Open Access Article
Open Access ArticleHighly efficient selective hydrogenation of levulinic acid to γ-valerolactone over Cu–Re/TiO2 bimetallic catalysts†
Yingxin Liu ab,
Kai Liua,
Meihua Zhanga,
Kaiyue Zhanga,
Jiao Maa,
Shuwen Xiaocd,
Zuojun Wei
ab,
Kai Liua,
Meihua Zhanga,
Kaiyue Zhanga,
Jiao Maa,
Shuwen Xiaocd,
Zuojun Wei *cd and
Shuguang Deng
*cd and
Shuguang Deng *b
*b
aCollege of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou 310014, P. R. China. E-mail: weizuojun@zju.edu.cn; Shuguang.Deng@asu.edu
bSchool for Engineering of Matter, Transport and Energy, Arizona State University, 551 E. Tyler Mall, Tempe, AZ 85287, USA
cKey Laboratory of Biomass Chemical Engineering of the Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, P. R. China
dInstitute of Zhejiang University-Quzhou, 78 Jiuhua Boulevard North, Quzhou 324000, P. R. China
First published on 22nd December 2021
Abstract
Highly active and thermally stable Cu–Re bimetallic catalysts supported on TiO2 with 2.0 wt% loading of Cu were prepared via an incipient wetness impregnation method and were applied for liquid phase selective hydrogenation of levulinic acid (LA) to γ-valerolactone (GVL) in H2. The effect of the molar ratios of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Re on the physico-chemical properties and the catalytic performance of the Cu–Re/TiO2 catalysts was investigated. Moreover, the influence of various reaction parameters on the hydrogenation of LA to GVL was studied. The results showed that the Cu–Re/TiO2 catalyst with a 1
Re on the physico-chemical properties and the catalytic performance of the Cu–Re/TiO2 catalysts was investigated. Moreover, the influence of various reaction parameters on the hydrogenation of LA to GVL was studied. The results showed that the Cu–Re/TiO2 catalyst with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of Cu to Re (Cu–Re(1
1 molar ratio of Cu to Re (Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2) exhibited the highest performance for the reaction. Complete conversion of LA with a 100% yield of GVL was achieved in 1,4-dioxane solvent under the reaction conditions of 180 °C, 4.0 MPa H2 for 4 h, and the catalyst could be reused at least 6 times with only a slight loss of activity. Combined with the characterization results, the high performance of the catalyst was mainly attributed to the well-dispersed Cu–Re nanoparticles with a very fine average size (ca. 0.69 nm) and the co-presence of Cu–Re bimetal and ReOx on the catalyst surface.
1)/TiO2) exhibited the highest performance for the reaction. Complete conversion of LA with a 100% yield of GVL was achieved in 1,4-dioxane solvent under the reaction conditions of 180 °C, 4.0 MPa H2 for 4 h, and the catalyst could be reused at least 6 times with only a slight loss of activity. Combined with the characterization results, the high performance of the catalyst was mainly attributed to the well-dispersed Cu–Re nanoparticles with a very fine average size (ca. 0.69 nm) and the co-presence of Cu–Re bimetal and ReOx on the catalyst surface.
1. Introduction
Increasing global energy demands and diminishing fossil fuel stores have provided the scientific community with an unprecedented power in developing new economically viable routes to sustainable energy.1,2 Biomass, as a clean, renewable and new carbon source with the advantages of abundant reserves, low price and short generation period, is considered to be an indispensable energy source for human development in the future. Biomass can be converted into biofuels and high value-added chemicals through different technologies.2,3 γ-Valerolactone (GVL) is one of the most important sustainable biomass-derived chemicals, which can be used to participate in various reactions, as well as being applied as a food flavor, lubricant, plasticizer and reaction solvent, owing to its excellent physical and chemical properties.4–7Typically, GVL can be obtained through hydrogenation and cyclization of biomass derivative levulinic acid (LA) over homogeneous or heterogeneous catalysts as shown in Scheme 1.8–10 In consideration of easy product separation and catalyst recycling, heterogeneous catalysts are preferred. Supported Ru, Rh, Pd, Pt, Au and Ir noble metal catalysts have been employed for the hydrogenation of LA into GVL.11–16 Among them, Ru-based catalysts have been proved to be one of the most active heterogeneous catalysts.17–21 Our previous work also showed that the Ru catalyst embedded in N-doped mesoporous carbon exhibited high performance for the hydrogenation of LA into GVL.22
At the same time, some researchers have focused on non-noble metal catalysts.23 The Cu-based non-noble metal catalysts, which are generally considered to be active for the selective hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds and relatively inactive for the hydrogenolysis of C–C bonds,24,25 have been reported to be effective for the hydrogenation of LA to GVL. For example, Hutchings et al.26 investigated the performance of Cu–ZrO2 catalyst for the hydrogenation of LA to GVL, and obtained over 90% of LA conversion after reaction 60 min at 200 °C and 35 bar H2. Xu et al.27 studied LA hydrogenation over Cu(30%)–WO3(10%)/ZrO2-CP-300 catalyst in ethanol and obtained the maximum GVL yield of 94% at 200 °C and 5 MPa H2 for 6 h. However, most of the copper monometallic catalysts exposed a lot of problems, such as a high copper loading and harsh reaction conditions.
O bonds and relatively inactive for the hydrogenolysis of C–C bonds,24,25 have been reported to be effective for the hydrogenation of LA to GVL. For example, Hutchings et al.26 investigated the performance of Cu–ZrO2 catalyst for the hydrogenation of LA to GVL, and obtained over 90% of LA conversion after reaction 60 min at 200 °C and 35 bar H2. Xu et al.27 studied LA hydrogenation over Cu(30%)–WO3(10%)/ZrO2-CP-300 catalyst in ethanol and obtained the maximum GVL yield of 94% at 200 °C and 5 MPa H2 for 6 h. However, most of the copper monometallic catalysts exposed a lot of problems, such as a high copper loading and harsh reaction conditions.
Recently, bimetallic catalysts have caused widespread concern due to their unique catalytic performance. It is widely believed that the addition of another metal will enhance the catalytic activity and stability of the catalyst by changing the electronic and geometric properties of the first metal.28,29 Zhang et al.30 reported that using CuAg/Al2O3 as catalyst and THF as a solvent, LA conversion could reach 100%, and GVL selectivity was up to 99% at 180 °C and 1.4 MPa H2 for 4 h. Cai et al.31 developed 10Cu–5Ni/Al2O3 bimetallic catalyst for the transfer hydrogenation of ethyl levulinate to GVL with 2-butanol as the hydrogen donor, and obtained a 97% yield of GVL in 12 h at 150 °C. Yanase et al.32 tested bimetallic Cu–Co/Al2O3 catalyst for gas-phase hydrogenation of LA to GVL and obtained a GVL productivity of 5.46 kgGVL kgcatalyst−1 h−1 with a GVL selectivity higher than 99% at 250 °C for 24 h.
In this study, we report the Cu–Re/TiO2 bimetallic catalyst for the liquid phase hydrogenation of LA to GVL. The choice of metal Re as the second component is mainly based on the following considerations. Firstly, ReOx as well as TiO2 has many oxygen vacancies, which facilitates the adsorption of oxygen-containing functional groups, such as –OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O,33,34 facilitating the catalytic conversion of LA. Secondly, Re oxophilic metal oxide can be partially reduced to a metallic state in a reducing atmosphere, and Re0 can serve as a second metal component to form a bimetal or alloy with another hydrogenation-active metal and provide a synergistic effect, which is beneficial to improve the hydrogenation catalytic performance and stability of the catalyst.35–37 Our previous work showed that the bimetallic catalyst Pt–Re exhibited high performance for hydrogenation of cinnamaldehyde to cinnamyl alcohol.38 And our recent study showed that bimetallic catalyst Fe–Re/TiO2 exhibited superior catalytic performance for LA hydrogenation to GVL compared to monometallic Fe and Re catalysts at similar metal content. Under optimized conditions, nearly full conversion of LA with a 95% yield of GVL could be achieved at 180 °C in water at a H2 pressure of 40 bar.39
O,33,34 facilitating the catalytic conversion of LA. Secondly, Re oxophilic metal oxide can be partially reduced to a metallic state in a reducing atmosphere, and Re0 can serve as a second metal component to form a bimetal or alloy with another hydrogenation-active metal and provide a synergistic effect, which is beneficial to improve the hydrogenation catalytic performance and stability of the catalyst.35–37 Our previous work showed that the bimetallic catalyst Pt–Re exhibited high performance for hydrogenation of cinnamaldehyde to cinnamyl alcohol.38 And our recent study showed that bimetallic catalyst Fe–Re/TiO2 exhibited superior catalytic performance for LA hydrogenation to GVL compared to monometallic Fe and Re catalysts at similar metal content. Under optimized conditions, nearly full conversion of LA with a 95% yield of GVL could be achieved at 180 °C in water at a H2 pressure of 40 bar.39
Here, Cu–Re bimetallic catalysts supported on TiO2 with different Cu/Re molar ratios were prepared via an incipient wetness impregnation method. A structure–activity relationship was extensively discussed based on various characterization results and activity testing results. Moreover, the effect of reaction parameters on LA conversion was investigated.
2. Experimental
2.1. Materials
Levulinic acid (99.0%) was purchased from Shanghai Jingchun Reagent Co., Ltd. Cu(NO3)2·3H2O (99.99%) was purchased from Shanghai Aibi Chemical Reagent Co., Ltd, China. NH4ReO4 (99.99%) was purchased from Shanghai Macklin Biochemical Co., Ltd. 1,4-Dioxane (99.0%), methanol (99.9%) and TiO2 (99.0%) were purchased from Shanghai Aladdin Reagent Co., Ltd. All the chemicals used in this work were analytical reagents and were used without further purification.2.2. Catalyst preparation
A series of Cu–Re/TiO2 bimetallic catalysts with different Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Re molar ratios were prepared by an incipient wetness impregnation method. In a typical procedure, an aqueous solution containing the required amount of Cu(NO3)2·3H2O and NH4ReO4 was added to the support TiO2 in a beaker. After impregnated for 24 h, the mixture was dried at 110 °C for 10 h and finally reduced at 500 °C in a tubular furnace under hydrogen flow for 3 h to obtain the target catalyst, which was denoted as Cu–Re(x
Re molar ratios were prepared by an incipient wetness impregnation method. In a typical procedure, an aqueous solution containing the required amount of Cu(NO3)2·3H2O and NH4ReO4 was added to the support TiO2 in a beaker. After impregnated for 24 h, the mixture was dried at 110 °C for 10 h and finally reduced at 500 °C in a tubular furnace under hydrogen flow for 3 h to obtain the target catalyst, which was denoted as Cu–Re(x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y)/TiO2, where x
y)/TiO2, where x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y means the molar ratio of Cu to Re. The molar ratio of Cu to Re was varied from (3
y means the molar ratio of Cu to Re. The molar ratio of Cu to Re was varied from (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to (1
1) to (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) by changing the Re content and using a fixed amount of Cu (2.0 wt%), in order to investigate the influence of Re content on the property of the Cu–Re/TiO2. Two monometallic catalysts 2.0 wt% Cu/TiO2 and 5.8 wt% Re/TiO2 were prepared by using the same method for comparison.
1) by changing the Re content and using a fixed amount of Cu (2.0 wt%), in order to investigate the influence of Re content on the property of the Cu–Re/TiO2. Two monometallic catalysts 2.0 wt% Cu/TiO2 and 5.8 wt% Re/TiO2 were prepared by using the same method for comparison.
2.3. Catalyst characterization
The specific surface area was determined by N2 adsorption at −196 °C with the Brunauer–Emmett–Teller (BET) method using an ASAP 2010 instrument (Micromeritics Instrument Co.). The pore size distribution and pore volume were measured at −196 °C by using Barrett–Joyner–Halenda (BJH) analysis from the desorption branch of the N2 adsorption–desorption isotherms. Prior to N2 physisorption, the samples were degassed under vacuum at 250 °C for 10 h.Transmission electron microscopy (TEM) images were obtained using a Tecnai G2 F30 S-Twin instrument (Philips-FEI Co., The Netherlands). Samples were prepared by dispersing the reduced catalyst powder in ethanol under ultrasound for 15–20 min and then dropping the suspension onto a copper grid coated with carbon film. The particle size distribution of the metal nanoparticles in each sample was determined from the corresponding TEM image by measuring the sizes of more than 100 particles.
H2 temperature-programmed reduction (H2-TPR) of the samples was performed on a Chemisorb FINESORB-3010 instrument. The dried catalyst samples were reduced at 10 °C min−1 under a gaseous mixture of 10 vol% hydrogen in nitrogen (gas flow rate: 60 mL min−1). Hydrogen consumption was monitored on a thermal conductivity detector (TCD).
X-ray photoelectron spectroscopy (XPS) spectra were obtained using an Escalab Mark II X-ray spectrometer (VG Co., United Kingdom) equipped with a magnesium anode (Mg Kα = 1253.6 eV). Energy corrections were performed using a 1s peak of the pollutant carbon at 284.6 eV. The sample was prepared by pressing the catalyst powder onto the surface with silver sol–gel.
2.4. Reaction procedure
The hydrogenation of LA to GVL was performed in a 25 mL stainless-steel autoclave equipped with magnetic stirring. In a typical reaction, 0.5 g of LA, 50 mg of catalyst and 10 mL of solvent 1,4-dioxane were introduced into the reactor. Then the reactor was sealed, purged with H2 five times, and pressurized with H2 to the required pressure. The autoclave was then heated to the required temperature and kept at this temperature for the required time under the continuous stirring speed of 1000 rpm to eliminate external diffusion. After the reaction, the autoclave was cooled to room temperature, the residual hydrogen gas was released and the reaction mixture was centrifuged. The solid catalyst was washed with 1,4-dioxane for the next cycle, and the separated liquid reaction solution was quantitatively analyzed with an Agilent 7890A gas chromatography equipped with an HP-5 capillary column (30.0 m × 0.32 mm × 0.25 μm) and a flame ionization detector (FID) using n-dodecane as an internal standard. The confirmation of the liquid products was performed on an Agilent 6890 GC system coupled to a mass spectrometer equipped with an Agilent 5973 quadrupole mass analyzer. The potential Cu and Re leach was detected by inductively coupled plasma-mass spectroscopy (ICP-MS, PerkinElmer Elan DRC-e).3. Results and discussion
3.1. Catalytic performance of Cu–Re/TiO2 catalysts
To ascertain the effect of Re on the performance of Cu/TiO2 catalyst, a series of Cu–Re/TiO2 catalysts with different Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Re molar ratios were prepared and tested for hydrogenation of LA into GVL in a batch reactor in 1,4-dioxane solvent at 180 °C and 4.0 MPa H2 for 3 h, and the results are shown in Fig. 1. Monometallic 2 wt% Cu/TiO2 showed almost no activity for the reaction, over which the conversion of LA and the selectivity to GVL was only 0.7% and 2.4%, respectively. Although monometallic 5.8 wt% Re/TiO2 showed good selectivity toward GVL (98.7%), its activity was low, with a LA conversion of 68.4%. It can be seen that the addition of Re remarkably improved the catalytic performance of the Cu-based catalysts for LA hydrogenation, and the performance of the Cu–Re/TiO2 bimetallic catalysts increased with the increase in Re
Re molar ratios were prepared and tested for hydrogenation of LA into GVL in a batch reactor in 1,4-dioxane solvent at 180 °C and 4.0 MPa H2 for 3 h, and the results are shown in Fig. 1. Monometallic 2 wt% Cu/TiO2 showed almost no activity for the reaction, over which the conversion of LA and the selectivity to GVL was only 0.7% and 2.4%, respectively. Although monometallic 5.8 wt% Re/TiO2 showed good selectivity toward GVL (98.7%), its activity was low, with a LA conversion of 68.4%. It can be seen that the addition of Re remarkably improved the catalytic performance of the Cu-based catalysts for LA hydrogenation, and the performance of the Cu–Re/TiO2 bimetallic catalysts increased with the increase in Re![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu molar ratio. It was noteworthy that the Cu–Re(1
Cu molar ratio. It was noteworthy that the Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 catalyst exhibited the highest activity and selectivity to GVL, giving a GVL yield as high as 97.5% at a LA conversion of 98.9%.
1)/TiO2 catalyst exhibited the highest activity and selectivity to GVL, giving a GVL yield as high as 97.5% at a LA conversion of 98.9%.
 | ||
Fig. 1 Hydrogenation of LA to GVL over Cu–Re/TiO2 catalysts with different Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Re molar ratios. Reaction conditions: 0.5 g LA, 50 mg catalyst, 10 mL 1,4-dioxane, 4.0 MPa H2, 180 °C, 3 h, and 1000 rpm. Re molar ratios. Reaction conditions: 0.5 g LA, 50 mg catalyst, 10 mL 1,4-dioxane, 4.0 MPa H2, 180 °C, 3 h, and 1000 rpm. | ||
3.2. Characterization of Cu–Re/TiO2 catalysts
N2 adsorption/desorption isotherms of support TiO2, monometallic Cu/TiO2 and Re/TiO2, and Cu–Re/TiO2 bimetallic catalysts are presented in Fig. 2, and the relative texture parameters (specific surface areas (SBET), pore volume and average pore diameter) of these samples are listed in Table 1. All the samples shown in Fig. 2 exhibited type IV isotherms, a typical characteristic for mesoporous materials.40 The SBET of TiO2 was kept no obvious change after supporting metal elements, while the pore volume and average pore diameter increased significantly, indicating a strong influence of the metal ions on TiO2 textural properties.41| Catalyst | SBETa (m2 g−1) | Vpb (cm3 g−1) | Dpb (nm) |
|---|---|---|---|
| a The specific surface area was calculated by using the BET method.b The pore size and pore volumes were derived from the adsorption branches of isotherms by using the BJH model. | |||
| TiO2 | 53 | 0.15 | 11.3 |
| Cu/TiO2 | 52 | 0.33 | 25.2 |
Cu–Re(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 1)/TiO2 |
60 | 0.39 | 26.0 |
Cu–Re(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 1)/TiO2 |
59 | 0.36 | 25.6 |
Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 1)/TiO2 |
60 | 0.32 | 25.6 |
| Re/TiO2 | 61 | 0.33 | 26.4 |
Fig. 3 shows the H2-TPR profiles of monometallic Cu/TiO2 and Re/TiO2, and bimetallic Cu–Re/TiO2 catalysts dried at 110 °C. In monometallic Cu/TiO2, two peaks occurred at 225 °C and 255 °C, which were likely attributed to the reduction of highly dispersed CuO species in intimate contact with TiO2 and the reduction of the oxide clusters with a structure similar to CuO, respectively.42 In the case of monometallic Re/TiO2, Re species reduced between 220 and 350 °C with a peak maximum at 288 °C, in agreement with the literature.43 On bimetallic Cu–Re(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 and Cu–Re(2
1)/TiO2 and Cu–Re(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 catalysts, two peaks were observed between 210 and 261 °C, and the peaks moved towards low temperature compared with the two monometallic catalysts Cu/TiO2 and Re/TiO2, which indicates that the addition of Re into Cu/TiO2 catalyst is of benefit to the reduction of metal oxide species. It is interesting to note that Cu–Re(1
1)/TiO2 catalysts, two peaks were observed between 210 and 261 °C, and the peaks moved towards low temperature compared with the two monometallic catalysts Cu/TiO2 and Re/TiO2, which indicates that the addition of Re into Cu/TiO2 catalyst is of benefit to the reduction of metal oxide species. It is interesting to note that Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 bimetallic catalyst displayed a main broad peak at 289 °C and two additional ones with low intensity. The main peak shifted to higher temperature compared with monometallic catalyst Cu/TiO2, likely corresponding to monometallic Re-containing sample, indicating that there was a strong interaction between Cu and Re on this sample and that most of Re atoms were distributed in the periphery of unreduced Cu atoms throughout the catalyst.44 Furthermore, the reduction bands of Cu–Re(1
1)/TiO2 bimetallic catalyst displayed a main broad peak at 289 °C and two additional ones with low intensity. The main peak shifted to higher temperature compared with monometallic catalyst Cu/TiO2, likely corresponding to monometallic Re-containing sample, indicating that there was a strong interaction between Cu and Re on this sample and that most of Re atoms were distributed in the periphery of unreduced Cu atoms throughout the catalyst.44 Furthermore, the reduction bands of Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 catalyst were changed to the symmetrical shape compared to those of other samples, which can be induced by a uniform reduction process caused by the homogeneity of metal particle size.44 Combined with the activity testing results of the catalysts as mentioned above, the improvement in the catalytic activity of the Cu–Re(1
1)/TiO2 catalyst were changed to the symmetrical shape compared to those of other samples, which can be induced by a uniform reduction process caused by the homogeneity of metal particle size.44 Combined with the activity testing results of the catalysts as mentioned above, the improvement in the catalytic activity of the Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 catalyst was probably attributed to the strong interaction between Cu and Re.
1)/TiO2 catalyst was probably attributed to the strong interaction between Cu and Re.
Fig. 4 displays the TEM images of the reduced Cu/TiO2 and Cu–Re/TiO2 catalysts. In the TEM image of monometallic Cu/TiO2 catalyst (Fig. 4(a)), only a few severely agglomerated Cu nanoparticles with a large average size (about 1.6 nm) were observed, which reduced the activity and selectivity of the catalyst significantly. For the three Cu–Re/TiO2 bimetallic catalysts (Fig. 4(b)–(d)), metal particles were smaller, with more narrow particle size distributions than Cu/TiO2, indicating that metal particles on Cu–Re bimetallic catalysts were uniformly distributed. In particular, Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 catalyst showed the largest metal dispersion and the smallest average metal particle size (0.69 nm). As shown in Fig. 4(d), the fringe spacing of Cu–Re(1
1)/TiO2 catalyst showed the largest metal dispersion and the smallest average metal particle size (0.69 nm). As shown in Fig. 4(d), the fringe spacing of Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (0.207 nm) is between those of Cu(110) (0.200 nm) and Re(101) (0.211 nm),45,46 indicating that a Cu–Re alloy was formed in the reduced Cu–Re(1
1) (0.207 nm) is between those of Cu(110) (0.200 nm) and Re(101) (0.211 nm),45,46 indicating that a Cu–Re alloy was formed in the reduced Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 catalyst and this was responsible for strong interaction between metallic Cu and Re species. The result is in good agreement with the TPR results.
1)/TiO2 catalyst and this was responsible for strong interaction between metallic Cu and Re species. The result is in good agreement with the TPR results.
 | ||
Fig. 4 TEM images of (a) Cu/TiO2, (b) Cu–Re(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2, (c) Cu–Re(2 1)/TiO2, (c) Cu–Re(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 and (d) Cu–Re(1 1)/TiO2 and (d) Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 catalysts. 1)/TiO2 catalysts. | ||
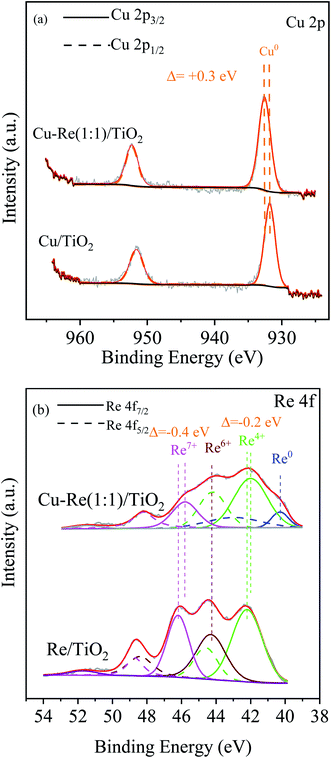
Fig. 5 shows the XPS spectra for Cu 2p (Fig. 5(a)) and Re 4f (Fig. 5(b)) levels of the reduced Cu/TiO2, Re/TiO2 and Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 catalysts, and the corresponding binding energies are listed in Table 2. The XPS data for Cu 2p on both Cu/TiO2 and Cu–Re(1
1)/TiO2 catalysts, and the corresponding binding energies are listed in Table 2. The XPS data for Cu 2p on both Cu/TiO2 and Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 catalysts reveal that the Cu species is essentially metallic Cu0.47 XPS analysis for Re 4f displays that Re is present in Re4+, Re6+ and Re7+ states in the case of monometallic Re/TiO2,48 and in Re0, Re4+ and Re7+ states in the case of bimetallic Cu–Re(1
1)/TiO2 catalysts reveal that the Cu species is essentially metallic Cu0.47 XPS analysis for Re 4f displays that Re is present in Re4+, Re6+ and Re7+ states in the case of monometallic Re/TiO2,48 and in Re0, Re4+ and Re7+ states in the case of bimetallic Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2,44 indicating that the reduction of Re species is not complete, in agreement with the literature.43,44 Compared to the corresponding monometallic catalysts, the binding energy for the Cu 2p3/2 descends to higher values by ca. 0.3 eV, while the binding energy of ReO2 for Re 4f7/2 shifts to lower values by ca. 0.2 eV, indicating a strong interaction may exist between Cu and Re species,49 per the TPR and TEM results.
1)/TiO2,44 indicating that the reduction of Re species is not complete, in agreement with the literature.43,44 Compared to the corresponding monometallic catalysts, the binding energy for the Cu 2p3/2 descends to higher values by ca. 0.3 eV, while the binding energy of ReO2 for Re 4f7/2 shifts to lower values by ca. 0.2 eV, indicating a strong interaction may exist between Cu and Re species,49 per the TPR and TEM results.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 catalysts
1)/TiO2 catalysts
| Catalyst | Cu 2p3/2 (2p1/2) (BE/eV) | Re 4f7/2 (4f5/2) (BE/eV) | |||
|---|---|---|---|---|---|
| Cu0 | Re0 | ReO2 | ReO3 | Re2O7 | |
| Cu/TiO2 | 931.7(951.6) | — | — | — | — |
Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 1)/TiO2 |
932.0(951.9) | 40.3(43.0) | 42.0(44.2) | — | 45.8(48.2) |
| Re/TiO2 | — | — | 42.2(44.6) | 44.3(48.7) | 46.2(48.4) |
3.3. Effects of various reaction conditions on hydrogenation of LA
The effect of the reaction temperature in the range of 140–200 °C on the conversion of LA and the selectivity to GVL over Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 catalyst was investigated, and the results are shown in Fig. 6. It can be seen that the conversion of LA increased from 22.0% at 140 °C to 53.4% at 160 °C, and the GVL selectivity increased from 78.4% to 89.9%. With the increase in the temperature to 180 °C, GVL selectivity increased to 98.6% with a LA conversion of 98.9%. Further increasing reaction temperature to 200 °C, both LA conversion and GVL selectivity were up to 100%. Considering energy-saving and GVL yield, 180 °C was chosen as the reaction temperature in the following study.
1)/TiO2 catalyst was investigated, and the results are shown in Fig. 6. It can be seen that the conversion of LA increased from 22.0% at 140 °C to 53.4% at 160 °C, and the GVL selectivity increased from 78.4% to 89.9%. With the increase in the temperature to 180 °C, GVL selectivity increased to 98.6% with a LA conversion of 98.9%. Further increasing reaction temperature to 200 °C, both LA conversion and GVL selectivity were up to 100%. Considering energy-saving and GVL yield, 180 °C was chosen as the reaction temperature in the following study.
 | ||
Fig. 6 Effect of reaction temperature on hydrogenation of LA to GVL over Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 catalyst. Reaction conditions: 0.5 g LA, 50 mg catalyst, 10 mL 1,4-dioxane, 4.0 MPa H2, 3 h, and 1000 rpm. 1)/TiO2 catalyst. Reaction conditions: 0.5 g LA, 50 mg catalyst, 10 mL 1,4-dioxane, 4.0 MPa H2, 3 h, and 1000 rpm. | ||
Fig. 7 compares LA hydrogenation results by reaction time over Cu/TiO2, Re/TiO2 and Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 catalysts. The bimetallic Cu–Re(1
1)/TiO2 catalysts. The bimetallic Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 catalyst exhibited the highest performance and produced a GVL yield of 97.5% after 3 h of reaction time. Extending the reaction time to 4 h, a GVL yield of 100% was achieved. In contrast, the monometallic Cu/TiO2 and Re/TiO2 catalysts showed a significantly lower activity, achieving a maximum GVL yield of 26.7% and 84.6%, respectively, after 4 h of reaction time. Combined with the characterization results of the catalysts, the excellent performance of Cu–Re(1
1)/TiO2 catalyst exhibited the highest performance and produced a GVL yield of 97.5% after 3 h of reaction time. Extending the reaction time to 4 h, a GVL yield of 100% was achieved. In contrast, the monometallic Cu/TiO2 and Re/TiO2 catalysts showed a significantly lower activity, achieving a maximum GVL yield of 26.7% and 84.6%, respectively, after 4 h of reaction time. Combined with the characterization results of the catalysts, the excellent performance of Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 catalyst for LA hydrogenation to GVL was mainly attributed to the following three aspects. Firstly, Cu–Re miscible phase was formed in the Cu–Re(1
1)/TiO2 catalyst for LA hydrogenation to GVL was mainly attributed to the following three aspects. Firstly, Cu–Re miscible phase was formed in the Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 catalyst during the reduction process, facilitating the synergistic interaction between copper and rhenium. Secondly, Cu–Re nanoparticles were highly dispersed on the TiO2 support with uniform and small size (0.69 nm), which increased the active sites on the surface of the catalyst, thereby improving the catalytic activity. Thirdly, the addition of Re was beneficial to the reduction of CuO to Cu0, and inhibiting the oxidation of metal Cu0. And the presence of ReOx species in the catalyst facilitated the adsorption of LA and the transformation of a reaction intermediate 4-hydroxyvaleric acid to GVL.33
1)/TiO2 catalyst during the reduction process, facilitating the synergistic interaction between copper and rhenium. Secondly, Cu–Re nanoparticles were highly dispersed on the TiO2 support with uniform and small size (0.69 nm), which increased the active sites on the surface of the catalyst, thereby improving the catalytic activity. Thirdly, the addition of Re was beneficial to the reduction of CuO to Cu0, and inhibiting the oxidation of metal Cu0. And the presence of ReOx species in the catalyst facilitated the adsorption of LA and the transformation of a reaction intermediate 4-hydroxyvaleric acid to GVL.33
Table 3 shows the effect of solvent on the performance of Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 catalyst for hydrogenation of LA to GVL. When methanol was used as a solvent, GVL yield was only 88.1%, and the reaction intermediate 4-hydroxyvaleric acid was the only by-product (11.9%). In the solvent-free conditions, the conversion of LA and the selectivity to GVL reached 97.3% and 97.6%, respectively. A higher yield of GVL (97.5%) was achieved by using 1,4-dioxane as solvent. H2O as solvent gave the highest yield of GVL. However, the stability of Cu–Re(1
1)/TiO2 catalyst for hydrogenation of LA to GVL. When methanol was used as a solvent, GVL yield was only 88.1%, and the reaction intermediate 4-hydroxyvaleric acid was the only by-product (11.9%). In the solvent-free conditions, the conversion of LA and the selectivity to GVL reached 97.3% and 97.6%, respectively. A higher yield of GVL (97.5%) was achieved by using 1,4-dioxane as solvent. H2O as solvent gave the highest yield of GVL. However, the stability of Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 catalyst in H2O was poor, and the catalytic activity of the recovered catalyst dropped down substantially, giving a lower yield of GVL (less than 50%) than the first cycle (100%).
1)/TiO2 catalyst in H2O was poor, and the catalytic activity of the recovered catalyst dropped down substantially, giving a lower yield of GVL (less than 50%) than the first cycle (100%).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 catalysta
1)/TiO2 catalysta
3.4. Recyclability of bimetallic Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 1)/TiO2 catalyst
1)/TiO2 catalyst
The recyclability of the bimetallic Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 catalyst in the hydrogenation of LA into GVL was examined by performing six consecutive catalytic runs. At each cycle, after hydrogenation reaction in 1,4-dioxane solvent at 180 °C and 4.0 MPa H2 for 3 h, the catalyst was centrifuged, washed with 1,4-dioxane, and reused. As shown in Fig. 8, the Cu–Re(1
1)/TiO2 catalyst in the hydrogenation of LA into GVL was examined by performing six consecutive catalytic runs. At each cycle, after hydrogenation reaction in 1,4-dioxane solvent at 180 °C and 4.0 MPa H2 for 3 h, the catalyst was centrifuged, washed with 1,4-dioxane, and reused. As shown in Fig. 8, the Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 catalyst exhibited excellent stability. After being recycled six times, Cu–Re(1
1)/TiO2 catalyst exhibited excellent stability. After being recycled six times, Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 still kept high activity and selectivity, achieving a GVL yield of 90.3%. Analysis of the reaction solution after the sixth run revealed no detectable leach of Cu and Re, which confirmed the high stability of the catalyst in 1,4-dioxane. The comparison with other Cu-containing catalysts (Table S1†) indicated that Cu–Re(1
1)/TiO2 still kept high activity and selectivity, achieving a GVL yield of 90.3%. Analysis of the reaction solution after the sixth run revealed no detectable leach of Cu and Re, which confirmed the high stability of the catalyst in 1,4-dioxane. The comparison with other Cu-containing catalysts (Table S1†) indicated that Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 developed in this work is a good catalyst for LA hydrogenation.
1)/TiO2 developed in this work is a good catalyst for LA hydrogenation.
 | ||
Fig. 8 Recyclability of Cu–Re(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TiO2 catalyst for hydrogenation of LA to GVL. Reaction conditions: 0.5 g LA, 50 mg catalyst, 10 mL 1,4-dioxane, 4.0 MPa H2, 180 °C, 3 h, and 1000 rpm. 1)/TiO2 catalyst for hydrogenation of LA to GVL. Reaction conditions: 0.5 g LA, 50 mg catalyst, 10 mL 1,4-dioxane, 4.0 MPa H2, 180 °C, 3 h, and 1000 rpm. | ||
4. Conclusions
Hydrogenation of levulinic acid (LA) to γ-valerolactone (GVL) is one of the most promising reactions in the fields of biomass conversion into high value-added chemicals and biofuels. Cu-based catalysts are active non-noble catalysts for the hydrogenation of LA to GVL. However, hydrogenation of LA over monometallic Cu catalysts generally needs to be carried out at harsh reaction conditions due to the low activity of the catalysts. To obtain an efficient Cu-based catalyst, a series of Cu–Re/TiO2 bimetallic catalysts prepared by an incipient wetness impregnation method were tested for the liquid phase hydrogenation of LA. The introduction of a suitable amount of Re into Cu/TiO2 was demonstrated to remarkably improve the catalytic performance for the hydrogenation of LA to GVL. The Cu–Re/TiO2 catalyst with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of Cu to Re achieved as high as 100% yield of GVL at 180 °C and 4.0 MPa H2 for 4 h. The catalyst could be reused at least 6 times with slight activity loss. TEM results showed that the addition of Re decreased the average size of the metal nanoparticles, improved the dispersion of Cu, and formed Cu–Re alloy. H2-TPR results showed that the presence of Re promoted the reduction of Cu and there is a strong interaction between Cu and Re. XPS results evidenced the co-presence of the metallic Re and ReOx, and revealed that the addition of Re inhibited the oxidation of metallic Cu. The co-presence of Cu–Re bimetal and ReOx species in the samples, and the improved Cu reducibility and dispersion were predominantly responsible for the good catalytic performance of Cu–Re/TiO2 catalysts.
1 molar ratio of Cu to Re achieved as high as 100% yield of GVL at 180 °C and 4.0 MPa H2 for 4 h. The catalyst could be reused at least 6 times with slight activity loss. TEM results showed that the addition of Re decreased the average size of the metal nanoparticles, improved the dispersion of Cu, and formed Cu–Re alloy. H2-TPR results showed that the presence of Re promoted the reduction of Cu and there is a strong interaction between Cu and Re. XPS results evidenced the co-presence of the metallic Re and ReOx, and revealed that the addition of Re inhibited the oxidation of metallic Cu. The co-presence of Cu–Re bimetal and ReOx species in the samples, and the improved Cu reducibility and dispersion were predominantly responsible for the good catalytic performance of Cu–Re/TiO2 catalysts.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Natural Science Foundation of China (21476211 and 21878269) and the Zhejiang Provincial Natural Science Foundation of China (LY18B060016).References
- P. Gallezot, Conversion of biomass to selected chemical products, Chem. Soc. Rev., 2012, 41(4), 1538–1558 RSC.
- L. T. Mika, E. Cséfalvay and Á. Németh, Catalytic conversion of carbohydrates to initial platform chemicals: chemistry and sustainability, Chem. Rev., 2018, 118(2), 505–613 CrossRef CAS PubMed.
- M. Besson, P. Gallezot and C. Pinel, Conversion of biomass into chemicals over metal catalysts, Chem. Rev., 2014, 114(3), 1827–1870 CrossRef CAS PubMed.
- D. M. Alonso, S. G. Wettstein and J. A. Dumesic, Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass, Green Chem., 2013, 15(3), 584–595 RSC.
- A. B. Jain and P. D. Vaidya, Kinetics of hydrogenation of furfuryl alcohol and γ-valerolactone over Ru/C catalyst, Energy Fuels, 2020, 34(8), 9963–9970 CrossRef CAS.
- Y. Zhao, Y. Fu and Q. X. Guo, Production of aromatic hydrocarbons through catalytic pyrolysis of γ-valerolactone from biomass, Bioresour. Technol., 2012, 114, 740–744 CrossRef CAS PubMed.
- G. H. Wang, X. Q. Liu, B. Yang, C. L. Si, A. M. Parvez, J. Jang and Y. H. Ni, Using green γ-valerolactone/water solvent to decrease lignin heterogeneity by gradient precipitation, ACS Sustainable Chem. Eng., 2019, 7(11), 10112–10120 CrossRef CAS.
- U. Omoruyi, S. Page, J. Hallett and P. W. Miller, Homogeneous catalyzed reactions of levulinic acid: To γ-valerolactone and beyond, ChemSusChem, 2016, 9(16), 2037–2047 CrossRef CAS PubMed.
- Z. H. Yu, X. B. Lu, C. Liu, Y. W. Han and N. Ji, Synthesis of γ-valerolactone from different biomass-derived feedstocks: Recent advances on reaction mechanisms and catalytic systems, Renewable Sustainable Energy Rev., 2019, 112, 140–157 CrossRef CAS.
- R. G. Weng, Z. H. Yu, J. Xiong and X. B. Lu, Effects of water in the heterogeneous catalytic valorization of levulinic acid into γ-valerolactone and its derivatives, Green Chem., 2020, 22(10), 3013–3027 RSC.
- S. G. Wettstein, J. Q. Bond, D. M. Alonso, H. N. Pham, A. K. Datye and J. A. Dumesic, RuSn bimetallic catalysts for selective hydrogenation of levulinic acid to γ-valerolactone, Appl. Catal., B, 2012, 117–118, 321–329 CrossRef CAS.
- L. E. Manzer, Catalytic synthesis of α-methylene-γ-valerolactone: a biomass-derived acrylic monomer, Appl. Catal., A, 2004, 272(1–2), 249–256 CrossRef CAS.
- J. Feng, M. Li, Y. H. Zhong, Y. L. Xu, X. J. Meng, Z. W. Zhao and C. G. Feng, Hydrogenation of levulinic acid to γ-valerolactone over Pd@UiO-66-NH2 with high metal dispersion and excellent reusability, Microporous Mesoporous Mater., 2020, 294, 109858 CrossRef CAS.
- M. Nemanashi, J. H. Noh and R. Meijboom, Hydrogenation of biomass-derived levulinic acid to γ-valerolactone catalyzed by mesoporous supported dendrimer-derived Ru and Pt catalysts: An alternative method for the production of renewable biofuels, Appl. Catal., A, 2018, 550, 77–89 CrossRef CAS.
- S. H. Zhu, Y. F. Xue, J. Guo, Y. L. Cen, J. G. Wang and W. B. Fan, Integrated conversion of hemicellulose and furfural into γ-valerolactone over Au/ZrO2 catalyst combined with ZSM-5, ACS Catal., 2016, 6(3), 2035–2042 CrossRef CAS.
- J. R. Wang, Y. Y. Wang, X. L. Tong, Y. W. Wang, G. Q. Jin and X. Y. Guo, Highly active Ir/SiC catalyst for aqueous hydrogenation of levulinic acid to γ-valerolactone, Catal. Commun., 2020, 139, 105971 CrossRef CAS.
- O. A. Abdelrahman, A. Heyden and J. Q. Bond, Analysis of kinetics and reaction pathways in the aqueous-phase hydrogenation of levulinic acid to form γ-valerolactone over Ru/C, ACS Catal., 2014, 4(4), 1171–1181 CrossRef CAS.
- J. J. Tan, J. L. Cui, G. Q. Ding, T. S. Deng, Y. L. Zhu and Y. W. Li, Efficient aqueous hydrogenation of levulinic acid to γ-valerolactone over a highly active and stable ruthenium catalyst, Catal. Sci. Technol., 2016, 6(5), 1469–1475 RSC.
- O. Mamun, M. Saleheen, J. Q. Bond and A. Heyden, Investigation of solvent effects in the hydrodeoxygenation of levulinic acid to c-valerolactone over Ru catalysts, J. Catal., 2019, 379(C), 164–179 CrossRef CAS.
- X. Q. Gao, S. H. Zhu, M. Dong, J. G. Wang and W. B. Fan, Ru nanoparticles deposited on ultrathin TiO2 nanosheets as highly active catalyst for levulinic acid hydrogenation to γ-valerolactone, Appl. Catal., B, 2019, 259, 118076 CrossRef.
- A. Hommes, A. J. ter Horst, M. Koeslag, H. J. Heeres and J. Yue, Experimental and modeling studies on the Ru/C catalyzed levulinic acid hydrogenation to γ-valerolactone in packed bed microreactors, Chem. Eng. J., 2020, 399, 125750 CrossRef CAS.
- Z. J. Wei, J. T. Lou, C. M. Su, D. C. Guo, Y. X. Liu and S. G. Deng, An efficient and reusable embedded Ru catalyst for the hydrogenolysis of levulinic acid to γ-valerolactone, ChemSusChem, 2017, 10(8), 1720–1732 CrossRef CAS PubMed.
- S. Dutta, I. K. M. Yu, D. C. W. Tsang, Y. H. Ng, Y. S. Ok, J. Sherwood and J. H. Clark, Green synthesis of gamma-valerolactone (GVL) through hydrogenation of biomass-derived levulinic acid using non-noble metal catalysts: A critical review, Chem. Eng. J., 2019, 372, 992–1006 CrossRef CAS.
- Z. He, H. Q. Lin, P. He and Y. Z. Yuan, Effect of boric oxide doping on the stability and activity of a Cu-SiO2 catalyst for vapor-phase hydrogenation of dimethyl oxalate to ethylene glycol, J. Catal., 2011, 277(1), 54–63 CrossRef CAS.
- J. L. Gong, H. R. Yue, Y. J. Zhao, S. Zhao, L. Zhao, J. Lv, S. P. Wang and X. B. Ma, Synthesis of ethanol via syngas on Cu/SiO2 Catalysts with Balanced Cu0-Cu+ Sites, J. Am. Chem. Soc., 2012, 134(34), 13922–13925 CrossRef CAS PubMed.
- I. Orlowski, M. Douthwaite, S. Iqbal, J. S. Hayward, T. E. Davies, J. K. Bartley, P. J. Miedziak, J. Hirayama, D. J. Morgan, D. J. Willock and G. J. Hutchings, The hydrogenation of levulinic acid to γ-valerolactone over Cu-ZrO2 catalysts prepared by a pH-gradient methodology, J. Energy Chem., 2019, 36, 15–24 CrossRef.
- Q. Xu, X. L. Li, T. Pan, C. G. Yu, J. Deng, Q. X. Guo and Y. Fu, Supported copper catalysts for highly efficient hydrogenation of biomass-derived levulinic acid and γ-valerolactone, Green Chem., 2016, 18(5), 1287–1294 RSC.
- T. G. Kelly and J. G. Chen, Metal overlayer on metal carbide substrate: unique bimetallic properties for catalysis and electrocatalysis, Chem. Soc. Rev., 2012, 41(24), 8021–8034 RSC.
- W. T. Yu, M. D. Porosoff and J. G. G. Chen, Review of Pt-based bimetallic catalysis: from model surfaces to supported catalysts, Chem. Rev., 2012, 112(11), 5780–5817 CrossRef CAS PubMed.
- L. Zhang, J. B. Mao, S. M. Li, J. M. Yin, X. D. Sun, X. W. Guo, C. S. Song and J. X. Zhou, Hydrogenation of levulinic acid into gamma-valerolactone over in situ reduced CuAg bimetallic catalyst: Strategy and mechanism of preventing Cu leaching, Appl. Catal., B, 2018, 232, 1–10 CrossRef CAS.
- B. Cai, X. C. Zhou, Y. C. Miao, J. Y. Luo, H. Pan and Y. B. Huang, Enhanced catalytic transfer hydrogenation of ethyl levulinate to γ-valerolactone over a robust Cu-Ni bimetallic catalyst, ACS Sustainable Chem. Eng., 2017, 5(2), 1322–1331 CrossRef CAS.
- D. Yanase, R. Yoshida, S. Kanazawa, Y. Yamada and S. Sato, Efficient formation of γ-valerolactone in the vapor-phase hydrogenation of levulinic acid over Cu-Co/alumina catalyst, Catal. Commun., 2020, 139, 105967 CrossRef CAS.
- Y. Takeda, Y. Nakagawa and K. Tomishige, Selective hydrogenation of higher saturated carboxylic acids to alcohols using a ReOx-Pd/SiO2 catalyst, Catal. Sci. Technol., 2012, 2(11), 2221–2223 RSC.
- M. D. Detwiler, P. Majumdar, X. K. Gu, W. N. Delgass, F. H. Ribeiro, J. Greeley and D. Y. Zemlyano, Characterization and theory of Re films on Pt(111) grown by UHV-CVD, Surf. Sci., 2015, 640, 2–9 CrossRef CAS.
- M. Tamura, K. Tokonami, Y. Nakagawa and K. Tomishige, Selective hydrogenation of crotonaldehyde to crotyl alcohol over metal oxide modified Ir catalysts and mechanistic insight, ACS Catal., 2016, 6(6), 3600–3609 CrossRef CAS.
- J. M. Keels, X. Chen, S. Karakalos, C. H. Liang, J. R. Monnier and J. R. Regalbuto, Aqueous-phase hydrogenation of succinic acid using bimetallic Ir-Re/C catalysts prepared by strong electrostatic adsorption, ACS Catal., 2018, 8(7), 6486–6494 CrossRef CAS.
- F. F. Yang, D. Liu, H. Wang, X. Liu, J. Y. Han, Q. F. Ge and X. L. Zhu, Geometric and electronic effects of bimetallic Ni-Re catalysts for selective deoxygenation of m-cresol to toluene, J. Catal., 2017, 349, 84–97 CrossRef CAS.
- Z. J. Wei, X. M. Zhu, X. S. Liu, H. Q. Xu, X. H. Li, Y. X. Hou and Y. X. Liu, Pt-Re/rGO bimetallic catalyst for highly selective hydrogenation of cinnamaldehyde to cinnamylalcohol, Chin. J. Chem. Eng., 2019, 27(2), 369–378 CrossRef CAS.
- X. M. Huang, K. T. Liu, W. L. Vrijburg, X. H. Ouyang, A. I. Dugulan, Y. X. Liu, M. W. G. M. T. Verhoeven, N. A. Kosinov, E. A. Pidko and E. J. M. Hensen, Hydrogenation of levulinic acid to γ-valerolactone over Fe-Re/TiO2 catalysts, Appl. Catal., B, 2020, 278, 119314 CrossRef CAS.
- K. Sing, D. Everett, R. Haul, L. Moscou, R. Pierotti, J. Rouquerol and T. Siemieniewsks, Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity, Pure Appl. Chem., 1985, 57(4), 603–619 CAS.
- V. G. Deshmane, S. L. Owen, R. Y. Abrokwah and D. Kuila, Mesoporous nanocrystalline TiO2 supported metal (Cu, Co, Ni, Pd, Zn, and Sn) catalysts: Effect of metal-support interactions on steam reforming of methanol, J. Mol. Catal. A: Chem., 2015, 408, 202–213 CrossRef CAS.
- X. Y. Jiang, G. H. Ding, L. P. Lou, Y. X. Chen and X. M. Zheng, Catalytic activities of CuO/TiO2 and CuO-ZrO2/TiO2 in NO + CO reaction, J. Mol. Catal. A: Chem., 2004, 218(2), 187–195 CrossRef CAS.
- B. Tapin, F. Epron, C. Especel, B. K. Ly, C. Pinel and M. Besson, Influence of the Re introduction method onto Pd/TiO2 catalysts for the selective hydrogenation of succinic acid in aqueous-phase, Catal. Today, 2014, 235, 127–133 CrossRef CAS.
- K. H. Kang, U. G. Hong, Y. Bang, J. H. Choi, J. K. Kim, J. K. Lee, S. J. Han and I. K. Song, Hydrogenation of succinic acid to 1,4-butanediol over Re-Ru bimetallic catalysts supported on mesoporous carbon, Appl. Catal., A, 2015, 490, 153–162 CrossRef CAS.
- R. A. Rather, S. Singh and B. Pal, A Cu+1/Cu0-TiO2 mesoporous nanocomposite exhibits improved H2 production from H2O under direct solar irradiation, J. Catal., 2017, 346, 1–9 CrossRef CAS.
- Z. F. Shao, C. Li, X. Di, Z. H. Xiao and C. H. Liang, Aqueous-phase hydrogenation of succinic acid to γ-butyrolactone and tetrahydrofuran over Pd/C, Re/C and Pd-Re/C catalysts, Ind. Eng. Chem. Res., 2014, 53(23), 9638–9645 CrossRef CAS.
- R. J. Shi, F. Wang, Tana, Y. Li, X. M. Huang and W. J. Shen, A highly efficient Cu/La2O3 catalyst for transfer dehydrogenation of primary aliphatic alcohols, Green Chem., 2010, 12(1), 108–113 RSC.
- A. Suknev, V. Zaikovskii, V. Kaichev, E. Paukshtis, E. Sadovskaya and B. Bal'zhinimaev, The nature of active sites in Pt-ReOX/TiO2 catalysts for selective hydrogenation of carboxylic acids to alcohols, J. Energy Chem., 2015, 24(5), 646–654 CrossRef.
- E. Hong, S. Bang, J. H. Cho, K. D. Jung and C. H. Shin, Reductive amination of isopropanol to monoisopropylamine over Ni-Fe/γAl2O3 catalysts: Synergetic effect of Ni-Fe alloy formation, Appl. Catal., A, 2017, 542, 146–153 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra05804e |
| This journal is © The Royal Society of Chemistry 2022 |