One-pot synthesis of α-sulfoximinophosphonate via Kabachnik–Fields reaction†
Abstract
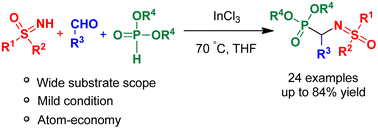
Herein, we disclose a novel approach for the synthesis of hitherto unknown α-sulfoximinophosphonate via the Kabachnik–Fields reaction of aldehyde, dialkylphosphite and sulfoximine in the presence of InCl3 in THF at 70 °C. α-Sulfoximinophosphonate is synthesized in good yields and its synthetic utilities are proved by functionalizing bromine through the Pd-catalyzed Suzuki–Miyaura cross-coupling reaction and reduction of a nitro group through the Béchamp reduction.


 Please wait while we load your content...
Please wait while we load your content...