Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Electro-organic synthesis: an environmentally benign alternative for heterocycle synthesis
Suman
Devi
a,
Jyoti
a,
Kiran
b,
Deepak
Wadhwa
*a and
Jayant
Sindhu
*b
aDepartment of Chemistry, Chaudhary Bansi Lal university, Bhiwani-127021, India. E-mail: deepakchem08@cblu.ac.in
bDepartment of Chemistry, COBS&H, CCSHAU, Hisar-125004, India. E-mail: jayantchem@gmail.com
First published on 19th August 2022
Abstract
Correction for ‘Electro-organic synthesis: an environmentally benign alternative for heterocycle synthesis’ by Suman Devi, et al., Org. Biomol. Chem., 2022, 20, 5163–5229.
The authors regret that there were a number of errors throughout their review. These are detailed below.
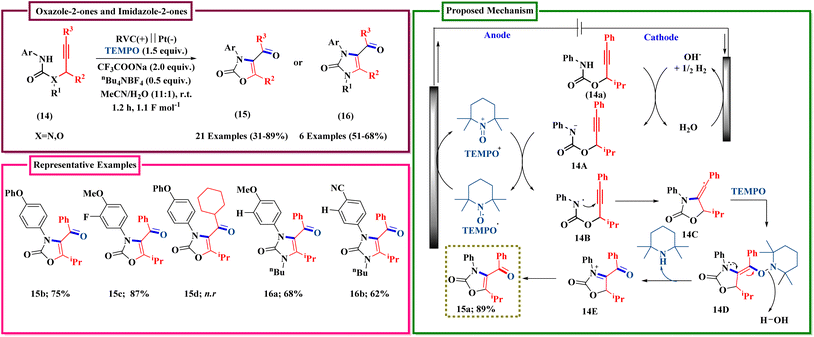
The structure of 15a was incorrect in Scheme 6. The correct scheme is shown below.
In the description of the work shown in Scheme 9, the sentence ‘The reaction was carried out in a continuous flow system furnished with a Pt rod as an anode and graphite as cathode.’, should be corrected to ‘The reaction was carried out in a continuous flow system furnished with a Pt rod as the cathode and graphite as the anode.’.
The notation of anode and cathode was incorrect in Scheme 13. Originally C and Ni were shown as cathode and anode, but in the reaction, they are working as anode and cathode, respectively. The correct scheme is shown below.
There were errors in structures 41A and 41B in Scheme 15. The correct scheme is shown below.
A double bond has been erroneously included in the aziridine ring in all the structures in Scheme 16. The correct scheme is shown below.
The notation of the anode and cathode was incorrect in Scheme 20. Originally RVC and Pt were shown as cathode and anode, but in the reaction, they are working as the anode and cathode, respectively. The correct scheme is shown below.
In Scheme 26 a double bond in the triazole rings was missing. The correct scheme is shown below.
 | ||
| Scheme 6 Synthesis of highly hindered triazolopyridinone by enantiospecific electrochemical rearrangement. | ||
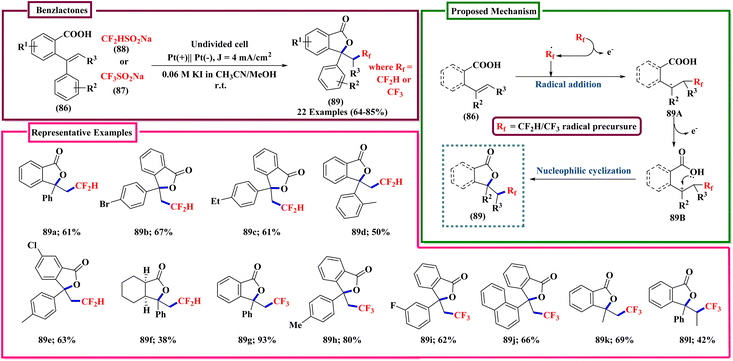
In Scheme 31 CF3SO2Na was incorrectly given as CF3SO3Na. The correct scheme is shown below.
In the description of the work shown in Scheme 32, the sentence ‘The reaction was performed in an undivided cell composed of Pt as cathode and carbon as an anode.’, should be corrected to ‘The reaction was performed in an undivided cell composed of Pt as the anode and carbon as the cathode.’. In addition, there were errors in the structures of intermediates 90C and 90D in Scheme 32. The correct scheme is shown below.
In Scheme 34, the intermediates 27 and 27A were incorrectly placed. The correct scheme is shown below.
There was an error in the structure of intermediate 102B in Scheme 37. The correct scheme is shown below.
The notation of anode and cathode was incorrect in Scheme 43. Originally C and Pt were shown as the cathode and anode, but in the reaction, they are working as the anode and cathode, respectively. The correct scheme is shown below.
 | ||
| Scheme 11 Synthesis of di-selenylated indolizine using 2-methylpyridine, diphenyl-diselenides and 2-bromoacetophenone. | ||
In the description of the work shown in Scheme 44, the sentence ‘The reaction was carried out in an undivided cell equipped with a Pt plate as anode and a carbon plate as cathode.’, should be corrected to ‘The reaction was carried out in an undivided cell equipped with a Pt plate as the cathode and a carbon plate as the anode.’. In addition, in Scheme 44 CF3SO2Na was incorrectly given as CF3SO3Na. The correct scheme is shown below.
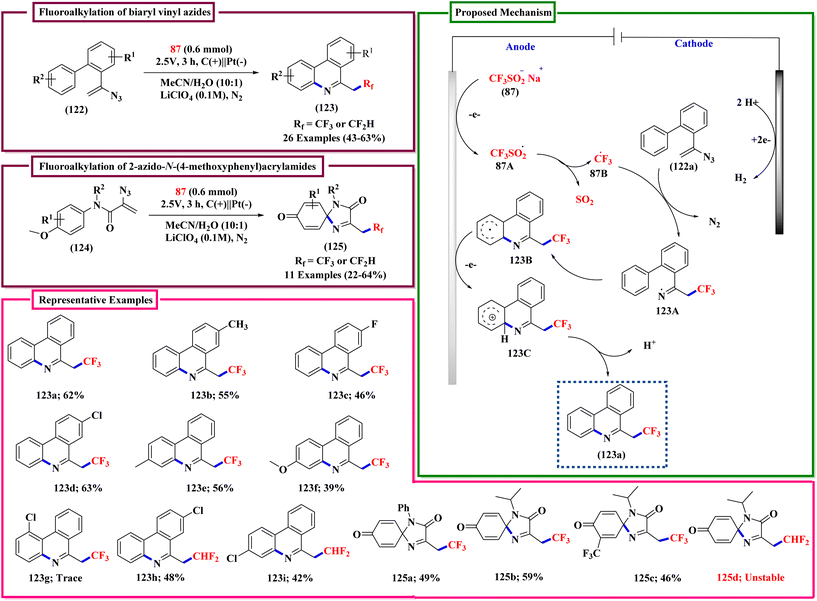 | ||
| Scheme 12 Synthesis of 6-trifluoroethyl phenanthridines and quinoline derivatives via fluorination and cyclization of vinyl azides. | ||
There were some charge related errors in Scheme 45. The correct scheme is shown below.
 | ||
| Scheme 13 Synthesis of CF3 containing imidazolines/oxazolidines using N-tosyl allylamine and acetonitrile. | ||
The structure of compound 162 was incorrect in Scheme 56. The correct scheme is shown below.
In the description of the work shown in Scheme 61, the sentence ‘The reaction was attempted in an undivided cell fitted with platinum as an cathode and graphite as an anode.’, should be corrected to ‘The reaction was attempted in an undivided cell fitted with platinum as the cathode and graphite as the cathode.’.
In Schemes 64 and 65 CF3SO2Na was incorrectly given as CF3SO3Na. The correct schemes are shown below.
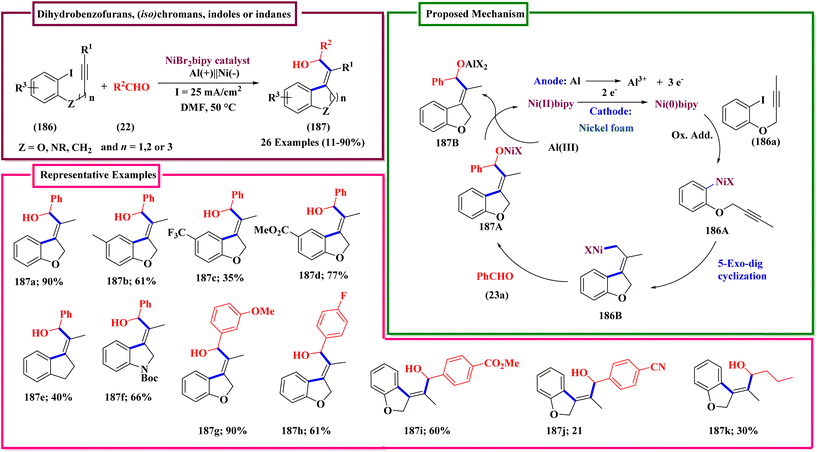
In the description of the work shown in Scheme 66, the sentence ‘The reaction was attempted in an undivided cell fitted with aluminium plate as cathode and nickel as anode.’, should be corrected to ‘The reaction was attempted in an undivided cell fitted with an aluminium plate as the anode and nickel foam as the cathode.’. The sentence ‘The reaction did not yield the desired product when Zn was used as cathode.’, should be corrected to ‘The reaction did not yield the desired product when Zn was used as the anode.’. In addition, the structure of intermediate 186a in Scheme 66 was incorrect. The correct scheme is shown below.
In the first sentence describing the work shown in Scheme 67, the work was attributed to the incorrect author and the incorrect reference was cited. The sentence ‘S. Ben Salah et al.142 reported the iron-catalysed oxidant-free electrochemical synthesis of quinazolinone (189) and benzimidazolone (191) via in situ formation of isocyanates. In order to find the efficient reaction conditions, the initial pre-screening of the reaction was attempted using 1,1′-(1,2-phenylene)bis(3-(benzyloxy)urea (188a) as model substrate’. Should be corrected to ‘D. Saha et al.3 reported the iron-catalysed oxidant-free electrochemical synthesis of quinazolinone (189) and benzimidazolone (191) via in situ formation of isocyanates. In order to find the efficient reaction conditions, the initial pre-screening of the reaction was attempted using 1,1′-(1,2-phenylene)bis(3-(benzyloxy)urea (188a) as the model substrate’, where the correct reference is ref. 3 as shown in the reference section below.
The notation of anode and cathode was incorrect Scheme 68. Originally RVC and Pt were shown as the cathode and anode, but in the reaction, they are working as the anode and cathode, respectively. The correct scheme is shown below.
 | ||
| Scheme 18 Synthesis of polycyclic isoquinolinones via dehydrogenative cycloaddition of amides and alkynes. | ||
In Scheme 69 a double bond was missing in the imidazole ring and also in the structures Ru-IV and Ru-V. The correct scheme is shown below.
References 112, 138, 142 and 146 were incorrect. The correct references are shown below as ref. 1–4 respectively.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
References
- C. Huang and H. C. Xu, Sci. China: Chem., 2019, 62, 1501–1503 CrossRef CAS.
- C. Huang, X. Y. Qian and H. C. Xu, Angew. Chem., Int. Ed., 2019, 58, 6650–6653 CrossRef CAS.
- D. Saha, I. M. Taily, S. Naik and P. Banerjee, Chem. Commun., 2021, 57, 631–634 RSC.
- X. Yi and X. Hu, Angew. Chem., Int. Ed., 2019, 58, 4700–4704 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |