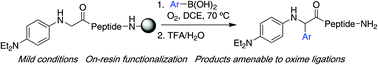
On-resin Cα-functionalization of N-arylglycinyl peptides with boronic acids†
Abstract
A late-stage α-C–H functionalization reaction of resin-bound, electron-rich N-aryl peptides with boronic acid nucleophiles under mild conditions is reported. We explore the impact of the N-arylglycinyl peptide structure on reactivity, and present a scope of the optimized reaction where both the peptide sequence and nature of boronic acid derivatives are varied.
- This article is part of the themed collection: New Talent


 Please wait while we load your content...
Please wait while we load your content...