Open Access Article
Open Access ArticleIonic liquids as effective additives to enhance the solubility and permeation for puerarin and ferulic acid†
Jing Yuan‡
 *a,
Ningning Zhou‡b,
Jieyu Wu
*a,
Ningning Zhou‡b,
Jieyu Wu b,
Tianxiang Yin
b,
Tianxiang Yin *b and
Yunbin Jia
*b and
Yunbin Jia *a
*a
aShanghai Sixth People's Hospital, Shanghai 201306, China. E-mail: yuanjing1323@163.com; jiayunbin@foxmail.com; Tel: +86 21 38297000
bSchool of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai 200237, China. E-mail: yintx@ecust.edu.cn; Fax: +86 21 64250804; Tel: +86 21 64252012
First published on 26th January 2022
Abstract
Ionic liquids, especially the cholinium-amino acid-based ionic liquids (CHAAILs), have recently been found to be effective ingredients in formulation of transdermal drug delivery system. In this work, we synthesized six CHAAILs, and investigated their ability to enhance the solubility and permeation of two active pharmaceutic ingredients (APIs), i.e. ferulic acid and puerarin. The solubility measurements showed that a low amount of CHAAILs can significantly increase the solubility of APIs. Moreover, the effective enhancement of permeation of APIs across a polyethersulfone (PES) membrane was achieved at low concentration (4 mg ml−1) of CHAAILs. It is more worthwhile that the presence of CHAAIL brings much less cytotoxicity as compared to traditional types of ionic liquids. Therefore, CHAAILs can be considered as great potential candidates of green and effective additives in transdermal drug delivery systems.
1. Introduction
Traditional Chinese Medicine (TCM) is a national treasure that the Chinese nation has developed in the struggle against diseases for thousands of years. It has made outstanding contributions to the prevention and treatment of various diseases. Transdermal drug delivery system (TDDS), as a safe and non-invasive drug administration method, has been widely applied in TCM, and possesses advantages such as avoidance of first pass hepatic elimination, sustainable therapeutic activity, reduction of side effects and improved patient compliance.1,2 However, during practical applications, some shortcomings were exposed. For instance, lots of the active pharmaceutical ingredients (APIs) are poorly soluble and the permeation efficiency of the API is low. This is also a longstanding challenge in the pharmaceutical industry. Therefore, different types of chemical enhancers and physical methods like microneedles or ultrasound, have been widely used to enhance the transdermal drug delivery of poorly soluble drugs.3–6 Besides, some smart carriers, like microemulsions, liposomes, etc., were also extensively explored to increase the transdermal drug delivery efficiency.7–9In recent decades, considerable attention has been paid to ionic liquids (ILs) as functional ingredients in drug delivery systems.10–13 They displayed high ability to solubilize both hydrophilic and hydrophobic compounds, which have been used in transdermal drug delivery systems alone or in forms of (micro-, nano-) emulsions to solubilize poorly soluble drugs (or as permeation enhancer simultaneously).14–20 More importantly, ILs have been shown to be effective solvents for transdermal delivery of macromolecules like protein and dextran.21–23 However, further application of ionic liquids in pharmaceutics was hindered by their possible toxicity since studies showed that imidazolium cation based or halide containing ionic liquids displayed much higher toxicity than commonly used organic solvents.24–26 Therefore, development of new type of ionic liquids with less toxicity from nature materials has been an important issue.
The development of cholinium-amino acid based ionic liquids (CHAAILs) provided a new strategy,27,28 which were shown to be much less toxic to various bacteria and considered as “practically harmless” and “truly green” ionic liquids.29,30 CHAAILs have been successfully used as reaction media for enzymatic synthesis, biosensors, extraction and separation agent, CO2 absorbent, etc.31–35 However, only few reports concerning the application of CHAAILs in drug delivery have been reported. For instance, Almeida et al.36,37 used cholinium glutanmate [Ch][Glu] and cholinium phenylalaninate [Ch][Phe] to formulate oil-in-water emulsions topical delivery systems, where CHAAILs have been proven to be able to considerably enhance the solubility of hydrophobic drugs with no influence on the activity of drugs and stability of formulates; Goto et al.38 constructed a new drug delivery system for paclitaxel (PTX) based on CHAAILs, i.e. PTX/CHAAILs/ethanol/Tween-8-/water, which was demonstrated as a potentially safer alternative due to fewer hypersensitivity reactions; Yuan et al. showed that two simple CHAAILs, i.e. cholinium glycinate [Ch][Gly] and cholinium analinate [Ch][Ala] can be acted as a good solubility and permeation enhancers for poor soluble drug ibuprofen.39
In order to further explore the potential applications of CHAAILs in drug delivery fields, herein, we synthesized a series of CHAAILs and evaluated their cytotoxicity. Moreover, the efficiencies of these CHAAILs on enhancing the solubility and permeation of two active pharmaceutic ingredients (APIs), i.e. ferulic acid and puerarin, were investigated. Ferulic acid (Scheme 1a) can be widely found in grains, flowers, beans, peanut and nuts etc. Puerarin (Scheme 1b) is the predominant bioactive compound extracted from the root of the plant Pueraria lobate. Ferulic acid and puerarin have been widely applied in Traditional Chinese Medicine due to their diverse biological functions.40–43
2. Materials and methods
2.1 Materials
Choline hydroxide (44% mass fraction, aqueous solution), glycine (Gly, ≥99%), L-alanine (Ala, ≥99%), serine (Ser, ≥99%), isoleucine (Ile,≥ 99%), aspartic acid (Asp, ≥99%), lysine (Lys, ≥99%), carbomer 940, and triethanolamine (>99%) were purchased from Aladdin and used as received. Ferulic acid (≥99%), and puerarin (≥99%) were purchased from Macklin and used as received. DMSO (≥99.9%), penicillin–streptomycin solution (100X), and L-glutamine (≥99%) were supplied by Aladdin. RPMI 1640 cell culture medium and fetal bovine serum (FBS) were purchased from Biological Industries. Phosphate buffered saline (PBS, pH = 7.4, 0.01 M) was purchased from Biorigin. CCK-8 was from Invigentech. A polyethersulfone (PES) membrane was supplied by Sartorius Stedim Biotech GmbH (Germany).2.2 Synthesis of ionic liquids
A typical synthesis process for CHAAIL was described as follows according to the previous report:39 in brief, the aqueous solution of choline hydroxide was added dropwise into the slight excess amino acid aqueous solution. The mixture was stirred under cooling with temperature keeping in the range of 0–5 °C for 24 h. The water was then removed by rotatory evaporation at 50 °C and a viscous liquid was obtained. Mixed solvent of acetonitrile and methanol (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) was used to wash the liquid and precipitate the excess amino acid. The precipitate was then filtered and the filtrate was evaporated to remove the remained mixed solvent. The obtained viscous liquid was dried under vacuum at 60 °C for one week and stored in vacuum desiccator in the presence of P2O5 before use. The water contents of six CHAAILs were determined to be less than 0.2 wt% by coulometric Karl-Fisher titration.
3, v/v) was used to wash the liquid and precipitate the excess amino acid. The precipitate was then filtered and the filtrate was evaporated to remove the remained mixed solvent. The obtained viscous liquid was dried under vacuum at 60 °C for one week and stored in vacuum desiccator in the presence of P2O5 before use. The water contents of six CHAAILs were determined to be less than 0.2 wt% by coulometric Karl-Fisher titration.
2.3 Solubility of APIs
The calibration curves of ferulic acid and puerarin were determined in PBS solution by UV-vis spectrometer (UV-2450, Shimadzu) with 1.0 cm quartz cell at 37.0 °C, where PBS buffer solution was used as the blank.The typical process for the determination of the solubility of API was as follows: excess amount of API was added to 10 ml PBS solution in the absence or presence of certain amount of CHAAIL. The mixture was then vigorously stirred at water bath with temperature being controlled at 37.0 ± 0.1 °C for 72 h. Thereafter, the mixture was centrifuged at 10000 rpm for 30 min and the upper supernatant was then carefully sucked and filtered by 0.22 μm syringe filter. The filtrate was diluted and analyzed by UV-vis spectrometer and the solubility of API was determined by the corresponding calibration curve. The process was repeated trice and the average value was adopted as the solubility of API at PBS solution in the presence or absence of CHAAIL.
2.4 Cytotoxicity evaluation
The CCK-8 assay was used to evaluate cell viability to indicate the safety of the CHAAILs. The L02 cell was cultured in RPMI 1640 supplemented with 10% FBS, 200 U ml−1 penicillin, 100 μg.ml−1 streptomycin and 0.3 g ml−1 L-glutamine under a humidified atmosphere of 95% CO2 at 37 °C. Cells were seeded at a density of approximately 104 cells per ml in a 96-well plate for 48 h. Thereafter, they were incubated with a series of CHAAILs solutions with various concentrations. The cytotoxicity were tested by the CCK-8 method,44,45 where the optical density was measured at 450 nm using a 96-well multi-scanner autoreader. The cell viability was displayed as the percentage compared to untreated cells (negative control). Three independent experiments were carried out and the average values were taken and reported with standard deviation.2.5 In vitro permeation behavior of API-gel
Certain concentrations of APIs in PBS solution in the absence and presence of 4 mg ml−1 CHAAILs were first prepared by precisely weighting. Aqueous solution of 1% Carbomer 940 was stirred gently and swollen overnight at 4 °C, which was followed by the addition of certain amount of PBS solution of APIs. The mixture was stirred and further neutralized by triethanolamine to pH = 7.4. The final concentration of ferulic acid and puerarin in corresponding gels were 2.12 mg g−1, and 3.21 mg g−1, respectively.Permeation investigation was performed in a glass Franz diffusion cell setup (TP-6, Jingtuo Sci. Instrument Co. Ltd, Tianjing, China) with 12 ml in the receptor compartment and 4 ml in the donor compartment. The available transfer area is 1.37 cm2. A polyethersulfone (PES) membrane with 150 μm thickness and 450 nm pore size was placed between the receptor and donor compartment and immobilized by a stainless-steel clamp. The donor compartment was filled with 0.75 g API-gels with or without CHAAILs. The receptor compartment was filled with a PBS solution and stirred by magnetic bar at a constant speed of 300 rpm, which was immersed in water bath with temperature being 37.0 ± 0.1 °C. The samples with a volume of 1 ml from the receptor were taken at certain intervals and the same amount of fresh PBS solution at the same temperature was supplemented. The samples were measured by UV-vis spectrometer to determine the concentration of API with the help of the calibration curve determined in the solubility measurements. The process was repeated trice and average values were adopted.
3. Results and discussions
The synthesized ionic liquids, cholinium glycinate [Ch][Gly], cholinium analinate [Ch][Ala], cholinium serinate [Ch][Ser], cholinium isoleucinate [Ch][Ile], cholinium asparticate [Ch][Asp], and cholinium lysinate [Ch][Lys], were characterized by 1HNMR (the spectra of CHAAILs are shown in Fig. S1 of the ESI†):[Ch][Ala]:1HNMR (400 MHz, D2O), δ: 1.14 (3H, d, J = 7.1, 0.8 Hz, CH3CH), 3.09 (9H, s, (CH3)3N), 3.21–3.27 (1H, m, CHNH2), 3.40–3.42 (2H, m, CH2N(CH3)3),3.94–3.97 (2H, m, CH2OH).
[Ch][Gly]:1HNMR (400 MHz, D2O), δ: 3.05 (2H, s, CH2NH2), 3.09 (9H, s, (CH3)3N), 3.40–3.42 (2H, m, CH2N(CH3)3), 3.96 (2H, qt, J = 6.0, 4.5, 3.4 Hz, CH2OH).
[Ch][Lys]:1HNMR (400 MHz, D2O), δ: 1.24 (2H, q, J = 8.6 Hz, CH2), 1.3–1.42 (2H, m, CH2), 1.43–1.6 (2H, m, CH2), 2.53 (2H, td, J = 7.1, 2.0 Hz, CH2), 3.08–3.17 (10H, m, (CH3)3N, (CH)NH2), 3.42 (2H, td, J = 5.1, 2.2 Hz, CH2N(CH3)3), 3.96 (2H, dq, J = 5.1, 2.6 Hz CH2OH).
[Ch][Ile]:1HNMR (400 MHz, D2O), δ: 0.74–0.84 (6H, m, CH3CH2CHCH3),1.04 (1H, ddt, J = 9.5, 6.7, 4.6 Hz, CH3CH2), 1.30 (1H, dtd, J = 14.9, 7.4, 4.2 Hz, CH3CH2), 1.57 (1H, ddt, J = 9.5, 6.7, 4.6 Hz, CH3CH2CH), 3,00 (1H, d, J = 5.3 Hz, CHNH2), 3.09 (9H, s, (CH3)3N), 3.40–3.42 (2H, m, CH2N(CH3)3), 3.94–3.97 (2H, m, CH2OH).
[Ch][Ser]:1HNMR (400 MHz, D2O), δ: 3.09 (9H, s, (CH3)3N), 3.23 (1H, dd, J = 5.8, 4.3 Hz, CHNH2), 3.40–3.42 (2H, m, CH2N(CH3)3), 3.55–3.65 (2H, m, CHCH2OH), 3.94–3.97 (2H, m, CH2OH).
[Ch][Asp]:1HNMR (400 MHz, D2O), δ: 2.59 (1H, dd, J = 17.5, 8.6 Hz, CH2CH), 2.72 (1H, dd, J = 17.5, 3.8 Hz, CH2CH), 3.09 (9H, s, (CH3)3N), 3.40–3.42 (2H, m, CH2N(CH3)3), 3.80 (1H, dd, J = 8.6, 3.8 Hz, CHNH2), 3.94–3.97 (2H, m, CH2OH).
3.1 Solubility of APIs
The calibration curves of APIs were determined to be A = 79.89C − 0.0017, and A = 71.37C − 0.0062 for ferulic acid and puerarin, respectively, where A and C refer to the absorbance and concentration in mg ml−1, respectively. The standard deviations for these calibration curves were estimated to be about 0.01. It should be mentioned that the presence of small amount CHAAILs resulted in negligible influence on the absorbance of APIs since CHAAILs present no obvious absorption at the maximum absorption wavelengths of APIs, i.e. 252 nm for puerarin and 286 nm for ferulic acid (the UV-vis spectra of APIs and CHAAILs are displayed in Fig. S2 of ESI†). The solubilities of APIs in PBS buffer solution (pH = 7.4, 0.01 M) were determined in the absence and presence of CHAAILs at different mass fractions from 0.1% wt to 1.6% wt. The results are displayed in Table 1, which clearly indicates the enhancement of API solubility in the presence of CHAAILs.| Ferulic acid | ||||||
|---|---|---|---|---|---|---|
| wILs | [Ch][Ser] | [Ch][Ile] | [Ch][Ala] | |||
| S mg ml−1 | δs mg ml−1 | S mg ml−1 | δs mg ml−1 | S mg ml−1 | δs mg ml−1 | |
| 0% | 3.4 | 0.1 | 3.4 | 0.1 | 3.4 | 0.1 |
| 0.4% | 7.6 | 0.2 | 7.1 | 0.1 | 7.9 | 0.1 |
| 0.7% | 10.2 | 0.2 | 9.1 | 0.2 | 10.7 | 0.1 |
| 1.0% | 12.6 | 0.3 | 12.5 | 0.3 | 14.4 | 0.2 |
| 1.3% | 15.9 | 0.3 | 15.3 | 0.3 | 18 | 0.2 |
| 1.6% | 18.7 | 0.4 | 16.7 | 0.3 | 20.6 | 0.3 |
| wILs | [Ch][Gly] | [Ch][Lys] | [Ch][Asp] | |||
|---|---|---|---|---|---|---|
| S mg ml−1 | δs mg ml−1 | S mg ml−1 | δs mg ml−1 | S mg ml−1 | δs mg ml−1 | |
| 0% | 3.4 | 0.1 | 3.4 | 0.1 | 3.4 | 0.1 |
| 0.4% | 8.1 | 0.1 | 9.5 | 0.2 | 6.8 | 0.1 |
| 0.7% | 11.4 | 0.2 | 13.9 | 0.3 | 9.6 | 0.2 |
| 1.0% | 14.2 | 0.2 | 18.9 | 0.4 | 12.4 | 0.3 |
| 1.3% | 17 | 0.3 | 22.3 | 0.5 | 14.8 | 0.3 |
| 1.6% | 19.4 | 0.2 | 26.7 | 0.5 | 17 | 0.4 |
| Puerarin | ||||||
|---|---|---|---|---|---|---|
| wILs | [Ch][Ser] | [Ch][Ile] | [Ch][Ala] | |||
| S mg ml−1 | δs mg ml−1 | S mg ml−1 | δs mg ml−1 | S mg ml−1 | δs mg ml−1 | |
| 0% | 4.9 | 0.1 | 4.9 | 0.1 | 4.9 | 0.1 |
| 0.4% | 12.4 | 0.3 | 8.9 | 0.2 | 10.1 | 0.2 |
| 0.7% | 15.9 | 0.4 | 14.2 | 0.3 | 16.5 | 0.3 |
| 1.0% | 22.4 | 0.5 | 19 | 0.4 | 18.1 | 0.4 |
| 1.3% | 26.7 | 0.5 | 23.7 | 0.5 | 24.8 | 0.5 |
| 1.6% | 30 | 0.7 | 26.5 | 0.5 | 25.1 | 0.6 |
| wILs | [Ch][Gly] | [Ch][Lys] | [Ch][Asp] | |||
|---|---|---|---|---|---|---|
| S mg ml−1 | δs mg ml−1 | S mg ml−1 | δs mg ml−1 | S mg ml−1 | δs mg ml−1 | |
| 0% | 4.9 | 0.1 | 4.9 | 0.1 | 4.9 | 0.1 |
| 0.4% | 13.7 | 0.3 | 14.5 | 0.3 | 9.3 | 0.2 |
| 0.7% | 17.4 | 0.4 | 18.6 | 0.4 | 13.9 | 0.3 |
| 1.0% | 23.4 | 0.5 | 31.8 | 0.6 | 17.2 | 0.3 |
| 1.3% | 26.6 | 0.6 | 41 | 0.8 | 21 | 0.4 |
| 1.6% | 33.6 | 0.7 | 45.4 | 0.9 | 23.4 | 0.5 |
In order to make it more clear to show the influence of the CHAAILs on the solubility of APIs, the plots of the solubility enhancement S/S0 (defined as the ratio of the solubility of API in the presence (S) and absence (S0) of CHAAILs) against the molar fraction of CHAAILs are displayed in Fig. 1.
 | ||
| Fig. 1 Plots of solubility enhancement of APIs (S/S0) against the molar fraction of CHAAILs for (a) ferulic acid, and (b) puerarin. | ||
It can be seen from Fig. 1 that (1) the solubility of APIs increases with the increasing concentration of CHAAIL in the studying concentration range; (2) at the same concentration of CHAAIL, the enhancements of solubility for two APIs are similar; (3) except for [Ch][Lys], other five CHAAILs show similar ability to increase the solubility of APIs. The high efficiency of [Ch][Lys] in increasing APIs' solubility might be ascribed to the fact that [Lys] anion possesses two –NH2 polar groups and longest hydrophobic chain among the investigated six amino acids, which would be more effective as a hydrotrope.
3.2 Cytotoxicity studies
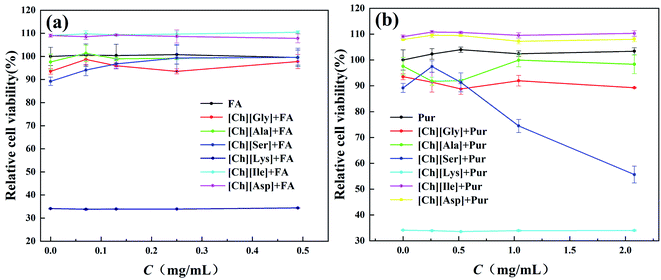
The L02 cells were exposed to CHAAILs at different concentrations directly for 72 h to evaluate the damage they cause. Decline in cell viability with the increase of CHAAILs concentration is clearly shown in Fig. 2. The IC50 values of these CHAAILs are determined and displayed in Fig. 2, which are much less toxic than those of commonly used imidazolium and pirydinium based ionic liquids.46 The low toxicity of these CHAAILs presented herein showed reasonable agreement with those results determined by Gouveia et al.25 Moreover, as shown in Fig. 2, [Ch][Ile], [Ch][Ser], [Ch][Gly], and [Ch][Ala] display similar cytotoxicity, while [Ch][Asp] and [Ch][Lys] show quite different results. Although a few researches have suggested that cation in ionic liquid dominates the toxicity of the ionic liquid,47,48 however, our research did suggest that anion also displayed important role on the cytotoxicity of ionic liquids. Some reports have indicated that the higher molecular weight of anion may bring about higher toxicity of CHAAILs on different bacteria.29,30 [Lys] anion presented highest molecular weight and the longest hydrophobic chain, which may result in relative higher toxicity of [Ch][Lys]. The situation for [Ch][Asp] was opposite, which displayed the lowest cytotoxicity due to the presence of another hydrophilic –COOH group that would not be favourable for the penetration into bilayer lipid membrane of the cell. In consideration of the IC50 values of all CHAAILs, the concentration of CHAAILs used in the following experiments was fixed to be 4 mg ml−1.Moreover, the evaluation of cytotoxicity of CHAAILs (4 mg ml−1) in the presence of different APIs concentrations were also performed to see if there were any synergistic effect between API and CHAAILs. The corresponding results are displayed in Fig. 3. It can be seen from Fig. 3 that for most CHAAILs, the co-existence of API and CHAAILs brings about no significant influence on the cell viability. However, obvious increase in the cytotoxicity was observed for mixture of puerarin and [Ch][Ser] when the concentration of puerarin was larger than 0.6 mg ml−1. It is more strange that the presence of [Ch][Lys] results in significant increase of cytotoxicity even at low APIs concentration. Certain special interaction between [Ch][Lys] and API may be speculated to account for this abnormal results, which needs further investigation.
3.3 Permeation behavior
The cumulative APIs diffusion per unit area in the absence and presence of 4 mg ml−1 CHAAILs across PES membrane over a period of 8 hours were determined and plotted in Fig. 4. | ||
| Fig. 4 Plots of cumulative APIs permeated as a function of time for (a) ferulic acid, and (b) puerarin. | ||
It is clearly indicated from Fig. 4 that the diffusion of APIs across the PES membrane in the presence of 4 mg ml−1 CHAAILs is quite different from that observed in PBS solution without CHAAILs. The cumulative permeated amount of APIs in the presence of CHAAILs displayed an obvious enhancement, especially for ferulic acid. It should be mentioned that two slopes can be observed in the plot of cumulative permeated amount against time for puerarin, which may suggest different behaviour and retention of puerarin in the PES membrane. These may be resulted form the different affinities and sizes of puerarin and the possible complex formed with CHAAILs. Thus, the DLS measurements were performed for the API solution in the presence and absence of [Ch][Ala]. The results were summarized in Fig. S3,† where the presence of different types of API aggregates was clearly indicated.
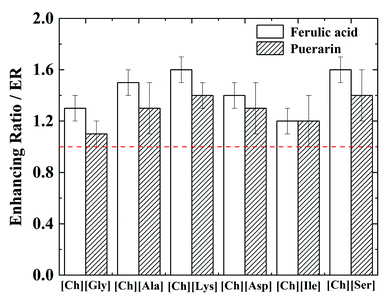
In order to make it more clear to investigate the influence of CHAAILs on the permeation behavior of APIs, the enhancing ratios ER (defined as the ratio of cumulative permeated APIs in 8 h in the presence and absence of CHAAILs, i.e. Q8h,with CHAAILs/Q8h,without CHAAILs) were calculated and plotted in Fig. 5.
It can be seen from Fig. 5 that an increase of 10–60% of the cumulative permeated amount of APIs can be achieved with the addition of 4 mg ml−1 CHAAILs. The increase efficiency varied for different CHAAILs, which may be due to that a series of factors may influence the enhancer efficiency like hydrophilicity, molecular size, substituent species and numbers, and the properties of drugs. It can be found that [Ch][Ser] and [Ch][Lys] displayed better ability to increase the permeated amount of APIs. This may be ascribed to the relative higher hydrophilicity of [Ser] and [Lys] anion. Moreover, the enhance efficiencies of all CHAAILs for ferulic acid were higher than those of puerarin, which may be resulted from the much less molecular weight of ferulic acid. Almeida et al.36 have showed that cholinium glutanmate [Ch][Glu] and cholinium phenylalaninate [Ch][Phe] displayed negligible effect on the permeation of caffeine and salicylic acid. This difference may be ascribed to the different CHAAILs, membranes, concentrations and model drugs used in these different investigations, since all these factors may influence the permeation behaviour.49,50 Wang et al.49 have systematically investigated the permeation enhance efficiency of imidazolium type ionic liquids, where the ER value ranges from 1.66 to 3.59 for different ionic liquids. However, considering the larger concentration of ionic liquid used (5% w/w) in their research, the CHAAILs presented herein (less than 1 wt%) displayed good performance acted as permeation enhancer for studied APIs.
4. Conclusions
To summarize, a series of CHAAILs were synthesized and investigated to show their ability to enhance the solubility and permeation of different APIs, i.e. ferulic acid and puerarin. The solubility measurements showed that slight amount of CHAAILs can significantly increase the solubility of APIs, which was attributed to the hydroptrope behaviour of these CHAAILs that can enhance the solubility of hydrophobic substance. Besides, the effective enhancement of permeation of APIs were achieved at low concentration (4 mg ml−1) of CHAAILs. It is more important that the addition of CHAAILs brought little cytotoxicity as suggested by CCK-8 assay using L02 cells. Thus, CHAAILs can be served as green and effective additives in transdermal drug delivery system for these poor water soluble drugs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Projects 21773063). The author Jing Yuan also thanks for the financial support from the scientific research project in Shanghai Sixth People's Hospital.References
- R. H. Guy, Pharm. Res., 1996, 13, 1765–1769 CrossRef CAS PubMed.
- M. R. Prausnitz, S. Mitragotri and R. Langer, Nat. Rev. Drug Discovery, 2004, 3, 115–124 CrossRef CAS PubMed.
- A. C. Williams and B. W. Barry, Adv. Drug Delivery Rev., 2004, 56, 603–618 CrossRef CAS PubMed.
- M. R. Prausnitz, Adv. Drug Delivery Rev., 2004, 56, 581–587 CrossRef CAS PubMed.
- J. A. Subramony, A. Sharma and J. B. Phipps, Int. J. Pharm., 2006, 317, 1–6 CrossRef CAS PubMed.
- A. Azagury, L. Khoury, G. Enden and J. Kost, Adv. Drug Delivery Rev., 2014, 72, 127–143 CrossRef CAS PubMed.
- M. Rodriguez-Aller, D. Guillarme, J. L. Veuthey and R. Gurny, J. Drug Delivery Sci. Technol., 2015, 30, 342–351 CrossRef CAS.
- X. L. Zhou, Y. Hao, L. P. Yuan, S. Pradhan, K. Shrestha, O. Pradhan, H. J. Liu and W. Li, Chin. Chem. Lett., 2018, 29, 1713–1724 CrossRef CAS.
- W. J. Lu, J. Yao, X. Zhu and Y. Qi, Biomed. Pharmacother., 2021, 134, 111103 CrossRef CAS PubMed.
- K. S. Egorova, E. G. Gordeev and V. P. Ananikov, Chem. Rev., 2017, 117, 7132–7189 CrossRef CAS PubMed.
- K. S. Egorova and V. P. Ananikov, J. Mol. Liq., 2018, 272, 271–300 CrossRef CAS.
- C. Agatemor, K. N. Ibsen, E. E. L. Tanner and S. Mitragotri, Bioeng. Transl. Med., 2018, 3, 7–25 CrossRef PubMed.
- Z. Sidat, T. Marimuthu, P. Kumar, L. C. du Toit, P. P. D. Kondiah, Y. E. Choonara and V. Pillay, Pharmaceutics, 2019, 11, 96 CrossRef CAS PubMed.
- M. Moniruzzaman, Y. Tahara, M. Tamura, N. Kamiya and M. Goto, Chem. Commun., 2010, 46, 1452–1454 RSC.
- M. Moniruzzaman, M. Tamura, Y. Tahara, N. Kamiya and M. Goto, Int. J. Pharm., 2010, 400, 243–250 CrossRef CAS PubMed.
- D. Dobler, T. Schmodts, I. Klingenhofer and F. Runkel, Int. J. Pharm., 2013, 441, 620–627 CrossRef CAS PubMed.
- S. B. M. Nor, P. M. Woi and S. H. Ng, J. Mol. Liq., 2017, 234, 30–39 CrossRef.
- S. Goindi, R. Kaur and R. Kaur, Int. J. Pharm., 2015, 495, 913–923 CrossRef CAS PubMed.
- D. Monti, E. Egiziano, S. Burgalassi, P. Chetoni, C. Chiappe, A. Sanzone and S. Tampucci, Int. J. Pharm., 2017, 516, 45–51 CrossRef CAS PubMed.
- Y. Poh, S. Ng and K. Ho, J. Mol. Liq., 2019, 273, 339–345 CrossRef CAS.
- E. E. Tanner, R. N. Ibsen and S. Mitragotri, J. Controlled Release, 2018, 286, 137–144 CrossRef CAS PubMed.
- Q. M. Qi and S. Mitragotri, J. Controlled Release, 2019, 311–312, 162–169 CrossRef CAS PubMed.
- X. Y. Wu, Z. J. Chen, Y. Li, Q. Yu, Y. Lu, Q. G. Zhu, Y. Li, D. P. An, J. P. Qi and W. Wu, Int. J. Pharm., 2019, 558, 380–387 CrossRef CAS PubMed.
- M. T. Garcia, N. Gathergood and P. Scammells, Green Chem., 2005, 7, 9–14 RSC.
- W. Gouveia, T. F. Jorge, S. Martins, M. Meireles, M. Carolino and C. Cruz, Chemosphere, 2014, 104, 51–56 CrossRef CAS PubMed.
- M. E. Heckenbach, F. N. Romero, M. D Green and R. U. Halden, Chemosphere, 2016, 150, 266–274 CrossRef CAS PubMed.
- Q. P. Liu, X. D. Hou, N. Li and M. H. Zong, Green Chem., 2012, 14, 304–307 RSC.
- S. de Santis, G. Masci, F. Casciotta, R. Caminiti, E. Scarpellini, M. Campetella and L. Gontrani, Phys. Chem. Chem. Phys., 2015, 17, 20687–20698 RSC.
- A. Yazdani, M. Sivapragasam, J. Leveque and M. Moniruzzaman, J. Microb. Biochem. Technol., 2016, 8, 415–421 CAS.
- X. D. Hou, Q. P. Liu, T. J. Smith, N. Li and M. H. Zong, PLoS One, 2013, 8, e59145 CrossRef CAS PubMed.
- Y. H. Bi, Z. Q. Duan, X. Q. Li, Z. Y. Wang and X. R. Zhao, J. Agric. Food Chem., 2015, 63, 1558–1561 CrossRef CAS PubMed.
- D. Zappi, R. Caminiti, G. M. Ingo, C. Sadun, C. Tortolini and M. L. Antonelli, Talanta, 2017, 175, 566–572 CrossRef CAS PubMed.
- R. Wang, Y. Chang, Z. Tan and F. Li, Sep. Sci. Technol., 2016, 51, 1093–1102 CrossRef.
- S. Bhattacharyya and F. U. Shah, ACS Sustainable Chem. Eng., 2016, 4, 5441–5449 CrossRef CAS.
- T. Q. To, K. Procter, B. A. Simmons, S. Subashchandrabose and R. Atkin, Faraday Discuss., 2018, 206, 93–112 RSC.
- T. S. de Almeida, A. Julio, N. Saraiva, A. S. Fernandes, M. E. M. Araujo, A. R. Baby, C. Rosado and J. P. Mota, Drug Dev. Ind. Pharm., 2017, 43, 1858–1865 CrossRef PubMed.
- R. Caparica, A. Julio, A. R. Baby, M. E. M. Araujo, A. S. Fernandes, J. G. Costa and T. S. de Almeida, Pharmaceutics, 2018, 10, 288 CrossRef CAS PubMed.
- M. R. Chowdhury, R. M. Moshikur, R. Wakabayashi, Y. Tahara, N. Kamiya, M. Moniruzzaman and M. Goto, Mol. Pharmaceutics, 2018, 15, 2484–2488 CrossRef CAS PubMed.
- J. Yuan, J. Y. Wu and T. X. Yin, J. Drug Delivery Sci. Technol., 2020, 60, 102037 CrossRef CAS.
- N. Kumar and V. Pruthi, Biotechnol. Rep., 2014, 4, 86–93 CrossRef PubMed.
- D. Li, Y. X. Rui, S. D. Guo, F. Luan, R. Liu and N. Zeng, Life Sci., 2021, 284, 119921 CrossRef CAS PubMed.
- K. H. Wong, G. Q. Li, K. M. Li, V. Razmovski-Naumovski and K. Chan, J. Ethnopharmacol., 2011, 134, 584–607 CrossRef PubMed.
- Y. X. Zhou, H. Zhang and C. Peng, Phytother. Res., 2014, 28, 961–975 CrossRef CAS PubMed.
- S. W. Li, W. T. Chen, L. G. Yao and Y. W. Guo, Steroids, 2018, 136, 17–21 CrossRef CAS PubMed.
- J. F. Liu, X. F. Huang, D. D. Liu, K. Y. Ji, C. Tao, R. Zhang and J. Chen, Phytomedicine, 2021, 91, 153678 CrossRef CAS PubMed.
- R. A. Kumar, N. Papaiconomou, J. M. Lee, J. Salminen, D. S. Clark and J. M. Prausnitz, Environ. Toxicol., 2009, 24, 388–395 CrossRef CAS PubMed.
- D. J. Couling, R. J. Bernot and K. M. Docherty, Green Chem., 2006, 8, 82–90 RSC.
- S. Stolte and J. Arning, Green Chem., 2007, 9, 760–767 RSC.
- D. Zhang, H. J. Wang, X. M. Cui and C. X. Wang, Pharm. Dev. Technol., 2017, 22, 511–520 CrossRef CAS PubMed.
- S.-F. Ng, J. Rouse, D. Sanderson and G. Eccleston, Pharmaceutics, 2010, 2, 209–223 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra07080k |
| ‡ These two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |