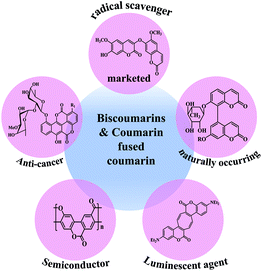
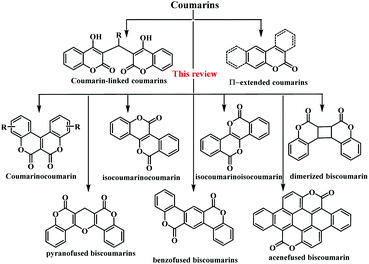
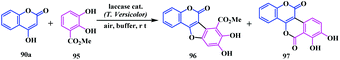Overview of coumarin-fused-coumarins: synthesis, photophysical properties and their applications
Manashi
Sarmah
a,
Kangkana
Chutia
a,
Dhiraj
Dutta
 ab and
Pranjal
Gogoi
ab and
Pranjal
Gogoi
 *ab
*ab
aApplied Organic Chemistry Group, Chemical Science and Technology Division, CSIR-North East Institute of Science and Technology, Jorhat 785006, Assam, India. E-mail: gogoipranj@yahoo.co.uk; gogoipranj@gmail.com
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad, U.P. 201002, India
First published on 16th November 2021
Abstract
Coumarin-fused-coumarins have attracted significant attention in the scientific world owing to their boundless applications in interdisciplinary areas. Various synthetic pathways have been developed to construct novel coumarin-fused-coumarin analogues by the fusion of modern methodologies with a classical Pechmann reaction or Knoevenagel condensation. Owing to their extended molecular framework, they possess interesting photophysical properties depending on the fused coumarin ring systems. This review highlights previously published reports on the synthetic strategies for structurally diverse coumarin-fused-coumarins. Furthermore, the scope of the synthesized biscoumarin-fused entities is described by highlighting their photophysical properties and applications.
1. Introduction
Heterocyclic compounds are considered as privileged skeletons owing to their fascinating and inevitable biological/natural relevance. From the realm of heterocyclic scaffolds, discrete structural units bearing a coumarin core have recently been found to be an interesting area of research. Coumarins have been widely explored as their molecular architecture ensures their prominent role in drug development owing to their plethora of pharmacological properties, such as their anticoagulant, antimicrobial, anti-inflammatory, antidiabetic, anticancer, anticonvulsant and antiproliferative activities.1 Despite their biological significance, they are also known for their impressive luminescent properties owing to their intrinsic charge transfer properties. They are generally known as optical brighteners and have a wide range of applications in the field of optoelectronics, dye-sensitized solar cells, cellular imaging and as fluorescent probes.2Recently, π-extended coumarins or coumarin-fused-heterocycles have gained considerable attention. The introduction of a heterocycle to a coumarin unit shows interesting photophysical and pharmacokinetic properties (Fig. 1).3 In this regard, coumarin-fused compounds and their conjugates have prevailed in the synthetic world with many applications. Different heterocycles conjugated with biscoumarins, such as biscoumarin-iminothiazole, dihydropyran and pyrazole or thiazolyl-pyrazole, have been synthesised4 and some of these complex conjugates show remarkable biological activities, such as the inhibition of alkaline phosphate, anti-cancer, anti-leishmanial, α-glucosidase inhibition, anti-oxidant, and anti-microbial properties.
Recently, researchers have particularly focused on the synthesis of novel coumarin-fused entities in which two or more coumarin units are embedded within the same molecular framework.5 In this regard, several synthetic methodologies have been developed in which these moieties are preferentially endorsed with tuneable pharmaceutical or photonic properties.
Following the synthetic methodology developed by Pechmann in 1884 for the construction of benzo-fused coumarin, various coumarin-fused/coumarin-linked analogues have been developed.6 Owing to the growing interest in this field, various research articles, including reviews on the synthesis and application of π-extended coumarins and biscoumarins, have been published. In this regard, Gryko et al. documented a collective survey of the π-extended coumarins highlighting the synthesis, optical properties and their applications.5 This review article is particularly focused on π-extended coumarins, therefore a thorough description of the biscoumarins or coumarin-fused-coumarins is not included. Furthermore, Mahmoodi et al. and Shahzad et al. published another review article based on synthetic strategies for the 3,3′-(methylene)bis(coumarins). They specifically highlighted the catalytic processes for the synthesis of biscoumarins.7a,b A mini review based on solvent-free approaches for the synthesis of biscoumarin has been reported by Chand.7c Additionally, Agarwal et al. reviewed eco-friendly protocols for the synthesis of biscoumarins, along with their biological applications.8 Indeed, all of these reports imply the biscoumarin moieties are connected with linkers. However, a summarized overview on the synthetic methodologies and applications of fused-biscoumarins, or simply coumarin-fused coumarins, has not been reported to date.
Here, we have attempted to include research articles depicting the synthesis of various coumarin-fused-coumarin analogues. In particular, emphasis has been given to the anticipated role of these moieties on biological applications based on their photo-physical properties. Furthermore, in this review article, we have undertaken a systematic journey to highlight the various types of fused-coumarins, such as coumarinocoumarin, isocoumarino-coumarin/isocoumarin, dimerized coumarinocoumarins and coumarinylcoumarins fused with different cyclic systems (e.g., benzo, pyrano, benzofuro and acene) (Scheme 1). With regards to the chemo/bio-viability of these extended coumarin derivatives, many researchers have been considerably attracted towards this field. Therefore, an up to date summary of these fused heterocyclic moieties would be beneficial for researchers working on the development of novel coumarin-fused organic materials for photonic applications.
1.1. Coumarinocoumarin derivatives
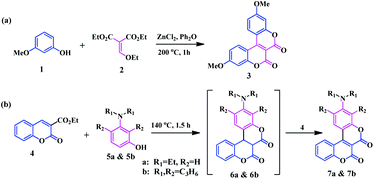
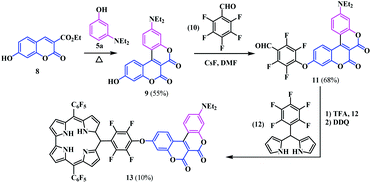
In the 1980s, Högberg and co-workers, reported some pyranone fused symmetrical coumarinocoumarins 3, in which two coumarin units were annulated with C3–C4 bonds.9 Regarding their synthesis, a Lewis acid catalysed O-acylation of 3-methoxyphenol 1 with diethyl ethoxymethylenemalonate 2, followed by ring closing and air-oxidation gave the coumarinocoumarins (Scheme 2a). Later, in 2006, Kovtun and co-workers developed another alternative process for the synthesis of a similar type of compound by the condensation of coumarin-3-carboxylic acid 4 with m-diethylaminophenol 5.10 In this process, the intensely fluorescent coumarinocoumarins 7a and b were derived by the oxidation of the intermediate 6 by another molecule of coumarin 4 instead of air oxidation (Scheme 2b).Thereafter, Gryko and co-workers aimed to develop a unique push–pull type coumarin architecture that displays strong fluorescence along with a bathochromic shift of the absorption with a significant Stokes shift.11 As such, following the general approach developed by Kovtun and group, they synthesized a novel biscoumarin moiety 9 bearing a hydroxyl group at the C-7 position of the coumarin core. The hydroxyl substituted biscoumarin was utilized to synthesize a corrole-coumarin dyad 13 possessing enhanced photophysical properties with a good photostability (Scheme 3). In this regard, the introduction of a strong electron-withdrawing group (pentafluorophenyl) to the coumarin core appears to be the best choice owing to its high solubility, lack of interference with spectroscopic properties and susceptibility to further transformations. The newly synthesised annulated biscoumarin moiety 9 was subjected to nucleophilic substitution with one fluorine atom of pentafluorobenzaldehyde 10. Then, condensation of the resulting coumarin-aldehyde 11 with dipyrromethane 12 leads to the complex corrole-coumarin dyad 13 in a 10% yield.
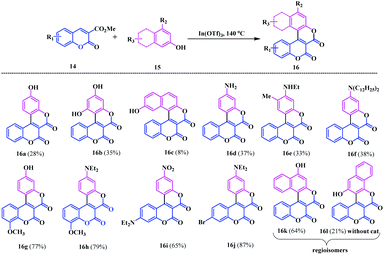
Again, in 2014, Gryko et al. introduced an interesting procedure to synthesize a similar type of V-shaped biscoumarin fused at the pyranone ring.12 They developed a combined strategy using the Högberg and Kovtun methods to utilize electron-rich phenols 15 and esters of the coumarin-3-carboxylic acids 14 in the presence of Lewis acids or bases to access functionalized π-expanded coumarins 16 (Scheme 4). They synthesised 16 V-shaped biscoumarin analogues bearing different substituents by using In(OTf)3 as a catalyst. Notably, the 3-aminophenols proved to an effective substrate, exclusively providing O-fused products without showing N-reactivity under the catalytic system.
However, in some cases, the dihydroxynaphthalenes led to two different regioisomers 16k and 16l with and without the addition of the catalyst In(OTf)3. Moreover, an interesting horseshoe-shaped coumarin system 18 with seven conjugated rings was synthesised using the reaction of coumarin bearing two pyranone units 17 with an electron rich phenol 5a (Scheme 5a). It is well known that π-expanded analogues have the highest probability to show interesting photophysical properties.13 In this respect, Gryko and co-workers, aimed to tune the skeletal structure of the synthesised biscoumarin moieties. They modified compound 16a using O-alkylation with tert-butyl bromoacetate and compound 16jvia a Sonogashira coupling reaction with arylacetylene 20 (Scheme 5b and c).
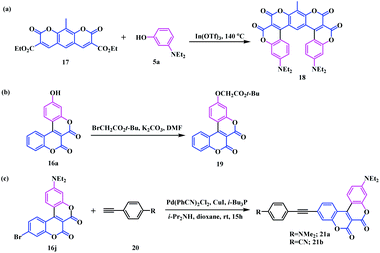 | ||
| Scheme 5 Synthesis of (a) horseshoe-shaped π-expanded coumarin system; (b) π-expanded alkoxycoumarins; and (c) the biscoumarin-arylacetylene framework. | ||
The newly developed biscoumarin framework shows intriguing photophysical properties. The biscoumarin analogue, bearing a C-7 hydroxyl group, shows a hypsochromic shift (λmax 444 nm). In contrast, the replacement of this hydroxyl group with a flexible linker (4-formyl)tetrafluorophenyl group 11 resulted in a bathochromic shift (λmax 455 nm). This influence of the linker was further confirmed using the strong absorption and emission of the corrole-coumarin dyad 13, which shows λem at 646 nm. This difference in the shift of the absorption and the emission results from the push–pull like behaviour of the respective coumarin nucleus and corrole linker.
Likewise, the optical properties of dyes 16, 18, 19 and 21 showed remarkable absorption and emission properties with respect to the solvent (Table 1).
| Biscoumarin | Solvent | λ abs (nm) | λ em (nm) | ϕ fl |
|---|---|---|---|---|
| 16a | DCM | 374 | 455 | 0.18 |
| 16b | DCM | 385 | 511 | 0.10 |
| 16k | DCM | 442 | 529 | 0.16 |
| 16l | DCM | 447 | 533 | 0.29 |
| 16c | DCM | 428 | 517 | 0.22 |
| DMSO | 428 | 506 | 0.02 | |
| 16d | DCM | 411 | 498 | 0.96 |
| DMSO | 438 | 534 | 0.68 | |
| 16e | DCM | 441 | 509 | 0.84 |
| DMSO | 454 | 552 | 0.27 | |
| 16f | DCM | 463 | 528 | 0.94 |
| DMSO | 464 | 562 | 0.02 | |
| 16g | DCM | 365 | 438 | 0.004 |
| DCM + (CH3)4NOH | 479 | 597 | 0.34 | |
| 16h | DCM | 458 | 530 | 0.91 |
| DMSO | 462 | 572 | 0.27 | |
| 16i | DCM | 494 | — | — |
| DMSO | 498 | — | — | |
| 16j | DCM | 466 | 540 | 0.90 |
| DMSO | 469 | 572 | 0.25 | |
| 18 | DCM | 458 | 542 | 0.67 |
| DMSO | 463 | 558 | 0.002 | |
| 19 | DCM | 365 | 440 | 0.008 |
| DMSO | 366 | 449 | 0.31 | |
| 21a | DCM | 459 | 691 | 0.04 |
| DMSO | 468 | — | — | |
| 21b | DCM | 476 | 572 | 0.69 |
| DMSO | 481 | 625 | 0.10 |
The coumarin-fused coumarins show enhanced intramolecular charge transfer (ICT) with respect to their electron donating substituents. It was observed that fused coumarins with amino substituents 16d–16f and 16h–16j displayed a stronger absorption/emission in comparison to the hydroxyl-substituted biscoumarin dyes. However, compound 16b, which possesses two hydroxyl groups, showed the emission maximum in the same region as the amine substituted biscoumarins. Regarding compound 16g, the presence of the MeO group at the C-8 position of the coumarin leads to non-radiative deactivation owing to additional stabilization of its anionic form. Consequently, a significant decrease in the fluorescence quantum yield was observed. For dyes 16c, 16k and 16l, moderate fluorescence quantum yields were observed. Additionally, modification of the biscoumarin framework 16j by expanding the chromophore revealed significant variation in the fluorescence behaviour. Furthermore, the O-alkylated product 19 showed a hypsochromic shift with respect to biscoumarin 16a with a strong fluorescence quantum yield in DMSO. However, a sharp decrease in the fluorescence quantum yield was observed in a non-polar solvent. On the other hand, the 7-alkoxy coumarins showed an increase in the Φfl value in polar media, which could be attributed to the least energetic π–π* transition.13b In contrast, the biscoumarin dye 18 showed a strong fluorescence in nonpolar media, but reduced fluorescence efficiency in DMSO. Consequently, analogous to the simple coumarin-fused coumarins, the modifications of their structures and study of their photophysical properties might eventually be beneficial for different biological or optoelectronic applications.
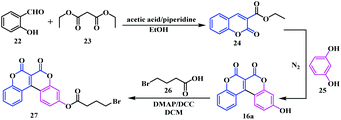
Owing to the growing interest in the unique photo and electro-optical properties of biscoumarin, very recently an interesting application of V-shaped biscoumarin has been reported by Hou and co-workers.14 They designed and synthetized an ICT-based probe 27 by condensation of the V-shaped biscoumarin 16a and 4-bromobutyric acid 26 (Scheme 6).
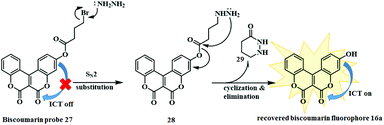
Indeed, this biscoumarin analogue 27 acts as a “turn on” fluorescent probe that facilitates the detection of hydrazine in water and gas by the naked eye. The biscoumarin 16a exhibited a push–pull electronic structure with an electron donating hydroxyl group and an electron-withdrawing carbonyl group at the 7 and 2 positions of the coumarin moiety respectively, thus revealing itself as an efficient fluorescent emitter. Upon introduction of the bromo-ester 26 to the fluorophore 16a, it prohibits the ICT process and consequently quenched the fluorescent emission. Later, upon reaction with hydrazine, a distinct increase in the intensity of the fluorescent emission (at 555 nm) was observed, signifying the reversal of the ICT process (Fig. 2). Simultaneously, the UV-vis absorption spectrum showed a red shift with a change from colourless to yellow providing “naked-eye” detection of hydrazine. Moreover, this biscoumarin probe could detect hydrazine with an excellent sensitivity over a wide pH range and the detection limit was as low as 4.2 ppb.
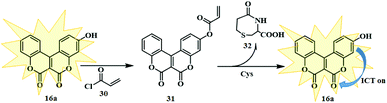
A similar type of off-on fluorescent probe was previously reported by Liu and co-workers.15 They adopted a similar reaction strategy to that mentioned above for the synthesis of a novel coumarinocoumarin probe named CCx (Scheme 6, Fig. 3). Indeed, the synthesised biscoumarin moiety (CCx) was efficiently designed to show the high selectivity for cysteine (Cys) and subsequently utilized in the detection of intracellular cysteine and the fluorescence imaging of Cys in HeLa cells. Moreover, CCx proved to be an effective biocompatible probe by offering in vivo fluorescence imaging in live cells. In addition, evaluation of the optical properties of CCx displayed a strong absorption at 445 nm and a fluorescence intensity at 535 nm with a 15-fold enhancement after the addition of Cys. Consequently, CCx showed a strong yellow fluorescence after the reaction with cysteine (Fig. 4). This variation is observed as a result of the photoinduced electron transfer (PET) effect caused by the presence of acrylates in the CCx framework. Furthermore, the detection limit of CCx for Cys was found to be 0.388 μM.
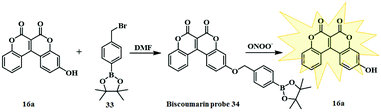
Recently, Zeng and co-workers synthesised another fluorescent probe by introducing borate 33 to the fluorophore 16a (Fig. 4).16 The fluorescent probe 34 shows an excellent response towards peroxynitrite ONOO− and could track endogenous and exogenous ONOO− fluctuations in living cells and zebrafish. Evaluation of the optical properties of the fluorescent probe 34 after the addition of ONOO− showed a strong fluorescent intensity at 540 nm and reached a maximum value within 5s. Furthermore, the detection limit of the probe for ONOO− was 4 nM.
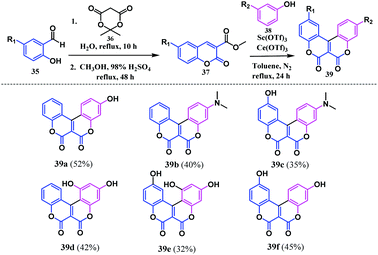
Liu et al. developed a similar synthetic strategy as Gryko and co-workers to access six coumarinocoumain analogues bearing hydroxyl or N,N-dimethylamino as the functional groups.17 Later, the significance of this novel combination of substituents in the biscoumarin moiety was investigated by determining their antioxidant potential in DNA oxidation. Regarding the synthesis, initially salicylaldehyde 35 was treated with Meldrum's acid 36 to form methyl coumarin-3-carboxylate 37. The oxidative annulation of this intermediate 37 in the presence of Ce(OTf)3, Sc(OTf)3 and m-substituted phenol 38 resulted in the coumarin-fused coumarin 39 (Scheme 7). Notably, the reaction was also investigated for ortho- and para-substituted phenols. Unfortunately, ortho- and para-substituted phenols with EDG [OH, N(CH3)2, NH2] or EWG (NO2, Br, and Cl) did not lead to the annulated coumarinocoumarins owing to the limitations that arise in the transesterification step and the C–C coupling of the phenol with the lactone ring.
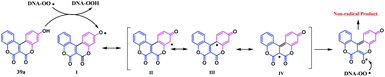
These synthesised coumarin-fused-coumarins 39a–f were investigated for their anti-oxidant potential towards DNA oxidation. Their inhibitory effect was evaluated using both biological (˙OH, Cu2+/glutathione (GSH), or 2,2′-azobis(2-amidinopropanehydrochloride) (AAPH)) and the chemical (2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonate) cationic radical (ABTS+˙), 2,2′-diphenyl-1-picrylhydrazyl radical (DPPH), or the galvinoxyl radical) systems. In general, the radical induced detection of DNA oxidation is possible after the reaction of the oxidized products with thiobarbituric acid. The lower the percentage of the thiobarbituric acid reactive substance (TBARS), the higher the inhibitory effect of the antioxidant towards DNA oxidation. The experimental TBARS percentages proved that these coumarin-fused-coumarins have a higher ability for inhibition of the ˙OH-induced oxidation of DNA, compared to Cu2+/GSH induced DNA oxidation. It was observed that the presence of the N(CH3)2-substituent in the biscoumarin skeleton shows a higher activity towards the ˙OH-induced oxidation of DNA. Furthermore, the inhibitory effect on AAPH induced oxidation of DNA by these compounds was significantly enhanced when a hydroxyl and N,N-dimethylamino groups are located on two different benzene rings. The process of inhibiting the AAPH-induced oxidation of DNA proceeds via the formation of a DNA-OO˙ radical derived from the DNA oxidation. This radical can abstract the hydrogen atom of 39a to form a phenoxyl radical I. The phenoxyl radical I undergoes a series of resonance rearrangements, leading to structures II, III and IV to form another phenoxyl radical that thereby quenches another DNA-OO˙ radical (Scheme 8).
Thus, the trapping of two radicals by structure 39a is possible owing to the presence of a hydroxyl group and the lactone motif of the biscoumarin skeleton. This indicates that with the increase in the hydroxyl group in the fused biscoumarin, the trapping of the radical increases. Furthermore, the radical scavenging property of the lactone ring increases with the increase in the electron donating effect. The presence of the –N(CH3)2 group improves the radical-scavenging properties, even in the absence of the hydroxyl group and likewise increases the ability to inhibit the AAPH-induced oxidation of DNA if both the –N(CH3)2 and hydroxyl groups are present at different positions on the benzene ring.
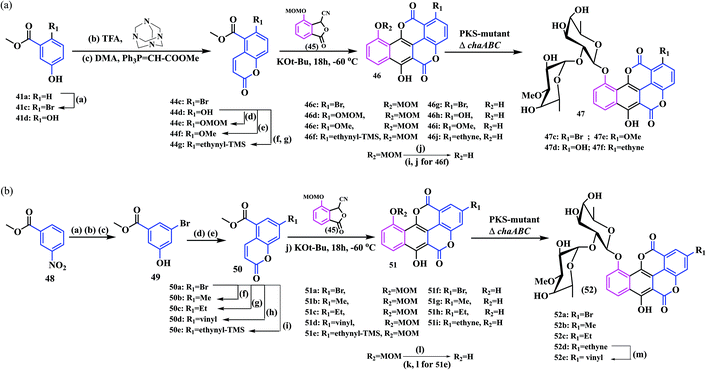
Hertweck and co-workers synthesized some complex coumarinocoumarin moieties, a class of natural product following both chemical and enzymatic pathways.18 They designed eleven coumarinocoumarin analogues by introducing different substituents in the coumarin core. This class of biscoumarins belong to the chartreusin family, which shows enhanced therapeutic activities with a notable photoactivation potential in visible light.19 Initially, they synthesised ethyl substituted chartreusins 40a and 40b following a series of combinatorial biosynthetic pathways (biosynthetic engineering). Next, in order to exchange the chartreusin ethyl group with a methyl substituent, they utilized a mutasynthetic route, that is a chemical process followed by biotransformation employing a ΔchaABC mutant which successfully allowed the exchange of the C-1 methyl with an ethyl group (Scheme 9). To accomplish this, they synthesised coumarins 44a and 44bvia a formylation-Wittig cascade reaction. Later, the fusion of the coumarins with pthalide 45 under Hauser annulation conditions afforded the 10-methoxymethyl-protected chartarins 46a and 46b in 92% and 66% yields, respectively.
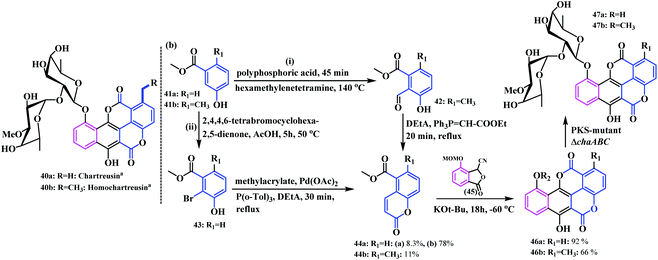 | ||
| Scheme 9 (a) Synthesis of 40a and 40b by combinatorial biosynthesis. (b) Synthetic pathway for chartreusin and norchartreusin. | ||
Thereafter, subsequent deprotection with BBr3 resulted in the (1 → 2) abeo chartreusin analogues 47a and 47b. With regards to the synthesis of norchartreusin 47a, the coumarin 44a formed was obtained in an unsatisfactory yield (8.3%) owing to the non-activation of the aromatic ring. Therefore, an alternative route following the halogenation-Heck reaction sequence using 2,4,4,6-tetrabromocyclohexa-2,5-dienone in acetic acid at 50 °C afforded the desired product in a good yield. Thereafter, the subsequent Heck reaction of 43 with methylacrylate provided the coumarin 44a in a 78% yield (Scheme 8).
Similarly, derivatives of 1-substituted and 2-substituted chartarins were also synthesized, as shown in Scheme 10a and b.
The synthesized chartreusins were evaluated for their biological activities using different cancer cell lines (human umbilical vein endothelial cells (HUVEC), human chronic myeloid leukaemia in blast crisis (K-562), human cervix carcinoma (HeLa), human colon adenocarcinoma (HT-29), and human melanoma (Mel-HO) cell lines). Interestingly, most of the chartreusin analogues showed activities against leukaemia and melanoma cell lines in contrast to HUVEC and HeLa cells. In addition, comparative studies of their derivatives revealed the different structure–activity relationships. The substituents in the C-1 and C-2 positions of chartreusin, along with homochartreusin 40b and chartreusin 40a showed strong cytostatic potentials. However, norchartreusin 47a was found to be inactive, which revealed the effect of the substituents on the coumarin skeleton. Furthermore, it was found that the 1-ethynyl and 1-methoxychartreusin analogues do not exhibit any cytostatic potential, whereas 1-vinylchartreusin (52e) and hydroxychartarin 46h exhibit strong antiproliferative effects against K-562 cells (GI50: 3.1 and 4.8 μM, respectively). Again, (1 → 2) abeo-ethynylchartreusin 52d, in contrast to the 1-ethynyl counterpart 46j, showed significant antiproliferative and cytotoxic activities. In addition, the derivatives 40b, 52b, and 52d showed potent antitumoral activities. Furthermore, the 2-vinyl derivative 52e, as well as the alkyne 52d, showed an enhanced antiproliferative activity when irradiated with blue light. Norchartreusin 47a shows strong antibacterial activity against Mycobacterium vaccae (2.49 μM). It is considered to be one of the target precursors in antibiotic research owing to the rise of tuberculosis and other related infectious diseases. The dependency of biological activities of the coumarinocoumarins (i.e., chartreusins) based on their different ring substituents are concisely highlighted in Fig. 5.
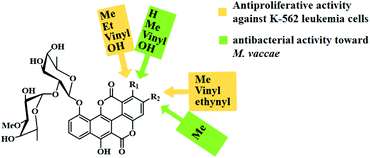 | ||
| Fig. 5 Overview of the biological activities depending on the ring substituents of the coumarin skeleton. | ||
Later, Chi and coworkers developed a unique approach by utilizing the carbene organocatalyst 55 to access a library of substituted coumarins.20 This methodology was extended to the metal-free total synthesis of several defucogilvocarcins M, E, and V via the chartreusin analogue. This protocol involves these N-heterocyclic carbene-catalysed remote-activation of α,β-unsaturated aldehydes 53 (enals) via the formal [5 + 5] cycloaddition reaction with furanose 54, which leads to diverse coumarin derivatives 57. Thereafter, Hauser annulation of biscoumarins 57 with furanone 58 afforded the desired chartreusin analogues 59a and b (Scheme 11a). Moreover, the synthesised coumarin 50b can be efficiently transformed to (1 → 2)-abeo-chartreusin 52b following the Hertweck methodology, which has a similar antitumor activity to the natural product chartreusin (Scheme 11b).
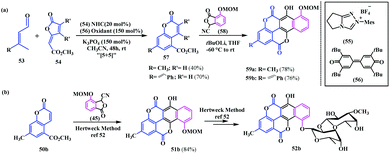 | ||
| Scheme 11 (a) NHC-catalysed synthesis of chartreusin. (b) Synthesis of bioactive (1 → 2)-abeo-chartreusin following the Hertweck methodology. | ||
1.2. Synthesis of coumarin-benzofused-coumarins
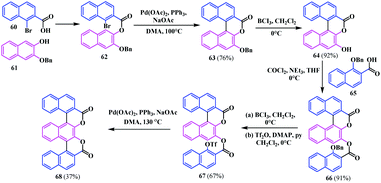
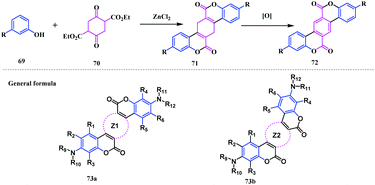
In addition to all the variants of the coumarin-fused-coumarins, in 1998 Bringmann and co-workers synthesised a naphthyl bridged coumarinocoumarin analogue 68 by Pd-catalysed coupling of an aryl-ester fragment.21 Initially, the bromo ester 62 was synthesised from L-bromo-2-naphthoic acid 60 and the 2,3-dihydroxynaphthalene derivative 61. Thereafter, the intramolecular coupling of 62 afforded the O-benzyl analogue 63. Subsequent deprotection, followed by esterification with 65, resulted in a benzoyl protected diester analogue 66. Again, deprotection followed by immediate triflation afforded a novel diester analogue 67, which effectively undergoes Pd-catalysed biaryl coupling leading to a novel and brilliant blue-green fluorescent naphthyl bridged biscoumarin 68 (Scheme 12).Later in 2003, Taniguchi and co-workers reported novel benzo-fused S/W-shaped biscoumarins.22 The synthesis was performed by following a double Pechmann reaction using an electron-rich phenol 69 with diethyl 2,5-dioxocyclo-hexane-1,4-dicarboxylate 70 in the presence of ZnCl2. This reaction proceeded via the generation of a partly unsaturated intermediate 71, which was easily transformed to the highly insoluble S-shaped benzocoumarinocoumarin 72 (Scheme 13).5,22
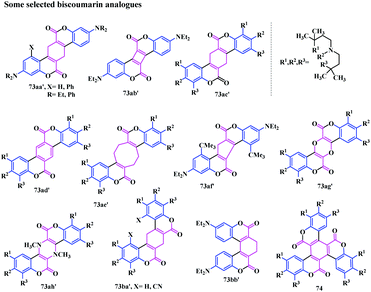
They synthesised a library of biscoumarins, having saturated a methylene group linked to the lactone skeleton, which upon dehydrogenation furnished the benzofused biscoumarin analogues (Fig. 6). Additionally, this reaction can also be performed by using substituted phenols with 1,3-diketones. The general formula 73a and 73b represents the synthesized biscoumarins (Scheme 12), in which R1 to R12 are either H, or other acceptable substitutes and Z1, Z2 are arbitrarily substituted 4 to 8 membered rings, which may or may not contain heteroatoms.
The synthesized fused-coumarin compounds can act both as light absorbing, as well as light emitting agents. The photophysical properties of the coumarin-fused-coumarins reveal that these compounds possess an absorption maximum in the visible region, particularly at 450 nm and a fluorescent maximum in the range of 400–500 nm with a large molar extinction co-efficient in the range of 1 × 104. These newly synthesized analogues have numerous applications as luminescent agents owing to their ability to emit visible light. Some of the noteworthy applications of these coumarin-fused-coumarins are their use in liquid crystal/plasma/brown tube displays, photochemical polymerization, solar cells, optical filters, dyeing, dye lasers and photo registries. Additionally, they can be used in several biological applications, such as substrate labelling in enzymatic reactions, antigen-antigen and complex protein formation reactions etc.
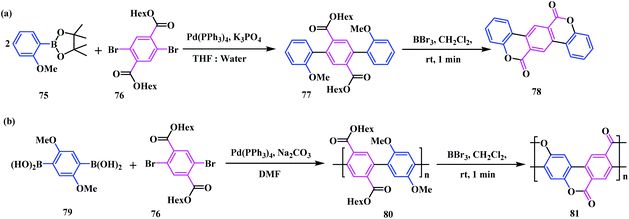
Considering a similar concept, Kim and co-workers developed a novel approach for the synthesis of the rigid and planar ladder-like conjugated biscoumarins 78 and 81.23 Initially, a di-ester analogue 77 was synthesized by reacting the boronic ester 75 with the dibromo ester 76 under an inert atmosphere. Next, BBr3 promoted cyclization of 77 in DCM led to the planar S-shaped biscoumarin compound 78 (Scheme 14a). Here, BBr3 stimulated the demethylation of the corresponding precursor 77. The in situ generated phenoxide ion of 77 acts as a nucleophile for acyl substitution with the neighbouring ester group to form the desired cyclized product 78. Again, this methodology was extended for the synthesis of a ladder-like polymeric system 81 by employing a Pd-catalysed cross coupling reaction of boronic acid 79 with the dibromo ester 76. Similarly, BBr3 promoted the cyclization of the precursor 80, resulting in a ladder-like extended coumarin fused coumarin polymer 81 (Scheme 14b).
From the UV-vis and fluorescence analysis, the planar and cyclized product showed a greater absorption maxima compared to those of the non-cyclized precursors. The λmax of the biscoumarin compound 78 showed a red shift in both the absorption and emission processes. Similarly, the conjugated ladder like polymer 81 showed an adsorption maximum at 390 nm in comparison to precursor 80, which was observed at 366 nm. This difference in the absorption maximum is due to the planarity of compound 81.
In addition, the synthesis of this novel cyclized ladder like polymeric fragment with extended conjugation and high planarity helps in the fabrication of low-cost semiconductors with improved properties. In many cases, the extended polymeric network possesses drawbacks regarding their solubility and purification. In this case, this shortcoming provides advantages, as the by-products were soluble, which could be easily separated from the sparingly soluble desired products. Furthermore, preparation of thin films of this ladder like coumarin-fused-coumarin polymer can be used as an active layer of an organic semiconductor for use in field-effect transistors.
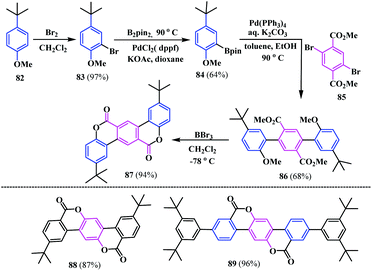
Dahl and co-workers developed a strategy for the synthesis of a similar type of benzo-fused biscoumarins 87–89 (Scheme 15). The fused coumarin moieties represent a crankshaft system composed of terphenyl dilactone. Firstly, they synthesized a crankshaft coumarin fused coumarin 87, as depicted in Scheme 14. Thereafter, similar isomeric coumarin-fused coumarins 88 and 89 were also synthesized. These three biscoumarins skeletons exhibit a high fluorescence and showed significant potential for use as pH-driven molecular switches and/or sensors with instant fluorescence attenuation at high pH values.
1.3. Synthesis of isocoumarinocoumarins
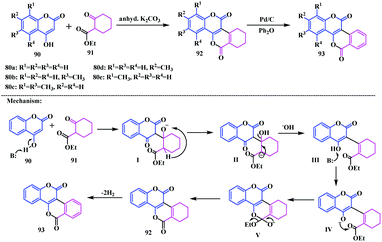
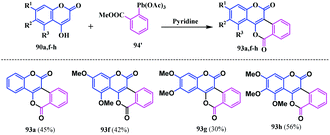
In the early 1980s, Merchant and coworkers reported structurally different biscoumarin analogues, these were coumarin-fused-isocoumarins 93 using a Pechmann condensation of 4-hydroxycoumarins 90 with ethylcyclohexanone-2-carboxylate 91 in the presence of anhydrous potassium carbonate.24 The tetrahydrobenzopyranone derivatives 92 thus formed were dehydrogenated in the presence of palladium charcoal under reflux conditions in diphenyl ether affording the isocoumarinocoumarin analogues, [2]benzopyrano [4,3-c][1]benzopyran-5,12-diones 93, as shown in Scheme 16.In 2006, Fedorov et al. reported another interesting methodology for the synthesis of a similar type of isocoumarinocoumarin analogue.25 They developed a cascade reaction strategy employing an organolead compound [2-(methoxycarbonyl)phenyllead triacetate] 94′ with 4-hydroxycoumarin 90 in the presence of pyridine. This reaction was performed with various 4-hydroxycoumarins including the dimethoxy and trimethoxy analogues affording the corresponding isocoumarinocoumarins 93a and 93f–h in moderate yields (30–56%) (Scheme 17).
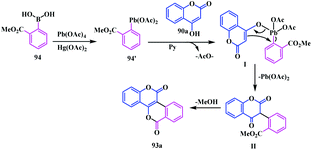
A plausible mechanism for this synthesis is shown in Scheme 16. In the initial step the organolead reagent 84′ was formed via transmetallation of phenyl boronic acid 84 with lead tetraacetate in the presence of a catalytic amount of mercuric acetate. This organolead reagent 84′ cannot be isolated and subsequently reacts with the unsubstituted 4-hydroxycoumarin 80a to form another intermediate I, which finally leads to an alpha-arylated product II. Later, the intramolecular transesterification of this compound II furnished the annulated product 93a (Scheme 18).
In some cases, this type of isocoumarinocoumarin was found as a side product. One such example was reported by Beifuss and co-workers, in which enzymatic oxidation of catechol 95 with substituted 4-hydroxycoumarin 90a leads to an important class of benzofuran compound 96, along with 7,8-dihydroxy-6H,11H-isochromeno[4,3-c]chromene-6,11-dione 97 as a side product with a yield of 29% (Scheme 19).26
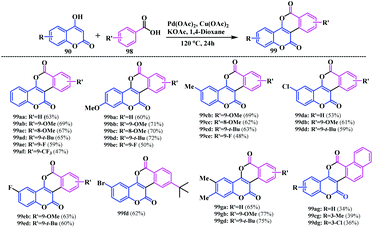
Recently, a noteworthy contribution towards the synthesis of coumarin-fused-isocoumarin was made by our group.27 This method represents the direct synthesis of isocoumarinocoumarin 99 using a Pd(II)-catalysed oxidative annulation reaction via double C–H activations. This cascade strategy was accomplished using 4-hydroxycoumarin 90 and arylcarboxylic acid 98 as the reacting partners. From the initial screening experiments, the reaction afforded a 63% yield using Pd(OAc)2 as a catalyst, and Cu(OAc)2 as an oxidant in the presence of KOAc in 1,4-dioxane at 120 °C (Scheme 20). Interestingly, this cascade strategy turned out to be an effective platform to access biscoumarin analogues with electronically diverse functionalities.
After synthesizing several coumarin-fused-isocoumarins, this strategy was further extended using 4-hydroxycoumarin 90a and 4-acetoxybenzoic acid 100 as substrates. Notably, this investigation delivers the hydrolysed biscoumarin 101 as a final product in an 18% yield (Scheme 21a). Based on the performed experiments and previously published literature, a plausible reaction mechanism for this Pd-catalysed cascade process has been proposed (Scheme 21b).
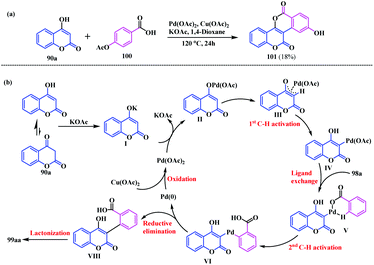 | ||
| Scheme 21 (a) Synthesis of biscoumarin using acetoxybenzoic acid as a reacting partner. (b) Plausible reaction mechanism for the synthesis of coumarin-fused isocoumarin. | ||
Initially, a Pd salt of 4-hydroxycoumarin II was formed via exchange of the alkoxide anion of 4-hydroxycoumarin I with Pd(OAc)2. Subsequently, the intermediate III leads first to C–H activation at the C-3 position, generating the C3–Pd species IV. Thereafter, ligand exchange of species IV with benzoate 98a forms the complex intermediate V. Consequently, a second C–H activation occurs at the benzoate moiety affording complex VI. Finally, the as formed species VI undergoes reductive elimination followed by lactonization to provide the desired isocoumarinocoumarin 99aa.
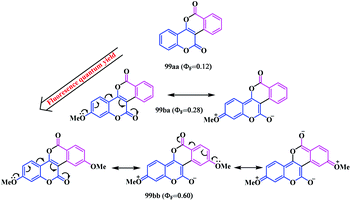
The photophysical properties of the synthesised biscoumarins were evaluated and appreciable quantum yields (0.12 to 0.60) were observed depending on their π-conjugation and the nature of the ring substituents (Fig. 7). A bathochromic shift of the absorption and emission were observed for the highly conjugated compounds 99ag and 99dg. Notably, the presence of an electron-donating group at the C-2 and/or C-9 position of the coumarin units exhibits a strong fluorescence quantum yield. Indeed, the quantum yield increases with the increasing number of electron donating groups in the peripheral ring of biscoumarin. This is a result of the greater delocalization of the π-bonds, as a result the ICT was enhanced throughout the molecule. A sharp variation in the quantum yield was observed upon increasing the electron donor –OMe groups at the C-2 and C-9 positions (99ba and 99bb) (Scheme 22). Similarly, the highly conjugated chloro-substituted compound 99dg also shows a significant increase in the quantum yield (Φ = 0.50) in contrast to its parent non-substituted analogue 99ag. However, biscoumarin moieties bearing alkyl group 99ga and 99gd did not show significant optical properties (Φ = 0.15 to 0.17).
1.4. Synthesis of isocoumarino-fused-isocoumarins
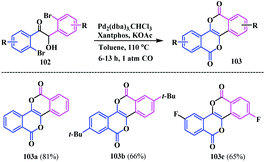
In 2018, Tani and Ogawa disclosed a novel synthetic strategy for the pyrano-fused tetracyclic π-systems 103. This protocol highlights a Pd-catalysed double carbonylative cyclization of the benzoins 102 to a novel bis-thieno-fused and bisisocoumarino core 103.28 After screening several experiments, this transformation proceeded efficiently using Pd2(dba)3·CHCl3 as the catalyst and Xantphos as the ligand in the presence of KOAc under 1 atm of CO in toluene at 110 °C. The optimized conditions afforded three isocoumarin-fused isocoumarins bearing different functionalities in the peripheral ring of the isocoumarin unit 103a–c (Scheme 23).The photophysical properties of the fused bisisocoumarin analogues were evaluated and the fluorescent quantum yields in DCM were found to be 0.009, 0.049 and 0.004, respectively, for 103a–c. However, the fluorescent quantum yields for these bis-isocoumarins were found to be enhanced in their crystalline state, and the Φcry for 103a–c were 0.21, 0.12 and 0.11, respectively.
Aggregation-induced-emission (AIE) has attracted significant attention in the field of optoelectronics.29 This phenomenon remains unexplored in many of the previously reported ring-fused polycyclic π-systems owing to their flexibility. In this regard, Tani and coworkers investigated the structure-AIE relationships for the synthesized biscoumarin analogues.30 The tight packing and planarity present in the fused crystals suppresses the molecular vibration, which in general is not suitable for AIE. However, the vibrational analysis and kinetic studies revealed that these synthesised isocoumarin-fused-isocoumarin analogues are AIE active. The carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching modes of the pyrone ring of these analogues are coupled strongly with non-radiative decay, which as a result makes it suitable for AIE activity. This decay pathway depends on the peripheral aromatic rings and is independent of the ring substituents of the isocoumarin core.
O stretching modes of the pyrone ring of these analogues are coupled strongly with non-radiative decay, which as a result makes it suitable for AIE activity. This decay pathway depends on the peripheral aromatic rings and is independent of the ring substituents of the isocoumarin core.
1.5. Synthesis of coumarin-pyranofused-coumarins
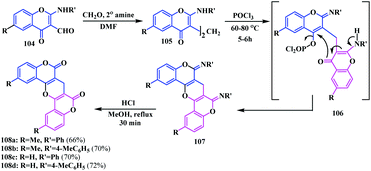
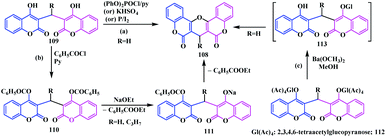
In addition to the coumarinocoumarins, biscoumarin fused with a pyrano-ring was also synthesized. In 2011, Bandyopadhyay and co-workers synthesised pyranofused-biscoumarins derivatives 108 using the chemoselective hydrolysis of diaryliminodicoumarins 107 (Scheme 24).31The reaction was initially performed by heating 2-arylaminochromone-3-carbaldehydes 104 with secondary amines (sarcosine, piperidine or diethylamine) in the presence of formalin in DMF. Thereafter, POCl3-induced dehydration followed by heating with HCl in methanol produced the desired pyranofused-biscoumarins 108 (7H-pyrano[3,2-c:5,6-c′]dicoumarins) (Scheme 24). The same biscoumarin motif was previously reported by Huebner's group by cyclization of dicoumarol 109 in the presence of (PhO)2POCl/pyridine or KHSO4 or P/I2 (Scheme 25a).32 Moreover, it was also synthesized from the substituted dicoumarol 109 in the presence of pyridine-acetic anhydride/benzoyl chloride,33 as well as from diglucoside 112 upon treatment with Ba(OCH3)2 in methanol34 (Scheme 25b and c).
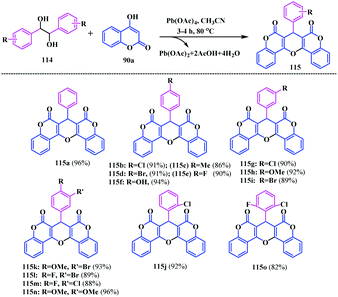
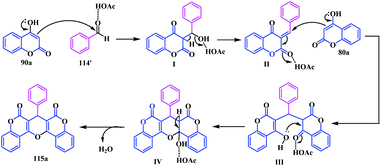
Shivashankar et al. presented a simple and efficient pseudo multicomponent reaction for the synthesis of the coumarin-pyrano fused coumarin analogues.35 They synthesized a diverse series of fused-pyrano-biscoumarins 115 by condensation of 4-hydroxycoumarin 90a (four equivalents) with electronically different 1,2-diols 114 in the presence of lead tetraacetate in acetonitrile at 80 °C (Scheme 26).
Regarding the mechanism of this reaction, it proceeds through a tandem process involving oxidation, condensation, cyclization, and dehydration (Scheme 27).
Initially, Pb(OAc)4 oxidizes 1,2-diol into benzaldehyde 114′ and forms acetic acid. The in situ generated acetic acid facilitates the acid catalysed condensation of benzaldehyde 114′ and 4-hydroxycoumarin 90a to afford benzylidene-chroman-2,4-dione I. Finally, the Michael addition of II with 4-hydroxycoumarin 90a, followed by cyclization and dehydration, yields the desired fused coumarin-pyrano-coumarin analogues 115 (Scheme 27).
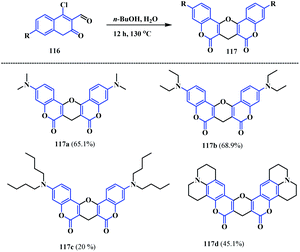
Another interesting methodology was developed by Yu and co-workers for the synthesis of pyrano-fused biscoumarins.36 They reported a facile additive- and column-free synthetic strategy for novel pyrano-fused biscoumarin fluorophores 117. This protocol highlights a novel dimerization of the coumarinyl aldehydes 116 in the presence of n-BuOH/H2O at 130 °C, resulting in four novel rigid and symmetrical pyrano-fused biscoumarins 117a–d (Scheme 28).
The synthesised coumarinyl pyrano-fused coumarins 117 showed interesting photophysical properties with absorption bands at λ(ε) = 366, 371, 372 and 385 nm, respectively, and λmax emission wavelengths at 426, 428, 429 and 448 nm, respectively, with a large Stokes shift. Owing to their better solubility and the higher fluorescence intensity of biscoumarin 117b, they was further explored for sensing G-quadruplexes (G 4s). Among the different G 4s (CM22, Ckit), anti-parallel G 4s (HRAS) and hybrid-type G 4s (22AG, G3T3, G4TTA, and Ckit*, 117b showed a six-fold enhancement in the fluorescent intensity with 22AG. From the molecular docking studies, it was revealed that G 4s (22AG) can bind with 117b through hydrogen bonds and π–π interactions. Consequently, its aggregation in aqueous solution was prevented and resulted in fluorescent enhancement via disaggregation induced emission. Additionally, the selectivity of 117b towards G 4s was studied by the interaction of 117b with a double-stranded DNA (ds-DNA) and 22AG in the same medium. Notably, the same enhancement of the fluorescence intensity was observed in the presence or absence of ds-DNA, signifying a good selectivity for G 4s over ds-DNA. However, a decrease in the fluorescence intensity of 117b was observed with single-stranded DNA (ss-DNA) and double-stranded DNA (ds-DNA), which additionally corroborates the fact that 117b can distinguish G 4s from other types of DNA. In conclusion, Yu et al. reported an interesting bio-chemical relationship between the synthesis of novel pyrano-fused-biscoumarin, which shows a good sensitivity and selectivity towards G 4s.
1.6. Synthesis of dimerized coumarin fused coumarins
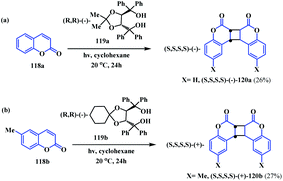
In 2005, Tanaka and Fujiwara reported an interesting host–guest complex formation strategy for the synthesis of some coumarin-fused-coumarins.37 They reported a highly enantioselective [2 + 2] photodimerization reaction of coumarin 118a and b with optically active host compounds 119a and b, which leads to optically active anti-head-to-head coumarin dimers 120a and b. Initially, they synthesised the optically active host compound 119.38 Photoirradiation of the optically active host (R,R)-(−)-119a and coumarin 118a in cyclohexane at 20 °C for 24 h resulted in a colourless 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion host–guest complex intermediate of (−)-119a and the coumarin dimer [(S,S,S,S)-(−)-120a]. Subsequent treatment with DMF/H2O afforded the pure (S,S,S,S)-(−)-120a crystals in a 26% yield (Scheme 29a). The photo dimerization reaction, using hydrocarbon and aromatic solvents, results in a mixture of enantiomers. One enantiomer of the dimer form complexes with the host and precipitates as inclusion crystals (−)-120a. Meanwhile, the opposite enantiomer of the coumarin dimer (+)-120a was obtained in solution. Similarly, photoirradiation of an equimolar mixture of 119b and 6-methylcoumarin 118b in cyclohexane resulted in the (S,S,S,S)-(+)-coumarin dimer (120b) in a 27% yield (Scheme 29b).
1 inclusion host–guest complex intermediate of (−)-119a and the coumarin dimer [(S,S,S,S)-(−)-120a]. Subsequent treatment with DMF/H2O afforded the pure (S,S,S,S)-(−)-120a crystals in a 26% yield (Scheme 29a). The photo dimerization reaction, using hydrocarbon and aromatic solvents, results in a mixture of enantiomers. One enantiomer of the dimer form complexes with the host and precipitates as inclusion crystals (−)-120a. Meanwhile, the opposite enantiomer of the coumarin dimer (+)-120a was obtained in solution. Similarly, photoirradiation of an equimolar mixture of 119b and 6-methylcoumarin 118b in cyclohexane resulted in the (S,S,S,S)-(+)-coumarin dimer (120b) in a 27% yield (Scheme 29b).
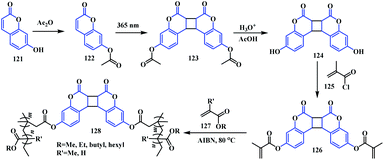
Coumarins have the potential to undergo the photo-reversible dimerization reaction above 300 nm and below 260 nm. Accordingly, Saito and co-workers designed a similar type of dimeric coumarin-fused-coumarin system 128, which was effectively used as a stimuli-responsive unit in a methylacrylate polymeric network.39 For the synthesis of the biscoumarin cross-linker 128, initially the phenolic group of the coumarin moiety 121 was protected using acetate. Thereafter, [2 + 2] photodimerization of the coumarinylacetate 122, followed by subsequent hydrolysis and esterification with methacryloyl chloride, resulted in the 7-methacryloyloxycoumarin fused coumarin cross-linker 126. Later, this dimer 126 was subjected to radical polymerization with a range of acrylate monomers 127 in the presence of 1% of AIBN as a radical initiator to form a series of coumarin-fused-coumarin crosslinked polyacrylates 128 (Scheme 30). Interestingly, these crosslinked polyacrylates show a photo-reversible self-healing characteristic. This was observed from the visual disappearance of surface scratches on thin films of the polymeric network owing to the application of UV light. Photo irradiation with 254 nm UV light leads to cleavage of the crosslinked polyacrylates forming a linear polymer, which increases molecular mobility. Furthermore, upon irradiation at 365 nm, photo-dimerization occurs, indicating the reformation of the crosslinked polymer.
1.7. Synthesis of coumarin-acene-fused-coumarins
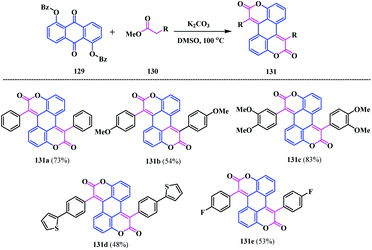
In 2014, Gryko and co-workers reported some interesting chemistry on coumarin by synthesizing two types of novel coumarin-fused-coumarins 131, 132 and 134 belonging to the perylene and pentacene family.40 This methodology highlights a modified Knoevenagel bis-condensation reaction between 1,5-dibenzoyloxyanthraquinone 129 and the phenylacetic acid methyl ester derivatives 130. It provides five bis-coumarinyl perylene analogues bearing electronically different substituents including heterocyclic functionalities 131a–e (Scheme 31).Notably, the biscoumarin analogue 131a, which is unstable in solution under sunlight or UV irradiation at 365 nm, undergoes light-driven dehydrogenation to form the pentacene-based (or coronene-like) coumarin-fused-coumarin framework 132 (Scheme 32a). However, oxidative aromatic coupling of 131b and 131c leads to a very poorly soluble product. The biscoumarin 132c was converted into a O-hexyl substituted analogue 133, which, on account of the steric hindrance, breaks the π−π interaction. The transformation of 131c to its corresponding hexyl ether motif was performed by four-fold demethylation in the presence of BBr3 followed by etherification using standard Williamson conditions. Thereafter, the photocyclization of 133 under a UV lamp at 365 nm resulted in oxacoronenone 134 in a 54% yield. In contrast, oxidative aromatic coupling of 133 in the presence of FeCl3, BF3·OEt2, and CH2Cl2 afforded 134 in an 87% yield (Scheme 32b).
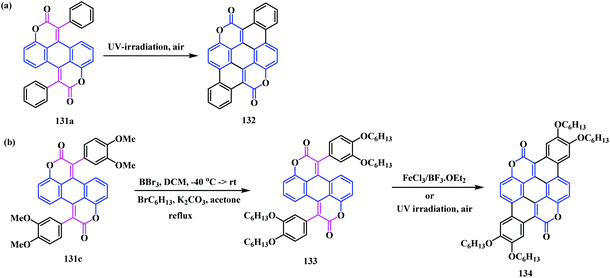 | ||
| Scheme 32 Synthesis of the pentacene-based coumarin-fused coumarin using (a) a light driven electrocyclization reaction, and (b) oxidative aromatic coupling. | ||
The introduction of the electron donating and withdrawing groups to the esters creates a significant impact on the spectral properties of these novel perylene/pentacene-based coumarin-fused coumarins. A very strong red shift was observed when an alkoxyl group was added to the planar dye 134. This is a result of the strong influence of the lone pair on the oxygen on the parent chromophores, which enhances the ICT. In particular, an increase in the fluorescence quantum yield was observed from the perylene unit 131c (Φfl = 0.10) to the pentacene structures 134 (Φfl = 0.90). This could result from the more flexible molecular structure of compounds 131a–e compared to the fully fused π-extended 134. In fact, the compounds 131a–e showed a moderate fluorescent quantum yield (Φfl = 0.10–0.31) with large Stokes shifts (5000–7000 cm−1). Additionally, the substituent orientation does not have a significant impact on the quantum yields of the perylene fused analogues. The pentacene biscoumarin compound 134 shows a strong hypsochromic shift owing to its π-expanded characteristics. Thus, these novel π-extended coumarin-fused-coumarins, with noteworthy photophysical properties, could be a significant contribution in the realm of optoelectronics and fluorescent imaging.
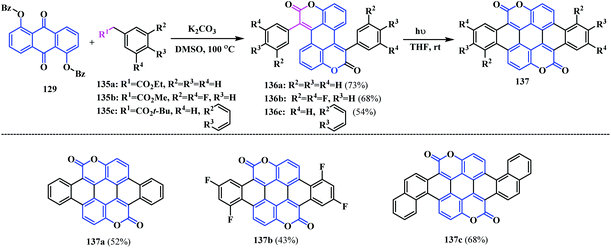
Again in 2017, Gryko and co-workers utilized the same reaction methodology for the synthesis of a similar type of acene fused-biscoumarins that possess different substituents in the phenyl acetic acid ester ring 135.41 the double Knoevenagel condensation of 1,5-dibenzoyloxyanthraquinone 129 with esters 135a, 135b and 135c gave biscoumarins 136a–c. Subsequent UV irradiation leads to double 6π-electrocyclization with concomitant oxidation leading to biscoumarin 137a–c (Scheme 33).
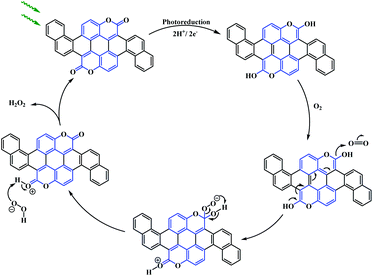
From the UV-Vis absorption and photoluminescence spectra, it can be seen that these acene-based coumarin-fused-coumarins show bathochromic shifts (∼50 nm). The optical properties of these fused compounds were found to be almost similar. However, a considerable change in the electrochemical behaviour was observed. Cyclic voltammetry analysis reveals that 137a and 137b showed a quasi-reversible reduction with irreversible oxidation. On the other hand, 137c showed both a quasi-reversible reduction and quasi-reversible 2e− oxidation, which is attributed to the greater π-extension. Consequently, the semiconducting properties of 137a and 137b fabricated on field-effect transistors (FETs) displayed n-type electron transport while both n- and p-type mobility was observed for 137c. They also studied the photocatalytic potential of the synthesised biscoumarin using the reduction of O2 to H2O2. Visible light irradiation of thin films of compound 137a–c in pure water produced 1–5 μg H2O2 per mg of compound within 1 h. The reaction proceeds via a 2e− reduction of the dissolved O2 to H2O2, with water as the sacrificial electron donor owing to its simultaneous oxidation. Additionally, the presence of two carbonyl functionalities in 137a–c offers an important catalytic site for selective 2e−/2H+ reduction (Scheme 34).
In 2019, Sahu and co-workers reported a unique regioisomeric pair of heptahelicene embedded coumarin-fused-coumarin analogues, 143 and 148.42 These heptahelicinyl biscoumarin analogues were synthesised by oxidative photocyclization of the E/Z isomers of a diarylolefin system. Initially, the Wittig olefination between 1,4-benzene dicarboxaldehyde 139 with suitable phosphonium salts (6-coumaryl)methyltriphenylphosphonium bromide 138 and (7-coumaryl)methyltriphenylphosphonium bromide 144 were performed to access the diarylolefins 140 and 145, respectively.43 Thereafter, coumarin-annulated tetrahelical carboxaldehydes 141 and 146 were formed by oxidative photocyclization of 140 and 145, respectively. Subsequently, the Wittig reaction of the tetrahelicenes 141 and 146 with the phosphonium salts 138 and 144 afforded the tetrahelicinyl olefin precursors 142 and 147. Consequently, oxidative photocyclization of 142 resulted in a mixture of 7-out-out heptahelical coumarin-fused-coumarins 143a and b in the ratio of 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (i.e. (a,b)
3 (i.e. (a,b)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (a,c) photocyclized product). However, the oxidative photocyclization of olefin 147 only occurs in the (a,b) position, leading to 7-in-in heptahelical coumarin-fused-coumarin as the exclusive product 148 (Scheme 35).
(a,c) photocyclized product). However, the oxidative photocyclization of olefin 147 only occurs in the (a,b) position, leading to 7-in-in heptahelical coumarin-fused-coumarin as the exclusive product 148 (Scheme 35).
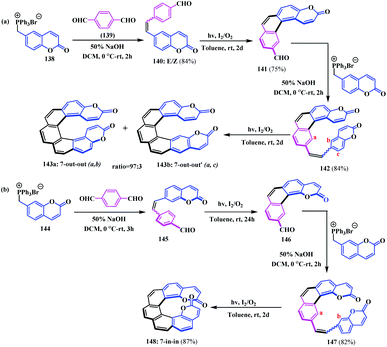 | ||
| Scheme 35 (a) & (b) Synthesis of the regioisomeric pairs of heptahelicene embedded coumarin-fused-coumarins. | ||
The fluorescence emission maxima of the regioisomeric heptahelicinal coumarin-fused-coumarins were found to be red shifted for the 7-in-in helicene compared to the 7-out-out heptahelicene. Moreover, the absorption and the fluorescence emission in both the cases tapered off at 450 and 650 nm, respectively, exhibiting fluorescence quantum yields of around Φf = 7–8%. Notably, the singlet lifetime (τ) and the fluorescent quantum yield of the 7-out-out heptahelicenal biscoumarin (Φf 7–8%, τ. 4.3–4.8 ns) is higher than the “in-in” regioisomer (Φf. 5%, τ. 3.2–3.9 ns). Additionally, the quantum yield (Φf) and singlet lifetime (τ) shows a significant variation with respect to the solvent. Amongst the different solvents (cyclohexane, acetonitrile and methanol), greater Φf and τ values were observed for methanol with a Stokes shift at 96 nm for the “in-in” regioisomer and 61 nm for the “out-out” isomer. Furthermore, these optically pure enantiomers showed the significant influence of the helicity depended on their chiroptical properties such as specific rotations, molar ellipticities, Cotton effects, and the anisotropic effect.
1.8. Synthesis of coumarinylbenzofuro-fused-coumarin
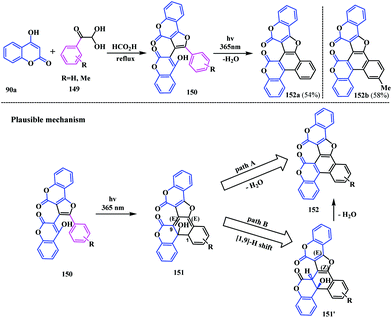
Recently, Komogortsev and coworkers formulated a novel photochemical approach for the synthesis of polycyclic biscoumarin analogues fused with the benzofuran unit 152.44 Initially, they synthesized a furopyrone moiety 150 using 4-hydroxycoumarin 90a and aryl glyoxals 149 in the presence of formic acid. Subsequently, photo-irradiation of 150 with UV light at 365 nm for 36 h in the presence of NMP at room temperature afforded the benzofuran embedded coumarin-fused-coumarin 152 (Scheme 36).The mechanism for the synthesis of the coumarinylbenzofuran-fused coumarin was assumed to follow one of two synthetic pathways. Initially, UV irradiation of 150 underwent 6π-electrocyclization leading to the formation of intermediate 151. The transformation of this intermediate to the biscoumarin 152 may proceed through two different pathways. Firstly, aromatization of the benzene ring with the elimination of a water molecule (Scheme 36, path A). Secondly, the intermediate 151 is converted to another intermediate 151′via a thermal suprafacial [1,9]-H sigmatropic shift. Then, elimination of the water molecule could lead to the final product 152 (Scheme 36, path B).
2. Conclusions
In this review, a systematic collection of structurally unique coumarinyl-fused-coumarin derivatives is presented. The synthetic strategies with different chemo/photo mediated routes using different reacting partners have been highlighted. Furthermore, the photophysical properties and structure–activity relationship of the synthesized coumarinyl-fused coumarin derivatives were described. They possess strong photo-responsive properties, and as a result they can act as light absorbing, as well as light emitting agents. These fused architectures have been used as fluorescent probes for the detection of hydrazine in water and gas using the naked eye, the detection of intracellular cysteine and the tracking of endogenous and exogenous ONOO− fluctuations in living cells. Further applications such as sensing of G 4s, use as radical scavengers and laser dyes, substrate labelling in enzymatic reactions, low-cost semiconductors, pH-driven molecular switches and so on have been observed. Notably, some of these compounds exhibit pharmacological properties, such as antioxidant, antiproliferative, antibacterial, and anticancer activities. However, in most of the reports, the relevance of these compounds is limited to their optical properties. Regarding practical applicability, these compounds require a significant amount of effort in order to achieve unique photophysical properties that have a high quantum yield. In this regard, there is still more scope to explore. Nevertheless, we believe that these fused architectures could be used in the synthesis of large π-extended coumarin-based conjugates, various coumarin-based heteroatom-doped π-extended sheets and so on, which could mimic graphene-like materials. We hope this review article will assist researchers to explore innovative design of novel coumarinyl-fused hybrids that could enable significant utilization in the practical world.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the CSIR, New Delhi, India for financial support (Project No. MLP-1015) and the Director, CSIR-NEIST, Jorhat, India for his interest in this work. M. S. acknowledges the DST-SERB for financial assistance in the form of NPDF (PDF/2019/000564). D. D. acknowledges the DST, New Delhi for the fellowship.Notes and references
- (a) D. Srikrishna, C. Godugu and P. K. Dubey, Mini-Rev. Med. Chem., 2018, 18, 113–141 CrossRef CAS; (b) I. Manolov, C. Maichle-Moessmer and N. Danchev, Eur. J. Med. Chem., 2006, 41, 882–890 CrossRef CAS; (c) C. Ito, M. Itoigawa, S. Onoda, A. Hosokawa, N. Ruangrungsi, T. Okuda, H. Tokuda, H. Nishino and H. Furukawa, Phytochemistry, 2005, 66, 567–572 CrossRef CAS PubMed; (d) S. S. Bhattacharyya, S. Paul, S. K. Mandal, A. Banerjee, N. Boujedaini and A. R. Khuda-Bukhsh, Eur. J. Med. Chem., 2009, 614, 128–136 CAS; (e) M. Musa, F. Khan and M. Cooperwood, Lett. Drug Des. Discovery, 2009, 6, 133–138 CrossRef CAS; (f) F. Borges, F. Roleira, N. Milhazes, L. Santana and E. Uriarte, Curr. Med. Chem., 2005, 12, 887–916 CrossRef CAS PubMed.
- (a) M. Cigaň, J. Donovalova, V. Szocs, J. Gaspar, K. Jakusova and A. Gaplovsky, J. Phys. Chem. A, 2013, 117, 4870–4883 CrossRef PubMed; (b) X. Liu, J. M. Cole, P. G. Waddell, T.-C. Lin, J. Radia and A. Zeidler, J. Phys. Chem. A, 2012, 116, 727–737 CrossRef CAS; (c) F. A. Mir, Optik, 2015, 126, 24–27 CrossRef CAS; (d) S. Kumar, P. Singh, R. Srivastava, R. R. Koner, A. Pramanik, J. Mathew, S. Sinha, M. Rawat, R. S. Anande and S. Ghosh, J. Mater. Chem. C, 2014, 2, 6637–6647 RSC; (e) A. Mishra, M. K. R. Fischer and P. Bäuerle, Angew. Chem., Int. Ed., 2009, 48, 2474–2499 CrossRef CAS; (f) G. Signore, R. Nifosi, L. Albertazzi, B. Storti and R. Bizzarri, J. Am. Chem. Soc., 2010, 132, 1276–1288 CrossRef CAS; (g) K. Tsukamoto, Y. Shinohara, S. Iwasaki and H. Maeda, Chem. Commun., 2011, 47, 5073–5075 RSC; (h) K. G. Reddie, W. H. Humphries, C. P. Bain, C. K. Payne, M. L. Kemp and N. Murthy, Org. Lett., 2012, 14, 680–683 CrossRef CAS PubMed.
- (a) F. Annunziata, C. Pinna, S. Dallavalle, L. Tamborini and A. Pinto, Int. J. Mol. Sci., 2020, 21, 4618 CrossRef CAS; (b) Ł. K. Ski, D. T. Gryko, A. L. Sobolewski and O. W. Morawski, Phys. Chem. Chem. Phys., 2018, 20, 14491–14503 RSC; (c) J. Snyder and K. Nakanishi, Tetrahedron Lett., 1981, 22, 5015–5018 CrossRef CAS; (d) F. Cheng, Z. Deng, Z. Guo, J. Chen and K. Zou, Nat. Prod. Res., 2013, 27, 1542–1547 CrossRef CAS.
- (a) A. Ibrar, S. Zaib, I. Khan, F. Jabeen, J. Iqbal and A. Saeed, RSC Adv., 2015, 5, 89919–89931 RSC; (b) N. K. N. A. Zawawi, M. Taha, N. Ahmat, N. H. Ismail, A. Wadood, F. Rahim and A. U. Rehman, Bioorg. Chem., 2015, 63, 36–44 CrossRef; (c) Neena, S. Nain, V. Bhardwaj and R. Kumar, Pharm. Chem. J., 2015, 49, 154–158 Search PubMed; (d) K. Kandasamy, S. Ganesabaskaran, M. P. Pachamuthu and A. Ramanathan, Spectrochim. Acta, Part A, 2015, 148, 184–188 CrossRef CAS PubMed; (e) N. O. Mahmoodi and S. Ghodsi, Res. Chem. Intermed., 2017, 43, 661–678 CrossRef CAS; (f) V. K. A. Kalalbandi, S. C. Bijjaragi and J. Seetharamappa, ChemistrySelect, 2018, 3, 3925–3929 CrossRef CAS.
- M. Tasior, D. Kim, S. Singha, M. Krzeszewski, K. H. Ahn and D. T. Gryko, J. Mater. Chem. C, 2015, 3, 1421–1446 RSC.
- (a) H. von Pechmann and W. Welsh, Ber. Dtsch. Chem. Ges., 1884, 17, 1646–1652 CrossRef; (b) H. D. Becker and H. Lingnert, J. Org. Chem., 1982, 47, 1095–1101 CrossRef CAS; (c) F. M. A. A. El-Taweel, S. Z. A. Sowellim and A. G. A. Elagamey, Bull. Chem. Soc. Jpn., 1995, 68, 905 CrossRef CAS; (d) Y. A. Jackson and K. S. C. Marriott, Heterocycles, 2002, 57, 1897–1900 CrossRef CAS.
- (a) D. Shahzad, A. Saeed, M. Faisal, F. A. Larik, S. Bilquees and P. A. Channar, Org. Prep. Proced. Int., 2019, 51, 199–239 CrossRef CAS; (b) N. O. Mahmoodi, F. G. Pirbasti and Z. Jalalifard, J. Chin. Chem. Soc., 2018, 65, 383–394 CrossRef CAS; (c) M. Chand, Arch. Org. Inorg. Chem. Sci., 2018, 2, 204–207 Search PubMed.
- P. Teli, A. Sethiya and S. Agarwal, ChemistrySelect, 2019, 4, 13772–13787 CrossRef CAS.
- T. Högberg, M. Vora, S. D. Drake, L. A. Mitscher and D. T. W. Chu, Acta Chem. Scand., Ser. B, 1984, 38, 359–366 CrossRef.
- E. M. Poronik, M. P. Shandura and Y. P. Kovtun, Chem. Heterocycl. Compd., 2006, 42, 410–411 CrossRef CAS.
- M. Tasior, R. Voloshchuk, Y. M. Poronik, T. Rowicki and D. T. Gryko, J. Porphyrins Phthalocyanines, 2011, 15, 1011–1023 CrossRef CAS.
- M. Tasior, Y. M. Poronik, O. Vakuliuk, B. Sadowski, M. Karczewski and D. T. Gryko, J. Org. Chem., 2014, 79, 8723–8732 CrossRef CAS.
- (a) S. Uchiyama, K. Takehira, T. Yoshihara, S. Tobita and T. Ohwada, Org. Lett., 2006, 8, 5869–5872 CrossRef CAS; (b) B. Valeur, Molecular Fluorescence Principles and Applications, Wiley VCH, Weinheim, Germany, 2002 Search PubMed.
- X. Jiang, M. Shangguan, Z. Lu, S. Yi, X. Zeng, Y. Zhang and L. Hou, Tetrahedron, 2020, 76, 130921 CrossRef CAS.
- C. Chen, L. Zhou, X. Huang and W. Liu, J. Mater. Chem. B, 2017, 5, 5892–5897 RSC.
- X. Zeng, Z. Li, J. Fu, C. Jiang, M. Ma, L. Zhu and X. Jin, Dyes Pigm., 2021, 186, 108982 CrossRef CAS.
- G.-L. Xi and Z.-Q. Liu, J. Agric. Food Chem., 2015, 63, 3516–3523 CrossRef CAS PubMed.
- N. Ueberschaar, Z. Xu, K. Scherlach, M. Metsä-Ketelä, T. Bretschneider, H.-M. Dahse, H. Görls and C. Hertweck, J. Am. Chem. Soc., 2013, 135, 17408–17416 CrossRef CAS.
- N. Ueberschaar, H.-M. Dahse, T. Bretschneider and C. Hertweck, Angew. Chem., Int. Ed., 2013, 52, 6185–6189 CrossRef CAS.
- X. Huang, T. Zhu, Z. Huang, Y. Zhang, Z. Jin, G. Zanoni and Y. R. Chi, Org. Lett., 2017, 19, 6188–6191 CrossRef CAS.
- G. Bringmann, A. Wuzik, J. Kraus, K. Peters and E.-M. Peters, Tetrahedron Lett., 1998, 39, 1545–1548 CrossRef CAS.
- M. Satsuki, S. Suga and Y. Taniguchi, PCT Int.Appl, WO2003020725A120030313, 2003 Search PubMed.
- I. Kim, T.-H. Kim, Y. Kang and Y.-b. Lim, Tetrahedron Lett., 2006, 47, 8689–8692 CrossRef CAS.
- J. R. Merchant, N. M. Koshti and K. M. Bakre, J. Heterocycl. Chem., 1981, 18, 1655–1658 CrossRef CAS.
- B. A. Maryasin, A. S. Shavyrin, J. P. Finet and A. Yu. Fedorov, Russ. Chem. Bull., Int. Ed., 2006, 55, 1612–1616 CrossRef CAS.
- H. Leutbecher, G. Greiner, R. Amann, A. Stolz, U. Beifuss and J. Conrad, Org. Biomol. Chem., 2011, 9, 2667–2673 RSC.
- K. Sharma, K. Neog and P. Gogoi, Org. Lett., 2020, 22, 73–77 CrossRef CAS PubMed.
- Y. Tani and T. Ogawa, Org. Lett., 2018, 20, 7442–7446 CrossRef CAS.
- (a) R. T. K. Kwok, C. W. T. Teung, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2015, 44, 4228–4238 RSC; (b) F. Hu, S. Xu and B. Liu, Adv. Mater., 2018, 30, 1801350–1801379 CrossRef; (c) X. Cai and B. Liu, Angew. Chem., Int. Ed., 2020, 59, 9868–9886 CrossRef CAS.
- Y. Tani and T. Ogawa, J. Mater. Chem. C, 2021, 9, 4281–4288 RSC.
- S. Maiti, S. K. Panja and C. Bandyopadhyay, J. Chem. Res., 2011, 35, 225–228 CrossRef.
- C. F. Huebner, W. R. Sullivan, M. A. Stahmann and K. P. Link, J. Am. Chem. Soc., 1943, 65, 2292 CrossRef CAS.
- M. A. Stahmann, L. H. Graf, C. F. Huebner, S. Roseman and K. P. Link, J. Am. Chem. Soc., 1944, 66, 900 CrossRef CAS.
- C. F. Huebner, S. A. Karjala, W. R. Sullivan and K. P. Link, J. Am. Chem. Soc., 1944, 66, 906 CrossRef CAS.
- N. Jagadishbabu and K. Shivashankar, J. Heterocycl. Chem., 2017, 54, 1543–1549 CrossRef CAS.
- H.-Z. He, K. Li, K.-K. Yu, P.-L. Lu, M.-L. Feng, S.-Y. Chen and X.-Q. Yu, Chem. Commun., 2020, 56, 6870–6873 RSC.
- K. Tanaka and T. Fujiwara, Org. Lett., 2005, 7, 3985–3988 CrossRef.
- F. Toda and K. Tanaka, Tetrahedron Lett., 1988, 29, 551 CrossRef CAS.
- M. Abdallh, M. T. Hearn, G. P. Simon and K. Saito, Polym. Chem., 2017, 8, 5875–5883 RSC.
- M. K. Węcławski, M. Tasior, T. Hammann, P. J. Cywiński and D. T. Gryko, Chem. Commun., 2014, 50, 9105–9108 RSC.
- M. K. Weclawski, M. Jakesova, M. Charyton, N. Demitri, B. Koszarna, K. Oppelt, S. Sariciftci, D. T. Gryko and E. Glowacki, J. Mater. Chem., 2017, 5, 20780–20788 RSC.
- A. Mukhopadhyay, K. Jana, T. Hossen, K. Sahu and J. N. Moorthy, J. Org. Chem., 2019, 84, 10658–10668 CrossRef CAS PubMed.
- J. N. Moorthy, S. Mandal, A. Mukhopadhyay and S. Samanta, J. Am. Chem. Soc., 2013, 135, 6872–6884 CrossRef CAS PubMed.
- C. V. Milyutin, B. V. Lichitsky, V. G. Melekhina, A. N. Komogortsev, A. N. Fakhrutdinov, M. E. Minyaev and M. M. Krayushkin, Tetrahedron Lett., 2020, 61, 152469 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |