Open Access Article
Open Access ArticleThe synthesis of anticancer sulfonated indolo[2,1-a]isoquinoline and benzimidazo[2,1-a]isoquinolin-6(5H)-ones derivatives via a free radical cascade pathway†
You-lu Pan‡
a,
Xiao-meng Gong‡a,
Rong-rong Haob,
Shen-xin Zenga,
Zheng-rong Shen *a and
Wen-hai Huang
*a and
Wen-hai Huang *a
*a
aKey Laboratory of Neuropsychiatric Drug Research of Zhejiang Province, Hangzhou Medical College, Hangzhou, Zhejiang, China
bHangzhou Chinese Academy of Sciences, Hangzhou Medical College, Advanced Medical Technology Institute, Hangzhou, Zhejiang, China
First published on 29th March 2022
Abstract
A facile CuBr2 induced radical relay addition/cyclization of activated alkenes with substituted-thiosulfonates has been achieved, leading to a broad range of sulfonated indolo[2,1-a]isoquinolines and benzimidazo[2,1-a]isoquinolin-6(5H)-ones in moderate to good yields. In particular, some compounds exhibit bioactivity against cancer cell lines. This protocol shows advantages of low-cost, base-free, simple operation, and broad functional group tolerance.
Introduction
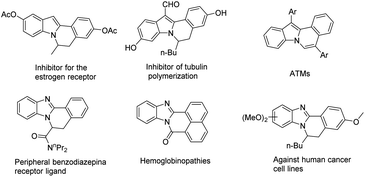
Nitrogen-containing heterocycles, for example, fused-indole and fused-benzimidazo derivatives, are commonly found in natural products and bioactive molecules.1 In particular, indolo[2,1-a]isoquinoline and benzimidazo[2,1-a]isoquinolin-6(5H)-ones derivatives containing the tetracyclic core structure (Fig. 1),2 with a wide range of biological properties, have attracted increasing attention from both synthetic and medicinal chemists. As indolo[2,1-a]isoquinoline and benzimidazo[2,1-a]isoquinolin-6(5H)-ones derivatives are an important series of molecules, huge efforts have been devoted to the assembly of these two special types of heterocyclic skeleton.In general, the traditional approach to prepare indolo[2,1-a]isoquinoline derivatives containing the tetracyclic core by using 2-arylindoles as materials is involved in a radical cyclization process, which is simple and convenient. For example, Xu's group developed a Fe(OTf)3-promoted tandem selenylation/cyclization to gain indolo[2,1-a]isoquinolin derivatives (Scheme 1a).3 Very recently, Lei's group disclosed an electrochemical radical cascade by applying Mn as the catalyst for the synthesis of indolo[2,1-a]isoquinoline derivatives from 2-arylindoles and boronic acid (Scheme 1b).1e On the other side, as for benzimidazo[2,1-a]isoquinolin-6(5H)-ones derivatives, it has been reported that a tandem phosphinoylation/cyclization of 2-arylbenzimidazoles with disubstituted phosphine oxides by using manganese(III) as the catalyst.4 Besides, Yu's group also reported a silver-catalyzed decarboxylative radical cascade cyclization toward benzimidazo[2,1-a]isoquinolin6(5H)-ones (Scheme 1c).5 Nevertheless, an unique approach for introducing a sulfone group into the indolo[2,1-a]isoquinolines and benzimidazo[2,1-a]isoquinolin-6(5H)-ones moiety has not been adequately studied.
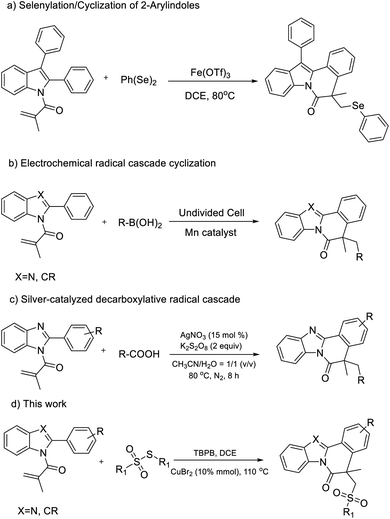 | ||
| Scheme 1 (a)–(c) The previous work to prepare the different derivatives of indolo[2,1-a]isoquinolines and benzimidazo[2,1-a]isoquinolin-6(5H)-ones; (d) the representative equation of our work. | ||
As an extremely valuable functional group, sulfonyl-containing compounds have obtained considerable interest due to their well-known biological activities and wide applications in pharmaceutical and food chemistry.6 Hence, the development of novel, versatile strategies to construct different useful skeletons bearing sulfonyl groups would be highly significant.7 Recently, the studies revealed that heterocyclic molecules containing sulfonyl-substituents exhibit unique bioactivities and chemical properties, and widely adopted in drug design.6d,8 Among the various sulfonate reagents, the incorporation of sulfonyl radicals, which generated in situ from thiosulfonates, have been disclosed in the literature.9 However, to the best of our knowledge, methods for the construction of molecules bearing both a sulfonyl group and a indolo[2,1-a]isoquinoline or benzimidazo[2,1-a]isoquinolin-6(5H)-ones motif by the usage of thiosulfonates have not yet been reported. Based on the significance of the sulfonyl group and our continued interest in the free radical process,10 in this article, we present an efficient CuBr2-catalysed sulfonation/cyclization with substituted-thiosulfonates for the synthesis of sulfonyl-substituted indolo[2,1-a]isoquinolines and benzimidazo[2,1-a]isoquinolin-6(5H)-ones, in which a C–S bond and C–C bond were constructed simultaneously under the oxidizing condition (TBPB).
Results and discussion
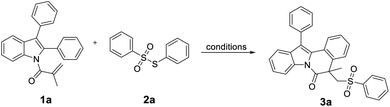
As shown in Table 1, the reaction was carried out between 1-(2,3-diphenyl-1H-indol-1-yl)-2-methylprop-2-en-1-one (1a) and S-phenyl benzenesulfonothioate (2a) to optimize the reaction condition. At first, 1a and 2a were dissolved in 1,2-dichloroethane (DCE) and treated with NiCl2 (catalyst, 10% mmol) and tert-butyl peroxybenzoate (TBPB, 3.0 equiv.) at 110 °C in pressure tube. However, just trace amounts of product 3a were detected (Table 1, entry 1). Subsequently, different catalysts were screened (Table 1, entries 2–4), and encouragingly, we obtained the product 3a in an isolated yield of 31% (Table 1, entry 4).| Entry | Catalyst (10% mmol) | Oxidant | Solvent | T (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction condition: 1a (0.1 mmol, 1.0 eq.), 2a (0.1 mmol, 1.0 eq.), catalyst (10% mmol), oxidant (3.0 eq.), N2, pressure tube.b Isolated yield.c N.R.: no reaction.d TBHP: 5.0–6.0 mol L−1 in decane.e TBPB (2.0 eq.).f Large scale: 1a (1.0 mmol, 1.0 eq.), 2a (1.0 mmol, 1.0 eq.), CuBr2 (10% mmol), TBPB (3.0 eq.), N2, pressure tube. | |||||
| 1 | NiCl2 | TBPB | DCE | 110 | Trace |
| 2 | (CH3COO)2Co | TBPB | DCE | 110 | N.R.c |
| 3 | CuI | TBPB | DCE | 110 | 23 |
| 4 | CuBr | TBPB | DCE | 110 | 31 |
| 5 | CuBr2 | TBPB | DCE | 110 | 85 |
| 6 | CuCl2 | TBPB | DCE | 110 | 62 |
| 7 | CuO | TBPB | DCE | 110 | 41 |
| 8 | — | TBPB | DCE | 110 | 25 |
| 9 | CuBr2 | TBHPd | DCE | 110 | 56 |
| 10 | CuBr2 | DTBP | DCE | 110 | 28 |
| 11 | CuBr2 | K2S2O8 | DCE | 110 | N.R. |
| 12 | CuBr2 | (NH4)2S2O8 | DCE | 110 | N.R. |
| 13 | CuBr2 | TBPB | ACN | 110 | 63 |
| 14 | CuBr2 | TBPB | DMF | 110 | 35 |
| 15 | CuBr2 | TBHP | Benzene | 110 | 21 |
| 16 | CuBr2 | TBHP | PhCF3 | 110 | 48 |
| 17 | CuBr2 | TBPB | EtOH | 110 | 27 |
| 18 | CuBr2 | TBPB | DMSO | 110 | 55 |
| 19 | CuBr2 | TBPB | DCE | 80 | 43 |
| 20 | CuBr2 | TBPBe | DCE | 110 | 42 |
| 21 | CuBr2 | TBPB | DCE | 110 | 72f |
Then, we further optimized the reaction condition to increase the yield of 3a. Initially, different copper catalysts, like CuBr2, CuCl2 and CuO, were screened to replace CuBr (Table 1, entries 5–7) for the reaction. Delightfully, CuBr2 was demonstrated to be the best choice for the reaction, with a dramatically increased yield of 85%. However, while the CuBr2 was absent from the reaction, the yield would be significantly decreased to 25%, indicating CuBr2 playing an essential role in the reaction process (Table 1, entry 8). Furthermore, different oxidants, such as tert-butyl hydroperoxide (TBHP), 2-(tert-butylperoxy)-2-methylpropane (DTBP), K2S2O8 and (NH4)2S2O8 (Table 1, entries 9–12), were also screened. Nevertheless, there was no better yield obtained than CuBr2. As exhibited in Table 1, different solvents, including acetonitrile (ACN), N,N-dimethylformamide (DMF), benzene, benzotrifluoride (PhCF3), ethanol and dimethyl sulfoxide (DMSO), were also employed into the reaction (entries 13–18). However, lower yields were gained and the results demonstrated that DCE was the best choice in our reaction. Additionally, while the reaction temperature was decreased to 80 °C, the yield of 3a was down to 43% (Table 1, entry 19), suggesting that proper temperature being required to initiate the chemical reaction. At last, when the amount of TBPB was reduced to 2.0 equiv., it would have a negative effect on the reaction (Table 1, entry 20). Hence, the adequate peroxide would promote the reaction process. According to the best condition screened above (Table 1, entry 5), we conducted the reaction of 1a on a large scale (1.0 mmol), and 3a was gained in an acceptable yield of 72% (Table 1, entry 21), demonstrating the good scalability of the developed reaction. In summary, the optimized reaction conditions were achieved for the CuBr2-catalysed sulfonation/cyclization, utilizing the oxidants TBPB in DCE at the temperature of 110 °C.
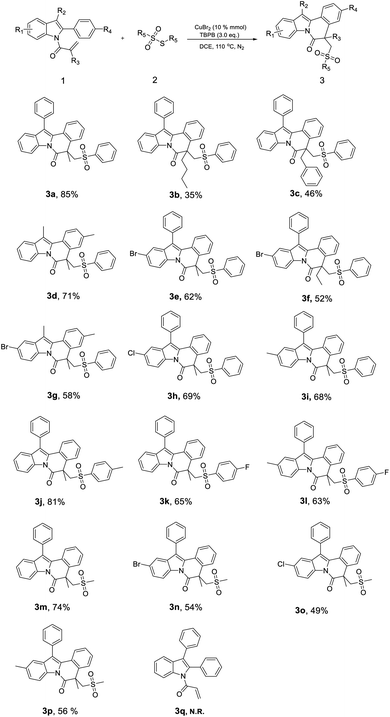
With the above optimized reaction conditions in hand, substrate scope studies were further performed, focusing on various 1-(2,3-diphenyl-1H-indol-1-yl)-2-methylprop-2-en-1-ones (Scheme 2). As exhibited in Scheme 2, the reaction was carried out between substituted 2-methyl-1-(2-phenyl-1H-indol-1-yl)prop-2-en-1-one (1) and various thiosulfonates (2) under our optimized reaction conditions to achieve the corresponding products in good yields. For example, we obtained the product 3a in a high yield of 85%. Nevertheless, when R3 is n-Bu or benzyl, it would produce 3b and 3c in a relatively lower yield of 35% and 46%, respectively, indicating the steric hindrance showing a negative role in the reaction. As displayed in Scheme 2, we got product 3d in a yield of 71%, and it suggested that the phenyl bearing 3-position of indole is better than methyl. Besides, while the –Br, –Cl and –CH3 was introduced at the 5-position of indole, it would acquire the corresponding products in a moderate yield (3e–3i).
 | ||
| Scheme 2 The reaction between different 2-methyl-1-(2-phenyl-1H-indol-1-yl)prop-2-en-1-ones (1) and sulfonothioates (2). | ||
On the other hand, substituted thiosulfonates were also reacted with compound 1a, and obviously, the electron-donating group (3j, 81%) would show more activity than the electron-withdrawing group (3k, 65%). At last, the reaction was performed between substituted indoles and S-methyl methanesulfonothioate. As displayed in Scheme 2, the yields of compounds 3m (74%), 3n (54%), 3o (49%) and 3p (56%) were lower than 3a (85%), 3e (62%), 3h (69%) and 3i (68%), respectively, indicating aromatic-substituted thiosulfonates maybe possess more activity than aliphatic group. Notably, while the starting material 1 was replaced by 1-(2,3-diphenyl-1H-indol-1-yl)prop-2-en-1-one (3q), there was no reaction occurred, demonstrating that acryl being unable to form crucial intermediate with another reagent under our reaction condition.
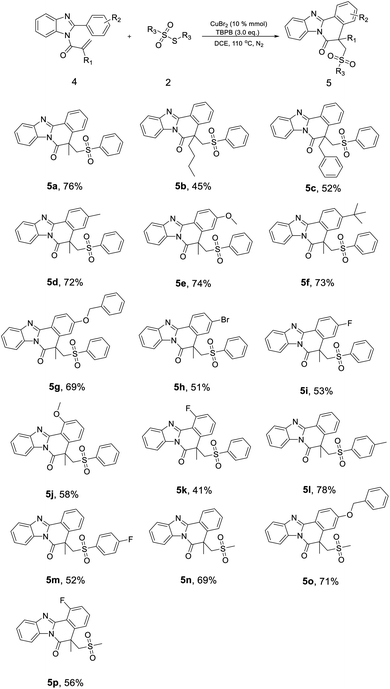
In addition to the above-mentioned, the reactions of 2-methyl-1-(2-phenyl-1H-benzo[d]imidazol-1-yl)prop-2-en-1-ones (4) with different thiosulfonates were also performed (Scheme 3). Obviously, the substituted compound 4 reacted with thiosulfonates also could achieve corresponding product in a relatively high yield, for example, product 5a (76%). In the beginning, two products 5b (45%) and 5c (52%) were produced in a moderate yield near 50%, which was lower than 5a. Subsequently, while the electron-donating group, like –CH3, –OCH3, –t-Bu and –OCH2Ph, was introduced at the para-position of benzene, we also could obtain the corresponding products, 5d (72%), 5e (74%), 5f (73%) and 5g (69%), respectively, in a relatively high yield (Scheme 3). By contrast, –Br and –F substituent groups would make the yield decreased, like 5h (51%) and 5i (53%). The results indicated that the electron-withdrawing groups at the para-position of phenyl would have a negative effect on the reaction process. Additionally, the ortho-substituted compound 4 also could react with thiosulfonates to produce corresponding products 5j (58%) and 5k (41%) in a moderate yield.
 | ||
| Scheme 3 The reaction between different 2-methyl-1-(2-phenyl-1H-benzo[d]imidazol-1-yl)prop-2-en-1-ones (4) and various sulfonothioates (2). | ||
On the other side, while the –Me and –F was introduced at para-position of phenyl in S-phenyl benzenesulfonothioate, the reaction also occurred to obtain products 5l (78%) and 5m (52%), demonstrating the electron-donating substituent group bearing S-phenyl benzenesulfonothioate also would promote the reaction process. At last, we also tested the S-methyl methanesulfonothioate as the sulfonate agent, and the products, 5n (69%), 5o (71%) and 5p (56%), were achieved in a moderate to high yield, indicating the reaction condition could be appropriate for a large scope of substrates.
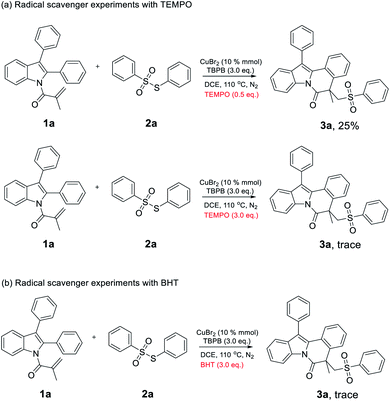
Based on the results discussed above, we carried out the primary control experiments to propose a possible mechanism. Initially, we added 0.5 equiv. of radical scavenger TEMPO (2,2,6,6-tetramethylpiperidine) into the reaction under standard conditions and gave product 3a in a decreased yield of 25% (Scheme 4a). Subsequently, 3.0 equiv. of TEMPO was added into the reaction and only trace amount of product 3a was detected (Scheme 4a). Additionally, another radical scavenger BHT (2,6-di-tert-butyl-4-methylphenol) with 3.0 equiv. was also mixed into the reaction and no good result was obtained (Scheme 4b).
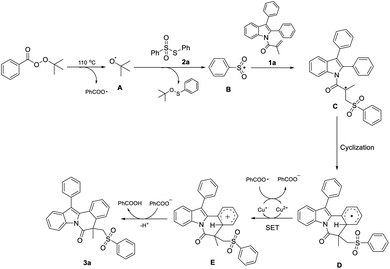
On the basis of control experiments and previous results,5,8c,11 a plausible mechanism for this transformation is proposed and shown in Scheme 5. Initially, in the presence of TBPB under heating conditions, tert-butoxyl radical A is formed via single electron transfer (SET) with the aid of a copper catalyst. Then, the S-phenyl benzenesulfonothioate is activated by the radical A to generate the radical B. Furthermore, the radical C was produced via the radical addition of 1-(2,3-diphenyl-1H-indol-1-yl)-2-methylprop-2-en-1-one (1a) with radical B. Subsequently, the radical C underwents an intramolecular cyclization to form intermediate D, which was further oxidized to form carbocation E through SET process. Finally, loss of a proton from E affords the desired product 3a.
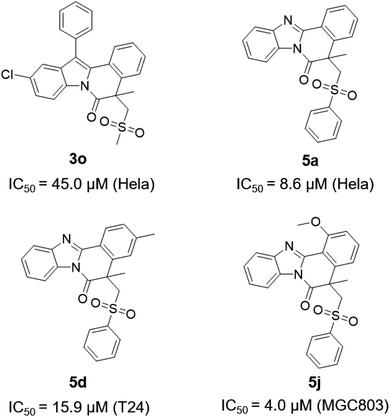
At last, the biological activities of these series of sulfonated indolo[2,1-a]isoquinolines and benzimidazo[2,1-a]isoquinolin-6(5H)-ones were explored in vitro, and antitumor activities of representative synthesized compounds were evaluated via the CCK8 assays of the MGC-803, T-24 and HeLa tumor cell lines. Paclitaxel (PTX) was applied as the positive control. As shown in Fig. 2, some representative compounds exhibited significant antitumor activities against the tumor cell lines. Notably, compound 5j displayed potent inhibitory bioactivity against MGC803 cell lines with IC50 values of 4.0 μM.
Conclusions
In summary, we have disclosed a practical TBPB induced radical relay addition/cyclization of activated alkenes with different thiosulfonates to synthesize various sulfonated indolo[2,1-a]isoquinolines and benzimidazo[2,1-a]isoquinolin-6(5H)-ones. Notably, some compounds exhibited bioactivity against different cancer cell lines. This reaction was performed in an easy operation, giving the target compounds in moderate to excellent yields. Further studies on the applications of these sulfonated derivatives in drug design are currently ongoing in our laboratory.Experimental section
General information
All reagents and solvents were commercially available and used directly without further purification. NMR spectra were recorded at room temperature on a Bruker Avance-400 or 500 spectrometer operating for 1H NMR and 13C NMR, using tetramethylsilane (TMS) as an internal standard. Chemical shifts are given in δ relative to TMS, and the coupling constants J are given in hertz. The peaks were internally referenced to CDCl3 (7.26 ppm) or residual undeuterated solvent signal (77.20 ppm for 13C NMR). The following abbreviations (or combinations thereof) were used to explain multiplicities: s = singlet, d = doublet, t = triplet, m = multiplet, brs = broad. High-resolution mass spectra (HRMS) were recorded on an Agilent 6200 LC/MS TOF using APCI or ESI in positive mode.Materials
The derivatives of substituted 2-methyl-1-(3-methyl-2-phenyl-1H-indol-1-yl)prop-2-en-1-ones were prepared via the reported methods,12 and the derivatives of substituted 2-methyl-1-(2-phenyl-1H-benzo[d]imidazol-1-yl)prop-2-en-1-ones were also prepared thought the previous published articles.4,13 Additionally, the substituted of acryloyl chlorides was synthesized following the published approaches.14Cell culture
The cell lines (HeLa, MGC803 and T24) and medium were purchased from American type culture collection (ATCC). HeLa and T24 were cultured in DMEM and McCOy's 5A medium, respectively. Besides, MGC803 was cultured in RPMI-1640 medium. All media were supplemented with 10% fetal bovine serum (EXCELL, FND500) and maintained at 37 °C in a humidified atmosphere of 5% CO2. The cell line was authenticated by STR profiling. Cell lines were monitored for mycoplasma contamination every 6 months.CCK-8 assay
Cell growth inhibition was typically assessed using the enhanced cell counting kit-8 (CCK-8, #C0042, Beyotime) assay. Cancer cells were first counted, and approximately 2500 cells per well were seeded in a 96-well cell culture plate (Corning Inc.). Then, after incubation at 37 °C in a humidified atmosphere with 5% CO2 for 24 h, the culture medium was replaced by a series of concentrations of drugs diluted with the corresponding culture fluid. Three replicates were made for each measurement. After co-incubation for 24 h, 10 μL of the CCK-8 reagent was added into each well, and OD at 450 nm was measured using a multifunction microplate reader (EnSpire) after incubation for 2 h at 37 °C. The percentage each concentration accounted for of the control was presented as cell viability. The IC50 value was calculated using SPSS.Characterization data of products
Colorless oil (40.6 mg, 85%). 1H NMR (500 MHz, CDCl3) δ 8.49 (d, J = 8.2 Hz, 1H), 7.53–7.41 (m, 6H), 7.37 (dd, J = 8.1, 1.1 Hz, 1H), 7.34–7.28 (m, 2H), 7.22–7.18 (m, 4H), 7.13 (dd, J = 7.9, 0.8 Hz, 1H), 6.99 (td, J = 7.7, 1.3 Hz, 1H), 6.95–6.85 (m, 1H), 4.52 (d, J = 14.5 Hz, 1H), 3.90 (d, J = 16.3 Hz, 1H), 1.61 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 170.59, 140.25, 134.49, 134.34, 134.06, 133.24, 132.42, 130.22, 129.35, 129.15, 128.86, 128.23, 128.05, 127.76, 127.40, 126.86, 126.01, 125.48, 125.19, 124.77, 120.98, 119.52, 116.89, 64.40, 46.69, 31.54. HRMS (ESI): calcd for C30H24NO3S [M + H]+ 478.1478, found 478.1460.
Yellow oil (18.2 mg, 35%). 1H NMR (400 MHz, CDCl3) δ 8.58 (d, J = 8.2 Hz, 1H), 7.63–7.48 (m, 7H), 7.45 (dd, J = 8.0, 1.0 Hz, 1H), 7.43–7.37 (m, 1H), 7.35–7.27 (m, 3H), 7.23 (t, J = 7.7 Hz, 2H), 7.17–7.12 (m, 1H), 7.05 (td, J = 7.6, 1.3 Hz, 1H), 7.01–6.96 (m, 1H), 4.57 (d, J = 14.6 Hz, 1H), 3.92 (d, J = 14.6 Hz, 1H), 2.34–2.11 (m, 1H), 1.89–1.72 (m, 1H), 1.19–1.00 (m, 2H), 0.97–0.79 (m, 2H), 0.67 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 170.20, 140.22, 134.10, 133.10, 133.00, 132.40, 130.24, 129.33, 128.77, 128.20, 128.04, 127.69, 127.33, 126.73, 126.65, 125.93, 125.23, 124.76, 120.91, 119.45, 116.99, 64.97, 50.47, 44.82, 25.27, 22.40, 13.57. HRMS (ESI): calcd for C33H30NO3S [M + H]+ 520.1947, found 520.1913.
Colorless oil (25.5 mg, 46%). 1H NMR (500 MHz, CDCl3) δ 8.53 (d, J = 8.2 Hz, 1H), 7.58–7.52 (m, 2H), 7.44–7.34 (m, 4H), 7.33–7.29 (m, 1H), 7.25 (dd, J = 14.9, 7.5 Hz, 3H), 7.17–7.13 (m, 1H), 7.14–7.08 (m, 1H), 7.08–7.01 (m, 2H), 6.88 (ddd, J = 11.2, 9.1, 4.2 Hz, 2H), 6.69 (t, J = 7.7 Hz, 2H), 6.69 (t, J = 7.7 Hz, 2H), 6.37 (d, J = 7.1 Hz, 2H), 4.78 (d, J = 14.6 Hz, 1H), 4.12 (d, J = 14.6 Hz, 1H), 3.33 (d, J = 12.4 Hz, 1H), 2.94 (d, J = 12.4 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 169.44, 140.76, 133.96, 133.90, 133.33, 133.12, 131.92, 131.82, 130.00, 129.50, 129.08, 128.91, 128.72, 127.95, 127.62, 127.56, 127.39, 127.28, 127.12, 127.02, 125.79, 125.21, 124.51, 120.44, 119.24, 116.58, 63.23, 52.79, 50.85. HRMS (ESI): calcd for C36H28NO3S [M + H]+ 554.1791, found 554.1775.
Colorless oil (30.5 mg, 71%). 1H NMR (400 MHz, CDCl3) δ 8.56–8.51 (m, 1H), 7.92 (d, J = 8.2 Hz, 1H), 7.62–7.54 (m, 1H), 7.48–7.42 (m, 2H), 7.40–7.32 (m, 3H), 7.27–7.22 (m, 2H), 7.14 (d, J = 8.1 Hz, 1H), 6.91 (s, 1H), 4.59 (d, J = 14.8 Hz, 1H), 3.96 (d, J = 14.7 Hz, 1H), 2.65 (s, 3H), 2.17 (s, 3H), 1.61 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 170.26, 140.43, 137.44, 134.29, 134.15, 133.08, 132.56, 129.44, 128.80, 128.66, 127.65, 125.58, 125.10, 124.36, 123.75, 118.27, 116.91, 114.24, 64.45, 46.46, 31.61, 21.31, 11.58. HRMS (ESI): calcd for C26H24NO3S [M + H]+ 430.1478, found 430.1477.
Colorless oil (34.5 mg, 62%). 1H NMR (400 MHz, CDCl3) δ 8.45 (d, J = 8.7 Hz, 1H), 7.65–7.37 (m, 11H), 7.29 (t, J = 7.7 Hz, 2H), 7.21 (d, J = 7.6 Hz, 1H), 7.12–7.06 (m, 1H), 6.99 (dt, J = 17.8, 5.3 Hz, 1H), 4.59 (d, J = 14.6 Hz, 1H), 3.99 (d, J = 14.6 Hz, 1H), 1.69 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 170.65, 140.18, 134.67, 134.22, 133.34, 132.98, 130.30, 130.13, 129.52, 128.91, 128.71, 128.51, 128.46, 127.73, 127.54, 126.89, 125.65, 124.74, 122.16, 119.93, 118.30, 118.25, 64.45, 46.64, 31.49. HRMS (ESI): calcd for C30H23BrNO3S [M + H]+ 556.0584, found 556.0532.
Yellow oil (29.7 mg, 52%). 1H NMR (400 MHz, CDCl3) δ 8.40 (d, J = 8.7 Hz, 1H), 7.44 (tdd, J = 17.8, 15.7, 6.6 Hz, 8H), 7.33–7.28 (m, 2H), 7.23–7.18 (m, 3H), 7.07 (d, J = 7.4 Hz, 1H), 7.03–6.97 (m, 1H), 6.94 (dd, J = 11.1, 4.1 Hz, 1H), 4.49 (d, J = 14.6 Hz, 1H), 3.88 (d, J = 14.6 Hz, 1H), 2.21 (dt, J = 14.5, 7.3 Hz, 1H), 1.82 (dq, J = 14.8, 7.4 Hz, 1H), 0.49 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 170.21, 140.21, 134.17, 133.35, 133.25, 132.85, 132.70, 130.48, 130.15, 129.50, 128.85, 128.67, 128.49, 128.45, 127.70, 127.49, 126.74, 126.49, 125.40, 122.12, 119.87, 118.32, 118.28, 64.67, 51.09, 38.31, 7.99. HRMS (ESI): calcd for C31H25BrNO3S [M + H]+ 570.0739, found 570.0732.
Yellow oil (29.4 mg, 58%). 1H NMR (400 MHz, CDCl3) δ 8.42 (d, J = 8.7 Hz, 1H), 7.92 (t, J = 13.7 Hz, 1H), 7.75–7.64 (m, 1H), 7.49–7.37 (m, 3H), 7.25 (dd, J = 8.5, 7.0 Hz, 2H), 7.20–7.11 (m, 1H), 7.19–7.08 (m, 1H), 6.91 (d, J = 13.9 Hz, 1H), 4.54 (d, J = 12.3 Hz, 1H), 3.96 (d, J = 14.8 Hz, 1H), 2.59 (s, 3H), 2.13 (s, 3H), 1.61 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 170.38, 140.35, 137.99, 134.47, 134.32, 133.18, 132.93, 130.66, 128.93, 128.72, 128.19, 127.70, 127.59, 125.27, 123.26, 121.15, 118.28, 117.81, 113.21, 64.49, 46.42, 31.54, 21.33, 11.54. HRMS (ESI): calcd for C26H23BrNO3S [M + H]+ 508.0583, found 508.0588.
Colorless oil (35.3 mg, 69%). 1H NMR (500 MHz, CDCl3) δ 8.43 (d, J = 8.7 Hz, 1H), 7.55–7.40 (m, 7H), 7.39–7.31 (m, 2H), 7.27 (ddd, J = 8.7, 4.0, 2.1 Hz, 1H), 7.24–7.19 (m, 2H), 7.16–7.11 (m, 2H), 7.03–6.99 (m, 1H), 6.95–6.89 (m, 1H), 4.50 (d, J = 14.8 Hz, 1H), 3.90 (d, J = 14.8 Hz, 1H), 1.61 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 170.61, 140.19, 134.66, 133.79, 133.36, 133.33, 132.62, 130.49, 130.43, 130.12, 129.51, 128.90, 128.50, 128.44, 127.72, 127.53, 126.89, 125.99, 125.63, 124.79, 120.05, 119.13, 117.93, 64.46, 46.62, 31.49. HRMS (ESI): calcd for C30H23ClNO3S [M + H]+ 512.1088, found 512.1029.
Yellow oil (33.4 mg, 68%). 1H NMR (400 MHz, CDCl3) δ 8.35 (d, J = 8.3 Hz, 1H), 7.53–7.41 (m, 7H), 7.31 (dd, J = 20.8, 7.7 Hz, 2H), 7.20–7.16 (m, 2H), 7.13 (t, J = 8.0 Hz, 2H), 7.02–6.95 (m, 2H), 6.90 (t, J = 7.6 Hz, 1H), 4.53 (d, J = 14.7 Hz, 1H), 3.90 (d, J = 14.6 Hz, 1H), 2.32 (s, 3H), 1.59 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 169.24, 139.12, 133.44, 133.36, 133.12, 132.17, 131.51, 131.42, 129.15, 128.28, 128.11, 127.77, 127.10, 126.85, 126.70, 126.30, 126.21, 125.77, 124.33, 124.20, 123.70, 119.73, 118.30, 115.45, 76.21, 63.26, 59.38, 45.51, 30.50, 28.67, 20.44, 20.05. HRMS (ESI): calcd for C31H26NO3S [M + H]+ 492.1633, found 492.1640.
Colorless oil (39.8 mg, 81%). 1H NMR (400 MHz, CDCl3) δ 8.53 (t, J = 33.1 Hz, 1H), 8.11 (d, J = 8.0 Hz, 1H), 7.64–7.35 (m, 9H), 7.27 (dd, J = 10.8, 5.7 Hz, 2H), 7.16 (t, J = 7.6 Hz, 1H), 7.01 (dd, J = 16.5, 8.0 Hz, 3H), 4.67 (d, J = 14.6 Hz, 1H), 3.95 (d, J = 14.6 Hz, 1H), 2.32 (s, 3H), 1.70 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 170.35, 144.57, 136.51, 134.49, 134.27, 134.05, 133.71, 132.34, 130.17, 129.37, 129.31, 129.25, 129.18, 128.50, 128.23, 128.07, 127.99, 127.35, 127.15, 125.92, 125.40, 125.07, 124.72, 120.85, 119.44, 116.86, 64.41, 46.53, 31.86, 21.33. HRMS (ESI): calcd for C31H26NO3S [M + H]+ 492.1634, found 492.1642.
Colorless oil (32.2 mg, 65%). 1H NMR (500 MHz, CDCl3) δ 8.52 (t, J = 36.9 Hz, 1H), 7.53–7.41 (m, 7H), 7.37 (dt, J = 7.7, 3.8 Hz, 1H), 7.35 (s, 1H), 7.25–7.19 (m, 2H), 7.13 (dt, J = 6.8, 3.4 Hz, 1H), 7.03 (tt, J = 10.3, 5.1 Hz, 1H), 6.96–6.91 (m, 1H), 6.88–6.79 (m, 2H), 4.49 (dd, J = 48.0, 23.0 Hz, 1H), 3.91 (d, J = 14.7 Hz, 1H), 1.61 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 169.42, 165.49, 163.45, 135.08, 133.36, 133.22, 132.89, 131.31, 129.72, 129.64, 129.11, 128.34, 127.94, 127.45, 127.24, 127.05, 126.43, 125.78, 125.10, 124.46, 124.17, 123.84, 120.11, 118.55, 115.74, 115.12, 114.94, 63.53, 45.65, 30.51. HRMS (ESI): calcd for C30H23FNO3S [M + H]+ 496.1383, found 496.1349.
Yellow oil (32.1 mg, 63%). 1H NMR (400 MHz, CDCl3) δ 8.33 (d, J = 8.3 Hz, 1H), 7.57–7.39 (m, 8H), 7.37–7.31 (m, 1H), 7.14 (dd, J = 9.9, 8.2 Hz, 2H), 7.05–6.89 (m, 3H), 6.83 (t, J = 8.5 Hz, 2H), 4.53 (d, J = 14.7 Hz, 1H), 3.90 (d, J = 14.6 Hz, 1H), 2.33 (s, 3H), 1.60 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 170.17, 169.12, 165.69, 163.14, 135.01, 134.98, 133.61, 133.29, 133.00, 131.46, 131.34, 129.70, 129.61, 129.10, 128.33, 127.95, 127.17, 126.91, 126.37, 125.76, 124.35, 124.23, 119.91, 118.38, 115.37, 115.11, 114.89, 76.21, 63.46, 59.39, 45.50, 30.50, 28.67, 20.44, 20.05. HRMS (ESI): calcd for C31H25FNO3S [M + H]+ 510.1539, found 510.1540.
Colorless oil (30.7 mg, 74%). 1H NMR (400 MHz, CDCl3) δ 8.79–8.38 (m, 1H), 7.51–7.30 (m, 8H), 7.26–7.19 (m, 3H), 7.02–6.95 (m, 1H), 4.44 (dd, J = 52.9, 15.1 Hz, 1H), 3.78 (dd, J = 35.6, 14.2 Hz, 1H), 2.74 (s, 3H), 1.63 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 169.87, 134.33, 133.28, 132.82, 131.37, 129.16, 128.26, 127.98, 127.39, 127.18, 126.53, 125.12, 125.00, 124.90, 124.24, 123.79, 120.24, 118.61, 115.62, 61.85, 46.03, 43.07, 30.06. HRMS (ESI): calcd for C25H22NO3S [M + H]+ 416.1321, found 416.1307.
Yellow oil (26.7 mg, 54%). 1H NMR (400 MHz, CDCl3) δ 8.43 (d, J = 8.4 Hz, 1H), 7.50–7.35 (m, 8H), 7.32 (d, J = 1.6 Hz, 1H), 7.25 (t, J = 7.6 Hz, 1H), 6.99 (t, J = 3.6 Hz, 1H), 4.36 (d, J = 14.8 Hz, 1H), 3.84 (d, J = 14.8 Hz, 1H), 2.59 (s, 3H), 1.66 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 169.90, 134.47, 133.18, 132.12, 131.94, 129.13, 129.08, 128.44, 127.80, 127.47, 126.67, 125.09, 125.05, 123.83, 121.23, 119.16, 117.27, 117.06, 61.97, 45.92, 43.04, 30.00. HRMS (ESI): calcd for C25H21BrNO3S [M + H]+ 494.0426, found 494.0426.
Yellow oil (22.0 mg, 49%). 1H NMR (400 MHz, CDCl3) δ 8.68–8.37 (m, 1H), 7.52–7.33 (m, 7H), 7.31–7.21 (m, 2H), 7.16 (d, J = 1.9 Hz, 1H), 7.04–6.92 (m, 1H), 4.35 (d, J = 14.7 Hz, 1H), 3.74 (d, J = 14.7 Hz, 1H), 2.60 (s, 3H), 1.69 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 169.86, 134.46, 132.74, 132.15, 131.58, 129.52, 129.24, 129.07, 128.43, 127.77, 127.46, 126.67, 125.10, 125.05, 123.87, 119.30, 118.21, 116.69, 61.95, 45.91, 43.05, 30.03. HRMS (ESI): calcd for C25H21ClNO3S [M + H]+ 450.0931, found 450.0929.
Yellow oil (24.1 mg, 56%). 1H NMR (400 MHz, CDCl3) δ 8.48 (d, J = 8.4 Hz, 1H), 7.61–7.47 (m, 5H), 7.44 (dd, J = 8.1, 4.9 Hz, 2H), 7.34–7.27 (m, 1H), 7.23 (dd, J = 8.4, 1.7 Hz, 1H), 7.10–7.02 (m, 2H), 4.44 (d, J = 14.7 Hz, 1H), 3.89 (d, J = 14.8 Hz, 1H), 2.69 (s, 3H), 2.39 (s, 3H), 1.73 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 170.64, 135.36, 134.63, 134.02, 132.61, 132.50, 130.22, 129.31, 129.08, 128.32, 128.18, 127.54, 127.44, 126.02, 125.88, 125.38, 121.13, 119.52, 116.30, 62.88, 60.45, 46.99, 44.09, 31.13, 29.73, 21.49, 21.11. HRMS (ESI): calcd for C26H24NO3S [M + H]+ 430.1477, found 430.1476.
Yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.44 (d, J = 7.8 Hz, 1H), 8.31–8.19 (m, 1H), 7.75 (t, J = 11.4 Hz, 1H), 7.40–7.29 (m, 7H), 7.22–7.14 (m, 2H), 7.12 (d, J = 7.9 Hz, 1H), 4.57–4.32 (m, 1H), 4.10–3.83 (m, 1H), 1.70 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 169.61, 148.21, 142.82, 138.75, 136.14, 132.38, 130.45, 130.30, 128.36, 127.99, 127.49, 126.59, 125.66, 125.25, 125.05, 124.75, 122.51, 121.84, 118.83, 114.80, 63.55, 45.94, 30.16. HRMS (ESI): calcd for C23H19N2O3S [M + H]+ 403.1117, found 403.1109.
Yellow oil (20.0 mg, 45%). 1H NMR (400 MHz, CDCl3) δ 8.46 (d, J = 7.0 Hz, 1H), 8.31–8.21 (m, 1H), 7.85–7.69 (m, 1H), 7.43–7.28 (m, 6H), 7.24–7.15 (m, 2H), 7.08 (d, J = 7.9 Hz, 1H), 4.45 (d, J = 14.7 Hz, 1H), 4.05 (m, J = 14.7 Hz, 1H), 2.28–2.08 (m, 1H), 1.88–1.68 (m, 1H), 1.05–0.91 (m, 2H), 0.87–0.74 (m, 2H), 0.64–0.53 (t, J = 7.6 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 169.36, 148.32, 142.59, 138.79, 134.78, 132.30, 130.40, 130.14, 127.96, 127.49, 126.53, 125.35, 125.10, 124.79, 123.33, 118.74, 114.85, 64.12, 50.01, 43.40, 24.18, 21.26, 12.43. HRMS (ESI): calcd for C26H25N2O3S [M + H]+ 445.1587, found 445.1585.
Colorless oil (24.9 mg, 52%). 1H NMR (500 MHz, CDCl3) δ 8.28–8.13 (m, 2H), 7.61 (dd, J = 5.8, 3.0 Hz, 1H), 7.44 (d, J = 7.5 Hz, 2H), 7.40–7.20 (m, 8H), 6.81 (t, J = 7.4 Hz, 1H), 6.68 (t, J = 7.7 Hz, 2H), 6.32 (t, J = 22.6 Hz, 2H), 4.69 (d, J = 14.7 Hz, 1H), 4.11 (t, J = 14.7 Hz, 1H), 3.34 (t, J = 18.0 Hz, 1H), 3.04 (d, J = 12.6 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 168.70, 147.79, 139.0, 133.76, 132.47, 131.08, 129.87, 128.15, 128.0, 127.58, 126.85, 126.77, 126.67, 126.14, 124.92, 124.84, 124.57, 123.68, 118.62, 114.47, 62.50, 51.95, 49.48. 13C NMR (126 MHz, CDCl3) δ 168.70, 147.79, 139.00, 133.76, 132.47, 131.08, 129.87, 128.15, 128.0, 127.58, 126.85, 126.77, 126.67, 126.14, 124.92, 124.84, 124.57, 123.68, 118.62, 114.47, 62.50, 51.95, 49.48. HRMS (ESI): calcd for C29H23N2O3S [M + H]+ 479.1430, found 479.1400.
Colorless oil (29.9 mg, 72%). 1H NMR (500 MHz, CDCl3) δ 8.35–8.24 (m, 2H), 7.75–7.74 (m, 1H), 7.45–7.26 (m, 5H), 7.20–7.13 (m, 3H), 6.77 (s, 1H), 4.49 (d, J = 14.9 Hz, 1H), 3.95 (d, J = 14.9 Hz, 1H), 2.08 (s, 3H), 1.64 (d, J = 49.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 170.76, 149.44, 143.94, 141.98, 139.92, 136.97, 133.31, 131.47, 129.61, 128.85, 127.53, 127.19, 126.17, 126.00, 125.58, 120.36, 119.77, 115.80, 64.56, 46.86, 31.15, 21.65. HRMS (ESI): calcd for C24H21N2O3S [M + H]+ 417.1274, found 417.1271.
Colorless oil (32.0 mg, 74%). 1H NMR (400 MHz, CDCl3) δ 8.44–8.28 (m, 1H), 8.28 (s, 1H), 7.85–7.64 (m, 1H), 7.41–7.30 (m, 5H), 7.23 (d, J = 8.0 Hz, 2H), 6.89 (dd, J = 8.8, 2.4 Hz, 1H), 6.51 (d, J = 2.4 Hz, 1H), 4.50 (d, J = 14.8 Hz, 1H), 4.00–3.79 (m, 1H), 3.64 (s, 3H), 1.59 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 169.58, 160.99, 148.35, 142.99, 138.88, 137.99, 132.24, 130.37, 127.89, 127.11, 126.59, 124.93, 124.26, 118.42, 114.72, 114.65, 113.38, 111.25, 63.53, 54.35, 46.08, 30.29. HRMS (ESI): calcd for C24H21N2O4S [M + H]+ 433.1223, found 433.1212.
Colorless oil (33.5 mg, 73%). 1H NMR (400 MHz, CDCl3) δ 8.34 (d, J = 8.3 Hz, 1H), 8.27–8.22 (m, 1H), 7.76–7.71 (m, 1H), 7.43–7.25 (m, 7H), 7.15 (dd, J = 9.4, 7.9 Hz, 2H), 4.51 (d, J = 14.8 Hz, 1H), 4.00 (d, J = 14.8 Hz, 1H), 1.63 (s, 3H), 1.18 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 169.89, 154.09, 148.39, 142.95, 138.84, 135.98, 132.47, 130.42, 127.90, 126.70, 125.00, 124.93, 124.48, 122.28, 119.23, 118.66, 114.72, 63.86, 46.20, 34.12, 30.40, 29.98. HRMS (ESI): calcd for C27H27N2O3S [M + H]+ 459.1743, found 459.1710.
Yellow oil (35.1 mg, 69%). 1H NMR (400 MHz, CDCl3) δ 8.35 (d, J = 8.7 Hz, 1H), 8.26–8.16 (m, 1H), 7.78–7.64 (m, 1H), 7.40–7.27 (m, 10H), 7.19 (dd, J = 9.5, 5.9 Hz, 2H), 7.01–6.89 (m, 1H), 6.56 (t, J = 10.8 Hz, 1H), 4.82 (q, J = 11.2 Hz, 2H), 4.45 (d, J = 18.3 Hz, 1H), 3.87 (d, J = 18.3 Hz, 1H), 1.56 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 169.56, 160.22, 148.31, 142.98, 138.90, 138.02, 134.77, 132.25, 130.37, 127.90, 127.74, 127.42, 127.08, 126.58, 124.93, 124.28, 118.43, 114.94, 114.66, 114.11, 112.09, 69.20, 63.56, 46.05, 30.16. HRMS (ESI): calcd for C30H25N2O4S [M + H]+ 509.1536, found 509.1536.
Colorless oil (24.5 mg, 51%). 1H NMR (400 MHz, CDCl3) δ 8.33–8.24 (m, 2H), 7.77–7.75 (m, 1H), 7.48–7.45 (m, 1H), 7.43–7.35 (m, 5H), 7.26 (t, J = 7.8 Hz, 2H), 7.14 (d, J = 1.6 Hz, 1H), 4.48 (d, J = 14.9 Hz, 1H), 3.90 (d, J = 14.9 Hz, 1H), 1.59 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 168.87, 147.34, 142.81, 138.58, 137.73, 133.36, 132.88, 130.96, 130.43, 128.96, 128.40, 128.10, 127.21, 126.57, 126.31, 125.23, 125.07, 124.92, 121.01, 118.93, 114.84, 63.33, 45.82, 29.86. HRMS (ESI): calcd for C23H18BrN2O3S [M + H]+ 481.0222, found 481.0251.
Colorless oil (22.3 mg, 53%). 1H NMR (400 MHz, CDCl3) δ 8.44 (dd, J = 8.8, 5.7 Hz, 1H), 8.28–8.20 (m, 1H), 7.80–7.67 (m, 1H), 7.44–7.33 (m, 5H), 7.25 (t, J = 7.7 Hz, 2H), 7.07 (td, J = 8.6, 2.4 Hz, 1H), 6.79 (dd, J = 9.5, 2.4 Hz, 1H), 4.45 (d, J = 14.8 Hz, 1H), 4.01 (d, J = 14.8 Hz, 1H), 1.67 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 170.07, 165.66, 163.13, 148.47, 143.86, 139.90, 139.82, 139.70, 133.71, 131.40, 129.12, 128.79, 128.70, 127.54, 126.18, 125.84, 119.85, 119.45, 119.42, 116.71, 116.49, 115.79, 113.96, 113.72, 64.40, 47.12, 31.06. HRMS (ESI): calcd for C23H18FN2O3S [M + H]+ 421.1023, found 421.1021.
Colorless oil (25.1 mg, 58%). 1H NMR (400 MHz, CDCl3) δ 8.38–8.14 (m, 1H), 8.01–7.76 (m, 1H), 7.56–7.39 (m, 2H), 7.41–7.28 (m, 2H), 7.31–7.30 (m, 4H), 6.98–6.83 (m, 1H), 6.83 (s, 1H), 4.47 (d, J = 14.8 Hz, 1H), 4.05 (s, 3H), 3.91 (d, J = 14.8 Hz, 1H), 1.58 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 169.51, 157.83, 146.32, 143.06, 138.87, 138.74, 132.38, 130.86, 129.30, 127.92, 126.63, 124.79, 124.74, 119.43, 118.05, 114.61, 110.94, 110.16, 63.58, 55.66, 45.93, 30.74. HRMS (ESI): calcd for C24H21N2O4S [M + H]+ 433.1233, found 433.1213.
Colorless oil (17.2 mg, 41%). 1H NMR (400 MHz, CDCl3) δ 8.32–8.20 (m, 1H), 7.95–7.83 (m, 1H), 7.45 (t, J = 7.2 Hz, 2H), 7.42–7.34 (m, 3H), 7.24 (dd, J = 15.0, 7.7 Hz, 3H), 7.14 (dd, J = 10.5, 8.4 Hz, 1H), 7.04 (d, J = 7.8 Hz, 1H), 4.50 (d, J = 14.7 Hz, 1H), 4.00 (dd, J = 36.8, 10.9 Hz, 1H), 1.62 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 169.02, 160.79, 158.18, 144.44, 144.36, 143.17, 138.75, 138.68, 132.60, 130.98, 130.89, 129.43, 128.08, 126.60, 125.26, 125.12, 121.74, 121.70, 119.64, 115.49, 115.27, 114.69, 111.34, 111.24, 63.54, 45.96, 30.47. HRMS (ESI): calcd for C23H18FN2O3S [M + H]+ 421.1023, found 421.0998.
Colorless oil (32.5 mg, 78%). 1H NMR (400 MHz, CDCl3) δ 8.44 (d, J = 7.8 Hz, 1H), 8.18 (d, J = 7.3 Hz, 1H), 7.72 (t, J = 20.2 Hz, 1H), 7.37 (tt, J = 20.2, 7.5 Hz, 4H), 7.22 (d, J = 8.2 Hz, 3H), 6.85 (t, J = 40.5 Hz, 2H), 4.48 (d, J = 14.7 Hz, 1H), 4.07–3.64 (d, J = 14.7 Hz, 1H), 2.11 (s, 3H), 1.69 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 169.44, 148.21, 143.83, 142.81, 136.16, 135.07, 130.44, 130.32, 128.46, 127.43, 126.89, 125.97, 125.17, 125.00, 124.68, 121.80, 118.83, 114.74, 63.45, 45.88, 30.54, 20.29. HRMS (ESI): calcd for C24H21N2O3S [M + H]+ 417.1274, found 417.1265.
Yellow oil (21.9 mg, 52%). 1H NMR (500 MHz, CDCl3) δ 8.45 (dd, J = 7.9, 1.1 Hz, 1H), 8.29–8.19 (m, 1H), 7.82–7.71 (m, 1H), 7.42–7.33 (m, 5H), 7.29–7.23 (m, 1H), 7.12 (dd, J = 17.2, 7.6 Hz, 1H), 6.91–6.82 (m, 2H), 4.46 (t, J = 25.1 Hz, 1H), 4.02–3.85 (m, 1H), 1.63 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 180.94, 169.52, 165.58, 163.53, 148.09, 142.88, 136.09, 134.77, 130.41, 130.31, 129.59, 129.52, 127.56, 125.62, 125.31, 125.15, 124.87, 121.95, 118.92, 115.37, 115.19, 114.72, 63.70, 45.95, 30.14. HRMS (ESI): calcd for C23H18FN2O3S [M + H]+ 421.1023, found 421.1017.
Yellow oil (23.5 mg, 69%). 1H NMR (400 MHz, CDCl3) δ 8.49 (d, J = 7.8 Hz, 1H), 8.33–8.18 (m, 1H), 7.81–7.71 (m, 1H), 7.59–7.42 (m, 3H), 7.37 (p, J = 7.4 Hz, 2H), 4.33 (d, J = 14.8 Hz, 1H), 3.87 (d, J = 14.8 Hz, 1H), 2.39 (s, 3H), 1.68 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 169.89, 148.16, 142.97, 136.97, 130.72, 130.42, 127.75, 125.77, 125.07, 125.02, 124.82, 122.07, 118.97, 114.65, 62.23, 46.14, 42.87, 29.79. HRMS (ESI): calcd for C18H17N2O3S [M + H]+ 341.0961, found 341.0954.
Yellow oil (31.7 mg, 71%). 1H NMR (400 MHz, CDCl3) δ 8.48 (d, J = 8.7 Hz, 1H), 8.34–8.27 (m, 1H), 7.81–7.75 (m, 1H), 7.40 (tdd, J = 14.0, 11.2, 7.1 Hz, 7H), 7.17 (dd, J = 8.8, 2.2 Hz, 1H), 7.05 (d, J = 2.1 Hz, 1H), 5.32–5.04 (m, 2H), 4.35 (d, J = 14.8 Hz, 1H), 3.75 (t, J = 35.0 Hz, 1H), 2.48 (s, 3H), 1.69 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 170.88, 161.54, 149.31, 144.05, 140.07, 135.95, 131.34, 128.86, 128.75, 128.52, 127.68, 126.01, 125.38, 119.59, 115.99, 115.54, 115.21, 112.93, 70.51, 63.24, 47.33, 43.83, 30.95. HRMS (ESI): calcd for C25H23N2O4S [M + H]+ 447.1379, found 447.1369.
Colorless oil (20.1 mg, 56%). 1H NMR (400 MHz, CDCl3) δ 8.33–8.23 (m, 1H), 7.90–7.84 (m, 1H), 7.52 (td, J = 8.1, 5.2 Hz, 1H), 7.42–7.37 (m, 2H), 7.31–7.21 (m, 2H), 4.40 (d, J = 14.7 Hz, 1H), 4.09–3.71 (d, J = 14.7 Hz, 1H), 2.78 (s, 3H), 1.70 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 169.50, 158.45, 143.29, 143.26, 139.68, 131.45, 131.35, 129.38, 125.31, 125.17, 120.88, 120.84, 119.76, 115.77, 115.56, 114.48, 61.83, 46.40, 43.27, 30.29. HRMS (ESI): calcd for C18H16FN2O3S [M + H]+ 359.0866, found 359.0859.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Zhejiang Provincial Key Research & Developement Plan (2021C03083), Health Commission of Zhejiang Province (WKJ-ZJ-1918), National Natural Science Funds of China (81803372), and Key Laboratory of Neuropsychiatric Drug Research of Zhejiang Province (2019E10021).References
- (a) A. J. Kochanowska-Karamyan and M. T. Hamann, Chem. Rev., 2010, 110, 4489 CrossRef CAS PubMed; (b) M. Somei and F. Yamada, Nat. Prod. Rep., 2004, 21, 278 RSC; (c) D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893 CrossRef CAS PubMed; (d) M. J. Taublaender, F. Glöcklhofer, M. Marchetti-Deschmann and M. M. Unterlass, Angew. Chem., Int. Ed., 2018, 57, 12270 CrossRef CAS PubMed; (e) Y. Yuan, Y. Zheng, B. Xu, J. Liao, F. Bu, S. Wang, J.-G. Hu and A. Lei, ACS Catal., 2020, 10, 6676 CrossRef CAS.
- (a) M. Leboeuf, A. Cave, A. Ranaivo and H. Moskowitz, Can. J. Chem., 1989, 67, 947 CrossRef; (b) R. Gastpar, M. Goldbrunner, D. Marko and E. von Angerer, J. Med. Chem., 1998, 41, 4965 CrossRef CAS PubMed; (c) T. Polossek, R. Ambros, S. Von Angerer, G. Brandl, A. Mannschreck and E. Von Angerer, J. Med. Chem., 1992, 35, 3537 CrossRef CAS PubMed; (d) R. Ambros, S. von Angerer and W. Wiegrebe, Arch. Pharm., 1988, 321, 743 CrossRef CAS PubMed.
- J. R. Zhang, H. Y. Liu, T. Fan, Y. Y. Chen and Y. L. Xu, Adv. Synth. Catal., 2020, 363, 497–504 CrossRef.
- S.-S. Jiang, Y.-T. Xiao, Y.-C. Wu, S.-Z. Luo, R.-J. Song and J.-H. Li, Org. Biomol. Chem., 2020, 18, 4843 RSC.
- K. Sun, S.-J. Li, X.-L. Chen, Y. Liu, X.-Q. Huang, D.-H. Wei, L.-B. Qu, Y.-F. Zhao and B. Yu, Chem. Commun., 2019, 55, 2861 RSC.
- (a) W. Li, G. Yin, L. Huang, Y. Xiao, Z. Fu, X. Xin, F. Liu, Z. Li and W. He, Green Chem., 2016, 18, 4879 RSC; (b) K. Sun, Z. Shi, Z. Liu, B. Luan, J. Zhu and Y. Xue, Org. Lett., 2018, 20, 6687 CrossRef CAS PubMed; (c) M. D. McReynolds, J. M. Dougherty and P. R. Hanson, Chem. Rev., 2004, 104, 2239 CrossRef CAS PubMed; (d) Y. Harrak, G. Casula, J. Basset, G. Rosell, S. Plescia, D. Raffa, M. G. Cusimano, R. Pouplana and M. D. Pujol, J. Med. Chem., 2010, 53, 6560 CrossRef CAS PubMed; (e) Q. Liu, F. Huang, X. Yuan, K. Wang, Y. Zou, J. Shen and Y. Xu, J. Med. Chem., 2017, 60, 10231 CrossRef CAS PubMed; (f) W. Dohle, F. L. Jourdan, G. Menchon, A. E. Prota, P. A. Foster, P. Mannion, E. Hamel, M. P. Thomas, P. G. Kasprzyk, E. Ferrandis, M. O. Steinmetz, M. P. Leese and B. V. L. Potter, J. Med. Chem., 2018, 61, 1031 CrossRef CAS PubMed; (g) Y. Huang, L. Huo, S. Zhang, X. Guo, C. C. Han, Y. Li and J. Hou, Chem. Commun., 2011, 47, 8904 RSC.
- (a) X.-X. Meng, Q.-Q. Kang, J.-Y. Zhang, Q. Li, W.-T. Wei and W.-M. He, Green Chem., 2020, 22, 1388 RSC; (b) Z. Yin, Y. Yu, H. Mei and J. Han, Green Chem., 2021, 23, 3256 RSC; (c) R. Ding, Y.-L. Liu, H. Hao, C.-Y. Chen, L. Liu, N.-S. Chen, Y. Guo and P.-L. Wang, Org. Chem. Front., 2021, 8, 3123 RSC; (d) Q. Liu, Y. Lv, R. Liu, X. Zhao, J. Wang and W. Wei, Chin. Chem. Lett., 2021, 32, 136 CrossRef CAS; (e) N. Zhou, M. Wu, K. Kuang, S. Wu and M. Zhang, Adv. Synth. Catal., 2020, 362, 5391 CrossRef CAS.
- (a) M. Teall, P. Oakley, T. Harrison, D. Shaw, E. Kay, J. Elliott, U. Gerhard, J. L. Castro, M. Shearman, R. G. Ball and N. N. Tsou, Bioorg. Med. Chem. Lett., 2005, 15, 2685 CrossRef CAS PubMed; (b) W.-M. Xu, F.-F. Han, M. He, D.-Y. Hu, J. He, S. Yang and B.-A. Song, J. Agric. Food Chem., 2012, 60, 1036 CrossRef CAS PubMed; (c) Y. Gu, L. Dai, J. Zhang, X. Lu, X. Liu, C. Wang, J. Zhang and L. Rong, J. Org. Chem., 2021, 86, 2173 CrossRef CAS PubMed.
- (a) J. Li, X.-E. Yang, S.-L. Wang, L.-L. Zhang, X.-Z. Zhou, S.-Y. Wang and S.-J. Ji, Org. Lett., 2020, 22, 4908 CrossRef CAS PubMed; (b) Q. Liang, P. J. Walsh and T. Jia, ACS Catal., 2020, 10, 2633 CrossRef CAS; (c) P. Mampuys, Y. Zhu, S. Sergeyev, E. Ruijter, R. V. A. Orru, S. Van Doorslaer and B. U. W. Maes, Org. Lett., 2016, 18, 2808 CrossRef CAS PubMed; (d) W. Kong, C. Yu, H. An and Q. Song, Org. Lett., 2018, 20, 4975 CrossRef CAS PubMed; (e) S. Huang, N. Thirupathi, C.-H. Tung and Z. Xu, J. Org. Chem., 2018, 83, 9449 CrossRef CAS PubMed.
- Y.-L. Liu, Y.-L. Pan, G.-J. Li, H.-F. Xu and J.-Z. Chen, Org. Biomol. Chem., 2019, 17, 8749 RSC.
- (a) S.-L. Zhou, L.-N. Guo, H. Wang and X.-H. Duan, Chem.–Eur. J., 2013, 19, 12970 CrossRef CAS PubMed; (b) J.-R. Zhang, H.-Y. Liu, T. Fan, Y.-Y. Chen and Y.-L. Xu, Adv. Synth. Catal., 2021, 363, 497 CrossRef; (c) R. Su, Y. Li, M.-Y. Min, X.-H. Ouyang, R.-J. Song and J.-H. Li, Chem. Commun., 2018, 54, 13511 RSC; (d) M. Zhang and X. Zeng, Org. Lett., 2021, 23, 3326 CrossRef CAS PubMed; (e) J. Li, Z. Wang, N. Wu, G. Gao and J. You, Chem. Commun., 2014, 50, 15049 RSC; (f) X.-J. Huang, F.-H. Qin, Y. Liu, S.-P. Wu, Q. Li and W.-T. Wei, Green Chem., 2020, 22, 3952 RSC; (g) Y. Tang, M. Yang, F. Wang, X. Hu and G. Wang, Tetrahedron Lett., 2021, 67, 152845 CrossRef CAS.
- (a) F.-L. Zeng, H.-L. Zhu, X.-L. Chen, L.-B. Qu and B. Yu, Green Chem., 2021, 23, 3677 RSC; (b) W. Kim, J. Koo and H. G. Lee, Chem. Sci., 2021, 12, 4119 RSC.
- H.-L. Zhu, F.-L. Zeng, X.-L. Chen, K. Sun, H.-C. Li, X.-Y. Yuan, L.-B. Qu and B. Yu, Org. Lett., 2021, 23, 2976 CrossRef CAS PubMed.
- (a) F. Weber and R. Brückner, Eur. J. Org. Chem., 2015, 2015, 2428 CrossRef CAS; (b) M. Fu, L. Chen, Y. Jiang, Z.-X. Jiang and Z. Yang, Org. Lett., 2016, 18, 348 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra06981k |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |