Synthesis of difluoromethyl carbinols from the Friedel–Crafts reaction of electron-rich arenes with difluorovinyl arylsulfonates†
Abstract
α,α-Difluoromethyl carbinols are important structural motifs in many therapeutic agents and functional materials. Herein, we developed a sustainable and efficient method for the synthesis of a series of α,α-difluoromethyl carbinols from the remarkable Friedel–Crafts reaction of electron-rich arenes including indoles, phenols and anilines with easily accessible 2,2-difluorovinyl arylsulfonates in trifluoroethanol. This simple and straightforward protocol takes advantage of the readily accessible difluoroacetaldehyde in situ from 2,2-difluorovinyl arylsulfonate in a step-economical manner. Meanwhile, this transformation is distinguished by its wide substrate scope and excellent functional group tolerance. Preliminary mechanistic studies suggest that trifluoroethanol is crucial to this reaction.
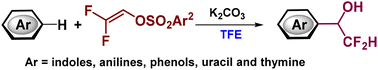


 Please wait while we load your content...
Please wait while we load your content...