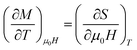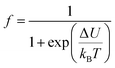Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Influence of Ni content on structural, magnetocaloric and electrical properties in manganite La0.6Ba0.2Sr0.2Mn1−xNixO3 (0 ≤ x ≤ 0.1) type perovskites
Ahmed
Dhahri
 *ab,
J.
Laifi
c,
Soumaya
Gouadria
d,
M.
Elhadi
b,
E.
Dhahri
*ab,
J.
Laifi
c,
Soumaya
Gouadria
d,
M.
Elhadi
b,
E.
Dhahri
 a and
E. K.
Hlil
e
a and
E. K.
Hlil
e
aLaboratoire de Physique Appliquée, Faculté des Sciences de Sfax, Université de Sfax, BP 1171, 3000, Tunisia. E-mail: dhahridhahri14@gmail.com
bDepartment of Physics, College of Science and Humanities – Dawadmi, Shaqra University, Riyadh, Saudi Arabia
cPhysics Department, College of Science, Jouf University, P.O. Box: 2014, Sakaka, Saudi Arabia
dDepartment of Physics, College of Science, Princess Nourah Bint Abdulrahman University, P.O. Box 84428, Riyadh 11671, Saudi Arabia
eUniv. Grenoble Alpes, CNRS, Grenoble INP, Institut Néel, 38000 Grenoble, France
First published on 1st February 2022
Abstract
We present a detailed study on the physical properties of La0.6Ba0.2Sr0.2Mn1−xNixO3 samples (x = 0.00, 0.05 and 0.1). The ceramics were fabricated using the sol–gel route. Structural refinement, employing the Rietveld method, disclosed a rhombohedral R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c phase. The magnetization vs. temperature plots show a paramagnetic–ferromagnetic (PM–FM) transition phase at the TC (Curie temperature), which decreases from 354 K to 301 K. From the Arrott diagrams M2vs. μ0H/M, we can conclude the phase transition is of the second order. Based on measurements of the isothermal magnetization around TC, the magnetocaloric effects (MCEs) have been calculated. The entropy maximum change (−ΔSM) values are 7.40 J kg−1 K−1, 5.6 J kg−1 K−1 and 4.48 J kg−1 K−1, whereas the relative cooling power (RCP) values are 232 J kg−1, 230 J kg−1 and 156 J kg−1 for x = 0.00, 0.05 and 0.10, respectively, under an external field (μ0H) of 5 T. Through these results, the La0.6Ba0.2Sr0.2Mn1−xNixO3 (0 ≤ x ≤ 0.1) samples can be suggested for use in magnetic refrigeration technology above room temperature. The electrical resistivity (ρ) vs. temperature plots exhibit a transition from metallic behavior to semiconductor behavior in the vicinity of TM–SC. The adiabatic small polaron hopping (ASPH) model is applied in the PM-semiconducting part (T > TMS). Throughout the temperature range, ρ is adjusted by the percolation model. This model is based on the phase segregation of FM-metal clusters and PM-insulating regions.
c phase. The magnetization vs. temperature plots show a paramagnetic–ferromagnetic (PM–FM) transition phase at the TC (Curie temperature), which decreases from 354 K to 301 K. From the Arrott diagrams M2vs. μ0H/M, we can conclude the phase transition is of the second order. Based on measurements of the isothermal magnetization around TC, the magnetocaloric effects (MCEs) have been calculated. The entropy maximum change (−ΔSM) values are 7.40 J kg−1 K−1, 5.6 J kg−1 K−1 and 4.48 J kg−1 K−1, whereas the relative cooling power (RCP) values are 232 J kg−1, 230 J kg−1 and 156 J kg−1 for x = 0.00, 0.05 and 0.10, respectively, under an external field (μ0H) of 5 T. Through these results, the La0.6Ba0.2Sr0.2Mn1−xNixO3 (0 ≤ x ≤ 0.1) samples can be suggested for use in magnetic refrigeration technology above room temperature. The electrical resistivity (ρ) vs. temperature plots exhibit a transition from metallic behavior to semiconductor behavior in the vicinity of TM–SC. The adiabatic small polaron hopping (ASPH) model is applied in the PM-semiconducting part (T > TMS). Throughout the temperature range, ρ is adjusted by the percolation model. This model is based on the phase segregation of FM-metal clusters and PM-insulating regions.
1. Introduction
Magnetic refrigeration (MR) technology based on the magnetocaloric effect (MCE) is advancing to become a suitable technology, compared to conventional gas refrigeration,1–3 due to a number of advantages.4 The MCE is generally characterized by two factors: change in entropy (ΔSM) and relative cooling power (RCP).Gadolinium (Gd) is a pure lanthanide element and is the first material that has a high MCE with a Curie temperature (TC) near room temperature (RT).5 Interestingly, further MCE investigations were performed for binary Gd–M compounds, such as Gd3SiGe2,6 which shows a MCE twice that of Gd.7 The researchers focused on finding new cheaper materials with larger MCEs. In this context, manganites with perovskite structure have certain advantages over Gd: their elements are not expensive, they are chemically stable, they have high resistivity and they exhibit a good MCE under low magnetic fields.8,9 Among these, ABO3 compounds are known as perovskite manganites. These materials have a general formula Re1−x3+Ax2+(Mn1−x3+Mnx4+)O32− where Re3+ is a rare earth element (Nd3+, La3+, Pr3+, Sm3+, …) and A2+ is an alkaline earth ion (Sr2+, Ba2+, Ca2+).10,11 They present some interesting properties, which make them very attractive materials for industrial applications.
The pure stoichiometric lanthanum manganite LaMnO3 is antiferromagnetic, insulating at 150 K, and the substitution of the rare earth element by a lower valence ion causes the oxidation of Mn3+ into Mn4+ to ensure electroneutrality in the material. It is followed by the appearance of macroscopic magnetization, i.e. a ferromagnetic coupling between the Mn3+ ions (t2g3e1g) and the Mn4+ ion (t2g3e0g). Substitution of La3+ by a divalent or monovalent ion can result in a wide Curie temperature range which can vary from 150 K to 375 K. Experimentally, manganites, in particular manganese oxides La1−xSrxMnO3 with x = 0.3, are well studied systems. They present an FM–PM transition accompanied by a metal–semiconductor transition close to TC. Several studies have been performed on the magnetocaloric properties of this compound, which exhibits a large change in magnetic entropy, with a narrow range of working temperatures in the vicinity of TC. In addition, several investigations have been carried out to estimate the substitution effects of the Mn site and these have shown that, for the La1−xSrxMnO3 family, even a low rate of substitution at the Mn site would induce a significant change in the properties of magnetotransport.12–14 The values of TC and ΔSM are generally affected by the partial substitution of manganese ions by certain transition metals, for example the In ion,15,16 the Al ion,17etc. On the other hand, the substitution of the rare earth La3+ by certain metals lead to a significant change in the magnetic and electrical properties.18,19
Indeed, the substitution of the Re site with divalent ions20 proves the oxidation of Mn3+ to Mn4+, which is the origin of the ferromagnetic character.21,22 The magnetic coupling between Mn4+ and Mn3+ is usually governed by the movement of the electron, for example between the two partly filled d layers with a strong Hund’s coupling on site.
The issue of replacing magnetic and non-magnetic ions in the Mn site is very important. For example, the partial substitution of Mn3+ ions with Ni2+ ions modifies the ratio of the Mn4+–O2–Mn3+ network and leads to a decrease of the double exchange (DE) interactions.23 Several studies have been carried out24–29 to explain the relationship between the magnetotransport and magnetic properties of Re1−xAxMnO3 substituted by different elements on the Mn site. Based on the information given in this article, we have carefully discussed the physical properties in La0.6Ba0.2Sr0.2Mn1−xNixO3 (0 ≤ x ≤ 0.1) compounds.
2. Experimental details
2.1 Preparation
The La0.6Ba0.2Sr0.2Mn1−xNixO3 ceramics were prepared using the sol–gel route. High purity precursors La(NO3)3·6H2O, Sr(NO3)2·6H2O, Mn(NO3)2·4H2O, Ba(NO3)2 and Ni(NO3)2·6H2O were weighed in stoichiometric proportions and then dissolved in distilled water with continuous stirring, whilst on a hot plate. The mixtures were dispersed in solutions containing a complexation agent (citric acid) and a polymerizing agent (ethylene glycol). The citric acid was used as a chelating agent and the ethylene glycol was used as a gelification agent. In order to form a homogenous yellowish gel, the solutions were heated on a hotplate at about 100 °C for 1 h under magnetic stirring. Then, to remove the excess solvent, the temperature was increased to 400 °C and the combustion led to a very fine and very homogeneous powder (black powder). At the end of this process, the calcinated powder was ground and pressed into pellets. The pellets were subjected to sintering at 900 °C for 24 hours in air.2.2 Characterization
X-ray powder diffraction (XRD) was used to examine the structural behavior of the samples. Using a “Panalytical X’Pert Pro” diffractometer, the XRD was conducted through Cu-Kα radiation (λCu = 1.54056 Å) with a 0.0167° step size and 19 ≤ 2θ ≤ 90° angular range. The refinement was analyzed by Rietveld’s program using FULLPROF software (version 0.2-March 1998-LLB-JRC).30 A Philips XL30 scanning electron microscope (SEM) and an energy dispersive X-ray (EDX) spectrometer working at 15 kV were used to carry out a morphological study of the compounds. The magnetization measurements were recorded with a BS1 and BS2 magnetometer, which was developed in the Louis Néel laboratory in Grenoble.3. Results and discussion
3.1 X-ray analysis
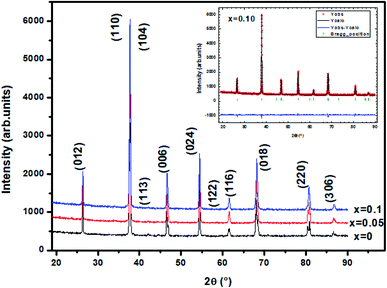
The XRD patterns of the La0.6Ba0.2Sr0.2Mn1−xNixO3 (LBSMNO) samples, recorded at room temperature (RT), are presented in Fig. 1. The analysis of these spectra indicates that all the compounds were successfully prepared with a good crystallinity and a single phase of La0.6Ba0.2Sr0.2Mn1−xNixO3; we have not detected a second phase. In the inset of Fig. 1, we show the crystalline structure of these samples. A good fit agreement between the simulation and the experimental pattern was observed.The patterns of our samples were indexed in the rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c) symmetry (no. 167), with (La, Ba, Sr): 6a (0, 0, 0.25), (Mn, Ni): 6b (0, 0, 0) and O: 18 (x, 0, 0.25). The different structural parameters are tabulated in Table 1. When the Ni substitution increases, the volume and the lattice parameters decrease. A similar behavior has also been previously observed.31
c) symmetry (no. 167), with (La, Ba, Sr): 6a (0, 0, 0.25), (Mn, Ni): 6b (0, 0, 0) and O: 18 (x, 0, 0.25). The different structural parameters are tabulated in Table 1. When the Ni substitution increases, the volume and the lattice parameters decrease. A similar behavior has also been previously observed.31
| x | |||
|---|---|---|---|
| 0.00 | 0.05 | 0.10 | |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c phase c phase |
|||
| a (Å) | 5.4971(4) | 5.4824(3) | 5.4785(1) |
| c (Å) | 13.4713(5) | 13.4572(1) | 13.4491(3) |
| V (Å3) | 352.54(1) | 350.30(2) | 349.58(4) |
| (O)Biso (Å2) | 0.85(2) | 0.54(3) | 2.61(6) |
| (O)x | 0.4472(1) | 0.4526(3) | 0.4435(5) |
| (La, Sr, Ba)Biso (Å2) | 0.967(1) | 0.352(3) | 0.853(5) |
| (Mn, Ni)Biso (Å2) | 0.368(2) | 0.267(7) | 1.812(6) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Discrepancy factors | |||
| R wp (%) | 2.62 | 3.51 | 4.11 |
| R p (%) | 3.22 | 1.09 | 4.20 |
| R F (%) | 5.172 | 6.532 | 2.235 |
| χ 2 (%) | 2.42 | 1.61 | 1.37 |
This decrease is explained by the fact that the average ionic radius of the manganese site (0.599 ≤ rMn+Ni ≤ 0.592) decreases, which can be assigned to the formulation of a higher level of Mn4+, compared to Mn3+.
Taking into account the neutrality of La0.63+Ba0.22+Sr0.22+(Mn0.6−y3+Mn0.4−x+y4+)Nix2+O32− (0 ≤ x ≤ 0.1), these results can be justified as follows: whenever the x ratio of Ni is increased, the proportion of Mn3+ weakens by y = 2x, while the ratio of Mn4+ increases by x. In Table 2, we have listed the (Mn/Ni)–O–(Mn/Ni) bond angles (θ(Mn/Ni)–O–(Mn/Ni)) and the (Mn/Ni)–O bond lengths (d(Mn/Ni)–O). It is noted that θ(Mn/Ni)–O–(Mn/Ni) decreases linearly with the increase of x, whereas d(Mn/Ni)–O increases, leading to a tilting of the BO6 octahedrons.
| x | 0.00 | 0.05 | 0.10 |
|---|---|---|---|
| θ (Mn/Ni)–O–(Mn/Ni) (°) | 166.2(1) | 164.98(9) | 162.82(6) |
| d (Mn/Ni)–O (Å) | 1.9571(1) | 1.9587(2) | 1.9633(1) |
| 〈rB〉 (Å) | 0.645 | 0.647 | 0.649 |
| t G | 0.974 | 0.973 | 0.972 |
| D | 0.286 | 0.285 | 0.283 |
| W (10−2) (arb. units) | 4.73 | 4.71 | 4.66 |
| D SC (nm) | 55 | 50 | 49 |
| 〈DSEM〉 ± σD (μm) | 1.33 ± 0.44 | 0.95 ± 0.38 | 0.79 ± 0.31 |
| D WH (nm) | 130 | 121 | 118 |
| p (%) | 7.26 | 7.32 | 7.35 |
The perovskite structure can be distorted from the ideal cubic structure, which greatly affects the properties. These distortions are principally given by the relationship between the ionic radius of the cations, defined by the tolerance factor tG:32
 | (1) |
In our work, the tolerance factor tG decreases with the increase of Ni (Table 2).
The rate of rhombohedric deformation D% can be calculated employing the expression: 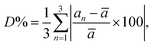 where
where 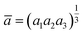 , a1 = a2 = a and
, a1 = a2 = a and 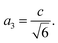
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35,36
35,36
From Table 2, the value of D decreases with the decrease in mean rMn+Ni.
On the other hand, the average crystallite size DSC can be calculated using Scherrer’s relationship:37
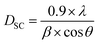 | (2) |
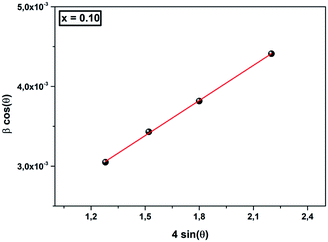
As in Scherrer’s method, the crystallite size values were determined from the Williamson–Hall equation:38
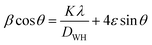 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ (y-axis) vs. 4 sin
θ (y-axis) vs. 4 sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ (x-axis) gives the strain (ε) and the crystallite size (DWH) can be calculated from the intercept of this line on the y-axis (Fig. 2). The calculated values are grouped in Table 2. We can deduce from this result that the average crystallite size determined by Williamson–Hall is greater than that obtained by Scherrer’s method, which is due to the broadening effect caused by the strain exhibited in this technique.
θ (x-axis) gives the strain (ε) and the crystallite size (DWH) can be calculated from the intercept of this line on the y-axis (Fig. 2). The calculated values are grouped in Table 2. We can deduce from this result that the average crystallite size determined by Williamson–Hall is greater than that obtained by Scherrer’s method, which is due to the broadening effect caused by the strain exhibited in this technique.
3.2 Morphological characterization
Fig. 3 presents the morphology of La0.6Ba0.2Sr0.2Mn1−xNixO3 (x = 0 and 0.1) as examples, demonstrated in the SEM images. It can be seen that the distribution of the grains is uniform over the entire surface and the grains are well joined, which shows that our samples are well formed.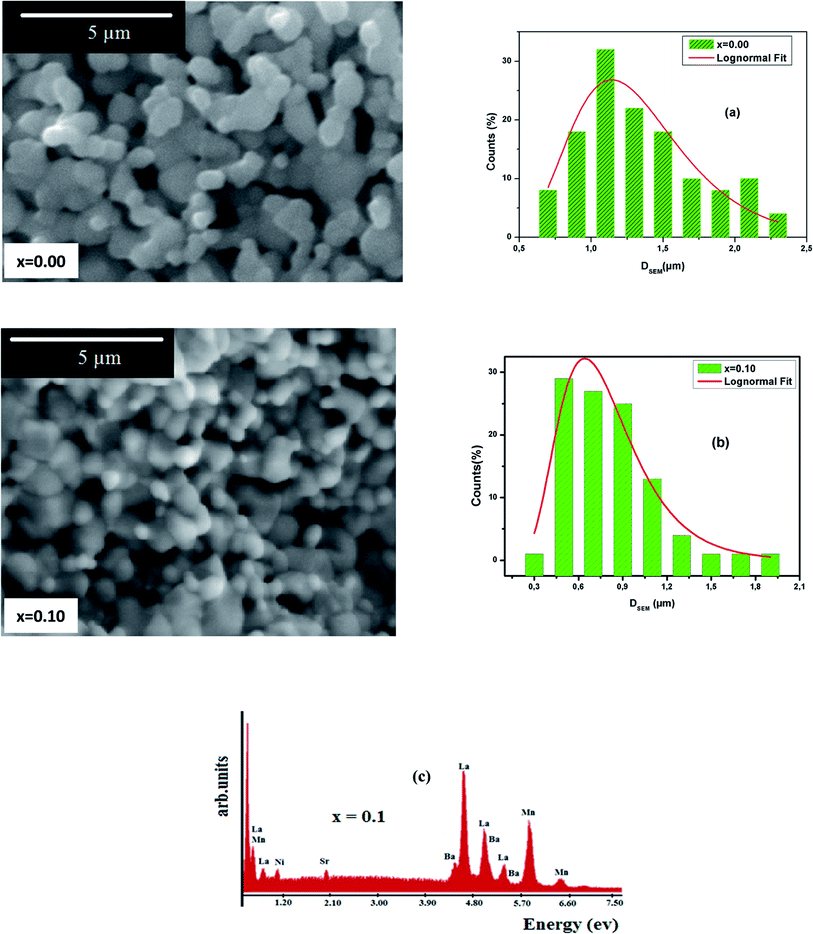 | ||
| Fig. 3 Typical SEM images for x = 0.00 and 0.10, (a) and (b) present the histograms of particle size and (c) shows the EDX analysis for x = 0.10. | ||
ImageJ software was employed to determine a statistical count of the grain size, which was performed on the SEM images. This technique consists of measuring the diameters of all the particles in the SEM image. Then, we adjusted these data using the log-normal function.
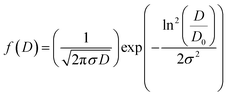 | (4) |
Where, σ is the median diameter obtained from the data dispersions and D0 is the median diameter obtained from the SEM images. Fig. 3(a) and (b) present the grain number (counts) versus the particle size. Using the fit results, the mean diameter 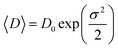 and standard deviation σD = 〈D〉[exp(σ2) − 1]1/2 were determined (Table 2).
and standard deviation σD = 〈D〉[exp(σ2) − 1]1/2 were determined (Table 2).
It is remarkable that the average size of the particles obtained is greater than the average size of the crystallites determined by XRD. This can be explained by the fact that each particle observed by SEM is made up of several crystallites. The energy dispersive X-ray microanalysis (EDX) spectrum of La0.6Ba0.2Sr0.2Mn0.9Ni0.1O3 is presented in Fig. 3(c) as an example. This technique confirms the composition and purity of the samples. The spectra reveal the homogeneous distribution of La, Ba, Sr, Mn, Ni and O atoms over a wide surface area.
To evaluate the porosity 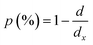 of the compounds, we calculated the X-ray density,
of the compounds, we calculated the X-ray density, 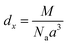 , where d is the bulk density, a is the lattice constant, M is the molecular weight and Na is Avogadro’s number39 (see Table 2).
, where d is the bulk density, a is the lattice constant, M is the molecular weight and Na is Avogadro’s number39 (see Table 2).
3.3 Magnetic properties
The evolution of M(T) measured at 0.05 T in cooled zero field (ZFC) and cooled field (FC) modes is presented in Fig. 4. It is observed that, in the low temperature region, the FC and ZFC curves diverge considerably for all samples, which proves the existence of typical spin glasses, which may also be ascribed to magnetic anisotropy. A spin glass-like state is generally prompted by the coexistence of competing AFM and FM interactions.40 With a decreasing temperature, a PM–FM phase transition was observed at the Curie temperature. TC is given at the lowest point of the first derivative of the curve M(T) (dM/dT) (inset Fig. 4). The TC values go from 354 K for x = 0.00 to 301 K for x = 0.10. This change has been attributed to the modification of the Mn–O–Mn bond angle. Doping with the slightly larger Ni2+ (rNi2+ = 0.69 Å) for Mn3+ (rMn3+ = 0.645 Å) decreases the mean value of the radius of the manganese-site, alters the Mn3+/Mn4+ ratio and decreases the bond angle θ(Mn/Ni)–O–(Mn/Ni). In the La0.6Ba0.2Sr0.2MnO3 sample, ferromagnetism is clarified by the DE interaction between the Mn3+ and Mn3+ ions. Taking into account the neutrality of the charges (La0.63+Ba0.22+Sr0.22+(Mn0.6−y3+Mn0.4−x+y4+)Nix2+O32−) of the samples, the substitution of Ni2+ transforms the average valence state of Mn3+ to Mn4+. This weakening of the Mn3+ ions essentially induces a reduction in the jumps of the electrons (eg) and the progressive suppression of the DE interaction subsequently leads to a reduction in ferromagnetism. Moreover, the competition between the AFM and FM interaction exchange is reinforced.41 On the other hand, when the Ni content increases, M decreases in the FM region and that is consistent with the results in ref. 16 and 42.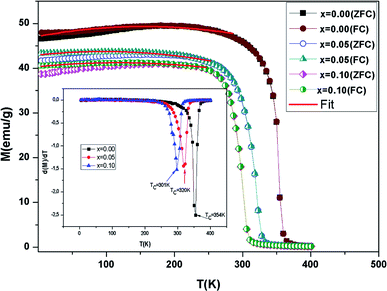 | ||
| Fig. 4 Evolution of magnetization vs. temperature under a magnetic field μ0H = 0.05 T for the compounds LBSMNO. The inset: dM/dT curve vs. T. | ||
The most important cause of the decrease in TC is the reduction of the one-electron bandwidth Wd. It is given as:43
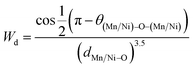 | (5) |
The increase in the Ni content results in a greater length of the d(Mn/Ni)–O bond and a reduction in the bond angle θ(Mn/Ni)–O–(Mn/Ni), and therefore a weaker bandwidth W (Table 2) which causes the decrease of TC.44,45 In fact, this reduction in W leads to a reduction in the FM coupling between neighboring manganese atoms.
In addition, it is interesting to understand the behavior of M vs. temperature in the FM region. In this context, and according to Lonzarich and Taillefer,46 magnetization obeys the theory of spin waves. At low temperatures, this theory is that the magnetization has multiplied in T3/2 (Bloch’s law) and over a wide range of temperatures in T2, yet in the vicinity of TC it varies as follows: (1 − T4/3/TC4/3)1/2.
In the FM region, the M data has been adjusted by this relation:
| M(T) = M0 + M3/2T3/2 + M2T2 | (6) |
For T > TC, the PM region, the Curie–Weiss law was used to analyse the inverse of magnetic susceptibility (χ−1 = H/M):
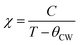 | (7) |
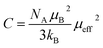 is the Curie constant and θCW is the paramagnetic-Curie temperature. These parameters were obtained using the fit of curve χm−1(T) (Fig. 5) and its values are also given in Table 3. The positive value of θCW suggests that the ferromagnetic interactions between the nearest neighbors are dominant in the system, which could be due to DE Mn3+–O2−–Mn4+ coupling. When x increased, this parameter decreased, which indicates the weakening of the ferromagnetic interactions.47 The values of θCW are higher than those of TC, which indicates the presence of magnetic inhomogeneity above TC.
is the Curie constant and θCW is the paramagnetic-Curie temperature. These parameters were obtained using the fit of curve χm−1(T) (Fig. 5) and its values are also given in Table 3. The positive value of θCW suggests that the ferromagnetic interactions between the nearest neighbors are dominant in the system, which could be due to DE Mn3+–O2−–Mn4+ coupling. When x increased, this parameter decreased, which indicates the weakening of the ferromagnetic interactions.47 The values of θCW are higher than those of TC, which indicates the presence of magnetic inhomogeneity above TC.
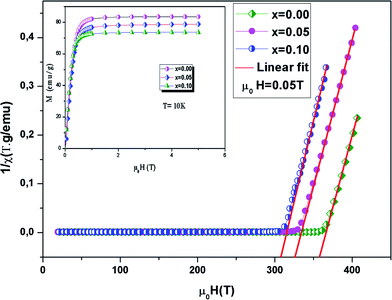 | ||
| Fig. 5 The inverse magnetic susceptibility χ−1versus temperature. The inset shows the field dependence of the magnetization curves at 10 K. | ||
| x | |||
|---|---|---|---|
| 0.00 | 0.05 | 0.10 | |
| T C (K) | 354 | 320 | 301 |
| θ CW (K) | 358 | 326 | 308 |
| μ expeff (μB) | 4.92 | 4.77 | 4.64 |
| μ theeff (μB) | 4.48 | 4.34 | 4.19 |
| μ 0 H C (10−3 T) | 9.7 | 8.9 | 7.7 |
| M s (emu g−1) | 84 | 79 | 75 |
| M r (emu g−1) | 13 | 8 | 5 |
| R | 0.066 | 0.10 | 0.15 |
The experimental effective moment μexpeff can be calculated using the parameter C and the values are given in Table 3.
The theoretical effective paramagnetic moment for La0.6Ba0.2Sr0.2Mn1−xNixO3 compositions could be calculated using the following expression: 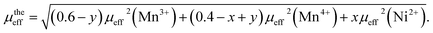
The percentage of Mn3+ and Mn4+ ions was calculated and checked by the conventional chemical method. The orbital moment is frozen (L = 0) for Mn3+ and Mn4+, so, the theoretical effective moment can be given as: 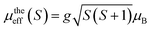 with S = 1.5 for (Mn4+, 3d3), 2 for (Mn3+, 3d4) and 4 for (Ni2+, 3d8) and g = 2. The calculated values of μtheeff(Mn3+), μtheeff(Mn4+) and Ni2+ are 4.90 μB, 3.78 μB and 2.828 μB, respectively. From Table 3, a difference between the experimental and theoretical values of μeff can be observed. This can be clarified by the presence of FM clusters within the PM phase.48
with S = 1.5 for (Mn4+, 3d3), 2 for (Mn3+, 3d4) and 4 for (Ni2+, 3d8) and g = 2. The calculated values of μtheeff(Mn3+), μtheeff(Mn4+) and Ni2+ are 4.90 μB, 3.78 μB and 2.828 μB, respectively. From Table 3, a difference between the experimental and theoretical values of μeff can be observed. This can be clarified by the presence of FM clusters within the PM phase.48
The evolution of magnetization vs. μ0H for La0.6Ba0.2Sr0.2Mn1−xNixO3 (0 ≤ x ≤ 0.1) at 10 K is depicted in the inset of Fig. 5. At μ0H = 1.5 T, the compounds exhibit a constant value of M. The magnetic moments determined by the magnetization data are obtained to be 3.56 μB per formula unit for x = 0.00, 3.35 μB per formula unit for x = 0.05 and 3.1 μB per formula unit for x = 0.1.
The calculated magnetic moment can be determined by: Msp = ((0.67 − 2x) × 4 + (0.4 + x) × 3 + 2x) μB = (3.88 − 3x) μB/f.u.
The magnetic moments of Ni2+, Mn3+ and Mn4+ have 2, 4 and 3 μB, respectively. The Msp values are 3.88 μB for x = 0.00, 3.73 μB for x = 0.05 and 3.58 μB for x = 0.10. This reduction can be attributed to competition between the ferromagnetic and antiferromagnetic interactions. In addition, the Ni2+ ion at the M site influences the valence states of the manganese ions, i.e. it decreases the Mn3+/Mn4+ ratio. This proves a reduction in the double Zener exchange (DE), which leads to a decrease in magnetization.
We analyzed the hysteresis loops at 10 K (μ0H = ±5 T) to better understand the magnetic properties at low temperatures (Fig. 6). The curves are similar, with hysteresis loops which are weak, with reasonably good coercive fields (μ0HC), which decrease with the increase of Ni content . This reduction can be assigned to the decrease in spin dependent electron hopping. In the weak μ0H region, M grew significantly and reached saturation as the field increased. The value of Ms (saturation magnetization) can be estimated at high μ0H at about 5 T. From Table 3, it was found that Ms decreased, which may be due to antiferromagnetic alignments. Likewise, the insertion of Ni in the Mn site modifies the valence states of the manganese ions and decreases the level of Mn3+/Mn4+, which in turn weakens the DE interaction. The inset of Fig. 6 shows a zoomed in view of the central portion of M versus μ0H, at small μ0H. The determined values of μ0HC with Ni substitution are summarized in Table 3. The weak hysteresis loop with large saturation values confirms the characteristic soft FM behavior of the compounds. In this context, it can be concluded that our compounds may be applicable to read and write processes in high density recording media or for information storage.49
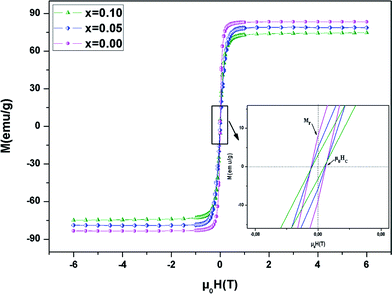 | ||
| Fig. 6 Magnetic hysteresis curves at T = 10 K for the LBSMNO samples. The inset shows a zoomed in view of the central portion of the hysteresis at a low field. | ||
The remanence ratio (R) is utilized to comprise the isotropic nature of our investigated compounds. The values of remanence varied in the range of 5–13 emu g−1. The ratio (R) is given by: R = Mr/Ms. We have summarized the values of R in Table 3. The small obtained values confirm the isotropic natures.50 In this context, for magnetic recording and memory devices,51 it is advantageous to have higher remanence ratios. The values of R reveal an increasing trend with Ni2+ substitution.
3.4 Magnetocaloric properties
We have shown previously that there is a phase transition around TC. Therefore, to calculate the change in magnetic entropy (−ΔSM), it is necessary to know the order of this transition.We have presented in Fig. 7(a–c), the external μ0H variation of the isothermal magnetization at different temperatures around TC. M increases rapidly at low μ0H and then achieves saturation, which shows ferromagnetic behavior. Above TC, thermally unsettled magnetic moments yield to increase magnetizations linearly at high temperatures, which means PM behavior. This phenomena demonstrates the magnetic phase transition.
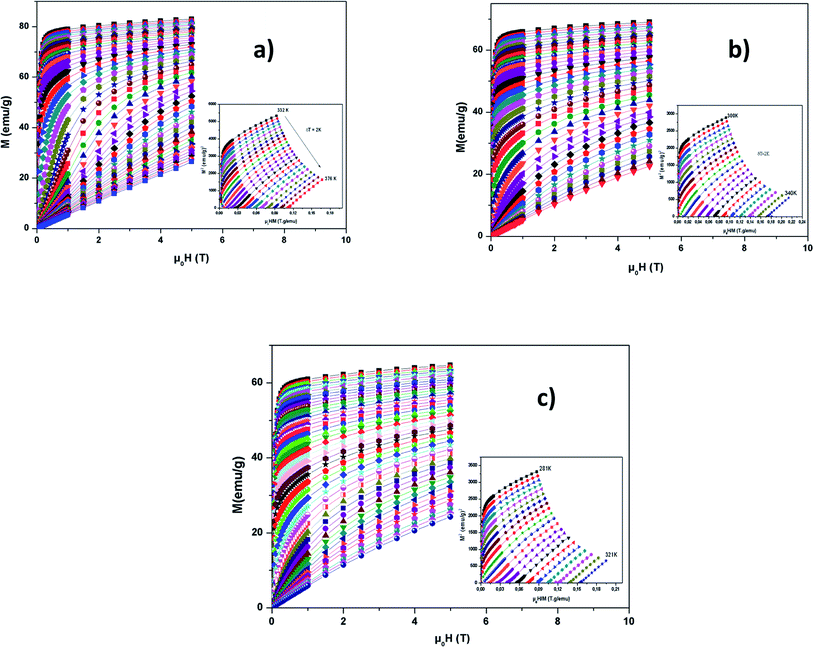 | ||
| Fig. 7 Magnetization versus field (M vs. μ0H) curves for LBSMNO compounds at 5 K. The inset: M2vs. μ0H/M plots around TC. | ||
In the inset of Fig. 7(a–c), we have presented the Arrott plots (M2vs. μ0H/M) for the La0.6Ba0.2Sr0.2Mn1−xNixO3 (0 ≤ x ≤ 0.1) compounds, from these curves we can conclude the nature of the magnetic phase transition. The slope of the curves is positive, so the transition is second order, according to Banerjee’s criteria.52 The MCE is an intrinsic characteristic of magnetic materials.53–55 Its principle is based on cooling or heating compounds when subjected to a magnetic field under adiabatic conditions, which is maximized when materials are close to their magnetic control temperature.
|ΔSM| can be determined, using Maxwell’s equations, by the formula:
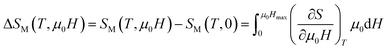 | (8) |
The peaks of the elaborated samples are the same as those of the experiment in the vicinity of TC where 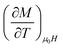 is the experimental value from the M(T) curve at μ0H.
is the experimental value from the M(T) curve at μ0H.
We can use the following expression:
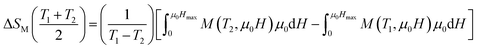 | (9) |
The predicted (−ΔSM) vs. temperature plots are shown in Fig. 8 at different μ0H values. It can be seen that (−ΔSM) depends on μ0H and the temperature until a maximum value (−ΔSmaxM) is reached around TC. (−ΔSM) increases with the increase of μ0H for each compound due to the spin effect, which becomes important with the increase of μ0H. The (−ΔSmaxM) for x = 0, 0.05 and 0.1 are 7.65, 5.44 and 4.45 J kg−1 K−1, respectively, at μ0H = 5 T. Although, when increasing the Ni ratio, (−ΔSmaxM) and TC decrease. This can be explicated by the lowering of the Mn3+/Mn4+ ratio, which goes from 1.5 (x = 0) to 0.8 (x = 0.1) and subsequently favors the DE interaction of Mn3+–O–Mn4+ over the superexchange (SE) interaction of Mn4+–O–Mn4+, Mn3+–O–Mn3+ and Ni2+–O–Ni2+.56 This result is strongly affected by structural parameters, such as decreasing the bond angles (Mn/Ni)–O–(Mn/Ni) and increasing the bond distances (Mn/Ni)–O.
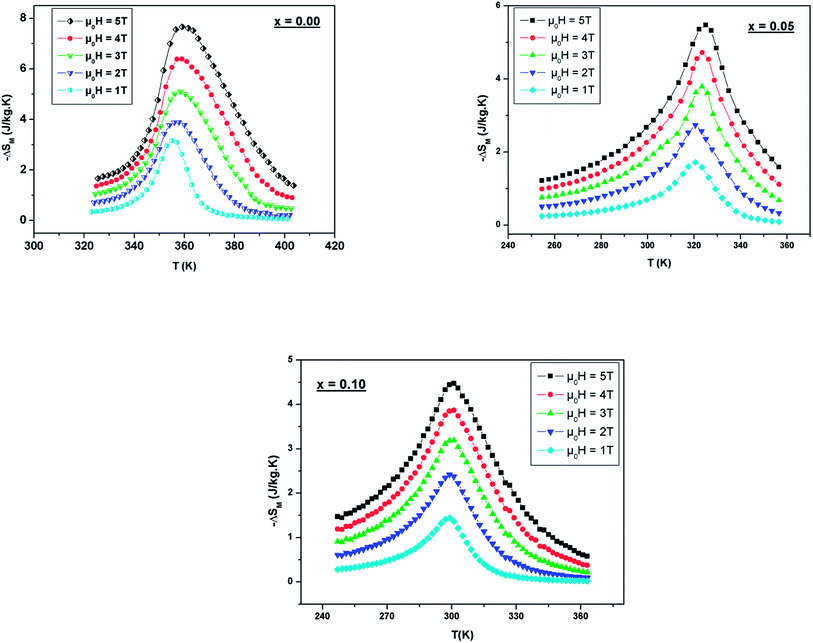 | ||
| Fig. 8 Temperature dependence of the magnetic entropy change under different external fields for LBSMNO manganites. | ||
(−ΔSmaxM) is not the only factor that determines the applicability of such a material, but also the temperature range over which it remains considerable is significant.
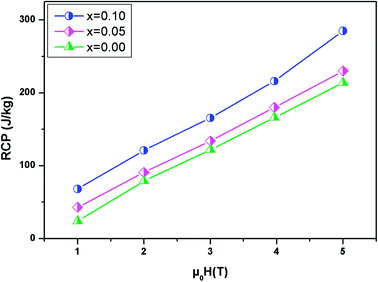
The relative cooling power (RCP) is another very important parameter along with (−delta), which defines the amount of heat that can be released between cold and hot sinks in an ideal refrigeration cycle and can be given by the following formula:57
| RCP = −ΔSmaxM × δTFWHM | (10) |
Fig. 9 present the relative cooling power as a function of μ0H for our compounds. The values of RCP increase with increasing μ0H and reache about 214 J kg−1 for x = 0, 230 J kg−1 for x = 0.05 and 285 J kg−1 for x = 0.1 at μ0H = 5 T. To better understand the performance of the MCE of our compounds, the values of (−ΔSmaxM) and RCP are compared to other manganites, as given in Table 4.58–62 It can be noted that our samples, especially for x = 0.1, have a suitable TC value, close to RT, and a relatively large magnetic entropy change to include other materials. This proves that our materials can be used in the field of magnetic refrigeration.
| Composition | T C | |ΔSmaxM| (J kg−1 K−1) | RCP (J kg−1) | μ 0 H (T) | Ref. |
|---|---|---|---|---|---|
| Gd | 293 | 5 | 153 | 2 | 46 |
| Gd | 293 | 9.5 | 410 | 5 | 47 |
| Gd5(Sr2Ge2) | 275 | 18.5 | 535 | 5 | 47 |
| La0.7Sr0.3Mn0.95Ti0.05O3 | 308 | 2.2 | 90 | 2 | 48 |
| La0.6Ba0.2Sr02MnO3 | 354 | 7.65 | 214 | 5 | This work |
| La0.6Ba0.2Sr0.2Mn0.95Ni0.05O3 | 320 | 5.44 | 230 | 5 | This work |
| La0.6Ba0.2Sr0.2Mn0.9Ni0.1O3 | 301 | 4.45 | 285 | 5 | This work |
| La0.6Ba0.2Sr02MnO3 | 354 | 3.88 | 82 | 2 | This work |
| La0.6Ba0.2Sr0.2Mn0.95Ni0.05O3 | 320 | 2.69 | 98 | 2 | This work |
| La0.6Ba0.2Sr0.2Mn0.9Ni0.1O3 | 301 | 2.37 | 121 | 2 | This work |
| La0.7Sr0.3Mn0.9Fe0.1O3 | 260 | 1.7 | 83 | 2 | 49 |
| La0.67Sr0.33Mn0.9Cr0.1O3 | 328 | 5 | — | 5 | 50 |
On the other hand, to affirm the nature of phase transition, Franco et al.63 proposed a phenomenological universal curve for the field dependence of ΔSM. We can construct the universal curve by normalizing all the ΔSmaxM: ΔSM(T, μ0H)/ΔSmaxM below and above TC, by imposing that the position of two additional reference points in the curve correspond to θ = ±1.
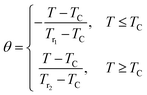 | (11) |
By referring to Banerjee’s criteria for a 2nd order PM–FM transition, all ΔSM curves at different μ0H values should merge into one curve with temperature scaling. If not, the samples follow a 1st order phase transition. Fig. 10 represents the evolution of ΔSM(T, μ0H)/ΔSmaxMvs. temperature θ at different μ0H values for x = 0.05, for example. In this figure, all the data collapses into a single master curve around TC, indicating the 2nd order nature of this phase transition. These results are in good accordance with those obtained by the Banerjee criterion discussed before. In addition, this universal curve can be used for practical purposes, such as extrapolating results to fields or temperatures not available in the laboratory, improving data resolution and deconvoluting the response of overlapping magnetic transitions.64
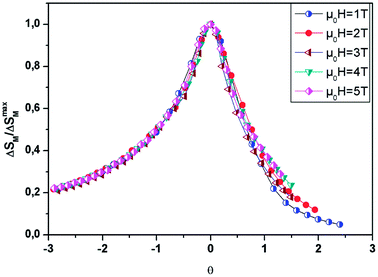 | ||
| Fig. 10 Universal behavior of the scaled ΔSM curves of the LBSM0.95N0.05O sample under various fields. | ||
We can adjust this curve by the Lorentzian function:
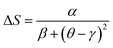 | (12) |
• α = 1.85 ± 0.03, β = 1.55 ± 0.02 and γ = 0.53 ± 0.04, for T ≤ TC.
• α = 1.16 ± 0.01, β = 1.12 ± 0.02 and γ = − 0.07 ± 0.03, for T > TC.
From eqn (7), the stance and magnitude of the peak, namely, (ΔSmaxM, TC) and Tr1 and Tr2 are the only ones that are to describe ΔS, where Tr2 > TC and Tr1 < TC. In the end, to transpose ΔS(θ) into the real ΔSM(T), we only use these values, which are fixed by the properties of the compounds.
It is essential to study how the MCE evolves over the ranges of applied magnetic fields and the desired temperatures, taking into account that the 2nd order transition has been proven for all samples.
The evolution of the ΔSMvs. the field is given by the expression, according to Parker and Oesterreicher:65 ΔSmaxM = b(μ0H)n, b is a constant and n is an exponent, which depends on the magnetic state of the sample. The exponent n can be expressed by the following expression:
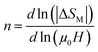 | (13) |
According to the mean field approach for conventional ferromagnetic compounds, a minimum value of “n” is 2/3 at TC. Below TC, n has been predicted to be 1 and the materials are in the FM state. However, above TC, it is equal to 2 in the PM zone, according to the Curie–Weiss law. Yet, recent experimental data indicates a deviation from n = 0.66, in the case of a few soft magnetic amorphous compounds. The temperature dependence of n is shown in Fig. 11. The values of n are found to be 0.67, 0.45 and 0.32 for x = 0.00, 0.05 and 0.10, respectively. For x = 0, the value is close to the values of the mean field model. However, for x = 0.05 and 0.1, these values do not coincide with the predicted value of the mean field of 0.66. This difference is probably due to local inhomogeneities around TC.66
3.5 Electrical properties
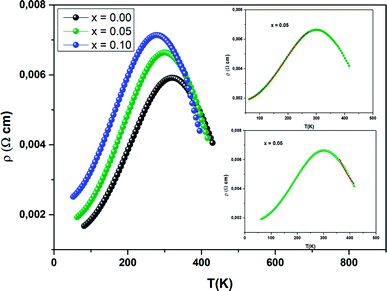
Fig. 12 presents the variation of electrical resistivity (ρ) vs. temperature (T) for our samples. All the samples are magnetically ordered and the resistivity exhibits a metallic behavior for low temperatures, resulting from a strong ferromagnetic coupling. Semiconductor behavior (SC) is reported at higher temperatures. It can be concluded that these samples undergo a semiconductor-to-metal (SC–M) transition at T = TM–SC. With increasing Ni concentration, this peak temperature TM–SC decreases (Table 5). From this table, one can see that TM–SC for all the doped materials is much lower than TC. From this result, it can be said that the transport properties are governed by the presence of inter-grain boundaries.| x = 0.00 | x = 0.05 | x = 0.10 | |
|---|---|---|---|
| ρ 0 (Ω cm) | 0.0011 | 0.0015 | 0.0022 |
| ρ 2 (× 10−7 Ω cm K−2) | 74.39 | 94.60 | 1.13 |
| ρ 5 (× 10−15 Ω cm K−5) | 72.73 | 1.28 | 2.32 |
| A (× 10−7 Ω cm) | 6.47 | 4.65 | 3.45 |
| E a/kB (K) | 2100 | 1390 | 780 |
| U 0/kB (K) | 8224 | 7822 | 6993 |
| T modC (K) | 305 | 297 | 275 |
| R 2 | 0.9998 | 0.9995 | 0.9999 |
To better understand the contribution of the different factors causing the conduction mechanism below the transition temperature (T < TM–SC), the ρ(T) curve was fitted using different theoretical models.
Conduction electrons meet different competitors, including scattering of the grain/domain boundary, electron–magnon scattering and electron–electron scattering. Using the following empirical relation,67 the electrical resistivity data is analyzed:
| ρFM = ρ0 + ρ2T2 + ρ5T5 | (14) |
The values of the electrical resistivity were adjusted using eqn (14) and in the inset of Fig. 13, we have given the best fit. In Table 5 we have grouped together the estimated values of the adjusted parameters.
Above (T > TM–SC), the resistivity is simulated by the SPH mechanism.69
ρ is expressed as in the adiabatic SPH model:
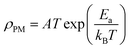 | (15) |
To understand the transport mechanism of the total resistivity over the whole temperature range, we used a phenomenological percolation model.70 For this model, resistivity is defined based on the contributions of the FM clusters in the PM region. Thus, the resistivity is expressed by the following expression:
| ρ = ρFMf + ρPM(1 − f) | (16) |
The volume fraction follows the Boltzmann distribution and this is expressed via the following equation:
With T = TmodC, f = fc = 0.5 where fc is called a percolation threshold.71 Where f < fc, the sample remains semiconducting and for f > fc it acquires a metallic phase.72
Hence, in the entire temperature range eqn (16), can be given as:
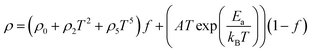 | (17) |
The data evaluated from eqn (17) are in agreement with the experimental results. It can be seen that the percolation model adequately describes the resistivity behavior over a wide range of temperatures, including the phase transition region. We have grouped the most suitable parameters in Table 5 and in Fig. 13, we have presented the fit of the data.
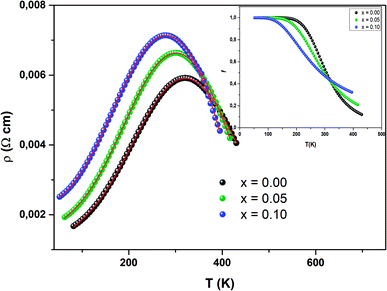 | ||
| Fig. 13 Temperature dependence of resistivity fitted according to eqn (16). The inset shows f(T) as function of temperature. | ||
The inset in Fig. 13 shows f(T) vs. temperature for all samples. Below TM–SC, the volume concentration of the ferromagnetic phase remains equal to 1. This proves the strong dominance of the FM part in this zone. Afterwards, f(T) starts to lower to zero, since the metallic state (FM) moves to a semiconductor state (PM). This result proves the validity of the percolation approach.
4. Conclusion
We have investigated the physical properties of polycrystalline La0.6Ba0.2Sr0.2Mn1−xNixO3 samples. Their crystal structures correspond to a rhombohedral structure, with the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c space group without any secondary phase. When the substitution rate increases, the unit cell volume decreases. The magnetic and electrical measurement data indicate that our compounds show FMM behaviour at low temperature (T < TM–SC) and PMS behaviour above TM–SC. This temperature decreases as the Ni substitution increases, due to the bandwidth reduction. The values of (−ΔSmaxM) at μ0H = 5 T are 7.40 J kg−1 K−1, 5.6 J kg−1 K−1 and 4.48 J kg−1 K−1 for x = 0.00, 0.05 and 0.10, respectively. The magnetocaloric performance of these samples indicates that the polycrystalline La0.6Ca0.1Sr0.3Mn1−xNixO3 compounds are good candidates for magnetic refrigeration at room temperature.
c space group without any secondary phase. When the substitution rate increases, the unit cell volume decreases. The magnetic and electrical measurement data indicate that our compounds show FMM behaviour at low temperature (T < TM–SC) and PMS behaviour above TM–SC. This temperature decreases as the Ni substitution increases, due to the bandwidth reduction. The values of (−ΔSmaxM) at μ0H = 5 T are 7.40 J kg−1 K−1, 5.6 J kg−1 K−1 and 4.48 J kg−1 K−1 for x = 0.00, 0.05 and 0.10, respectively. The magnetocaloric performance of these samples indicates that the polycrystalline La0.6Ca0.1Sr0.3Mn1−xNixO3 compounds are good candidates for magnetic refrigeration at room temperature.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by Princess Nourah Bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R184), Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia.References
- B. Arun, V. Akshay, G. R. Mutta, C. Venkatesh and M. Vasundhara, Mixed rare earth oxides derived from monazite sand as an inexpensive precursor material for room temperature magnetic refrigeration applications, Mater. Res. Bull., 2017, 94, 537–543 CrossRef.
- R. Gauß, G. Homm and O. Gutfleisch, The resource basis of magnetic refrigeration, J. Ind. Ecol., 2017, 7, 1291–1300 Search PubMed.
- A. R. Shelke, A. V. Ghule, Y. P. Lee, C. D. Lokhande and N. G. Deshpande, Investigations on magnetic properties and magnetocaloric effects in electron-doped La1−xZrxMnO3, J. Alloys Compd., 2017, 692, 522–528 CrossRef.
- J. R. Gómez, R. F. Garcia, A. D. M. Catoira and M. R. Gómez, Magnetocaloric effect: a review of the thermodynamic cycles in magnetic refrigeration, Renewable Sustainable Energy Rev., 2013, 17, 74–82 CrossRef.
- K. A. Gschneidner and V. K. Pecharsky, Magnetocaloric materials, Annu. Rev. Mater. Sci., 2000, 30, 387 CrossRef.
- V. K. Pecharsky and K. A. Gschneidner Jr., Magnetocaloric effect and magnetic refrigeration, J. Magn. Magn. Mater., 1999, 200, 44–56 CrossRef.
- V. Chaudhary, X. Chen and R. V. Ramanujan, Iron and manganese based magnetocaloric materials for near room temperature thermal management, Prog. Mater. Sci., 2019, 100, 64–98 CrossRef.
- A. Biswas, T. Samanta, S. Banerjee and I. Das, Appl. Phys. Lett., 2008, 92, 212502 CrossRef.
- M. H. Phan and S. Yu, Review of the magnetocaloric effect in manganite materials, J. Magn. Magn. Mater., 2007, 308, 325–340 Search PubMed.
- M. Khlifi, E. Dhahri and E. K. Hlil, J. Alloys Compd., 2014, 587, 771–777 Search PubMed.
- G. F. Wang, L. R Li, Z. R. Zhao, X. Q. Yu and X. F. Zhang, Ceram. Int., 2014, 40, 16449–16454 Search PubMed.
- Y. Zhang and X. Xu, AIP Adv., 2020, 10, 035220 CrossRef.
- Y. Zhan and X. Xu, J. Magn. Magn. Mater., 2020, 512, 166998 Search PubMed.
- Y. Zhang and X. Xu, RSC Adv., 2020, 10, 20646 RSC.
- A. Belkahla, K. Cherif, H. Belmabrouk, A. Bajahzar, J. Dhahri and E. K. Hlil, Solid State Commun., 2019, 294, 16–22 CrossRef.
- M. Dhahri, J. Dhahri and E. K. Hlil, J. Magn. Magn. Mater., 2017, 434, 100–104 CrossRef.
- E. Amal, F. I. H. Rhouma, J. Dhahri and E. K. Hlil, Appl. Phys. A, 2017, 123, 358 CrossRef.
- S. Bouzidi, M. A. Gdaiem, A. Dhahri, J. Dhahri and E. K. Hlil, J. Mater. Sci.: Mater. Electron., 2020, 31, 11548–11559 Search PubMed.
- E. Bouzaiene, A. H. Dhahri, J. Dhahri and E. K. Hlil, Inorg. Chem. Commun., 2021, 132, 108824 Search PubMed.
- M. Khlifi, M. Bejar, O. El Sadek, E. Dhahri, A. M. Ahmed and E. K. Hlil, J. Alloys Compd., 2011, 509(27), 7410–7415 CrossRef CAS.
- K. Cherif, J. Dhahri, E. Dhahri, M. Oumezzine and H. Vincent, J. Solid State Chem., 2002, 163, 466–471 CrossRef CAS.
- A. Dhahri, E. Dhahri and E. K. Hlil, J. Alloys Compd., 2017, 727, 449–459 CrossRef CAS.
- J. L Garcia-Munoz, C. Frontera, O. Beran, N. Bellido, J. Hernandez-Velasco and C. Ritter, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 014409–0144919 CrossRef.
- J. Dhahri, A. Dhahri, M. Oumezzine and E. Dhahri, J. Magn. Magn. Mater., 2008, 320, 2613–2617 CrossRef CAS.
- E. Tka, K. Cherif, J. Dhahri and E. Dhahri, J. Alloys Compd., 2011, 509, 8047–8055 CrossRef CAS.
- S. Mnefgui, N. Zaidi, A. Dhahri, E. K. Hlil and J. Dhahri, J. Solid State Chem., 2014, 215, 193–200 CrossRef CAS.
- S. Jin, H. Li, K. Chu, X. Yu, X. Guan, X. Pu, X. Gu and X. Liu, High Room-temperature TCR of La0.7(K0.25Sr0.05)MnO3:xAg2O Composites Obtained at Optimized Ag2O Ratio, J. Alloys Compd., 2021, 873, 159762–159772 CrossRef CAS.
- X. Liu, TCR and MR room-temperature enhancing mechanism of La0.7K0.3-xSrxMnO3 ceramics for uncooling infrared bolometers and magnetic sensor devices, Ceram. Int., 2021, 47, 18931–18941 CrossRef.
- X. Yu, H. Li, K. Chu, X. Pu, X. Gu, S. Jin, X. Guan and X. Liu, A comparative study on high TCR and MR of La0.67Ca0.33MnO3 polycrystalline ceramics prepared by solid-state and sol-gel methods, Ceram. Int., 2021, 47, 13469–13479 CrossRef CAS.
- H. M. Rietveld, J. Appl. Crystallogr., 1969, 2, 65–71 CrossRef CAS.
- S. Kuharuangrong, Ceram. Int., 2004, 30, 273 CrossRef CAS.
- V. M. Goldschmidt, Geochem. Verteilungsgesetz. Der Elm., 1927, vol. 7, p. 8 Search PubMed.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751 CrossRef.
- J. Cibert, J.-F. Bobo and U. Luders, C. R. Phys., 2005, 6, 977 Search PubMed.
- N. Dhahri, A. Dhahri, K. Cherif, J. Dhahri, K. Taibi and E. Dhahri, J. Alloys Compd., 2010, 496, 69–74 Search PubMed.
- M. Dhahri, A. Zaidi, K. Cherif, J. Dhahri and E. K. Hlil, J. Alloys Compd., 2017, 691, 578–586 CrossRef CAS.
- A. Dhahri, M. Jemmali, E. Dhahri and M. A. Valente, J. Alloys Compd., 2015, 638, 221–227 Search PubMed.
- G. K. Williamson and W. H. Hall, Acta Metall., 1953, 1, 22 Search PubMed.
- A. Benali, A. Souissi, M. Bejar, E. Dhahri, M. F. P. Graça and M. A. Valente, Chem. Phys. Lett., 2015, 637, 7–12 Search PubMed.
- M. S. Kim, J. B. Yang, Q. Cai, X. D. Zhou, W. J. James, W. B. Yelon, P. E. Parris, D. Buddhikot and S. K. Malik, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 014433 CrossRef.
- P. Thamilmaran, M. Arunachalam, S. Sankarrajan and K. Sakthipandi, J. Magn. Magn. Mater., 2015, 396, 181–189 CrossRef CAS.
- Z. H. Wang, J. W. Cai, B. G. Shen, X. Chen and W. S. Zhan, J. Phys.: Condens. Matter, 2000, 12, 601–610 Search PubMed.
- P. G. Radaelli, G. Iannone, M. Marezio, H. Y. Hwang, S.-W. Cheong, J. D. Jorgensen and D. N. Argyriou, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 56, 8265 Search PubMed.
- P. A. Joy, C. Raj Sankar and S. K. Date, J. Phys.: Condens. Matter, 2002, 14, L663 CrossRef CAS.
- A. Dhahri, E. Dhahri and E. K. Hlil, Appl. Phys. A, 2014, 116, 2077–2085 CrossRef CAS.
- G. G. Lonzarich and L. Taillefer, J. Phys. C: Solid State Phys., 1985, 18, 4339 CrossRef CAS.
- I. Sfifir, A. Ezaami, W. Cheikhrouhou-Koubaa and A. Cheikhrouhou, Structural, magnetic and magnetocaloric properties in La0.7-xDyxSr0.3MnO3 manganites (x = 0.00, 0.01 and 0.03), J. Alloys Compd., 2017, 696, 760 Search PubMed.
- H. Terashita and J. J. Neumeier, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 71, 225408 Search PubMed.
- K. El Maalam, M. Ben Ali, H. El Moussaoui, O. Mounkachi, M. Hamedoun, R. Masrour, E. K. Hlil and A. Benyoussef, J. Alloys Compd., 2015, 622, 761–764 CrossRef CAS.
- S. Thankachan, B. P. Jacob, S. Xavier and E. M. Mohammed, Phys. Scr., 2013, 87, 025701 Search PubMed.
- S. E. Shirsath, S. S. Jadhav, B. G. Toksha, S. M. Patange and K. M. Jadhav, J. Appl. Phys., 2011, 110, 013914 CrossRef.
- W. Chen, L. Y. Nie, W. Zhang, Y. J. Shi, J. J. Hu, A. J. Li and Y. W. Du, J. Alloys Compd., 2005, 395, 23 CrossRef CAS.
- H. Fu, R. L. Hadimani, Z. Ma, M. X. Wang, B. H. Teng and D. C. Jiles, Magnetocaloric effect in GdCoxAl2−x system for (0.15 ≤ x ≤ 1)compositions, J. Appl. Phys., 2014, 115, 17A914 Search PubMed.
- O. Gutfleisch, M. A. Willard, E. Bruck, C. H. Chen, S. G. Sankar and J. P. Liu, Magnetic Materials and Devices for the 21st Century: Stronger, Lighter, and More Energy Efficient, Adv. Mater., 2011, 23, 821 Search PubMed.
- M. A. Hamad, Theoretical J. Therm. Theoretical work on magnetocaloric effect in ceramic and sol–gel La0.67Ca0.33MnO3, J. Therm. Anal. Calorim., 2013, 111, 1251–1254 CrossRef CAS.
- O. Toulemonde, F. Studer and B. Raveau, Solid State Commun., 2001, 118, 107 Search PubMed.
- S. Mnefgui, A. Dhahri, N. Dhahri, J. Dhahri and E. I. K. Hlil, J. Magn. Magn. Mater., 2013, 340, 91 CrossRef CAS.
- E. Bruck, O. Tegus, D. T. C. Thanh and K. H. J. Buschow, J. Magn. Magn. Mater., 2007, 310, 2793–2799 Search PubMed.
- K. A. Gschneidner Jr, V. K. Pecharsky and A. O. Tsokol, Rep. Prog. Phys., 2005, 68, 1479–1539 Search PubMed.
- D. I. N. H. Nam, N. V. Dai, L. V. Hong, N. X. Phuc, S. C. Yu, M. Tachibana and E. Takayama-Muromachi, J. Appl. Phys., 2008, 103, 043905–043909 Search PubMed.
- S. K. Barik, C. Krishnamoorthi and R. Mahendiran, J. Magn. Magn. Mater., 2011, 323, 1015–1021 Search PubMed.
- Y. Sun, W. Tong and Y. Zhang, J. Magn. Magn. Mater., 2001, 232, 205–208 Search PubMed.
- Q. Y. Dong, H. W. Zhang, J. R. Sun, B. G. Shen and V. Franco, J. Appl. Phys., 2008, 103, 1161 Search PubMed.
- V. Franco, J. S. Blázquez, B. Ingale and A. Conde, The magnetocaloric effect and magnetic refrigeration near room temperature: materials and models, Annu. Rev. Mater. Res., 2012, 42, 305 Search PubMed.
- H. Oesterreicher and F. T. Parker, J. Appl. Phys., 1984, 55, 4334 Search PubMed.
- V. Franco, J. S. Blázquez and A. Conde, The influence of Co addition on the magnetocaloric effect of Nanoperm-type amorphous alloys, Appl. Phys. Lett., 2006, 100, 064307 Search PubMed.
- A. Urushibara, Y. Moritomo, T. Arima, A. Asamitsu, G. Kido and Y. Tokura, Phys. Rev. B: Condens. Matter Mater. Phys., 1995, 51, 14103–14108 Search PubMed.
- R. Mahendiran, R. Mahensh, A. K. Raychan dhuri and C. N. R. Rao, Solid State Commun., 1996, 99, 149–152 Search PubMed.
- B. S. Nagaraja, A. Rao and G. S. Okram, Structural, electrical, magnetic and thermal studies on Eu1−xSrxMnO3 (0.2 ≤ x ≤ 0.5) manganites, J. Alloys Compd., 2016, 683, 308–317 Search PubMed.
- G. Li, H. D. Zhou, S. L. Feng, X. J. Fan and X. G. Li, Competition between ferromagnetic metallic and paramagnetic insulating phases in manganites, J. Appl. Phys., 2002, 92, 1406 CrossRef CAS.
- B. Kurniawan, S. Winarsih, A. Imaduddin and A. Manaf, Correlation between microstructure and electrical transport properties of La0.7(Ba1-xCax)0.3MnO3 (x 1/4 0 and 0.03) synthesized by sol-gel, Phys. B, 2018, 532, 161–165 Search PubMed.
- A. Dhahri, M. Jemmali, E. Dhahri and E. K. Hlil, Electrical transport and giant magnetoresistance in La0.75Sr0.25Mn1-xCrxO3 (0.15, 0.20 and 0.25) manganite oxide, Dalton Trans., 2015, 44, 5620–5627 Search PubMed.
| This journal is © The Royal Society of Chemistry 2022 |