Peptide macrocyclisation via late-stage reductive amination†
Abstract
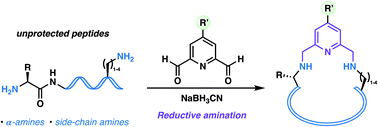
A two-component reductive amination approach to the synthesis of peptide macrocycles is reported which leverages the inherent reactivity of proteinogenic amine nucleophiles. Unprotected peptides bearing α-amine and side chain amine motifs undergo two-fold reductive amination reactions with 2,6-pyridinedialdehyde linkers in aqueous media to afford macrocyclic peptide products with backbone embedded pyridine motifs. Dialdehyde staples bearing valuable azide and alkyne handles also enable the post-cyclisation modification of peptides using copper-catalysed azide–alkyne cycloaddition (CuAAC) chemistry.
- This article is part of the themed collection: New Talent


 Please wait while we load your content...
Please wait while we load your content...