T. Praczyk and J. Pernak, J. Mol. Liq., 2019, 276, 431–440, DOI:10.1016/j.molliq.2018.12.013.
- R. F. M. Elshaarawy, F. H. A. Mustafa, A. R. Sofy, A. A. Ahmed and C. Janiak, J. Environ. Chem. Eng., 2019, 7, 102800, DOI:10.1016/j.jece.2018.11.044.
- R. F. M. Elshaarawy and C. Janiak, Tetrahedron, 2014, 70, 8023, DOI:10.1016/j.tet.2014.08.034.
- R. Yankova and I. Tankov, J. Mol. Liq., 2021, 323, 114634, DOI:10.1016/j.molliq.2020.114634.
- T. Yamada and M. Mizuno, ACS Omega, 2021, 6, 1709, DOI:10.1021/acsomega.0c05769.
- W. Huang, R. A. Wheeler and R. Frech, Spectrochim. Acta, Part A, 1994, 50, 985, DOI:10.1016/0584-8539(94)80147-9.
- F. M. Vitucci, F. Trequattrini, O. Palumbo, J.-B. Brubach, P. Roy and A. Paolone, Vib. Spectrosc., 2014, 74, 81, DOI:10.1016/j.vibspec.2014.07.014.
- N. Nikfarjam, M. Ghomi, T. Agarwal, M. Hassanpour, E. Sharifi, D. Khorsandi, M. Ali, F. Rossi, A. Rossetti, E. Nazarzadeh, N. Rabiee, D. Afshar, M. Vosough, T. Kumar, V. Mattoli, E. Lichtfouse, F. R. Tay and P. Makvandi, Adv. Funct. Mater., 2021, 2104148, DOI:10.1002/adfm.202104148.
- H. Galbraith and T. B. Miller, J. Appl. Microbiol., 1973, 36, 647, DOI:10.1111/j.1365-2672.1973.tb04150.x.
- P. Mester, M. Wagner and P. Rossmanith, Ecotoxicol. Environ. Saf., 2015, 111, 96, DOI:10.1016/j.ecoenv.2014.08.032.
- K. I. Assaf and W. M. Nau, Angew. Chem., Int. Ed., 2018, 57, 13968, DOI:10.1002/anie.201804597.
- J. Łuczak, C. Jungnickel, I. Łacka, S. Stolte and J. Hupka, Green Chem., 2010, 12, 593–601, 10.1039/b921805j.
- S. Anvari, H. Hajfarajollah, B. Mokhtarani, M. Enayati, A. Sharifi and M. Mirzaei, J. Mol. Liq., 2016, 221, 685–690, DOI:10.1016/j.molliq.2016.05.093.
- R. F. M. Elshaarawy, H. R. Z. Tadros, R. M. Abd El-Aal, F. H. A. Mustafa, Y. A. Soliman and M. A. Hamed, J. Environ. Chem. Eng., 2016, 4, 2754, DOI:10.1016/j.jece.2016.05.016.
- R. F. M. Elshaarawy, I. M. Eldeen and E. M. Hassan, J. Mol. Struct., 2017, 1128, 162, DOI:10.1016/j.molstruc.2016.08.059.
- S. Stolte, J. Arning, U. Bottin-Weber, M. Matzke, F. Stock, K. Thiele, M. Uerdingen, U. Welz-Biermann, B. Jastorff and J. Ranke, Green Chem., 2006, 8, 621, 10.1039/b602161a.
- A. M. Shawky, N. A. Ibrahim, M. A. S. Abourehab, A. N. Abdalla and A. M. Gouda, J. Enzyme Inhib. Med. Chem., 2021, 36, 15, DOI:10.3109/14756366.2015.1057716.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra07128a |
| This journal is © The Royal Society of Chemistry 2022 |