Brønsted acid promoted substrate-dependent regiodivergent alkynylcyclopropane–cyclopentadiene rearrangement assisted by the internal carbonyl group†
Abstract
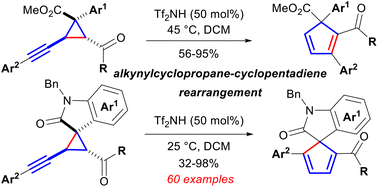
Direct ring expansion of alkynylcyclopropanes in a manner similar to the venerable vinylcyclopropane–cyclopentene rearrangement would form a highly strained cyclic allene intermediate and thus their rearrangement to cyclopentadienes is underdeveloped. Brønsted acid promoted ring expansion of alkynylcyclopropanes with the vicinal acyl and ester or spiro amide groups to cyclopentadienes, under the assistance of the internal carbonyl groups to successfully avoid such inaccessible intermediates, has been realized under mild conditions with broad functionality tolerance and substrate-dependent regioselectivity. Facile valence tautomeric interconversion between the oxepinium and cyclopropa-3,4-dihydropyrylium species generated from addition of the carbonyl group to the alkynyl substituent facilitates the inversion of the cyclopropane stereocenter, which affords an opportunity for the trans-aligned carbonyl group to cooperate with the opposite substituents to prompt the alkynylcyclopropane–cyclopentadiene rearrangement.


 Please wait while we load your content...
Please wait while we load your content...