Fabrication of diverse multicompartment micelles by redispersion of triblock terpolymer bulk morphologies†
Giada
Quintieri
ab,
Daniel
Schlattmann
ab,
Monika
Schönhoff
 ab and
André H.
Gröschel
ab and
André H.
Gröschel
 *ab
*ab
aPhysical Chemistry, University of Münster, Corrensstr. 28-30, 48149 Münster, Germany. E-mail: andre.groeschel@uni-muenster.de
bCenter for Soft Nanoscience (SoN), University of Münster, Busso-Peus-Str. 10, 48149 Münster, Germany
First published on 23rd August 2022
Abstract
Redispersing block copolymer (BCP) bulk films in selective solvents is a simple and efficient method to prepare BCP micelles and polymersomes. While ABC triblock terpolymers are known to form multicompartment micelles (MCMs) with intricate nanoarchitecture, this is typically done by solvent exchange instead of redispersion of bulk films despite obvious advantages of greatly reduced solvent usage. Here, we provide guidelines on how to form MCMs with defined shapes and inner structure through direct redispersion of terpolymer bulk morphologies in selective plasticizing solvents. For this purpose, we redisperse a series of polystyrene-b-polybutadiene-b-poly(tert-butyl methacrylate) (PS-b-PB-b-PT) triblock terpolymers in acetone/isopropanol mixtures, where PT is always soluble, PB always insoluble, and PS will range from soft (high acetone content) to kinetically frozen (high isopropanol content). We investigate the effect of solvent mixtures, block composition, and thermal annealing on MCM shape and core morphology. Additionally, we performed terpolymer blend experiments to open up a simple route to further diversify the range of accessible MCM morphologies.
Introduction
MCMs have attracted considerable interest over the last two decades in the scientific community, after the concept was first introduced by Ringsdorf in 1999.1 Indeed, MCMs represent a step forward from the more simplistic core–shell micelles and continue to blur the boundaries towards the structural complexity of biological nanostructures (e.g., the shape and patchy surface of virus nanoparticles). Today, MCMs have become vital components in the research on nanomedicine, catalysis, and nanotechnology.2–7A common method to produce MCMs is the solution self-assembly of three different blocks in which A is often denoted as the corona and B/C are incompatible solvophobic core segments.8 MCMs are predominantly obtained from linear ABC triblock terpolymers,9,10 ABC miktoarm star terpolymers,11,12 or blends of two diblock copolymers (AB/BC or AB/CD).13–16 Besides self-assembly of premade terpolymers, chain-extension of solvophilic precursors by polymerization-induced self-assembly (PISA) is another strategy17,18 leading to compartmentalized morphologies. Very recently, this concept was adapted to the polymerization-induced particle assembly of AB diblock micelles into triblock terpolymer clusters with high fraction of tetrahedral nanostructures.19 We previously demonstrated that a hierarchical self-assembly process by step-wise solvent exchange allows the formation of very homogeneous MCMs with predictable morphology thereby gaining access to patchy and striped spheres, cylinders, sheets and polymersomes.20–24 However, this technique is usually accompanied by a special preparation protocol and requires excessive amounts of solvents. A more straightforward way of preparing the wealth of MCMs with comparable quality would be desirable to become more attractive for applications.
The concept of nanostructure formation by film rehydration was first introduced in 1969![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 and exemplified on the rehydration of phospholipid films in order to form thin-walled vesicles. The methodology was then extended to AB diblock copolymers, i.e., thin films made of PEO-b-PCL were first formed by vacuum evaporation of organic solvents followed by rehydration of the bulk morphology and thermal annealing to obtain giant polymersomes.26 The microphase behavior of AB diblock copolymers is usually easier to be controlled and the morphology is mostly affected by the molar fraction of the blocks. On the other hand, ABC triblock terpolymers show a higher level of complexity due to the competition of three different interaction parameters χA, χB, χC as well as two distinct volume fractions ϕA, ϕB, (ϕC = 1 − ϕA − ϕB).27–30 ABC triblock terpolymers are known to form more complicated bulk morphologies,30 which also have been the prime source for Janus nanostructure.31 At the same time, in solution, the redispersion of triblock and tetrablock bulk films has been used before, but mainly for the formation of polymersomes.32–34 On the other hand, bulk film redispersion of linear ABC triblock terpolymers has not been studied much with the aim to form MCMs,35 but might represent an alternative route towards narrowly distributed nanostructures.
25 and exemplified on the rehydration of phospholipid films in order to form thin-walled vesicles. The methodology was then extended to AB diblock copolymers, i.e., thin films made of PEO-b-PCL were first formed by vacuum evaporation of organic solvents followed by rehydration of the bulk morphology and thermal annealing to obtain giant polymersomes.26 The microphase behavior of AB diblock copolymers is usually easier to be controlled and the morphology is mostly affected by the molar fraction of the blocks. On the other hand, ABC triblock terpolymers show a higher level of complexity due to the competition of three different interaction parameters χA, χB, χC as well as two distinct volume fractions ϕA, ϕB, (ϕC = 1 − ϕA − ϕB).27–30 ABC triblock terpolymers are known to form more complicated bulk morphologies,30 which also have been the prime source for Janus nanostructure.31 At the same time, in solution, the redispersion of triblock and tetrablock bulk films has been used before, but mainly for the formation of polymersomes.32–34 On the other hand, bulk film redispersion of linear ABC triblock terpolymers has not been studied much with the aim to form MCMs,35 but might represent an alternative route towards narrowly distributed nanostructures.
Here, we explore the redispersion of linear polystyrene-b-polybutadiene-b-poly(tert-butyl methacrylate) (PS-b-PB-b-PT or SBT) triblock terpolymers in acetone/isopropanol (Ace/IPA) mixtures where PT will form the corona, PB the core compartments, and PS the inner core. Thereby, we especially focus on soft/plasticizing solvent conditions (for PS) by continuously increasing the acetone content in 10 vol% steps, because a highly swollen core-forming block could allow for dramatic structural reorganization as well as structural optimization. Indeed, the solvent composition plays a key role in the control of the MCM shape as well as the core morphology. We also blended SBT triblock terpolymers with different block compositions to further expand the MCM library without the necessity for additional polymer synthesis.
Experimental section
Materials and methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA mixtures to give a final concentration of 2 g L−1. The Ace
IPA mixtures to give a final concentration of 2 g L−1. The Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA compositions was varied between 0%–100% Ace in 10 vol% steps. We prepared two sets of samples. One set was stirred at room temperature for 7 days and the other one was thermally annealed at 50 °C for 7 days while stirring.
IPA compositions was varied between 0%–100% Ace in 10 vol% steps. We prepared two sets of samples. One set was stirred at room temperature for 7 days and the other one was thermally annealed at 50 °C for 7 days while stirring.
Characterization
Nuclear Magnetic Resonance ( 1 H-NMR) spectra were recorded on a Magritek NMR (60 MHz) spectrometer with deuterated CDCl3 as solvent.Dynamic Light Scattering (DLS) measurements were performed on an ALV/CGS-3 (ALV-LSE-5004 correlator) goniometer system using a 35 mW He–Ne laser operating at a wavelength of λ = 633 nm. Sample temperature was always kept at 293.1 ± 0.2 K with an equilibration time of at least 10 min prior to each measurement to suppress convection. Toluene was used in the matching bath and the temperature was monitored with the goniometers built-in sensor. The observation angle was set to θ = 90°. All samples were prepared with a concentration of 5 g L−1 of polymer in the corresponding solvent composition. Refractive index and viscosity of the solvent mixtures were taken from previous work.23 To analyse the obtained correlation functions the CONTIN algorithm was applied by using the ALV Correlator Software (V.3.0.5.9).
Transmission Electron Microscopy (TEM) measurements were performed on a JEOL JEM-1400 Plus, operating at an accelerating voltage of 120 kV, a point resolution of 0.38 nm as well as a line resolution of 0.2 nm. Images were recorded with 16-bit 4096 × 4096 Pixel CMOS digital camera and processed with FIJI open-source software package.37 For sample preparation, one drop of the polymer solution (c = 0.5 g L−1) was deposited on a carbon-coated copper grid (200 mesh, Science Services) and excess solution was blotted after 30 s using filter paper and dried at room temperature. The samples were stained with OsO4 to enhance the contrast.
Results and discussion
Effect of thermal annealing
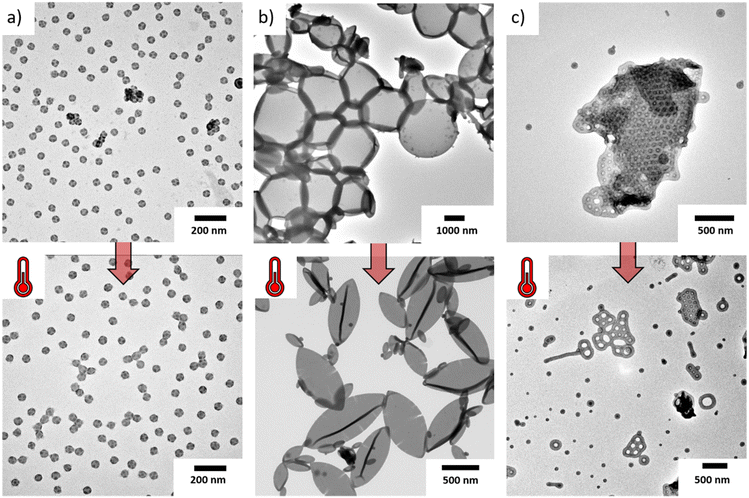
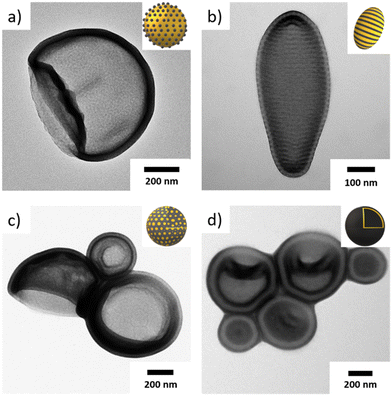
Before we start discussing the effect of block composition on structural features of the MCMs, we first studied the effect of the solvent composition on the morphology. More specifically, we chose Ace/IPA mixtures as re-dispersing solvent, because PT remains soluble in all compositions and serves as corona, while PB remains insoluble at all compositions forming the core compartments. Since PB has a Tg < 0 °C, it should not contribute to kinetic trapping. PS on the other hand has a Tg = 100 °C and potentially prevents re-dispersion of the bulk morphology depending on its weight fraction and the solvent composition. Pure IPA is a non-solvent for PS, while Ace is a θ-solvent, having a plasticizing effect on it, and thus, allowing for substantial structural reorganization upon re-dispersion.20,22,23,38–41We therefore went ahead and compared MCMs prepared through redispersion at room temperature for 7 days with those that were heated to 50 °C for 7 days. NMR measurements verified that thermal annealing did not noticeably affect the solvent compositions (variations <3 vol%) (Fig. S1 and S2†). The TEM images in Fig. 1 and DLS measurements (Fig. S3†) show the evolution of some examples before and after thermal annealing (the corresponding bulk morphologies can be found in Fig. S4†). For TEM, all samples were stained with OsO4 to enhance the contrast of the PB domain, which appears darkest. Fig. 1a shows S507B537T358 in Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 80
IPA 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v), which forms a sphere-on-sphere morphology, i.e. spherical MCMs with a PS core (grey) and spherical domains of PB (dark grey). Since this structure is entirely different than the lamella-lamella bulk morphology of S507B537T358 and thermal annealing did not further alter the structure, we assume to have reached thermodynamic equilibrium conditions in this solvent composition. This conclusion is also supported by DLS measurements (Fig. S3a and b†) where the samples retain a narrow size distribution before and after annealing. Solvent composition alone is however not a guarantee to reach equilibrium conditions as demonstrated on S307B525T76 (Fig. 1b). There, we obtained a core–shell gyroid morphology in bulk that first transformed into round polymersomes without noticeable membrane morphology in Ace
20 (v/v), which forms a sphere-on-sphere morphology, i.e. spherical MCMs with a PS core (grey) and spherical domains of PB (dark grey). Since this structure is entirely different than the lamella-lamella bulk morphology of S507B537T358 and thermal annealing did not further alter the structure, we assume to have reached thermodynamic equilibrium conditions in this solvent composition. This conclusion is also supported by DLS measurements (Fig. S3a and b†) where the samples retain a narrow size distribution before and after annealing. Solvent composition alone is however not a guarantee to reach equilibrium conditions as demonstrated on S307B525T76 (Fig. 1b). There, we obtained a core–shell gyroid morphology in bulk that first transformed into round polymersomes without noticeable membrane morphology in Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 80
IPA 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v), but after thermal annealing, developed into ellipsoidal polymersomes with PB cylinders on the PS membrane. Further overview images and close-ups of the PB cylinder morphology are summarized in Fig. S5 of the ESI.† We previously analysed these morphologies in detail with electron tomography and found that the anisotropy of the PB cylinders promoted the formation of topological defects leading to deformation of the polymersomes into elongated, ellipsoidal shapes.42 DLS measurements (Fig. S3c and d† dark blue curve) showed a broadening of the size distribution in Ace
20 (v/v), but after thermal annealing, developed into ellipsoidal polymersomes with PB cylinders on the PS membrane. Further overview images and close-ups of the PB cylinder morphology are summarized in Fig. S5 of the ESI.† We previously analysed these morphologies in detail with electron tomography and found that the anisotropy of the PB cylinders promoted the formation of topological defects leading to deformation of the polymersomes into elongated, ellipsoidal shapes.42 DLS measurements (Fig. S3c and d† dark blue curve) showed a broadening of the size distribution in Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 80
IPA 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v) after thermal annealing. The full width at half maximum (FWHM) and maximum at main peak are increased by factors of five and two, respectively, indicating changes in size and morphology. This difference in size and shape was also clearly observed in TEM. Thermal annealing and the plasticising properties of acetone allow for vast structural reorganization of the PS membrane, in this case leading to fission of larger spherical polymersomes (kinetic product) to the smaller ellipsoidal polymersomes (thermodynamic product). Reducing the solvent quality for the same polymer, i.e. S307B525T76 in Ace
20 (v/v) after thermal annealing. The full width at half maximum (FWHM) and maximum at main peak are increased by factors of five and two, respectively, indicating changes in size and morphology. This difference in size and shape was also clearly observed in TEM. Thermal annealing and the plasticising properties of acetone allow for vast structural reorganization of the PS membrane, in this case leading to fission of larger spherical polymersomes (kinetic product) to the smaller ellipsoidal polymersomes (thermodynamic product). Reducing the solvent quality for the same polymer, i.e. S307B525T76 in Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 30
IPA 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 (v/v), the high Tg = 100 °C of the PS domain does not allow redispersion at room temperature. Fig. 1c shows an ordered structure with hexagonal perforations resembling the bulk morphology. After thermal annealing, the bulk film separated completely into perforated sheets with PS core and PB shell (high genus bilayers).
70 (v/v), the high Tg = 100 °C of the PS domain does not allow redispersion at room temperature. Fig. 1c shows an ordered structure with hexagonal perforations resembling the bulk morphology. After thermal annealing, the bulk film separated completely into perforated sheets with PS core and PB shell (high genus bilayers).
Considering again the bulk morphologies of the SBTs (Fig. S4†), thermal annealing is certainly necessary to avoid kinetic trapping and fully develop the MCMs morphology, especially at higher IPA content and high weight fraction of PS. Throughout the rest of the manuscript we annealed all samples to discuss effects of solvent and block lengths on reproducible morphologies.
Controlling the MCM shape
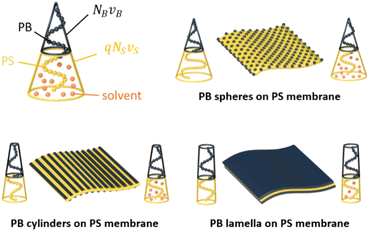
In this section, we discuss how the shape of the MCMs can be controlled by the solvophilic-to-solvophobic balance (SSB), i.e. the volume ratio of corona over the core domains. We define| SSB = Vcorona/Vcore = NTνT/(qNS2/3νS + NB2/3νB) |
A transition between morphologies should thus be possible via two pathways (Scheme 1): (1) by synthetically reducing the corona length (NT) and (2) by increasing the core volume either through block lengths (NS, NB) and/or selective de-/swelling (q). In the following we will discuss both pathways.
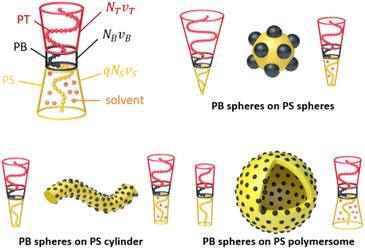 | ||
| Scheme 1 Controlling MCM shape. The shape of MCMs during redispersion is determined by the SBB that can be altered either synthetically by changing block lengths or by selective solvent de-/swelling. | ||
For pathway (1), we synthesized four SBTs with decreasing NT and thus decreasing Vcorona, while keeping the core volume comparable, i.e. all SBTs were re-dispersed in the same solvent mixture (Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 90
IPA 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
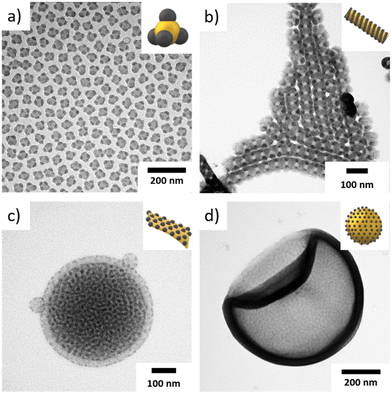
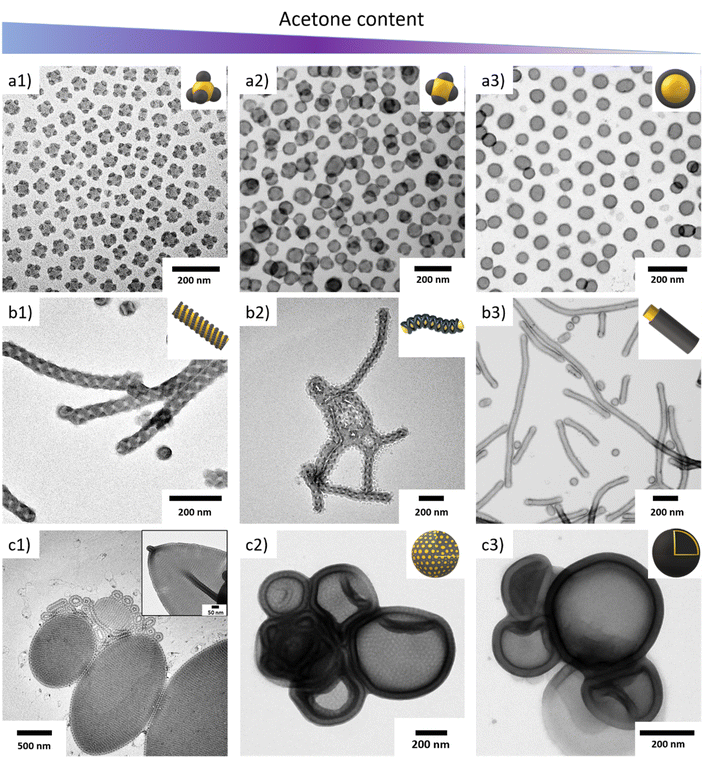
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (v/v)) and have comparable segment volumes (Fig. 2). S512B547T464 with an SSB = 1.27 formed MCMs with a spheres-on-sphere morphology. These MCMs are very uniform in size with a core diameter of Dsph = 50 nm and a high fraction of MCMs carried four spherical PB domains with a size of dPB = 20 nm in a tetrahedral arrangement (Fig. 2a, Fig. S6†). The overall narrow size distribution was also confirmed by DLS measurements (Fig. S3f†). It should be noted that nanoparticles with tetrahedral surface structure are rather rare19 and have not been observed in our previous works on solution self-assembly of ABC triblock terpolymers.20,23 These particles are of particular interest for higher hierarchical self-assembly. If it was possible to utilize these PB domains as surface patches for directional particle–particle interaction, a colloidal diamond lattice with nanoscale resolution would become accessible. Decreasing the SSB to 0.60 (S298B747T161 in Fig. 2b) reduced the surface curvature and we observed a transition to cylindrical MCMs with a core diameter of Dcyl = 60 nm and a double helical arrangement of the PB domain (Fig. S7†). This double helix has a thickness of dPB = 25 nm and a pitch of also about 25 nm. The reduced curvature and the large NB = 747 caused the spherical PB domains to overlap and merge into PB cylinders which wrap around the larger cylindrical PS core in a helical manner. This core morphology does not solely depend on NB, but rather on both NB and NS, but we will discuss these dependences later. Further lowering the SSB to 0.38 (S539B173T139 in Fig. 2c, overview TEM analysis in Fig. S8†), we observed bilayer discs with a diameter distribution of about Dbil = 250–350 nm. Bilayer discs can be identified in TEM due to a higher electron contrast in the centre (dark) as compared to lower electron contrast at the edges (bright). This is typical for bilayers, as the centre consists of a two tightly packed polymer layers, whereas the edge is merely a splayed monolayer.48–50
10 (v/v)) and have comparable segment volumes (Fig. 2). S512B547T464 with an SSB = 1.27 formed MCMs with a spheres-on-sphere morphology. These MCMs are very uniform in size with a core diameter of Dsph = 50 nm and a high fraction of MCMs carried four spherical PB domains with a size of dPB = 20 nm in a tetrahedral arrangement (Fig. 2a, Fig. S6†). The overall narrow size distribution was also confirmed by DLS measurements (Fig. S3f†). It should be noted that nanoparticles with tetrahedral surface structure are rather rare19 and have not been observed in our previous works on solution self-assembly of ABC triblock terpolymers.20,23 These particles are of particular interest for higher hierarchical self-assembly. If it was possible to utilize these PB domains as surface patches for directional particle–particle interaction, a colloidal diamond lattice with nanoscale resolution would become accessible. Decreasing the SSB to 0.60 (S298B747T161 in Fig. 2b) reduced the surface curvature and we observed a transition to cylindrical MCMs with a core diameter of Dcyl = 60 nm and a double helical arrangement of the PB domain (Fig. S7†). This double helix has a thickness of dPB = 25 nm and a pitch of also about 25 nm. The reduced curvature and the large NB = 747 caused the spherical PB domains to overlap and merge into PB cylinders which wrap around the larger cylindrical PS core in a helical manner. This core morphology does not solely depend on NB, but rather on both NB and NS, but we will discuss these dependences later. Further lowering the SSB to 0.38 (S539B173T139 in Fig. 2c, overview TEM analysis in Fig. S8†), we observed bilayer discs with a diameter distribution of about Dbil = 250–350 nm. Bilayer discs can be identified in TEM due to a higher electron contrast in the centre (dark) as compared to lower electron contrast at the edges (bright). This is typical for bilayers, as the centre consists of a two tightly packed polymer layers, whereas the edge is merely a splayed monolayer.48–50
The small NB = 173 led to spherical PB domains that decorate the PS surface in a hexagonal pattern. Since both sides of the sheet are visible at the same time (imaging in transmission) the hexagonal arrangement gives the impression of a Moiré pattern. Finally, at SSB = 0.25 (S539B173T89, Fig. 2d, overview TEM analysis in Fig. S9†), we obtained polymersomes. The NT was reduced to such an extent that dense chain packing in the core led to quasi planar arrangements. The PB domains are again spherical due to the short NB = 173 and decorate the in- and outside of the polymersome. Overall, these results demonstrate that – just like in solvent exchange methods – the shape of terpolymer-based MCMs can be predicted also for bulk film redispersion by considering the corona and core volume.
Next, we followed pathway (2) and changed the core volume while keeping NT constant. For that, S307B525T76 was re-dispersed and annealed in solvent compositions with decreasing q, i.e. with increasing IPA contents (Fig. 3, see also Fig. S4 & S10† for more solvent compositions and overview images). At a high Ace content, the PS domain is highly swollen, which increases the core volume qNS2/3νS and in turn decreases the SSB. In Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 90
IPA 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (v/v), S307B525T76 has the highest q and thus smallest SSB = 0.28.
10 (v/v), S307B525T76 has the highest q and thus smallest SSB = 0.28.
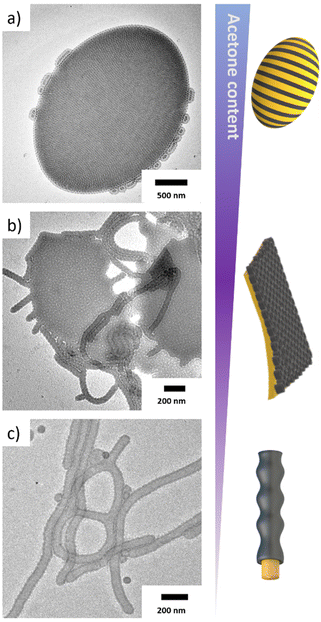
The expanded core promoted parallel packing of chains at the core/solvent interface and fostered the formation of polymersomes with a low curvature PS membrane (Fig. 3a, see also Fig. S5†). In case of S307B525T76, the NB = 525 led to PB cylinders on the PS membrane, which caused a deformation of the polymersomes into elongated, ellipsoidal shapes.42,51 Such anisotropic particles could of interest in nanomedicine, as the non-spherical shapes might be beneficial for higher cell uptake.52,53 While this structure persisted at Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 80
IPA 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v) (compare Fig. 1b), the structure changed to bilayer sheets at Ace
20 (v/v) (compare Fig. 1b), the structure changed to bilayer sheets at Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 70
IPA 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (v/v) (Fig. 3b). The shrinking core volume increased the SSB = 0.38 stabilizing higher average core curvature, which is why also cylinders were observed alongside the bilayer sheets. The transitions from polymersome to sheets with cylinders attached (or vice versa) is reminiscent to the formation of diblock copolymer octopi and jelly-fish intermediates.50,54 The PB microdomain adopts a perforated structure noticeable on the bright spherical spots where only the underlying PS membrane is visible. The observed pattern is reminiscent of either a hexagonally perforated shell or a bicontinous core morphology with interpenetrating PS/PB domains. Further increase of the IPA content to Ace
30 (v/v) (Fig. 3b). The shrinking core volume increased the SSB = 0.38 stabilizing higher average core curvature, which is why also cylinders were observed alongside the bilayer sheets. The transitions from polymersome to sheets with cylinders attached (or vice versa) is reminiscent to the formation of diblock copolymer octopi and jelly-fish intermediates.50,54 The PB microdomain adopts a perforated structure noticeable on the bright spherical spots where only the underlying PS membrane is visible. The observed pattern is reminiscent of either a hexagonally perforated shell or a bicontinous core morphology with interpenetrating PS/PB domains. Further increase of the IPA content to Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 40
IPA 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 (v/v) gives an SSB = 0.65 and a complete transition to cylindrical MCMs. These have a Dcyl = 70 nm and a core–shell morphology, where PB completely engulfs the PS core (Fig. 3c). Despite changing to SBTs with different NS or NB (S307B379T82 and S305B523T64) or further decreasing q, we were not able to develop spherical MCMs, which suggests that a larger NT is required to stabilize the high curvature core. On closer inspection of the structure in Fig. 3c, the PB shell showed some irregularities or undulations and did not seem to be fully developed, yet. Further reduction of the acetone content did not improve the shell homogeneity, but instead prevented the complete redispersion of the bulk film. As we will show later, a complete PB shell can be obtained by increasing the PB content in the terpolymer.
60 (v/v) gives an SSB = 0.65 and a complete transition to cylindrical MCMs. These have a Dcyl = 70 nm and a core–shell morphology, where PB completely engulfs the PS core (Fig. 3c). Despite changing to SBTs with different NS or NB (S307B379T82 and S305B523T64) or further decreasing q, we were not able to develop spherical MCMs, which suggests that a larger NT is required to stabilize the high curvature core. On closer inspection of the structure in Fig. 3c, the PB shell showed some irregularities or undulations and did not seem to be fully developed, yet. Further reduction of the acetone content did not improve the shell homogeneity, but instead prevented the complete redispersion of the bulk film. As we will show later, a complete PB shell can be obtained by increasing the PB content in the terpolymer.
Controlling the core morphology
We next discuss how the core morphology is affected by the volume fraction of the PB block, ϕPB. An increase in ϕPB should lead to a transition from PB spheres to cylinders, bicontinous, and lamellae, in line with ABC triblock terpolymers self-assembled through solvent exchange.23 The core morphology is coarsely governed by| ϕB = NB2/3νB/(qNS2/3νS + NB2/3νB) |
We first investigated core morphologies after synthetically increasing NB, while maintaining a similar SSB ≈ 0.2 and keeping the solvent composition constant at Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 80
IPA 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v). Under these conditions, the four selected SBTs all resulted in polymersomes with different membrane morphology due to the different ϕB (Fig. 4). Starting with a ϕPB = 0.03 (S539B173T89), spherical PB domains formed on the inside and outside of the polymersome membrane (Fig. 4a, see also Fig. S8†). In an analogy to the bulk case, this morphology resembles a classical bcc sphere morphology (although a defect-free bcc lattice would not be stable on a sphere). Increasing the ϕPB to 0.09 (S310B383T57, in Ace
20 (v/v). Under these conditions, the four selected SBTs all resulted in polymersomes with different membrane morphology due to the different ϕB (Fig. 4). Starting with a ϕPB = 0.03 (S539B173T89), spherical PB domains formed on the inside and outside of the polymersome membrane (Fig. 4a, see also Fig. S8†). In an analogy to the bulk case, this morphology resembles a classical bcc sphere morphology (although a defect-free bcc lattice would not be stable on a sphere). Increasing the ϕPB to 0.09 (S310B383T57, in Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 90
IPA 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (v/v)) led to a morphological transition from PB spheres to cylinders (Fig. 4b, see also Fig. S5†). As before, polymersomes with an anisotropic cylinder morphology in the membrane adopt an elongated shape. A subsequent increase of the ϕPB = 0.13 (S305B523T64) resulted in a bicontinous membrane (Fig. 4c, see also Fig. S9†), and finally, a lamellar (core–shell) membrane at ϕPB = 0.18 (S300B754T57) (Fig. 4d, see also Fig. S10†). It can be noticed that while all morphologies are found, they form at smaller ϕPB values as the corresponding classical bulk morphologies. For simplicity we assume that the volume of the PB microdomain is unaffected by the Ace or IPA content, which is simply based on the interaction parameters. However, some swelling by the solvent (e.g. through osmotic pressure) likely increased the PB volume, which is why the presented values for ϕPB are probably underestimated.
10 (v/v)) led to a morphological transition from PB spheres to cylinders (Fig. 4b, see also Fig. S5†). As before, polymersomes with an anisotropic cylinder morphology in the membrane adopt an elongated shape. A subsequent increase of the ϕPB = 0.13 (S305B523T64) resulted in a bicontinous membrane (Fig. 4c, see also Fig. S9†), and finally, a lamellar (core–shell) membrane at ϕPB = 0.18 (S300B754T57) (Fig. 4d, see also Fig. S10†). It can be noticed that while all morphologies are found, they form at smaller ϕPB values as the corresponding classical bulk morphologies. For simplicity we assume that the volume of the PB microdomain is unaffected by the Ace or IPA content, which is simply based on the interaction parameters. However, some swelling by the solvent (e.g. through osmotic pressure) likely increased the PB volume, which is why the presented values for ϕPB are probably underestimated.
Nevertheless, the morphological trend from spheres to cylinders to bicontinous and lamellar can still be controlled by increasing values of ϕPB and follows the same trend as in bulk.
Next, we discuss the change of the core morphology by solvent composition. For that we re-dispersed the same SBT in different Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA mixtures while keeping the shape constant (Fig. 5 and Fig. S11†). We discuss the effect of solvent composition on three different SBTs that form spheres, cylinders and polymersomes. For instance, S512B547T464 forms narrowly-dispersed tetrahedral MCMs with a PS core of Dsph = 50 nm and PB domains of dPB = 20 nm in Ace
IPA mixtures while keeping the shape constant (Fig. 5 and Fig. S11†). We discuss the effect of solvent composition on three different SBTs that form spheres, cylinders and polymersomes. For instance, S512B547T464 forms narrowly-dispersed tetrahedral MCMs with a PS core of Dsph = 50 nm and PB domains of dPB = 20 nm in Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 90
IPA 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (v/v) (Fig. 5a). The observed MCM shape and core morphology are expected from the SSB = 1.27 and the ϕB = 0.07. Increasing the IPA content to Ace
10 (v/v) (Fig. 5a). The observed MCM shape and core morphology are expected from the SSB = 1.27 and the ϕB = 0.07. Increasing the IPA content to Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 60
IPA 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 (v/v), the spherical MCM shape persists (SSB = 2.08), however, the previously spherical PB domains start to spread on the PS core as a result of increase in ϕB = 0.08 and lower PS compatibility with the solvent (Fig. 5a2). The interfacial energy between solvent and PS increased with increasing IPA content, while it remained more or less the same for PB. Energetically, it should therefore be beneficial, if PB spreads onto the PS core to prevent the increasingly unfavourable PS/solvent interface. The transition to spherical core–shell-corona MCMs is completed in Ace
40 (v/v), the spherical MCM shape persists (SSB = 2.08), however, the previously spherical PB domains start to spread on the PS core as a result of increase in ϕB = 0.08 and lower PS compatibility with the solvent (Fig. 5a2). The interfacial energy between solvent and PS increased with increasing IPA content, while it remained more or less the same for PB. Energetically, it should therefore be beneficial, if PB spreads onto the PS core to prevent the increasingly unfavourable PS/solvent interface. The transition to spherical core–shell-corona MCMs is completed in Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 10
IPA 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 (v/v) (SSB = 5.58, ϕB = 0.12). MCMs still display a core diameter of Dsph = 50 nm, but now covered by a complete PB shell with a thickness of dPB = 10 nm (Fig. 5a3). Note that the dimension of PB continuously decreased, because the four spherical PB domains progressively flattened to cover the entire core surface. Another example is given by S298B747T161 that demonstrated a double helix on cylinder morphology in Ace
90 (v/v) (SSB = 5.58, ϕB = 0.12). MCMs still display a core diameter of Dsph = 50 nm, but now covered by a complete PB shell with a thickness of dPB = 10 nm (Fig. 5a3). Note that the dimension of PB continuously decreased, because the four spherical PB domains progressively flattened to cover the entire core surface. Another example is given by S298B747T161 that demonstrated a double helix on cylinder morphology in Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 90
IPA 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (v/v) (SSB = 0.60 and ϕB = 0.16) with a core diameter Dcyl = 60 nm and a helix thickness of dPB = 25 nm (Fig. 5b1). At a composition of Ace
10 (v/v) (SSB = 0.60 and ϕB = 0.16) with a core diameter Dcyl = 60 nm and a helix thickness of dPB = 25 nm (Fig. 5b1). At a composition of Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 70
IPA 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (v/v) (SSB = 0.79 and ϕB = 0.21) the MCMs are still cylinders, but the PB domains changed into a ribbon-like morphology, possibly a perforated shell (Fig. 5b2). A perforated shell could be realized if PB form two double helices instead of one (one left handed and one right handed). The soft PB helices could easily merge into a perforated shell as previously observed in bulk for PB cylinders in lamella-cylinder morphologies.55 Again, a complete transition to core–shell-corona cylinders can be achieved at higher IPA contents of Ace
30 (v/v) (SSB = 0.79 and ϕB = 0.21) the MCMs are still cylinders, but the PB domains changed into a ribbon-like morphology, possibly a perforated shell (Fig. 5b2). A perforated shell could be realized if PB form two double helices instead of one (one left handed and one right handed). The soft PB helices could easily merge into a perforated shell as previously observed in bulk for PB cylinders in lamella-cylinder morphologies.55 Again, a complete transition to core–shell-corona cylinders can be achieved at higher IPA contents of Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 40
IPA 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 (v/v) (SSB = 1.31 and ϕB = 0.33) (Fig. 5b3). The core diameter decreased slightly to Dcyl = 45 nm, while the PB shell thickness was in the range of dPB = 15 nm. Finally, Fig. 5c1 shows elongated polymersomes with a PB cylinder morphology for S310B383T57 in Ace
60 (v/v) (SSB = 1.31 and ϕB = 0.33) (Fig. 5b3). The core diameter decreased slightly to Dcyl = 45 nm, while the PB shell thickness was in the range of dPB = 15 nm. Finally, Fig. 5c1 shows elongated polymersomes with a PB cylinder morphology for S310B383T57 in Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 90
IPA 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (v/v) (SBB = 0.22 and ϕB = 0.08). An increase of the IPA content to Ace
10 (v/v) (SBB = 0.22 and ϕB = 0.08). An increase of the IPA content to Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 70
IPA 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (v/v) maintains the polymersome shape (SSB = 0.29), but alters the membrane morphology to bicontinous (ϕB = 0.12) (Fig. 5c2). At an Ace
30 (v/v) maintains the polymersome shape (SSB = 0.29), but alters the membrane morphology to bicontinous (ϕB = 0.12) (Fig. 5c2). At an Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 30
IPA 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 (v/v) the membrane morphology fully transitioned and core–shell-corona polymersome are observed (SBB = 0.60 and ϕB = 0.15) (Fig. 5c3).
70 (v/v) the membrane morphology fully transitioned and core–shell-corona polymersome are observed (SBB = 0.60 and ϕB = 0.15) (Fig. 5c3).
MCMs by polymer blending
Lastly, we show that redispersion of bulk films can be used as a tool for the formation of a large variety of MCM morphologies without the need of further polymer synthesis. Specifically, we have performed a polymer blending study in which two SBT bulk films were re-dispersed in Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA mixture in a ratio of 1
IPA mixture in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
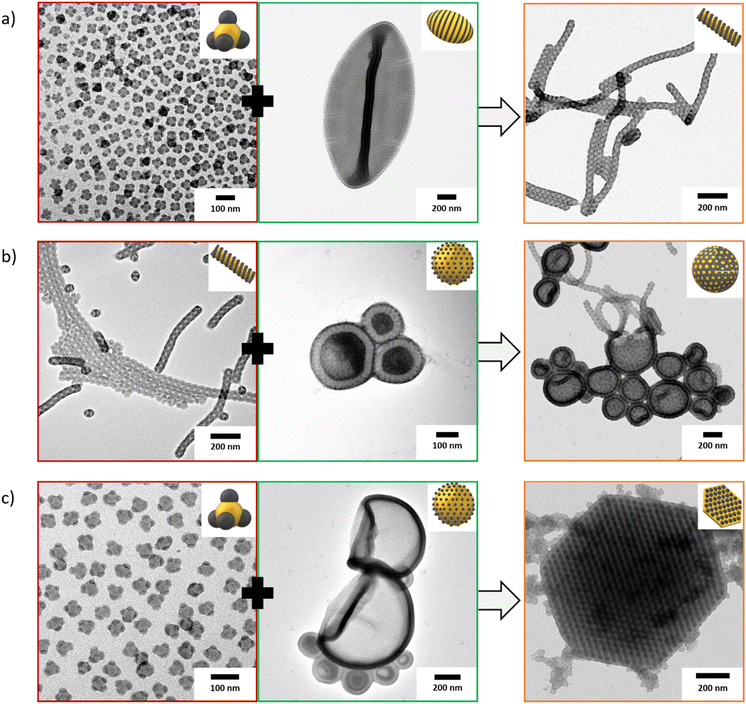
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) and at a concentration of 2 g L−1. The dispersion was thermally annealed for 7 days at 50 °C to allow the bulk films to melt and SBTs to mix into a blended structure. The morphology of the blended structure should then correspond to the averaged SSB and ϕB of both employed SBTs. We prepared three different blends of different SBTs (Table 1) and the resulting MCMs are summarized in Fig. 6. First, we mixed spheres-on-sphere MCMs (S512B547T464) with cylinders-on-polymersomes (S305B523T64) in Ace
1 (w/w) and at a concentration of 2 g L−1. The dispersion was thermally annealed for 7 days at 50 °C to allow the bulk films to melt and SBTs to mix into a blended structure. The morphology of the blended structure should then correspond to the averaged SSB and ϕB of both employed SBTs. We prepared three different blends of different SBTs (Table 1) and the resulting MCMs are summarized in Fig. 6. First, we mixed spheres-on-sphere MCMs (S512B547T464) with cylinders-on-polymersomes (S305B523T64) in Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 90
IPA 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (v/v) (Fig. 6a). The theoretical composition calculated from the respective chain lengths resulted in an apparent SSB = 0.64 and ϕB = 0.09, which should lead to the formation of cylindrical MCMs with a double helix morphology. We indeed identified such MCMs after blending, confirming that equilibration is possible and that the combined SBTs seem to be able to mix and melt during thermal annealing despite both polymers being supplied as bulk films. Next, we mixed double helix cylinders (S298B747T161) with spheres-on-polymersomes (S539B173T89) in Ace
10 (v/v) (Fig. 6a). The theoretical composition calculated from the respective chain lengths resulted in an apparent SSB = 0.64 and ϕB = 0.09, which should lead to the formation of cylindrical MCMs with a double helix morphology. We indeed identified such MCMs after blending, confirming that equilibration is possible and that the combined SBTs seem to be able to mix and melt during thermal annealing despite both polymers being supplied as bulk films. Next, we mixed double helix cylinders (S298B747T161) with spheres-on-polymersomes (S539B173T89) in Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 90
IPA 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (v/v) to yield a theoretical composition of SSB = 0.38 and ϕB = 0.07, and thus, bilayer sheets with a cylinder morphology.
10 (v/v) to yield a theoretical composition of SSB = 0.38 and ϕB = 0.07, and thus, bilayer sheets with a cylinder morphology.
| Polymers | Blend | |||||||
|---|---|---|---|---|---|---|---|---|
| Polymer1a | SSB1 | ϕ B,1 | Polymer2a | SSB2 | ϕ B,2 | Composition (theo.)a | SSBtheo | ϕ B,theo |
| a Subscripts denote the degree of polymerization. b Calculations based on q = 7.4 in 90 v% acetone. c Calculations based on q = 6.8 in 80 v% acetone. | ||||||||
| S512B547T464 | 1.27b | 0.08b | S305B523T64 | 0.24b | 0.12b | S371B531T191 | 0.64b | 0.10b |
| S298B747T161 | 0.60b | 0.16b | S539B173T89 | 0.25b | 0.02b | S429B433T122 | 0.38b | 0.07b |
| S512B547T464 | 1.38c | 0.08c | S539B173T139 | 0.42c | 0.03c | S529B309T258 | 0.77c | 0.05c |
However, instead we identify jelly-fish polymersomes with a bicontinous membrane and cylinder micelles attached as tentacles (Fig. 6b). This difference might be explained by an incomplete morphological transition due to a higher PS content of the polymersomes forming SBT. Lastly, we selected an SBT that forms spheres-on-spheres (S512B547T464) and mixed them with spheres-on-polymersomes (S539B173T139) in Ace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 80
IPA 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v) (Fig. 6c). The blended polymers with a theoretical composition of SSB = 0.77 and ϕB = 0.05 formed micro-sized prismatic platelets containing hexagonally packed core–shell cylinders with a diameter of around 25 nm.
20 (v/v) (Fig. 6c). The blended polymers with a theoretical composition of SSB = 0.77 and ϕB = 0.05 formed micro-sized prismatic platelets containing hexagonally packed core–shell cylinders with a diameter of around 25 nm.
Surrounding the hexagonal platelets, we observe spherical micelles, which may be attributed to remaining spherical MCMs that did not undergo the mix and melt process, and instead, agglomerated around the platelets as an effect of drying on the TEM grid. This blend morphology also reveals the limitations of this process, because S539B173T139 forms a core–shell cylinder bulk morphology that probably did not fully disintegrate under our experimental conditions. Instead, the S512B547T464 with a large and swollen PT corona likely dispersed first and served as surfactant to stabilize the micron-sized pieces of the bulk film. The pronounced hexagonal shape is remarkable and probably originates from the hexagonal inner order of the cylinder arrangement. The stability of these structures is under current investigation, as we still identify them in large quantity despite prolonged thermal annealing times.
Conclusions
In summary, we have provided guidelines to prepare libraries of MCMs with controlled shape and core morphology by straightforward bulk film redispersion. The shape and core morphology are both affected by the length of the respective polymer blocks; however, they are also affected by the solvent composition so that shape and core morphology can be altered for the same triblock terpolymer simply by changing the solvent composition. For our SBT system a decrease in acetone content led to less swollen PS cores and therefore MCMs with higher core curvature (sphere, cylinder, polymersome). The core morphology is likewise affected by the solvent due to a lowered affinity of PS towards the solvent, enhancing the interaction with the PB phase. We further showed that redispersion of two bulk films with different morphologies provides a route to a larger range of MCMs morphologies without the need of polymer synthesis. In particular, we have formed hexagonal plates with hexagonally packed PB cylinders. These microparticles inspire the formation of porous hexosomes after removal of the PB phase (e.g. ozonolysis) and might be relevant for applications requiring addressable surfaces and high surface area.56Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the Imaging Center Essen (IMCES) at the university clinic Essen where the TEM data was recorded. GQ and AHG are grateful for financial support from the DFG through the Emmy Noether Program (GR5075/2-1).References
- H. Ringsdorf, P. Lehmann and R. Weberskirch, Book of Abstracts, 217th ACS National Meeting, American Chemical Society, 1999 Search PubMed.
- A. C. Rinkenauer, A. Schallon, U. Günther, M. Wagner, E. Betthausen, U. S. Schubert and F. H. Schacher, ACS Nano, 2013, 7, 9621–9631 CrossRef CAS PubMed.
- M. Wu, Y. Zhu and W. Jiang, Angew. Chem., Int. Ed., 2018, 57, 3578–3582 CrossRef CAS PubMed.
- C. V. Synatschke, T. Nomoto, H. Cabral, M. Förtsch, K. Toh, Y. Matsumoto, K. Miyazaki, A. Hanisch, F. H. Schacher, A. Kishimura, N. Nishiyama, A. H. E. Müller and K. Kataoka, ACS Nano, 2014, 8, 1161–1172 CrossRef CAS PubMed.
- T. Nomoto, S. Fukushima, M. Kumagai, K. Machitani, Arnida, Y. Matsumoto, M. Oba, K. Miyata, K. Osada, N. Nishiyama and K. Kataoka, Nat. Commun., 2014, 5(1), 3545 CrossRef PubMed.
- R. J. R. W. Peters, M. Marguet, S. Marais, M. W. Fraaije, J. C. M. Van Hest and S. Lecommandoux, Angew. Chem., Int. Ed., 2014, 53, 146–150 CrossRef CAS PubMed.
- T. P. Lodge, A. Rasdal, Z. Li and M. A. Hillmyer, J. Am. Chem. Soc., 2005, 127, 17608–17609 CrossRef CAS PubMed.
- A. H. Gröschel and A. H. E. Müller, Nanoscale, 2015, 7, 11841–11876 RSC.
- Z. Ma, H. Yu and W. Jiang, J. Phys. Chem. B, 2009, 113, 3333–3338 CrossRef CAS PubMed.
- F. Schacher, A. Walther, M. Ruppel, M. Drechsler and A. H. E. Müller, Macromolecules, 2009, 42, 3540–3548 CrossRef CAS.
- H. Huo, J. Liu, S. Kannan, L. Chen, Y. Zhao, L. Zhang, G. Chang, Q. Zhang and F. Liu, Macromolecules, 2021, 54, 35–43 CrossRef CAS.
- Z. Li, E. Kesselman, Y. Talmon, M. A. Hillmyer and T. P. Lodge, Science, 2004, 306, 98–101 CrossRef CAS PubMed.
- V. V. Palyulin and I. I. Potemkin, Macromolecules, 2008, 41, 4459–4463 CrossRef CAS.
- G. Li, L. Shi, R. Ma, Y. An and N. Huang, Angew. Chem., Int. Ed., 2006, 45, 4959–4962 CrossRef CAS PubMed.
- I. K. Voets, A. De Keizer, P. De Waard, P. M. Frederik, P. H. H. Bomans, H. Schmalz, A. Walther, S. M. King, F. A. M. Leermakers and M. A. Cohen Stuart, Angew. Chem., Int. Ed., 2006, 45, 6673–6676 CrossRef CAS PubMed.
- I. K. Voets, A. De Keizer, M. A. Cohen Stuart, J. Justynska and H. Schlaad, Macromolecules, 2007, 40, 2158–2164 CrossRef CAS.
- M. J. Derry, L. A. Fielding and S. P. Armes, Prog. Polym. Sci., 2016, 52, 1–18 CrossRef CAS.
- F. D'Agosto, J. Rieger and M. Lansalot, Angew. Chem., Int. Ed., 2020, 59, 8368–8392 CrossRef PubMed.
- D. Li, N. Liu, M. Zeng, J. Ji, X. Chen and J. Yuan, Polym. Chem., 2022, 3529–3538 RSC.
- A. H. Gröschel, F. H. Schacher, H. Schmalz, O. V. Borisov, E. B. Zhulina, A. Walther and A. H. E. Müller, Nat. Commun., 2012, 3, 710 CrossRef PubMed.
- A. H. Gröschel, A. Walther, T. I. Löbling, J. Schmelz, A. Hanisch, H. Schmalz and A. H. E. Müller, J. Am. Chem. Soc., 2012, 134, 13850–13860 CrossRef PubMed.
- A. H. Gröschel, A. Walther, T. I. Löbling, F. H. Schacher, H. Schmalz and A. H. E. Müller, Nature, 2013, 503, 247–251 CrossRef PubMed.
- T. I. Löbling, O. Borisov, J. S. Haataja, O. Ikkala, A. H. Gröschel and A. H. E. Müller, Nat. Commun., 2016, 7, 12097 CrossRef PubMed.
- D. Coban, O. Gridina, M. Karg and A. H. Gröschel, Macromolecules, 2022, 55, 1354–1364 CrossRef CAS.
- J. P. Reeves and R. M. Dowben, J. Cell. Physiol., 1969, 73, 49–60 CrossRef CAS PubMed.
- W. Qi, P. P. Ghoroghchian, G. Li, D. A. Hammer and M. J. Therien, Nanoscale, 2013, 5, 10908–10915 RSC.
- F. S. Bates and G. H. Fredrickson, Phys. Today, 1999, 52, 32–38 CrossRef CAS.
- U. Breiner, U. Krappe, T. Jakob, V. Abetz and R. Stadler, Polym. Bull., 1998, 40, 219–226 CrossRef CAS.
- U. Breiner, U. Krappe, V. Abetz and R. Stadler, Macromol. Chem. Phys., 1997, 198, 1051–1083 CrossRef CAS.
- R. Stadler, C. Auschra, J. Beckmann, U. Krappe, I. Voigt-Martin and L. Leibler, Macromolecules, 1995, 28, 3080–3097 CrossRef CAS.
- A. Walther and A. H. E. Müller, Chem. Rev., 2013, 113, 5194–5261 CrossRef CAS PubMed.
- C. Zhou, M. A. Hillmyer and T. P. Lodge, Macromolecules, 2011, 44, 1635–1641 CrossRef CAS.
- A. K. Brannan and F. S. Bates, Macromolecules, 2004, 37, 8816–8819 CrossRef CAS.
- M. T. Popescu, M. Korogiannaki, K. Marikou and C. Tsitsilianis, Polymer, 2014, 55, 2943–2951 CrossRef CAS.
- W. Zhao, D. Chen, Y. Hu, G. M. Grason and T. P. Russell, ACS Nano, 2011, 5, 486–492 CrossRef CAS PubMed.
- T. I. Löbling, P. Hiekkataipale, A. Hanisch, F. Bennet, H. Schmalz, O. Ikkala, A. H. Gröschel and A. H. E. Müller, Polymer, 2015, 72, 479–489 CrossRef.
- J. Schindelin, I. Arganda-Carreras, E. Frise, V. Kaynig, M. Longair, T. Pietzsch, S. Preibisch, C. Rueden, S. Saalfeld, B. Schmid, J. Y. Tinevez, D. J. White, V. Hartenstein, K. Eliceiri, P. Tomancak and A. Cardona, Nat. Methods, 2012, 9, 676–682 CrossRef CAS PubMed.
- G. Quintieri and A. H. Gröschel, Polym. Chem., 2021, 12, 1429–1438 RSC.
- S. Jain and F. S. Bates, Science, 2003, 300, 460–464 CrossRef CAS PubMed.
- Y. Mai and A. Eisenberg, Chem. Soc. Rev., 2012, 41, 5969–5985 RSC.
- T. L. Nghiem, T. I. Löbling and A. H. Gröschel, Polym. Chem., 2018, 9, 1583–1592 RSC.
- T. I. Gröschel, C. K. Wong, J. S. Haataja, M. A. Dias and A. H. Gröschel, ACS Nano, 2020, 14, 4829–4838 CrossRef PubMed.
- D. Han, X. Li, S. Hong, H. Jinnai and G. Liu, Soft Matter, 2012, 8, 2144–2151 RSC.
- S. Schrage, R. Sigel and H. Schlaad, Macromolecules, 2003, 36, 1417–1420 CrossRef CAS.
- L. Zhang and A. Eisenberg, J. Am. Chem. Soc., 1996, 118, 3168–3181 CrossRef CAS.
- L. Zhang and A. Eisenberg, Science, 1995, 268, 1728–1731 CrossRef CAS PubMed.
- J. N. Israelachvili, D. J. Mitchell and B. W. Ninham, J. Chem. Soc., Faraday Trans. 2, 1976, 72, 1525–1568 RSC.
- C. K. Wong, M. H. Stenzel and P. Thordarson, Chem. Soc. Rev., 2019, 48, 4019–4035 RSC.
- L. Zhang and A. Eisenberg, Macromolecules, 1999, 32, 2239–2249 CrossRef CAS.
- A. Blanazs, J. Madsen, G. Battaglia, A. J. Ryan and S. P. Armes, J. Am. Chem. Soc., 2011, 133, 16581–16587 CrossRef CAS PubMed.
- C. K. Wong, M. Heidelmann, M. Dulle, X. Qiang, S. Förster, M. H. Stenzel and A. H. Gröschel, J. Am. Chem. Soc., 2020, 142, 10989–10995 CrossRef CAS PubMed.
- C. K. Wong, A. D. Martin, M. Floetenmeyer, R. G. Parton, M. H. Stenzel and P. Thordarson, Chem. Sci., 2019, 10, 2725–2731 RSC.
- L. Florez, C. Herrmann, J. M. Cramer, C. P. Hauser, K. Koynov, K. Landfester, D. Crespy and V. Mailänder, Small, 2012, 8, 2222–2230 CrossRef CAS PubMed.
- M. Antonietti and S. Förster, Adv. Mater., 2003, 15, 1323–1333 CrossRef CAS.
- A. Steinhaus, D. Srivastva, A. Nikoubashman and A. H. Gröschel, Polymers, 2019, 11, 1107 CrossRef PubMed.
- S. J. Jeon, G. R. Yi and S. M. Yang, Adv. Mater., 2008, 20, 4103–4108 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2nr03874a |
| This journal is © The Royal Society of Chemistry 2022 |