Discovery of gargantulides B and C, new 52-membered macrolactones from Amycolatopsis sp. Complete absolute stereochemistry of the gargantulide family†
Abstract
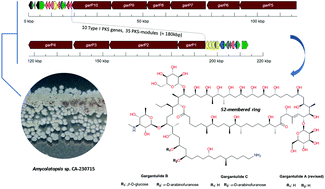
Gargantulides B and C, two new and highly complex 52-membered glycosylated macrolactones, were isolated from Amycolatopsis sp. strain CA-230715 during an antibacterial screening campaign. The structures of these giant macrolides were elucidated by 2D NMR spectroscopy and shown to be related to gargantulide A, although containing additional β-glucopyranose and/or α-arabinofuranose monosaccharides separately attached to their backbones. Genome sequencing allowed the identification of a strikingly large 216 kbp biosynthetic gene cluster, among the largest type I PKS clusters described so far, and the proposal of a previously unreported biosynthetic pathway for gargantulides A–C. The absolute configurations of gargantulides B and C were assigned based on a combination of NMR and bioinformatics analysis of ketoreductase and enoylreductase domains within the multimodular type I PKS. In addition, the absolute stereochemistry of gargantulide A has now been revised and completed. Gargantulides B and C display potent antibacterial activity against a set of drug-resistant Gram-positive bacteria and moderate activity against the clinically relevant Gram-negative pathogen Acinetobacter baumannii.


 Please wait while we load your content...
Please wait while we load your content...