Water-promoted regio-selective trifluoromethylation of vinyl conjugated diazoacetates†
Xinxin
Han
a,
Xin
Liu
a,
Yueyun
Bao
a,
Hunahuan
Song
a,
Yu-Rou
Zhao
a,
Xiaoying
Wang
a,
Junjie
Zhang
a,
Le
Liu
 a,
Xin-Hua
Duan
a,
Xin-Hua
Duan
 a,
Jinbo
Hu
a,
Jinbo
Hu
 b and
Mingyou
Hu
*a
b and
Mingyou
Hu
*a
aSchool of Chemistry, Xi'an Key Laboratory of Sustainable Energy Material Chemistry, and MOE Key Laboratory for Nonequilibrium Synthesis and Modulation of Condensed Matter, Xi'an Jiaotong University, Xi'an 710049, China
bKey Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai 200032, China. E-mail: mingyouhu@xjtu.edu.cn
First published on 1st December 2021
Abstract
An efficient trifluoromethylation reaction of vinyldiazoacetates under mild reaction conditions was depicted. Pre-generated CuCF3 was used as a trifluoromethylation reagent and water acted as both a promoter and proton source. The reaction is compatible with a broad scope of functional groups and easy to scale-up. The wide functional group tolerance of the reaction enables further synthetic manipulation of the products.
Introduction
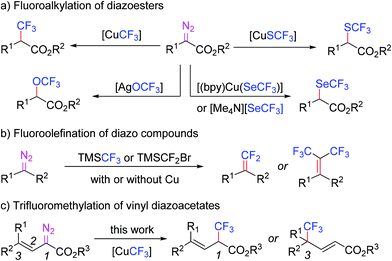
Fluorinated organic compounds often show improved metabolic stability, lipophilicity and bioavailability compared to their non-fluorinated analogues.1 Therefore, they are useful motifs in pharmaceuticals, agrochemicals and materials science.2 Moreover, fluorinated functional groups are usually used as versatile surrogates in many indispensable transformations.3 The trifluoromethyl group is a representative fluorinated moiety used to decorate molecules; in this regard, its installation has aroused a great deal of interest.3,4 Therefore, numerous synthetically useful approaches have been developed to constitute C–CF3 bonds, while the increasing demand for diverse trifluoromethylated compounds still needs to be met.5Diazo compounds are well-established carbene precursors, which proved to be effective in the incorporation of fluorine into molecules.6–12 Previously, we developed a water-promoted trifluoromethylation of diazo esters using pre-generated CuCF3 as a trifluoromethylating agent.7a In that work, we found that water played a crucial role in activating CuCF3 by dissociating redundant ligands (I−), as well as a proton source to quench the reaction intermediate. Considering the merit of reaction efficacy, this protocol has been successfully applied to trifluoromethylthiolation,8 trifluoromethoxylation,9 trifluoromethylselenolation,10gem-difluoroalkenylation,11etc. (Scheme 1). Despite its synthetic potential, the substrate scope is mainly limited to aryl/alkyl diazo esters, and/or diaryl diazomethanes. It intrigued our interest in exploring variable synthetically convertible functionalities to demonstrate the prowess for further manipulation. The displacement of aryl/alkyl groups with a vinyl group while preserving the ester group renders operationally stable donor–acceptor alkenyl conjugated diazo esters and enables their further modulation.13,14 Nevertheless, since the allylic carbene tends to undergo conjugative isomerisation, a plausible 1,3-selective trifluoromethylation may occur, leading to increased complexity.14 Herein, we report our discovery on the trifluoromethylation of vinyl diazoacetates.
Results and discussion
To test the feasibility of the trifluoromethylation of vinylconjugated diazoacetates, we embarked on the investigation by virtue of previously reported conditions. Ethyl phenylethenyl diazoacetate (1a) was chosen as a model substrate, pre-generated CuCF3 (from CuI, TMSCF3 and CsF 1/1.1/1.1) was used as a trifluoromethylation reagent, and N-methylpyrrolidone (NMP) was used as the solvent. To our disappointment, when 44 equivalents of H2O (1/15 to NMP in volume)7a were added, no desired product was observed. We quickly realized that the substrate is less reactive than phenyl diazoacetate, a popularly used substrate in previous work.7–12 Then, we attempted to increase the quantity of H2O and were excited to observe the desired product 2a on adding 1/6 H2O (in volume to NMP, vide infra), although in only 6% yield (Table 1, entry 5), while smaller quantities of H2O resulted in no desired reaction at all (Table 1, entries 2–4). When H2O 1/5 to NMP in volume was used, the yield increased steeply to 54% (Table 1, entry 6). Whereas when 1/4 of H2O was used, the yield was slightly lower than when 1/5 of H2O was used; this is probably owing to the fast decomposition of CuCF3 in the presence of excessive amounts of water (Table 1, entry 7).7a Further optimization showcased that shortening the reaction time to 3 h resulted in the highest yield of 89% (Table 1, entries 7–10), which indicated that the product would decompose with extended reaction time, while 1.5 h does not drive the reaction completely to the end (Table 1, entry 11). It was confirmed by 19F NMR that by extending the reaction time over 3 h, more side-products were observed. Notably, improving the reaction temperature and using DMF instead of NMP rendered inferior results (Table 1, entries 12 and 13). It is worth mentioning that the trifluoromethylation occurred solely on the carbenic carbon in all cases, and no 3-trifluoromethylation product was detected.| Entry | Time (h) | Temp. (°C) | H2O/NMP (V/V) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), CuI (0.75 mmol), CsF (0.825 mmol), and TMSCF3 (0.825 mmol). b Yields were determined by 19F NMR with PhOCF3 as an internal standard. c DMF was used as a solvent. | ||||
| 1 | 12 | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
0 |
| 2 | 12 | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
0 |
| 3 | 12 | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
0 |
| 4 | 12 | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
0 |
| 5 | 12 | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
5 |
| 6 | 12 | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
54 |
| 7 | 12 | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
39 |
| 8 | 8 | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
60 |
| 9 | 5 | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
64 |
| 10 | 3 | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
89 (82) |
| 11 | 1.5 | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
59 |
| 12 | 3 | 40 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
41 |
| 13c | 3 | 25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
72 |
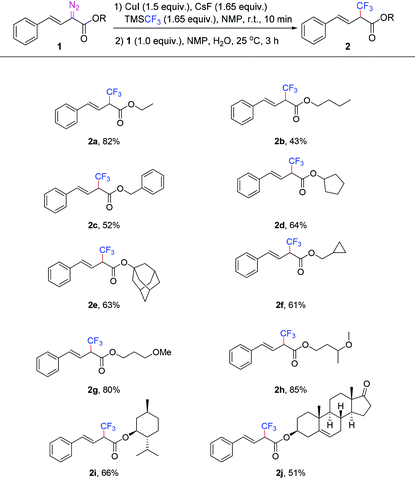
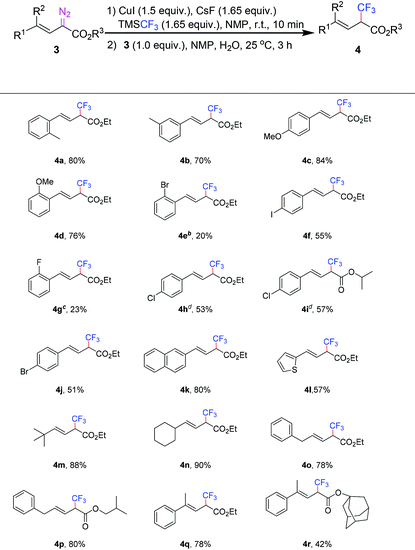
With the optimized conditions in hand (Table 1, entry 10), we examined the generality of this trifluoromethylation reaction using various vinyl diazoacetates, and moderate to high yields could be obtained (Table 2). Elongating the ester alkyl chain, or using cyclic alcohol esters resulted in a slight yield drop, which was probably caused by the steric hindrance from the ester moiety. This current reaction is different from the previous trifluoromethylation of α-diazo esters (Table 2, 2a–2j).7a The substrate bearing the cyclopropyl group was well tolerated under standard reaction conditions, and none of the ring-opened side-products were detected (Table 2, 2f). When substrates with ether groups were employed, the ether groups were kept intact, and good yields were obtained (Table 2, 2g and 2h). When vinyl diazoacetates derived from L-menthol and dehydroepiandrosterone were subjected to the standard conditions, the reactions worked smoothly to give 66% and 51% yield, respectively (Table 2, 2i and 2j).
| a Unless otherwise noted, reactions were performed on a 0.5 mmol scale by adding 1 and water to the pre-generated CuCF3/NMP solution. Yields refer to isolated products. |
|---|
|
Encouraged by the above results, we continued to study vinyl diazoacetates with diverse substituents on the vinyl moiety (Table 3). The aryl vinyl diazoacetates bearing alkyl, methoxy, and halogen substituents on the aromatic ring reacted smoothly to afford the trifluoromethylation products in medium to good yields (Table 3, 4a–4l). The methyl and methoxy substituents at the ortho-, meta-, or para-position of the aryl ring only slightly influenced the product yields (Table 3, 4a–4d), while the halogen substituents at the ortho-position of the aryl ring afforded lower yields than those at the para-position (Table 3, 4e–4j). It is worth noting that halogen substituents fluoro, chloro, bromo and iodo are all compatible under the reaction conditions (Table 3, 4e–4j), and no carbon–halogen bond cleaved products were observed. Moreover, the thiophenyl group was well tolerated, and a moderate yield of 57% was obtained (Table 3, 4l). Intriguingly, when alkyl or benzyl substituted vinyl diazoacetates were subjected to standard reaction conditions, excellent product yields could be obtained (Table 3, 4m–4p), perhaps owing to the improved stability of vinyl diazoacetates with less π-conjugation, which differentiates the current trifluoromethylation from the previous hydroboronation.13 The aforementioned results intrigued our interest in testing more complicated substrates; to our delight, trisubstituted vinyl diazoacetates 3q and 3r afforded the corresponding products 4q and 4r in 78% and 42% yield, respectively (Table 3, 4q and 4r). By comparison, the lower yield of 4r than 4q is probably ascribed to the combined steric effect of both substituents on vinyl and ester moieties.
| a Unless otherwise noted, reactions were performed on a 0.5 mmol scale by adding 3 and water to the pre-generated “CuCF3”. Yields refer to the isolated yields of analytically pure products. b The reaction was performed at 35 °C and the reaction time was 7 h. c The reaction was performed at 35 °C and the reaction time was 5 h. d The reaction was performed at 35 °C. |
|---|
|
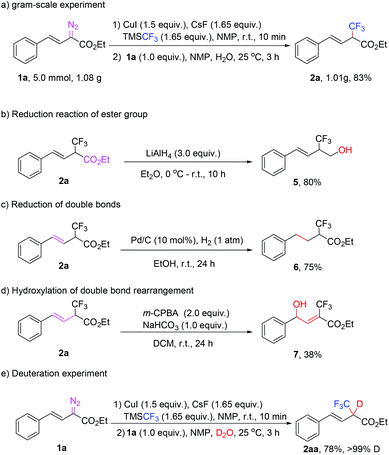
To further explore the practicability of the reaction, a gram scale experiment using 1a as the substrate was performed under standard conditions, and the reaction worked smoothly to afford 2a in 83% yield (Scheme 2(a)). Moreover, reduction of the ester with LiAlH4 and the vinyl group with Pd/C–H2 was carried out successfully to give 80% and 75% yield, respectively (Schemes 2b and c). Unexpectedly, when 2a was exposed to m-CPBA oxidation, the normal epoxidation product was not detected, instead a double bond-hydroxylation induced isomerisation product 7 was obtained in 38% yield (Scheme 2(d)). To clarify the importance of water, we also conducted a D2O-mediated reaction, and the deuterium substituted product was obtained in 78% yield, which indicated that water not only acts as a promoter, but also as a proton source (Scheme 2(e)).15
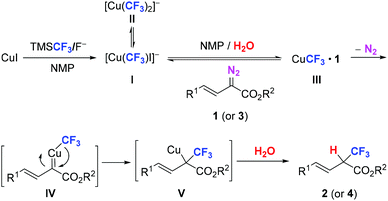
Based on the experimental results, a plausible mechanism was proposed as shown in Scheme 3. Compared to previous work,7a,15 the primary difference was that the reactivity of substrates used in this work was generally lower than that of the previously used ones, which indicates that more water was essential to promote the reaction. The reaction was initiated by pre-generated CuCF3, which existed as an equilibrium of (trifluoromethyl)copper species I and IIvia ligand redistribution on copper. Then, water was added to promote iodide anion dissociation of CuCF3 to increase its reactivity, subsequently forming a diazo compound coordinated complex III in the presence of diazo compound 1 (or 3). Complex III underwent nitrogen gas extrusion to form trifluoromethylcopper–carbene intermediate IV, and migration of CF3 to the carbenic carbon atom of IV resulted in intermediate V. It was then hydrolysed to afford product 2 (or 4).
Conclusions
In summary, we described an efficient trifluoromethylation reaction of vinyl diazoacetates; the vinyl moiety enables further synthetic manipulation of the products. In comparison with the reaction of phenyl diazoacetates, vinyl diazoacetates require more water as a promoter. The reaction is not sensitive to the steric effect and showcases robust functional group tolerance. Moreover, the gram scale experiment showed no yield drop at all. Interestingly, conventional reductive operation affords the corresponding reduced products, while oxidation with m-CPBA results in a double bond migration hydroxylated product. Replacement of H2O with D2O afforded a deuterated product with a similar yield, demonstrating the role of H2O as both a promoter and proton source.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank the National Natural Science Foundation of China (21901196), the Natural Science Basic Research Plan in Shaanxi Province of China (2020JQ-016), and Xi'an Jiaotong University (71211920000001) for financial support. We also thank Chao Feng and Lu Bai at the Center for Instrumental Analysis of Xi'an Jiaotong University for their assistance with NMR and HRMS analyses. We thank professor Zhenghu Xu (Shandong University) for the kind discussion.Notes and references
- (a) K. Müller, C. Faeh and F. Diederich, Fluorine in Pharmaceuticals: Looking Beyond Intuition, Science, 2007, 317, 1881–1886 CrossRef PubMed; (b) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Fluorine in medicinal chemistry, Chem. Soc. Rev., 2008, 37, 320–330 RSC; (c) W. K. Hagmann, The Many Roles for Fluorine in Medicinal Chemistry, J. Med. Chem., 2008, 51, 4359–4569 CrossRef CAS PubMed; (d) J.-P. Bégué and D. Bonnet-Delpon, Bioorganic and Medicinal Chemistry of Fluorine, John Wiley & Sons, Inc., Hoboken, NJ, 2008 CrossRef.
- (a) N. A. Meanwell, Synopsis of Some Recent Tactical Application of Bioisosteres in Drug Design, J. Med. Chem., 2011, 54, 2529–2591 CrossRef CAS PubMed; (b) N. A. Meanwell, Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design, J. Med. Chem., 2018, 61, 5822–5880 CrossRef CAS PubMed; (c) J. Wang, M. S. Rosello, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011), Chem. Rev., 2014, 114, 2432–2506 CrossRef CAS PubMed; (d) T. Furuya, A. S. Kamlet and T. Ritter, Catalysis for Fluorination and Trifluoromethylation, Nature, 2011, 473, 470–477 CrossRef CAS; (e) R. Britton, V. Gouverneur, J.-H. Lin, M. Meanwell, C. Ni, G. Pupo, J.-C. Xiao and J. Hu, Contemporary Synthetic Strategies in Organofluorine Chemistry, Nat. Rev. Methods Primers, 2021, 1, 47 CrossRef; (f) F. Wang and P. Tang, Recent Advances in Trifluoromethoxylation Reactions, Chin. J. Org. Chem., 2020, 40, 1805–1813 CrossRef CAS.
- For selected examples, see: (a) D. Mandal, R. Gupta, A. K. Jaiswal and R. D. Young, Frustrated Lewis-Pair-Meditated Selective Single Fluoride Substitution in Trifluoromethyl Groups, J. Am. Chem. Soc., 2020, 142, 2572–2578 CrossRef CAS PubMed; (b) K. Fuchibe, H. Hatta, K. Oh, R. Oki and J. Ichikawa, Lewis Acid Promoted Single C–F Bond Activation of the CF3 Group: SN1′-Type 3,3-Difluoroallylation of Arenes with 2-Trifluoromethyl-1-alkenes, Angew. Chem., Int. Ed., 2017, 56, 5890–5893 CrossRef CAS PubMed; (c) T. Kawamoto, R. Sasaki and A. Kamimura, Synthesis of α-Trifluoromethylated Ketones from Vinyl Triflates in the Absence of External Trifluoromethyl Sources, Angew. Chem., Int. Ed., 2017, 56, 1342–1345 CrossRef CAS PubMed; (d) K. Takahashi, Y. Ano and N. Chatani, Fluoride Anion-initiated bis-Trifluoromethylation of Phenyl Aromatic Carboxylates with (Trifluoromethyl)- trimethylsilane, Chem. Commun., 2020, 56, 11661–11664 RSC; (e) F. Xiao, S. Yuan, H. Huang, F. Zhang and G. Deng, Copper-Catalyzed Three-Component Domino Cyclization for the Synthesis of 4-Aryl-5-(arythio)-2-(trifluoromethyl)oxazoles, Org. Lett., 2019, 21, 8533–8536 CrossRef CAS; (f) J. B. I. Sap, N. J. W. Straathof, T. Knauber, C. F. Meyer, M. Médebielle, L. Buglioni, C. Genicot, A. A. Trabanco, T. Noël, C. W. am Ende and V. Gouverneur, Organophotoredox Hydrodefluorination of Trifluoromethylarenes with Translational Applicability to Drug Discovery, J. Am. Chem. Soc., 2020, 142, 9181–9187 CrossRef CAS PubMed; (g) Y.-C. Luo, F.-F. Tong, Y. Zhang, C.-Y. He and X. Zhang, Visible-Light-Induced Palladium-Catalyzed Selective Defluoroarylation of Trifluoromethy- larenes with Arylboronic Acids, J. Am. Chem. Soc., 2021, 143, 13971–13979 CrossRef CAS PubMed.
- For reviews on trifluoromethylation reactions, see: (a) F. Qing, Recent Advances of Trifluoromethylation, Chin. J. Org. Chem., 2012, 32, 815–824 CrossRef CAS; (b) H. Xiao, Z. Zhang, Y. Fang, L. Zhu and C. Li, Radical trifluoromethylation, Chem. Soc. Rev., 2021, 50, 6308–6319 RSC; (c) C. Zhang, Recent Advances in Trifluoromethylation of Organic Compounds Using Umemoto's Reagents, Org. Biomol. Chem., 2014, 12, 6580–6589 RSC; (d) A. J. Fernandes, A. Panossian, B. Michelet, A. Martin-Mingot, F. R. Leroux and S. Thibaudeau, CF3-substituted Carbocations: Underexploited Intermediates with Great Potential in Modern Synthetic Chemistry, Beilstein J. Org. Chem., 2021, 17, 343–378 CrossRef CAS PubMed; (e) J. Nie, H.-C. Guo, D. Cahard and J.-A. Ma, Asymmetric Construction of Stereogenic Carbon Centers Featuring a Trifluoromethyl Group from Prochiral Trifluoromethylated Substrates, Chem. Rev., 2011, 111, 455–529 CrossRef CAS PubMed; (f) A. Studer, A “Renaissance” in Radical Trifluorome- thylation, Angew. Chem., Int. Ed., 2012, 51, 8950–8958 CrossRef CAS PubMed; (g) L. Chu and F.-L. Qing, Oxidative Trifluoromethylation and Trifluoromethylthiolation Reactions Using (Trifluoromethyl)- trimethylsilane as a Nucleophilic CF3 Source, Acc. Chem. Res., 2014, 47, 1513–1522 CrossRef CAS PubMed; (h) G. Wang, X. He, J. Dai and H. Xu, Recent Advances in Copper-Promoted Trifluoromethylation Reactions, Chin. J. Org. Chem., 2014, 34, 837–851 CrossRef CAS; (i) S. Wang and W. Wang, Recent Advances of the Construction of Trifluoromethylated Quaternary Carbon Center, Chin. J. Org. Chem., 2020, 40, 1901–1911 CrossRef CAS.
- (a) E. Merino and C. Nevado, Addition of CF3 across Unsaturated Moieties: A Powerful Functionalization Tool, Chem. Soc. Rev., 2014, 43, 6598–6608 RSC; (b) T. B. Vaishak, P. K. Soumya, P. V. Saranya and G. Anilkumar, Recent Advances and Prospects in the Iron Catalyzed Trifluoromethylation Reactions, Catal. Sci. Technol., 2021, 11, 4690–4701 RSC; (c) S. B. Vallejo and A. Postigo, Metal-Mediated Radical Perfluoroalkylation of Organic Compounds, Coord. Chem. Rev., 2013, 257, 3051–3069 CrossRef; (d) M. Shimizu and T. Hiyama, Modern Synthetic Methods for Fluorine-Substituted Target Molecules, Angew. Chem., Int. Ed., 2005, 44, 214–231 CrossRef CAS PubMed; (e) J.-A. Ma and D. Cahard, Asymmetric Fluorination, Trifluoromethylation, and Perfluoroalkylation Reactions, Chem. Rev., 2008, 108, PR1–PR43 CrossRef CAS; (f) O. A. Tomashenko and V. V. Grushin, Aromatic Trifluoromethylation with Metal Complexes, Chem. Rev., 2011, 111, 4475–45521 CrossRef CAS PubMed; (g) X.-F. Wu, H. Neumann and M. Beller, Recent Developments on the Trifluoromethylation of (Hetero)Arenes, Chem. – Asian J., 2012, 7, 1744–1754 CrossRef CAS; (h) P. Poutrel, M. V. Lvanona, X. Pannecoucke, P. Jubault and T. Poisson, Copper-Catalyzed Enantioselective Formation of C-CF3 Centers from β-CF3-Substituted Acrylates and Acrylonitriles, Chem. – Eur. J., 2019, 25, 15262–15266 CrossRef CAS PubMed.
- For reviews on diazo compounds, see: (a) M. L. Hossain and J. Wang, Cu(I)-Catalyzed Cross-Coupling of Diazo Compounds with Terminal Alkynes: An Efficient Access to Allenes, Chem. Rec., 2018, 18, 1548–1559 CrossRef CAS PubMed; (b) F. Wei, C. Song, Y. Ma, L. Zhou, C.-H. Tung and Z. Xu, Gold Carbene Chemistry from Diazo Compounds, Sci. Bull., 2015, 60, 1479–1492 CrossRef CAS; (c) J. R. Fulton, V. K. Aggarwal and J. Vicente, The Use of Tosylhydrazone Salts as a Safe Alternative for Handling Diazo Compounds and Their Applications in Organic Synthesis, Eur. J. Org. Chem., 2005, 1479–1492 CrossRef CAS. For selected examples, also see: (d) Y. Su, G. Liu, J. Liu, L. Tram, H. Qiu and M. Dolye, Radical-Mediated Strategies for the Functionalization of Alkenes with Diazo Compounds, J. Am. Chem. Soc., 2020, 142, 13846–13855 CrossRef CAS PubMed; (e) M. Álvarez, M. Besora, F. Molina, F. Maseras and T. Belderrain, Two Copper-Carbenes from One Diazo Compound, J. Am. Chem. Soc., 2021, 143, 4837–4843 CrossRef; (f) S. Wu, H.-S. Song and C.-P. Zhang, Fluoroalkylation of Diazo Compounds with Diverse Rfn Reagents, Chem. – Asian J., 2020, 15, 1660–1677 CrossRef CAS PubMed; (g) H. D. Khanal, R. S. Thombal, S. M.-B. Maezono and Y. R. Lee, Designs and Strategies for the Halo-Functionalization of Diazo Compounds, Adv. Synth. Catal., 2018, 360, 3185–3212 CrossRef CAS.
- (a) M. Hu, C. Ni and J. Hu, Copper-Mediated Trifluoromethylation of α-Diazo Esters with TMSCF3: The Important Role of Water as a Promoter, J. Am. Chem. Soc., 2012, 134, 15257–15260 CrossRef CAS PubMed; (b) Q. Ma and G. C. Tsui, Trifluoromethylation of α-Diazoesters and α-Diazoketones with Fluoroform-Derived CuCF3: Synergistic Effects of Co-solvent and Pyridine as a Promoter, Org. Chem. Front., 2019, 6, 27–31 RSC; (c) X.-Q. Hu, J.-B. Han and C.-P. Zhang, Cu-Mediated Trifluoromethylation of Aromatic α-Diazo Esters with the Yagupolskii-Umemoto Reagent, Eur. J. Org. Chem., 2017, 324–331 CrossRef CAS.
- (a) X. Wang, Y. Zhou, G. Ji, G. Wu, M. Li, Y. Zhang and J. Wang, Trifluoromethylthiolation of Diazo Compounds through Copper Carbene Migratory Insertion, Eur. J. Org. Chem., 2014, 3093–3096 CrossRef CAS; (b) M. Hu, J. Rong, W. Miao, C. Ni, Y. Han and J. Hu, Copper-Mediated Trifluoromethylthiolation of α-Diazoesters, Org. Lett., 2014, 16, 2030–2033 CrossRef CAS PubMed; (c) Q. Lefebvre, E. Fava, P. Nikolaienko and M. Rueping, Hydrotrifluoromethylthiolation of α-Diazo Esters-Synthesis of α-SCF3 Substituted Esters, Chem. Commun., 2014, 50, 6617–6619 RSC; (d) E. Emer, J. Twilton, M. Tredwell, S. Calderwood, T. L. Collier, B. Liégault, M. Taillefer and V. Gouverneur, Diversity-Oriented Approach to CF3CHF-, CF3CFBr-, CF3CF2-, (CF3)2CH-, and CF3(SCF3)CH-Substituted Arenes from 1-(Diazo-2,2,2-trifluoroethyl)arenes, Org. Lett., 2014, 16, 6004–6007 CrossRef CAS PubMed.
- G.-F. Zha, J.-B. Han, X.-Q. Hu, H.-L. Qin, W.-Y. Fang and C.-P. Zhang, Silver-mediated direct trifluoromethoxylation of α-diazo esters via the OCF3 anion, Chem. Commun., 2016, 52, 7458–7461 RSC.
- (a) C. Matheis, T. Krause, V. Bragoni and L. Goossen, Trifluoromethylthiolation and Trifluoromethylselenolation of α-Diazo Esters Catalyzed by Copper, Chem. – Eur. J., 2016, 22, 12270–12273 CrossRef CAS PubMed; (b) T. Dong, J. He, Z.-H. Li and C.-P. Zhang, Catalyst- and Additive-Free Trifluoromethylselenolation with [Me4N][SeCF3], ACS Sustainable Chem. Eng., 2018, 6, 1327–1333 CrossRef CAS; (c) T. Chen, Y. You and Z. Weng, Copper-Mediated Synthesis of α-Trifluoromethylselenolated Esters, J. Fluorine Chem., 2018, 216, 43–46 CrossRef CAS.
-
(a) M. Hu, Z. He, B. Gao, L. Li, C. Ni and J. Hu, Copper-Catalyzed gem-Difluoroolefination of Diazo Compounds with TMSCF3 via C–F Bond Cleavage, J. Am. Chem. Soc., 2013, 135, 17302–17305 CrossRef CAS PubMed;
(b) M. Hu, C. Ni, L. Li, Y. Han and J. Hu,
gem-Difluoroolefination of Diazo Compounds with TMSCF3 or TMSCF2Br: Transition-Metal-Free Cross-Coupling of Two Carbene Precursors, J. Am. Chem. Soc., 2015, 137, 14496–14501 CrossRef CAS PubMed;
(c) Z. Zhang, W. Yu, C. Wu, C. Wang, Y. Zhang and J. Wang, Reaction of Diazo Compounds with Difluorocarbene: An Efficient Approach towards 1,1-Difluoroolefins, Angew. Chem., 2016, 128, 281–285 CrossRef;
(d) J. Zheng, J.-H. Lin, L.-Y. Yu, Y. Wei, X. Zheng and J.-C. Xiao, Cross-Coupling between Difluorocarbene and Carbene-Derived Intermediates Generated from Diazo Compounds for the Synthesis of gem-Difluoroolefins, Org. Lett., 2015, 17, 6150–6153 CrossRef CAS PubMed;
(e) Q. Wang, C. Ni, M. Hu, Q. Xie, Q. Liu, S. Pan and J. Hu, From C1 to C3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Copper-Catalyzed gem-Bis(trifluoromethyl)olefination of α-Diazo Esters with TMSCF3, Angew. Chem., Int. Ed., 2020, 59, 8507–8511 CrossRef CAS PubMed.
Copper-Catalyzed gem-Bis(trifluoromethyl)olefination of α-Diazo Esters with TMSCF3, Angew. Chem., Int. Ed., 2020, 59, 8507–8511 CrossRef CAS PubMed. - (a) M. V. Ivanova, A. Bayle, T. Besset, X. Pannecoucke and T. Poisson, Copper Salt-Controlled Divergent Reactivity of [Cu]CF2PO(OEt)2 with α-Diazocarbonyl Derivatives, Angew. Chem., Int. Ed., 2016, 55, 14141–14145 CrossRef CAS PubMed; (b) W. Yuan, L. Eriksson and K. J. Szabó, Rhodium-Catalyzed Geminal Oxyfluorination and Oxytrifluoro-Methylation of Diazocarbonyl Compounds, Angew. Chem., Int. Ed., 2016, 55, 8410–8415 CrossRef CAS PubMed.
- D. Drikerman, R. Möβel, W. A. Jammal and I. Vilotijevic, Synthesis of Allylboranes via Cu(I)-Catalyzed B–H Insertion of Vinyldiazoacetates into Phosphine–Borane Adducts, Org. Lett., 2020, 22, 1091–1095 CrossRef PubMed.
- For examples of the 1,3-selective reaction of vinyl diazoacetates, see: (a) Y. Sevryugina, B. Weaver, J. Hansen, J. Thompson, H. M. L. Davies and M. A. Petrukhina, Influence of Electron-Deficient Ruthenium(I) Carbonyl Carboxylates on the Vinylogous Reactivity of Metal Carbenoids, Organometallics, 2008, 27, 1750–1757 CrossRef CAS; (b) C. Qin and H. M. L. Davies, Silver-Catalyzed Vinylogous Fluorination of Vinyl Diazoacetates, Org. Lett., 2013, 15, 6152–6154 CrossRef CAS PubMed; (c) R. Kajihara, S. Harada, J. Ueda and T. Nemoto, Synthesis of Functionalized Iodoalkenes Using a Multicomponent Reaction Triggered by Electrophilic Iodination of Alkenyldiazoacetates, Tetrahedron Lett., 2018, 59, 1906–1908 CrossRef CAS.
- M. Hu, Q. Xie, X. Li, C. Ni and J. Hu, Copper-Mediated Deuterotrifluoromethylation of α-Diazo Esters, Chin. J. Chem., 2016, 34, 469–472 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1qo01654g |
| This journal is © the Partner Organisations 2022 |