Palladium-catalysed stereoselective [3 + 2] annulation of vinylethylene carbonates and tryptanthrin-based ketones†
Yang
Fan‡
ab,
Qing-Zhu
Li‡
b,
Jun-Long
Li
 *b,
Bin
Zhang
b,
Zhen
Dai
b,
Ke
Xie
b,
Rong
Zeng
b,
Liang
Zou
*c and
Xiang
Zhang
*b
*b,
Bin
Zhang
b,
Zhen
Dai
b,
Ke
Xie
b,
Rong
Zeng
b,
Liang
Zou
*c and
Xiang
Zhang
*b
aCollege of Pharmacy, Dali University, Dali 671003, PR China
bAntibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, School of Pharmacy, Chengdu University, Chengdu 610106, PR China. E-mail: lijunlong709@hotmail.com; XZhang_CDU@hotmail.com
cSchool of Food and Biological Engineering, Chengdu University, Chengdu 610106, PR China. E-mail: zouliangcdu@126.com
First published on 22nd November 2021
Abstract
The palladium-catalysed [3 + 2] annulation of vinylethylene carbonates (VECs) and ketones remains challenging in organic synthesis. Herein, we successfully achieved the [3 + 2] annulation of tryptanthrin-based ketones and VECs for the efficient synthesis of indoloquinazolinone derivatives with generally excellent yields and good diastereoselectivity. Notably, the asymmetric version of this [3 + 2] annulation can also be achieved by using a chiral spiroketal-based diphosphine ligand. In addition, preliminary biological studies reveal that some of the products exhibit promising antibacterial activity.
Introduction
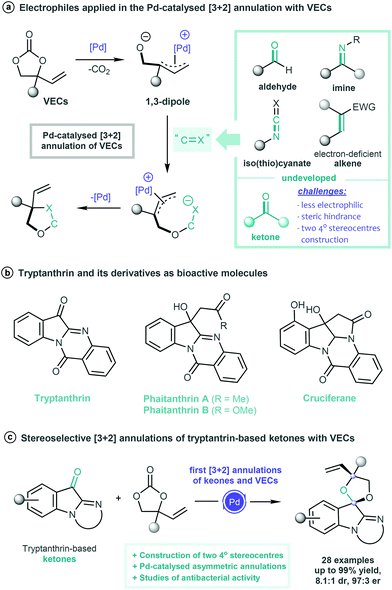
Transition-metal-catalysed annulation processes are powerful tools for building diverse biologically important organic molecules.1 In particular, stereoselective reactions involving zwitterionic π-allyl palladium species have stimulated great interest among synthetic chemists, and significant achievements have been made over the past decades.2 As easily accessible and versatile building blocks, vinylethylene carbonates (VECs)3 can readily undergo decarboxylative activation in the presence of palladium catalysts and generate highly reactive zwitterionic π-allyl palladium complexes, thereby enabling a series of interesting chemical transformations.4 In 2014, Zhang and co-workers used aldehydes for the palladium-catalysed [3 + 2] annulation of VECs, and the zwitterionic π-allyl palladium generated in situ served as a 1,3-dipole to react with various aldehydes in a sequential 1,2-addition/cyclisation fashion.5 Since then, a series of palladium-catalysed [3 + 2] annulation reactions of VECs have been developed by using diverse unsaturated systems, such as imines,6 isocyanates,7 isothiocyanates8 and electron-deficient alkenes.9 However, using ketones, which are important carbonyl substrates, in [3 + 2] cyclisation remains challenging probably because they have low electrophilic properties, steric hindrance effect and two quaternary carbon centres that are difficult to construct in a single step (Scheme 1a).Therefore, catalytic systems that allow ketones to participate in the stereoselective annulation reactions of VECs are highly desirable.
Indoloquinazolinones are key structural motifs in a variety of natural products and biologically active compounds.10 Tryptanthrin and its analogues, such as phaitanthrin A and B and cruciferane, consist of indoloquinazolinone cores, which exhibit a broad spectrum of biological activities (Scheme 1b).11 Therefore, substantial efforts have been made to develop various valuable transformations on the basis of tryptanthrin cores and enrich the structural diversity of biologically interesting scaffolds.12 Encouraged by these achievements and our continuing interest in the construction and modification of biologically relevant frameworks,13 we envisioned that the unprecedented ketone-participated [3 + 2] annulation of VECs might be achieved by using tryptanthrins as a highly reactive electrophile. In this work, a method for the palladium-catalysed stereoselective [3 + 2] annulation of VECs and tryptanthrin-based ketones has been developed. With this method, a series of indoloquinazolinone compounds bearing two quaternary carbon centres can be easily synthesised with excellent yields and good diastereoselectivity. In addition, the asymmetric catalytic cyclisation has been achieved by using a chiral spiroketal-based diphosphine ligand, producing the target products with high enantioselectivity. Preliminary biological studies reveal that some of the obtained indoloquinazolinone molecules show promising antibacterial activity (Scheme 1c).
Results and discussion
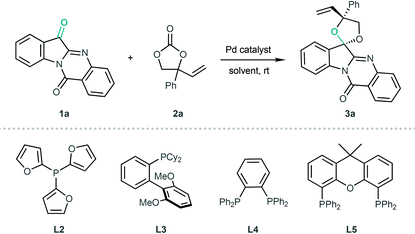
Initially, attempts to verify the concept of Pd-catalysed [3 + 2] annulation of VECs and ketones were unsuccessful despite using several ketone substrates, such as trifluoroacetophenone and isatins. Then, we tested tryptanthrin 1a as a highly reactive ketone in the reaction with VEC 2a. As shown in Table 1, 1a triggered the target [3 + 2] cyclisation in the presence of Pd(PPh3)4 in toluene at ambient temperature, and the desired product 3a was obtained in 11% yield with 3.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereoselectivity (Table 1, entry 1). With this encouraging result, we subsequently examined the effect of the solvent, and DCM was identified as the optimal solvent that could dramatically improve the yield and diastereoselectivity (Table 1, entries 2–7). We further evaluated ligand effects by using various phosphines in combination with Pd2(dba)3·CHCl3. As shown in entries 8–11, inferior results were generally observed after monophosphine ligands L1–L3 and bisphosphine ligand L4 were screened. Although Xantphos L5 gave the product 3a in 99% yield, poor diastereoselectivity was observed (Table 1, entry 12).
1 diastereoselectivity (Table 1, entry 1). With this encouraging result, we subsequently examined the effect of the solvent, and DCM was identified as the optimal solvent that could dramatically improve the yield and diastereoselectivity (Table 1, entries 2–7). We further evaluated ligand effects by using various phosphines in combination with Pd2(dba)3·CHCl3. As shown in entries 8–11, inferior results were generally observed after monophosphine ligands L1–L3 and bisphosphine ligand L4 were screened. Although Xantphos L5 gave the product 3a in 99% yield, poor diastereoselectivity was observed (Table 1, entry 12).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Pd source | Ligand | Solvent | Yieldb (%) | drc |
|---|---|---|---|---|---|
| a Reactions were performed with 0.10 mmol of 1a, 0.15 mmol of 2a and 5 mol% of Pd(PPh3)4 (or 2.5 mol% of Pd2(dba)3·CHCl3/10 mol% of ligand) in 1.0 mL solvent at room temperature for 12 h. b Isolated yields. c Determined by 1H NMR analysis of the crude reaction mixture. ND: not determined; Cy: cyclohexyl. | |||||
| 1 | Pd(PPh3)4 | — | Toluene | 11 | 3.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | Pd(PPh3)4 | — | THF | <5 | ND |
| 3 | Pd(PPh3)4 | — | MeCN | 58 | 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | Pd(PPh 3 ) 4 | — | DCM | 96 |
7.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1
|
| 5 | Pd(PPh3)4 | — | CHCl3 | 63 | 7.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | Pd(PPh3)4 | — | DCE | 48 | 6.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | Pd(PPh3)4 | — | PhCl | 62 | 4.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | Pd2(dba)3·CHCl3 | PCy3L1 | DCM | <5 | ND |
| 9 | Pd2(dba)3·CHCl3 | L2 | DCM | 34 | 5.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | Pd2(dba)3·CHCl3 | L3 | DCM | 35 | 7.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | Pd2(dba)3·CHCl3 | L4 | DCM | 47 | 7.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | Pd2(dba)3·CHCl3 | L5 | DCM | 99 | 4.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
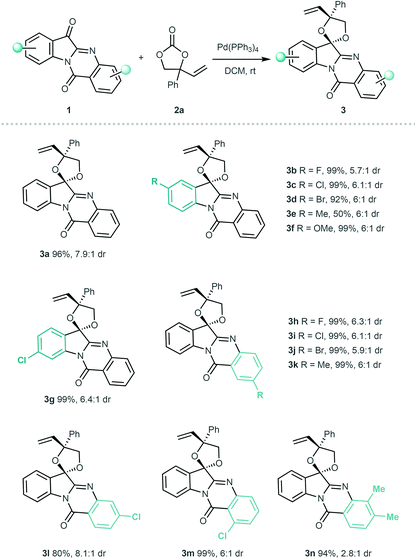
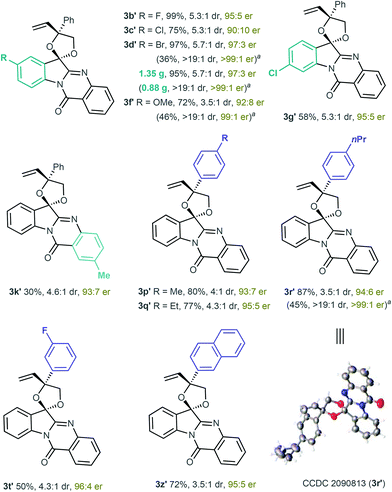
After establishing the optimal conditions, we then investigated the generality and limitations of the [3 + 2] annulation by using various tryptanthrin-based ketone substrates. As shown in Table 2, substrates 1 with diverse electronic and steric properties on the indole skeleton readily participated in the [3 + 2] annulation, affording the desired products 3a–3g in excellent yields (up to 99% yield) with reasonable diastereoselectivity. Electron-withdrawing and electron-donating substituents on the quinazolinone ring at different positions of 1 were well tolerated, providing the products 3h–3m in 80%–99% yields with up to 8.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereoselectivity. Dimethyl-substituted 1 was also a suitable substrate and generated the product 3n in an excellent yield but with decreased diastereoselectivity.
1 diastereoselectivity. Dimethyl-substituted 1 was also a suitable substrate and generated the product 3n in an excellent yield but with decreased diastereoselectivity.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a,b
a,b
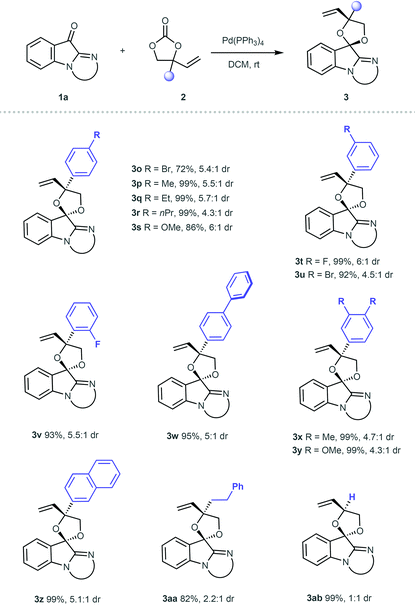
Various substituted VECs 2 were subsequently investigated in the reaction with ketone 1a under the catalysis of Pd(PPh3)4 (Table 3). VECs 2 featuring either an electron-donating or electron-withdrawing group at the para-position of the benzene ring worked well under standard conditions, generating the corresponding products 3o–3s in 72%–99% yields with good diastereoselectivity. VECs bearing a fluorine or bromine atom at the meta-position of the benzene ring were also suitable substrates for the production of 3t and 3u in excellent yields. The reactions proceeded smoothly for the ortho-fluoro-substituted VEC, and 3v was obtained in 93% yield. The biphenyl VEC could also participate in the reaction, and the corresponding 3w was obtained in 95% yield with 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. The reactions with disubstituted VECs smoothly provided products 3x and 3y in excellent yields. The reaction was compatible with naphthyl VEC, and afforded product 3z efficiently. Furthermore, the alkyl-substituted VEC was tested, and 3aa was produced in a good yield with decreased diastereoselectivity. The reaction with VEC bearing a single vinyl substituent generated the product in an extremely high yield, but the diastereoselective control remained a limitation for this method (3ab).
1 dr. The reactions with disubstituted VECs smoothly provided products 3x and 3y in excellent yields. The reaction was compatible with naphthyl VEC, and afforded product 3z efficiently. Furthermore, the alkyl-substituted VEC was tested, and 3aa was produced in a good yield with decreased diastereoselectivity. The reaction with VEC bearing a single vinyl substituent generated the product in an extremely high yield, but the diastereoselective control remained a limitation for this method (3ab).
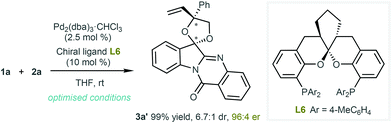
Subsequently, we turned our attention to the enantioselective synthesis and used a chiral ligand in the presence of a Pd2(dba)3·CHCl3 pre-catalyst. After a large number of chiral phosphine ligands were screened for the [3 + 2] reaction,14L6 was selected as the optimal ligand to produce 3a′ with satisfactory results (99% yield, 6.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and 96
1 dr and 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 er, see Scheme 2). This asymmetric catalytic system was found to be robust and exhibited good compatibility. As shown in Scheme 3, more than 10 examples of enantioenriched products were readily synthesised under the established catalytic system. The absolute configuration of 3r′ was determined through X-ray analysis.15 Notably, the optically pure products could be easily obtained through simple recrystallisation, as exemplified in 3d′, 3f′ and 3r′ in Scheme 3.
4 er, see Scheme 2). This asymmetric catalytic system was found to be robust and exhibited good compatibility. As shown in Scheme 3, more than 10 examples of enantioenriched products were readily synthesised under the established catalytic system. The absolute configuration of 3r′ was determined through X-ray analysis.15 Notably, the optically pure products could be easily obtained through simple recrystallisation, as exemplified in 3d′, 3f′ and 3r′ in Scheme 3.
 | ||
| Scheme 3 Scope of Pd-catalytic asymmetric synthesis. aThe data in parentheses refer to the results after recrystallisation. | ||
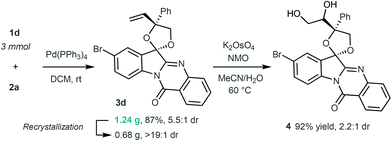
In addition, the practicability and synthetic usefulness of the [3 + 2] annulation was further demonstrated with a scale-up experiment. Under the standard conditions, product 3d was produced on a 3 mmol scale without yield loss, and 0.68 g of the single diastereoisomer was obtained after recrystallisation. On treating the product 3d with K2OsO4 and NMO (4-methylmorpholine N-oxide), the 1,2-diol 4 could be smoothly synthesised through oxidative dihydroxylation of the terminal alkene moiety (Scheme 4).
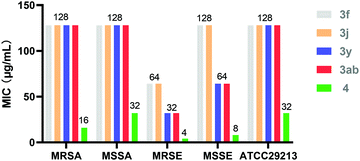
The synthesised indoloquinazoline compounds were screened for in vitro antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA, clinic isolates), methicillin-sensitive Staphylococcus aureus (MSSA, clinic isolates), methicillin-resistant Staphylococcus epidermidis (MRSE, clinic isolates), methicillin-sensitive Staphylococcus epidermidis (MSSE, clinic isolates) and Staphylococcus aureus (ATCC25923) with the broth microdilution method.16 The preliminary experimental result indicated that compounds 3f, 3j, 3y and 3ab exhibited specific bioactivity against MRSE, with minimum inhibitory concentration (MIC) values ranging from 32 μg mL−1 to 64 μg mL−1. In addition, diol 4 showed promising inhibition effects on the tested bacteria, with the MIC value ranging from 4 μg mL−1 to 32 μg mL−1 (Fig. 1).
Conclusions
In summary, we have developed the first example of palladium catalysed [3 + 2] annulation of VECs and ketones. In this work, tryptanthrins have been found to be a reactive ketone substrate that allows the catalytic construction of the indoloquinazoline skeleton. Moreover, the asymmetric version of the [3 + 2] annulation was achieved by using a chiral spiroketal-based diphosphine ligand, and the products were obtained with satisfactory enantioselectivity. In addition, the indoloquinazoline compounds were also tested for in vitro antibacterial activity, and some of the compounds showed promising bioactivity against Staphylococcus. Investigation into the interesting transformations of tryptanthrins and related medicinal applications is currently underway in our laboratory, and the results will be reported in due course.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
Financial support from the NSFC (21871031 and 22071011), the Science & Technology Department of Sichuan Province (2021YJ0404), the Longquan Talents Program, and the start-up funding from Chengdu University is gratefully acknowledged.Notes and references
- For selected reviews on transition-metal-catalysed annulations, see: (a) I. Nakamura and Y. Yamamoto, Transition-Metal-Catalyzed Reactions in Heterocyclic Synthesis, Chem. Rev., 2004, 104, 2127–2198 CrossRef CAS PubMed; (b) H.-W. Frühauf, Metal-Assisted Cycloaddition Reactions in Organotransition Metal Chemistry, Chem. Rev., 1997, 97, 523–596 CrossRef PubMed; (c) M. Lautens, W. Klute and W. Tam, Transition Metal-Mediated Cycloaddition Reactions, Chem. Rev., 1996, 96, 49–92 CrossRef CAS PubMed; (d) L. Song and E. V. V. der Eycken, Transition Metal-Catalyzed Intermolecular Cascade C–H Activation/Annulation Processes for the Synthesis of Polycycles, Chem. – Eur. J., 2021, 27, 121–144 CrossRef CAS PubMed; (e) S. Das and A. Dutta, Recent advances in transition-metal-catalyzed annulations for the construction of a 1-indanone core, New J. Chem., 2021, 45, 4545–4568 RSC.
- For selected reviews on zwitterionic π-allyl palladium mediated annulations, see: (a) L.-N. Guo, X.-H. Duan and Y.-M. Liang, Palladium-Catalyzed Cyclization of Propargylic Compounds, Acc. Chem. Res., 2011, 44, 111–122 CrossRef CAS PubMed; (b) H. Ohno, Synthesis and Applications of Vinylaziridines and Ethynylaziridines, Chem. Rev., 2014, 114, 7784–7814 CrossRef CAS PubMed; (c) N. De and E. J. Yoo, Recent Advances in the Catalytic Cycloaddition of 1,n-Dipoles, ACS Catal., 2018, 8, 48–58 CrossRef CAS; (d) J. He, J. Ling and P. Chiu, Vinyl Epoxides in Organic Synthesis, Chem. Rev., 2014, 114, 8037–8128 CrossRef CAS PubMed; (e) J. Wang, S. A. Blaszczyk, X. Li and W. Tang, Transition Metal-Catalyzed Selective Carbon–Carbon Bond Cleavage of Vinylcyclopropanes in Cycloaddition Reactions, Chem. Rev., 2021, 121, 110–139 CrossRef CAS PubMed; (f) B. Niu, Y. Wei and M. Shi, Recent advances in annulation reactions based on zwitterionic π-allyl palladium and propargyl palladium complexes, Org. Chem. Front., 2021, 8, 3475–3501 RSC.
- For selected reviews on VECs, see: (a) A. Khan and Y. J. Zhang, Palladium-Catalyzed Asymmetric Decarboxylative Cycloaddition of Vinylethylene Carbonates with Electrophiles: Construction of Quaternary Stereocenters, Synlett, 2015, 26, 853–860 CrossRef CAS; (b) W. Guo, J. E. Gómez, À. Cristòfol, J. Xie and A. W. Kleij, Catalytic Transformations of Functionalized Cyclic Organic Carbonates, Angew. Chem., Int. Ed., 2018, 57, 13735–13747 CrossRef CAS PubMed; (c) J. E. Gómez and A. W. Kleij, Chapter Three-Catalytic nonreductive valorization of carbon dioxide into fine chemicals, Adv. Organomet. Chem., 2019, 71, 175–226 CrossRef; (d) L. Zuo, T. Liu, X. Chang and W. Guo, An Update of Transition Metal-Catalyzed Decarboxylative Transformations of Cyclic Carbonates and Carbamates, Molecules, 2019, 24, 3930 CrossRef CAS PubMed; (e) Q.-Z. Li, Y. Liu, M.-Z. Li, X. Zhang, T. Qi and J.-L. Li, Palladium-catalysed decarboxylative annulations of vinylethylene carbonates leading to diversely functionalised heterocycles, Org. Biomol. Chem., 2020, 18, 3638–3648 RSC.
- For selected examples of the transformations of VECs, see: (a) Z.-Q. Rong, L.-C. Yang, S. Liu, Z. Yu, Y.-N. Wang, Z. Y. Tan, R.-Z. Huang, Y. Lan and Y. Zhao, Nine-Membered Benzofuran-Fused Heterocycles: Enantioselective Synthesis by Pd-Catalysis and Rearrangement via Transannular Bond Formation, J. Am. Chem. Soc., 2017, 139, 15304–15307 CrossRef CAS PubMed; (b) S. Singha, T. Patra, C. G. Daniliuc and F. Glorius, Highly Enantioselective [5+2] Annulations through Cooperative N-Heterocyclic Carbene (NHC) Organocatalysis and Palladium Catalysis, J. Am. Chem. Soc., 2018, 140, 3551–3354 CrossRef CAS PubMed; (c) P. Das, S. Gondo, P. Nagender, H. Uno, E. Tokunaga and N. Shibata, Access to benzo-fused nine-membered heterocyclic alkenes with a trifluoromethyl carbinol moiety via a double decarboxylative formal ring-expansion process under palladium catalysis, Chem. Sci., 2018, 9, 3276–3281 RSC; (d) Y. Wei, S. Liu, M.-M. Li, Y. Li, Y. Lan, L.-Q. Lu and W.-J. Xiao, Enantioselective Trapping of Pd-Containing 1,5-Dipoles by Photogenerated Ketenes: Access to 7-Membered Lactones Bearing Chiral Quaternary Stereocenters, J. Am. Chem. Soc., 2019, 141, 133–137 CrossRef CAS PubMed; (e) X. Zhang, X. Li, J.-L. Li, Q.-W. Wang, W.-L. Zhou, Y.-Q. Liu, Z.-Q. Jia, F. Peng and B. Han, Regiodivergent construction of medium-sized heterocycles from vinylethylene carbonates and allylidenemalononitriles, Chem. Sci., 2020, 11, 2888–2894 RSC; (f) Q.-S. Dai, Y.-M. Tao, X. Zhang, H.-J. Leng, H. Huang, P. Xiang, Q.-Z. Li, Q.-W. Wang and J.-L. Li, Palladium-catalysed cyclisation of vinylethylene carbonates and anhydrides: a new approach to diverse medium-sized bislactones, Chem. Commun., 2020, 56, 12439–12442 RSC; (g) B. M. Trost and A. Aponick, Palladium-Catalyzed Asymmetric Allylic Alkylation of meso- and dl-1,2-Divinylethylene Carbonate, J. Am. Chem. Soc., 2006, 128, 3931–3933 CrossRef CAS PubMed; (h) Y. J. Zhang, J. H. Yang, S. H. Kim and M. J. Krische, anti-Diastereo- and Enantioselective Carbonyl (Hydroxymethyl) allylation from the Alcohol or Aldehyde Oxidation Level: Allyl Carbonates as Allylmetal Surrogates, J. Am. Chem. Soc., 2010, 132, 4562–4563 CrossRef CAS PubMed; (i) A. Cai, W. Guo, L. Martínez-Rodríguez and A. W. Kleij, Palladium-Catalyzed Regio- and Enantioselective Synthesis of Allylic Amines Featuring Tetrasubstituted Tertiary Carbons, J. Am. Chem. Soc., 2016, 138, 14194–14197 CrossRef CAS PubMed; (j) A. Khan, S. Khan, I. Khan, C. Zhao, Y. Mao, Y. Chen and Y. J. Zhang, Enantioselective Construction of Tertiary C–O Bond via Allylic Substitution of Vinylethylene Carbonates with Water and Alcohols, J. Am. Chem. Soc., 2017, 139, 10733–10741 CrossRef CAS PubMed; (k) W. Guo, L. Martínez-Rodríguez, R. Kuniyil, E. Martin, E. C. Escudero-Adán, F. Maseras and A. W. Kleij, Stereoselective and Versatile Preparation of Tri- and Tetrasubstituted Allylic Amine Scaffolds under Mild Conditions, J. Am. Chem. Soc., 2016, 138, 11970–11978 CrossRef CAS PubMed; (l) W. Guo, L. Martínez-Rodríguez, E. Martin, E. C. Escudero-Adán and A. W. Kleij, Highly Efficient Catalytic Formation of (Z)-1,4-But-2-ene Diols Using Water as a Nucleophile, Angew. Chem., Int. Ed., 2016, 55, 11037–11040 CrossRef CAS PubMed; (m) W. Guo, R. Kuniyil, J. E. Gómez, F. Maseras and A. W. Kleij, A Domino Process toward Functionally Dense Quaternary Carbons through Pd-Catalyzed Decarboxylative C(sp3)–C(sp3) Bond Formation, J. Am. Chem. Soc., 2018, 140, 3981–3987 CrossRef CAS PubMed; (n) H. Wang, S. Qiu, S. Wang and H. Zhai, Pd-Catalyzed Umpolung of π–Allylpalladium Intermediates: Assembly of All-Carbon α-Vinyl Quaternary Aldehydes through C(sp3)–C(sp3) Coupling, ACS Catal., 2018, 8, 11960–11965 CrossRef CAS; (o) R. Zeng, J.-L. Li, X. Zhang, Y.-Q. Liu, Z.-Q. Jia, H.-J. Leng, Q.-W. Huang, Y. Liu and Q.-Z. Li, Diastereoselective Construction of 6,8-Dioxabicyclo[3.2.1]octane Frameworks from Vinylethylene Carbonates via Palladium-Organo Relay Catalysis, ACS Catal., 2019, 9, 8256–8262 CrossRef CAS; (p) T. Qi, S. Fu, X. Zhang, T.-H. Liu, Q.-Z. Li, C. Gou and J.-L. Li, Theoretical insight into the origins of chemo- and diastereo-selectivity in the palladium-catalysed (3+2) cyclisation of 5-alkenyl thiazolones, Org. Chem. Front., 2021, 8, 6203–6214 RSC.
- A. Khan, R. Zheng, Y. Kan, J. Ye, J. Xing and Y. J. Zhang, Palladium-Catalyzed Decarboxylative Cycloaddition of Vinylethylene Carbonates with Formaldehyde: Enantioselective Construction of Tertiary Vinylglycols, Angew. Chem., Int. Ed., 2014, 53, 6439–6442 CrossRef CAS PubMed.
- (a) L. Yang, A. Khan, R. Zheng, L. Y. Jin and Y. J. Zhang, Pd-Catalyzed Asymmetric Decarboxylative Cycloaddition of Vinylethylene Carbonates with Imines, Org. Lett., 2015, 17, 6230–6233 CrossRef CAS PubMed; (b) Y. Liu, Q.-W. Huang, Q.-Z. Li, H.-J. Leng, Q.-S. Dai, R. Zeng, Y.-Q. Liu, X. Zhang, B. Han and J.-L. Li, Highly Chemo- and Diastereoselective Construction of Quaternary Stereocenters through Palladium-Catalyzed [3+2] Cyclization of 5-Alkenyl Thiazolones, Org. Lett., 2019, 21, 7478–7483 CrossRef CAS PubMed; (c) J.-U. Park, H.-I. Ahn, H.-J. Cho, Z. Xuan and J. H. Kim, Asymmetric Synthesis of N-Fused 1,3-Oxazolidines via Pd-Catalyzed Decarboxylative (3+2) Cycloaddition, Adv. Synth. Catal., 2020, 362, 1836–1840 CrossRef CAS; (d) X. Gao, D. Zhu, F. Jiang, J. Liao, W. Wang, Y. Wu, L. Zheng and H. Guo, Palladium-catalyzed stereoselective (3+2) cycloaddition of vinylethylene carbonates with cyclic N-sulfonyl ketimines, Org. Biomol. Chem., 2021, 19, 4877–4881 RSC.
- A. Khan, J. Xing, J. Zhao, Y. Kan, W. Zhang and Y. J. Zhang, Palladium-Catalyzed Enantioselective Decarboxylative Cycloaddition of Vinylethylene Carbonates with Isocyanates, Chem. – Eur. J., 2015, 21, 120–124 CrossRef CAS PubMed.
- W. Xiong, S. Zhang, H. Li, Z. Zhang and T. Xu, Pd-Catalyzed Decarboxylative Cycloaddition of Vinylethylene Carbonates with Isothiocyanates, J. Org. Chem., 2020, 85, 8773–8779 CrossRef CAS PubMed.
- (a) A. Khan, L. Yang, J. Xu, L. Y. Jin and Y. J. Zhang, Palladium-Catalyzed Asymmetric Decarboxylative Cycloaddition of Vinylethylene Carbonates with Michael Acceptors: Construction of Vicinal Quaternary Stereocenters, Angew. Chem., Int. Ed., 2014, 53, 11257–11260 CrossRef CAS PubMed; (b) L.-C. Yang, Z.-Q. Rong, Y.-N. Wang, Z. Y. Tan, M. Wang and Y. Zhao, Construction of Nine-Membered Heterocycles through Palladium-Catalyzed Formal [5+4] Cycloaddition, Angew. Chem., Int. Ed., 2017, 56, 2927–2931 CrossRef CAS PubMed; (c) K. Liu, I. Khan, J. Cheng, Y. J. Hsueh and Y. J. Zhang, Asymmetric Decarboxylative Cycloaddition of Vinylethylene Carbonates with β-Nitroolefins by Cooperative Catalysis of Palladium Complex and Squaramide, ACS Catal., 2018, 8, 11600–11604 CrossRef CAS; (d) L.-C. Yang, Z. Y. Tan, Z.-Q. Rong, R. Liu, Y.-N. Wang and Y. Zhao, Palladium-Titanium Relay Catalysis Enables Switch from Alkoxide-π-Allyl to Dienolate Reactivity for Spiro-Heterocycle Synthesis, Angew. Chem., Int. Ed., 2018, 57, 7860–7864 CrossRef CAS PubMed; (e) I. Khan, C. Zhao and Y. J. Zhang, Pd-Catalyzed asymmetric decarboxylative cycloaddition of vinylethylene carbonates with 3-cyanochromones, Chem. Commun., 2018, 54, 4708–4711 RSC; (f) X. Gao, M. Xia, C. Yuan, L. Zhou, W. Sun, C. Li, B. Wu, D. Zhu, C. Zhang, B. Zheng, D. Wang and H. Guo, Enantioselective Synthesis of Chiral Medium-Sized Cyclic Compounds via Tandem Cycloaddition/Cope Rearrangement Strategy, ACS Catal., 2019, 9, 1645–1654 CrossRef CAS; (g) J. Wang, L. Zhao, Q. Rong, C. Lv, Y. Lu, X. Pan, L. Zhao and L. Hu, Asymmetric Synthesis of 3,3′-Tetrahydrofuryl Spirooxindoles via Palladium-Catalyzed [3+2] Cycloadditions of Methyleneindolinones with Vinylethylene Carbonates, Org. Lett., 2020, 22, 5833–5838 CrossRef CAS PubMed; (h) C. Zhao, I. Khan and Y. J. Zhang, Enantioselective Total Synthesis of Furofuran Lignans via Pd-Catalyzed Asymmetric Allylic Cycloadditon of Vinylethylene Carbonates with 2-Nitroacrylates, Chem. Commun., 2020, 56, 12431–12434 RSC; (i) H. Zhang, X. Gao, F. Jiang, W. Shi, W. Wang, Y. Wu, C. Zhang, X. Shi and H. Guo, Palladium-Catalyzed Asymmetric [3+2] Cycloaddition of Vinylethylene Carbonates with 2-Arylidene-1,3-Indandiones: Synthesis of Tetrahydrofuran-Fused Spirocyclic 1,3-Indandiones, Eur. J. Org. Chem., 2020, 4801–4804 CrossRef CAS; (j) H.-P. Lv, X.-P. Yang, B.-L. Wang, H.-D. Yang, X.-W. Wang and Z. Wang, Chiral Bidentate Phosphoramidite-Pd Catalyzed Asymmetric Decarboxylative Dipolar Cycloaddition for Multistereogenic Tetrahydrofurans with Cyclic N-Sulfonyl Ketimine Moieties, Org. Lett., 2021, 23, 4715–4720 CrossRef CAS PubMed; (k) Q.-W. Huang, T. Qi, Y. Liu, X. Zhang, Q.-Z. Li, C. Gou, Y.-M. Tao, H.-J. Leng and J.-L. Li, Lewis Acid/Brønsted Base-Assisted Palladium Catalysis: Stereoselective Construction of Skeletally Diverse SpiroKetolactams from Vinylethylene Carbonates, ACS Catal., 2021, 11, 10148–10158 CrossRef CAS; (l) C. Zhao, B. H. Shah, H. Li, X. Wu and Y. J. Zhang, Palladium-catalyzed allylic cycloaddition of vinylethylene carbonates with 3-nitrochromone, Asian J. Org. Chem., 2021, 10, 545–548 CrossRef CAS; (m) B. Yan, L. Zuo, X. Chang, T. Liu, M. Cui, Y. Liu, H. Sun, W. Chen and W. Guo, Kinetically Controllable Pd-Catalyzed Decarboxylation Enabled [5+2] and [3+2] Cycloaddition toward Carbocycles Featuring Quaternary Carbons, Org. Lett., 2021, 23, 351–357 CrossRef CAS PubMed; (n) Y. Zheng, T. Qin and W. Zi, Enantioselective Inverse Electron Demand (3+2) Cycloaddition of Palladium-Oxyallyl Enabled by a Hydrogen-Bond-Donating Ligand, J. Am. Chem. Soc., 2021, 143, 1038–1045 CrossRef CAS PubMed.
- For selected reviews, see: (a) S. B. Mhaske and N. P. Argade, The chemistry of recently isolated naturally occurring quinazolinone alkaloids, Tetrahedron, 2006, 62, 9787–9826 CrossRef CAS; (b) U. A. Kshirsagar, Recent developments in the chemistry of quinazolinone alkaloids, Org. Biomol. Chem., 2015, 13, 9336–9352 RSC.
- (a) C.-W. Jao, W.-C. Lin, Y.-T. Wu and P.-L. Wu, Isolation, Structure Elucidation, and Synthesis of Cytotoxic Tryptanthrin Analogues from Phaius mishmensis, J. Nat. Prod., 2008, 71, 1275–1279 CrossRef CAS PubMed; (b) A. M. Tucker and P. Grundt, The chemistry of tryptanthrin and its derivatives, ARKIVOC, 2012, 2012, 546–569 Search PubMed; (c) R. Kaur, S. K. Manjal, R. K. Rawal and K. Kumar, Recent synthetic and medicinal perspectives of tryptanthrin, Bioorg. Med. Chem., 2017, 25, 4533–4552 CrossRef CAS PubMed; (d) M. Chen, L. Gan, S. Lin, X. Wang, L. Li, Y. Li, C. Zhu, Y. Wang, B. Jiang, J. Jiang, Y. Yang and J. Shi, Alkaloids from the Root of Isatis indigotica, J. Nat. Prod., 2012, 75, 1167–1176 CrossRef CAS PubMed; (e) C.-F. Chang, Y.-L. Hsu, C.-Y. Lee, C.-H. Wu, Y.-C. Wu and T.-H. Chuang, Isolation and Cytotoxicity Evaluation of the Chemical Constituents from Cephalantheropsis gracilis, Int. J. Mol. Sci., 2015, 16, 3980–3989 CrossRef CAS PubMed.
- For selected examples, see: (a) B. Rai, R. D. Shukla and A. Kumar, Zinc oxide-NP catalyzed direct indolation of in situ generated bioactive tryptanthrin, Green Chem., 2018, 20, 822–826 RSC; (b) A. Kamal, B. V. S. Reddy, B. Sridevi, A. Ravikumar, A. Venkateswarlu, G. Sravanthi, J. P. Sridevi, P. Yogeeswari and D. Sriram, Synthesis and biological evaluation of phaitanthrin congeners as anti-mycobacterial agents, Bioorg. Med. Chem. Lett., 2015, 25, 3867–3872 CrossRef CAS PubMed; (c) M. Beyrati and A. Hasaninejad, One-pot, three-component synthesis of spiroindoloquinazoline derivatives under solvent-free conditions using ammonium acetate as a dual activating catalyst, Tetrahedron Lett., 2017, 58, 1947–1951 CrossRef CAS; (d) W.-L. Chen, C.-X. Liu, H.-P. Xiao and J. Jiang, Ni(acac)2-Catalyzed Addition Reactions of Aryl- and Alkylboronic Acids to Tryptanthrins, Synlett, 2016, 27, 1989–1992 CrossRef CAS; (e) X. Zhang, Q.-Z. Li, Q.-F. Huang, X.-L. He, E.-D. Ma, Z.-B. Wang, X. Feng, J.-L. Li and Q.-W. Wang, Wittig /Michael Cascade Reaction for Efficient Synthesis of Indoloquinazoline Derivatives Bearing a Phosphorous Ylide Moiety, Chin. J. Synth. Chem., 2021, 29, 91–99 Search PubMed.
- (a) B. Han, X.-H. He, Y.-Q. Liu, G. He, C. Peng and J.-L. Li, Asymmetric organocatalysis: an enabling technology for medicinal chemistry, Chem. Soc. Rev., 2021, 50, 1522–1586 RSC; (b) Q. Li, L. Zhou, X.-D. Shen, K.-C. Yang, X. Zhang, Q.-S. Dai, H.-J. Leng, Q.-Z. Li and J.-L. Li, Stereoselective Construction of Halogenated Quaternary Carbon Centers by Brønsted Base Catalyzed [4+2] Cycloaddition of α-Haloaldehydes, Angew. Chem., Int. Ed., 2018, 57, 1913–1917 CrossRef CAS PubMed; (c) Q.-Z. Li, X. Zhang, R. Zeng, Q.-S. Dai, Y. Liu, X.-D. Shen, H.-J. Leng, K.-C. Yang and J.-L. Li, Direct Sulfide-Catalyzed Enantioselective Cyclopropanations of Electron-Deficient Dienes and Bromides, Org. Lett., 2018, 20, 3700–3704 CrossRef CAS PubMed; (d) Q.-Z. Li, X. Zhang, K. Xie, Q.-S. Dai, R. Zeng, Y.-Q. Liu, Z.-Q. Jia, X. Feng and J.-L. Li, Diastereodivergent synthesis of cyclopropanes via on-water [2+1] annulations of diazo compounds with electron-deficient alkenes, Green Chem., 2019, 21, 2375–2379 RSC; (e) X. Zhang, Q.-F. Huang, W.-L. Zou, Q.-Z. Li, X. Feng, Z.-Q. Jia, Y. Liu, J.-L. Li and Q.-W. Wang, Synthetic approach to skeletally diverse nitrogen heterocycles from dicyano-2-methylenebut-3-enoates, Org. Chem. Front., 2019, 6, 3321–3326 RSC; (f) J.-L. Li, Y.-Q. Liu, W.-L. Zou, R. Zeng, X. Zhang, Y. Liu, B. Han, Y. He, H.-J. Leng and Q.-Z. Li, Radical Acylfluoroalkylation of Olefins through N-Heterocyclic Carbene Organocatalysis, Angew. Chem., Int. Ed., 2020, 59, 1863–1870 CrossRef CAS PubMed.
- For detailed optimisation screening for asymmetric synthesis, see the ESI.†.
- CCDC 2090813 (3r′) contains the supplementary crystallographic data for this paper, and for more details, see the ESI.†.
- The bioactivity was assessed by using the broth dilution method; for relative references, see: (a) L. Ouyang, Y. Huang, Y. Zhao, G. He, Y. Xie, J. Liu, J. He, B. Liu and Y. Wei, Preparation, antibacterial evaluation and preliminary structure-activity relationship (SAR) study of benzothiazol- and benzoxazol-2-amine derivatives, Bioorg. Med. Chem. Lett., 2012, 22, 3044–3049 CrossRef CAS PubMed; (b) Farhanullah, T. Kang, E.-J. Yoon, E.-C. Choi, S. Kim and J. Lee, 2-[2-Substituted-3-(3,4-dichlorobenzylamino)propylamino]-1H-quinolin-4-ones as Staphylococcus aureus methionyl-tRNA synthetase inhibitors, Eur. J. Med. Chem., 2009, 44, 239–250 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data for new compounds. CCDC 2090813. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1qo01543e |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2022 |