Open Access Article
Open Access ArticleDNA/RNA sequencing using germanene nanoribbons via two dimensional molecular electronic spectroscopy: an ab initio study†
M.
Reza Rezapour
 * and
Blanca
Biel
* and
Blanca
Biel

Department of Atomic, Molecular and Nuclear Physics, Faculty of Science, Campus de Fuente Nueva, University of Granada, 18071 Granada, Spain. E-mail: rezapour@ugr.es
First published on 3rd March 2022
Abstract
Developing fast, reliable, and cost effective, yet practical DNA/RNA sequencing methods and devices is a must. In this regard, motivated by the recently introduced two-dimensional electronic molecular spectroscopy (2DMES) technique for molecular recognition, and the compatibility of 2D layers of group IV elements with the current technology of manufacturing electronic devices, we investigate the capability of germanene nanoribbons (GeNRs) as a feasible, accurate, and ultra-fast sequencing device under the application of 2DMES. We show that by employing 2DMES, not only can GeNRs unambiguously distinguish different nucleobases to sequence DNA/RNA, they are also capable of recognizing methylated nucleobases that could be related to cancerous cell growth. Our calculations indicate that, compared to frequently used graphene layers, germanene provides more distinct adsorption energies for different nucleobases which implies its better ability to recognize various molecules unambiguously. By calculating the conductance sensitivity of the system for experimental purposes, we also show that the introduced sequencing device possesses a high sensitivity and selectivity characteristic. Thus, our proposed system would be a promising device for next-generation DNA sequencing technologies and would be realizable using the current protocols of fabricating electronic devices.
Introduction
2D materials have progressed rapidly across a diversity of scientific and engineering subfields, including next-generation nanoelectronics, owing to their fascinating electrical, optical, chemical, thermal, and structural properties.1–7 Among the 2D materials, graphene has been the epitome of all such materials.8,9 Graphene and graphene nanoribbons have diverse applications in various sectors from electronics to energy, to health and to the environment.10–25 The wide application range of graphene has motivated experimental research to explore other 2D compounds, such as hexagonal boron-nitride,26,27 silicene,28,29 germanene,30 stanene,31 phosphorene,32,33 and transition metal dichalcogenides,34,35 Among graphene-analogue 2D monolayers, those composed of group IV elements such as silicene36–38 and germanene39–41 are of particular interest due to their full adaptability with the currently in-use protocols of device fabrication and integration in the present silicon and germanium based semiconductor technologies. They also show excellent flexibility and compressibility relative to graphene, while strain engineering has been proposed to modify their electronic and thermal properties. Such aspects make these materials an acceptable candidate for creating nano-electromechanical systems and sensors that can integrate well with the current generation of device technology.42 In line with the efforts for the practical exploitation of these 2D materials, hydrogen-terminated germanene was synthesized by adding covalently bonded hydrogen on both sides of germanene.43 Recently, Chen et al. showed that thermally annealed germanene has structural stability at room temperature and exhibits a highly conductive metallic state.44 However, for nanoelectronics applications, the material requirement is to be semiconductive and with a tunable band gap. Since the quantum confinement is a known technique for band gap modulation, GeNR in both armchair GeNR (AGeNRs) and zigzag GeNR (ZGeNR) forms were studied.44 It was shown that the band gap of AGeNR varies in an oscillatory motion by increasing its width, whereas ZGeNR shows a ferromagnetic behavior along its edges.45,46It should be noted that the crystal structure of germanene does not exactly resemble that of graphene, as it exhibits a buckled honeycomb structure instead of the flat honeycomb phase of graphene.41,47 Intriguingly, unlike carbon atoms in graphene, in germanene, germanium atoms tend to adopt sp3 hybridization over sp2, which makes it highly chemically active on the surface and hence promising for applications such as batteries,48 catalysts,49 and sensors.50 This feature also allows the electronic states of germanene to be easily tuned by chemical functionalization51 such as adsorption,52,53 which is an important approach for molecular sensing and DNA sequencing.54 It is worth mentioning that although the interaction of various gas molecules with germanene monolayers has been extensively studied,55–57 its interaction with biomolecules such as DNA/RNA nucleobases has only been very limitedly investigated.
A pioneer study on the application of group IV elemental monolayers as potential biosensors has been conducted by Amorim and Scheicher,58 who comprehensively investigated silicene as a promising building block of a DNA sequencing device. Later, Hussain, et al. also studied the adsorption characteristics of DNA nucleobases, aromatic amino acids and heterocyclic molecules on 2D silicene and germanene monolayers.59 On the other hand, the technique called two dimensional molecular electronic spectroscopy (2DMES), introduced in our previous works,60 has been shown to be efficient in unambiguously recognizing and distinguishing various molecules including DNA/RNA bases and their mutated forms. In this technique, once a molecule is adsorbed onto a monolayer, the relative differential conductance (Δg with respect to the differential conductance of the pristine monolayer) of the molecule–monolayer system is mapped with respect to bias and gate voltages. The observed dips in the electron transmission spectrum of the complex, which emerged due to the Fano resonance,61 are shown to be unique electronic fingerprints of the adsorbed molecule and thus can be utilized for molecular sensing and DNA/RNA sequencing.
Motivated by all these findings, in this work, we investigate the capability of AGeNR as a nano-biosensor for DNA/RNA sequencing under the application of the 2DMES technique. For this purpose, first, we briefly study the structural and electronic characteristics of AGeNRs. Next, the adsorption of various nucleobases onto AGeNR and the thermodynamic stability of the obtained nucleobases–AGeNR complexes are examined. The selected nucleobases are adenine (A), cytosine (C), guanine (G), thymine (T), uracil, 5-methylcytosine (5mC), and 5-hydroxymethylcytosine (5hmC), in which the latter two were selected because of their potential role in cancer cell growth. In the following step, we calculate transmission profiles of the introduced nucleobase–AGeNR systems and show that once a DNA/RNA base is adsorbed onto the AGeNR's surface, the ballistic electron transfer mode exhibits a new path with dips at certain energies. To investigate the possibility of uniquely assigning the emerged Fano dips as molecular fingerprints to each nucleobase in an AGeNR based sequencing device, we calculate and plot Δg spectra for each nucleobase–AGeNR system for a certain range of bias and gate voltages. Comparing the obtained 2D and three-dimensional (3D) Δg maps indicates that AGeNR can be employed to unambiguously recognize and distinguish various nucleobases provided 2DMES is applied. By calculating the conductance sensitivity, we also show that AGeNR is able to recognize various nucleobases with acceptable sensitivity.
Computational methods
Our first-principles calculations are performed based on density functional theory (DFT). The Vienna ab initio simulation package (VASP)62 is used to optimize all the geometries and investigate the electronic structures of the systems. To treat the exchange–correlation effects, we employ the generalized gradient approximation (GGA) of Perdew, Burke, and Ernzerhof (PBE).63 The plane-augmented wave (PAW) method is used to describe the electron–ion interactions and the Kohn and Sham orbitals are expanded in a plane wave basis set.64Since the nucleobase–AGeNR interaction in the proposed system is governed by van der Waals (vdW) forces,65,66 we also include a functional accounting for dispersion effects, representing these forces in accordance with the method developed by Tkatchenko–Scheffler (TS),67 which is implemented on VASP.68
The cutoff energy for the grid-mesh is 500 Ry in the calculations and a k-point mesh of 1 × 1 × 64 is employed along the x, y, and z directions, respectively. All the structures are fully relaxed until energy and forces are converged to 10−5 eV and 0.01 eV Å−1, respectively. To investigate the transport characteristics of the systems, DFT combined with nonequilibrium Green's function (NEGF),69,70 as implemented in TranSIESTA,71 is utilized. The transmission is given by:
| Tσ(E,Vb) = Tr[ΓLGΓRG†] | (1) |
 | (2) |
 , which is a shifted value relative to the Fermi level of neutral system EF.
, which is a shifted value relative to the Fermi level of neutral system EF.
Results and discussion
Atomic and electronic structure
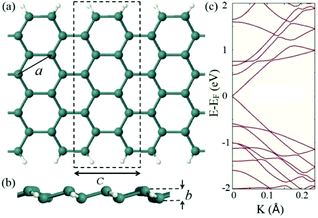
To perform the study, we chose an 8-AGeNR, in which 8 stands for the number of dimer lines along the width of the AGeNR. For simplicity, from now on 8-AGeNR will be denoted as AGeNR unless otherwise specified. The dangling bonds on the edges of AGeNR are hydrogen-terminated. Fig. 1a and b show the front and side views of the chosen AGeNR schematically, in which the lattice constant, a, in similarity with 2D germanene, the buckling height, b, and the primitive unit cell (dashed-lines rectangle) with cell length c, are specified. The thermodynamic stability of germanene nanoribbons has already been discussed in the literature, where it has been shown that the low buckled form of germanene is energetically more favorable and behaves as a semi-metal.72–74 Our calculated values for a, b, and c are 3.96 Å, 0.64 Å, and 6.96 Å, respectively, in agreement with previous studies.44,72Fig. 1c depicts the calculated band structure of AGeNR. The plotted energy band diagram represents the semi-metal nature of the system with a band gap of 0.036 eV, in agreement with the band gap behavior of low buckled AGeNRs with a width of the group 3p + 2 (p is an integer number).44,46Adsorption configurations
Initially, each nucleobase is deposited at the middle of the AGeNR, on different adsorption sites with different orientations, yet parallel to the AGeNR surface at a distance of 3 Å. Here, we follow the results on the adsorption behavior of nucleobases on germanene obtained from previous studies.59,74 The separation distance is defined between nucleobase and AGeNR (d) as the distance between the nearest atoms of the two subsystems. Although the initial positions and orientations of the nucleobase are arbitrarily chosen, however, since the interaction between the nucleobase and the substrate is mediated by weak vdW forces,75 the variation of adsorption energy for different nucleobase–AGeNR configurations would be negligible.76Fig. 2 shows the fully relaxed configurations of the studied nucleobase–AGeNR complexes. It is inferred from Fig. 2 that except for adenine, which is stacked on AGeNR in parallel, other nucleobases take tilted orientations towards the AGeNR surface. It is also noteworthy that, unlike the adsorption of nucleobases on silicene, in which C and G chemically bond with the substrate (a process of an exothermic nature),58 here all the bases exhibit physisorption interaction with the AGeNR, which might be due to the endothermic nature of dissociative adsorption of some oxygen containing molecules on the germanene surface.77 | ||
| Fig. 2 Optimized structures of the studied nucleobase–AGeNR systems. The color key of the atoms is illustrated in the bottom right panel. | ||
To investigate the relative stability of nucleobases on top of the AGeNR surface, we calculate the adsorption energy (Ead) using the following equation:
| Ead = EAGeNR+nb − (EAGeNR + Enb) | (3) |
| A | C | G | T | U | 5mC | 5hmC | |
|---|---|---|---|---|---|---|---|
| E ad (eV) | −0.73 | −0.58 | −0.85 | −0.61 | −0.50 | −0.55 | −0.54 |
| d (Å) | 2.98 | 3.05 | 2.76 | 3.02 | 3.11 | 3.05 | 3.06 |
In order to further investigate the adsorption of nucleobases on AGeNR and the nature of the interaction between two segments of the system, we calculate and plot the charge density redistribution of the studied nucleobase–AGeNR complexes as depicted in Fig. S1 in the ESI.† It can be seen from the plotted isosurfaces that, in all the studied nucleobase–AGeNR systems, the final charge density is concentrated mainly on the nucleobase and there is no complex charge density redistribution between nucleobases and AGeNR. This clearly indicates that the interaction between the two parts of the system is ruled by weak and purely non-covalent forces which confirms the adsorption nature of the nucleobases onto the AGeNR's surface to be physisorption.
Charge transport properties
On the basis of the above analyses, we calculate the charge transport properties for all the introduced nucleobase–AGeNR structures along the AGeNR's axis direction. Fig. 3a illustrates a schematic representation of the proposed two-probe transport configuration, which can be modeled as a Fano–Anderson chain. Thus, peaks/dips in the transmission profile of each nucleobase–AGeNR system due to the Fano effect can be expected. Fig. 3b shows the calculated transmission profiles of the studied nucleobase–AGeNR structures as well as the bare AGeNRs at zero bias voltage (Vb).It is followed from Fig. 3b that the adsorption of a nucleobase onto the AGeNR surface results in sharp reductions in the transmission spectrum of AGeNR at certain energies that can be controlled by the application of gate voltage (Vg). Since the transport characteristics of a nucleobase attached on the AGeNR surface strongly depend on the frontier molecular orbital (MO) energy levels, the emerged dips in the transmission profile can be assigned to each molecule uniquely and hence utilized for recognition and sequencing purposes. This is confirmed by investigating the projected density of states (PDOS) of the nucleobase–AGeNR systems, as shown in the inset of Fig. 3b, which is plotted with respect to the DOS of pristine AGeNR. As shown in the plotted PDOS profiles, adsorbed molecules induce strong states to the electronic structure of AGeNR in the energy values consistent with the energy values of the emerged transmission reductions. One can see that except for uracil, the other nucleobases provide at least one energetically distinct transmission reduction, and hence distinguishable from those of the other nucleobases. Fig. 3b shows that the four canonical bases of A, C, G, and T, once adsorbed on the AGeNR, provide Fano dips at E − EF = −0.42 (as well as −0.70), −0.98, −0.46 (as well as −1.84) and −1.07 eV, respectively, which makes these bases unambiguously distinguishable. In the case that methylated C derivatives i.e. 5mC and 5hmC are attached to the AGeNR's surface, transmission reductions arise at E − EF = −1.03, −0.95 eV respectively. For the case of U adsorbed on the AGeNR, a transmission reduction appears at E − EF = −1.03 eV, whose emerged Fano dip is minor compared to those of the other studied systems due to the lower Ead of U and the larger d. Besides, the molecular fingerprint of U is situated within the energy range of the transmission reductions of C, 5mC, and T which prevents unambiguous discrimination of U. However, in the following we show that although molecular fingerprints of some nucleobases are energetically adjacent, they can be recognized and distinguished without any ambiguity by employing the 2DMES technique.
Application of 2DMES
In practice, it is the conductance of a biosensor that is measured. Thus, we calculate Δg of the proposed sensing system at varying Vb and Vg (E − EF equally). It is also worth noting that since the 1D-conductance changes by a unit of quantum conductance, measuring it rather than the current provides more accurate distinction of the variation of the biosensor's signal. The 2D and 3-dimensional (3D) views of the calculated Δg (−Δg for visual aids) with respect to Vb and Vg for different nucleobase–AGeNR systems are depicted in Fig. 4. The illustrated plots explicitly represent distinct features of Δg graphs for various studied systems where the variation of the Δg surface for each system is different. It means that, even in the case of two nucleobases providing Fano dips at contiguous energies, their Δg maps in the vicinity of their molecular fingerprints would still be different, which enables their unambiguous recognition and distinction. From the experimental view, it is noteworthy that the required time for measurements is the number of the selected bias voltages times the time for a gate voltage sweep. However, not many bias voltages are necessary to distinguish nucleobases; only measurements at two or three different bias voltages would be sufficient. Therefore, the time required for a reliable measurement is of a similar order of magnitude to the case when the bias voltage is not controlled. On the other hand, as it can be seen from Fig. 4, sweeping electron transport channels in an energy range of 1 eV would be enough to provide a Δg map for each nucleobase that includes its unique and distinct electronic fingerprints. This is in contrast to the 1D plot of the transmission spectrum (or eventually the current–voltage graphs as shown in Fig. S3 in ESI†) which might be unable to distinctly recognize different nucleobases due to the overlap of their corresponding molecular fingerprints. This is the reason why the 2DMES technique can clearly recognize the molecular fingerprints, while the 1D current measurement can hardly provide accurate information on sensing molecules. For practical use, variations of Δg spectral maps for various nucleobases can be stored in a database. Then, through data search and analysis, the characterization of a molecule would be possible using the obtained Δg spectroscopy data. | ||
| Fig. 4 2D and 3D maps of differential conductance, Δg, for different nucleobase–AGeNR systems with respect to electron channel energies (E − EF) at varying bias voltages Vb. | ||
To provide further insight on the susceptibility of the introduced sequencing method in recognizing different nucleobases, we also calculate the conductance sensitivity (S) of the proposed system. In general, S can be defined as S(%) = |g − g0|/g0 where g and g0 are the zero-bias conductance of a biosensor with and without the adsorbed molecule, respectively. Fig. 5 illustrates the calculated sensitivity for all the studied nucleobase–AGeNR systems at two different Vg values. The plotted histograms in Fig. 5 show that, in practice, the introduced germanene based sequencing device possesses the capability of recognizing different nucleobases distinctly using the measured conductance data at various corresponding Vg values. For instance, at Vg = −1.03 V, the proposed biosensor would exhibit larger sensitivities for G and A with S(%) = 29.8% and 19.3%, respectively, where the order of nucleobases in terms of the sensitivity of the device is G > A > T > C > 5mC > 5hmC > U. However, at Vg = −1.47 V, 5mC and C with S(%) = 57.3% and 31.7%, respectively, provide higher sensitivity where the order of nucleobases is as 5mC > C > U > A > G > T > 5hmC. Therefore, by sweeping Vg in a certain range, the proposed biosensor can recognize and distinguish various nucleobases despite their different interaction strength with AGeNR.
 | ||
| Fig. 5 Sensitivity histograms of AGeNR for different nucleobases at (a) Vg = −1.03 V and (b) Vg = −1.47 V. | ||
Conclusions
In summary, we studied the molecular sensing and DNA/RNA sequencing properties of armchair type germanene nanoribbon by employing the 2DMES technique. Towards this goal, we first investigated the adsorption behaviors of canonical DNA/RNA bases as well as the methylated forms of cytosine on an AGeNR. Our calculations show that the order of nucleobases in terms of their adsorption energies onto the AGeNR's surface is G > A > T > C > 5mC > 5hmC > U which exhibits more distinct adsorption energies than graphene with G > A ≈ T ≈ C > U. Then, we showed that once a nucleobase is attached onto an AGeNR, electronic fingerprints of the adsorbed base in the form of Fano dips emerge in the transmission spectrum of the nucleobase–AGeNR structure, a feature that can be utilized to recognize and distinguish the adsorbed base. In order to avoid ambiguity in recognizing various bases and also supply a feasible method to perform the required practical measurements, we employ the 2DMES technique to provide Δg spectra of the introduced complexes with respect to Vb and Vg. The calculated 2D and 3D Δg maps for different studied systems, in contrast with 1D current–voltage profiles, exhibit explicitly distinct features which enable unambiguous recognition of nucleobases. This means that, in practice, providing the Δg spectrum for each nucleobase while the nucleobase is translocating the width of AGeNR can be utilized for unambiguous molecular recognition. The provided Δg maps for various nucleobases can be stored in a data set for DNA/RNA sequencing purpose. Also, in order to demonstrate the admissible susceptibility of AGeNR to recognize various bases, we calculate the conductance sensitivity of the proposed system and show that AGeNR can provide large sensitivities for different nucleobases at different Vg values, making it applicable for molecular sensing usages. Under the practical conditions, where the aqueous environment is present, it is expected that water molecules will affect the transport characteristics of a nano-biosensor. However, it has been shown that germanene is hydrophobic.80 Therefore, similarly to graphene, we expect that interaction between a nucleobase and the AGeNR would be stronger than that existing between the water molecules and the AGeNR. Hence, the stacking structures would not be significantly influenced by solvent effects. However, a QM/MM study (which is beyond the scope of this manuscript) would better elucidate the solvent effect. Given that germanene is compatible with current technology of fabricating electronic devices, our proposed device in combination with the 2DMES technique can be a promising feasible nano-biosensor for DNA/RNA sequencing and cancerous DNA recognition as well as molecular sensing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
MRR is funded by H2020 Marie Skłodowska-Curie Actions, 841673. Financial supports from AEI and FEDER under project MAT2017-88258-R (AEI/FEDER, UE) and from the Programa Operativo FEDER of Andalucía 2014-2020 under project B-FQM-272-UGR20 are gratefully acknowledged by BB. MRR gratefully acknowledges Prof. Geunsik Lee from the Ulsan National Institute of Science and Technology (UNIST) for providing computational resources of UNIST Supercomputing center. Red Española de Supercomputación (RES) and the Alhambra supercomputer of the University of Granada are also acknowledged for providing computational time and facilities.References
- M. Zeng, Y. Xiao, J. Liu, K. Yang and L. Fu, Chem. Rev., 2018, 118, 6236–6296 CrossRef CAS PubMed.
- K. Khan, A. K. Tareen, M. Aslam, R. Wang, Y. Zhang, A. Mahmood, Z. Ouyang, H. Zhang and Z. Guo, J. Mater. Chem. C, 2020, 8, 387–440 RSC.
- D. Akinwande, C. Huyghebaert, C. H. Wang, M. I. Serna, S. Goossens, L. J. Li, H. S. Philip Wong and F. H. L. Koppens, Nature, 2019, 573, 507–518 CrossRef CAS PubMed.
- C. Liu, H. Chen, S. Wang, Q. Liu, Y. G. Jiang, D. W. Zhang, M. Liu and P. Zhou, Nat. Nanotechnol., 2020, 15, 545–557 CrossRef CAS PubMed.
- A. Cresti, N. Nemec, B. Biel, G. Niebler, F. Triozon, G. Cuniberti and S. Roche, Nano Res., 2008, 1, 361–394 CrossRef CAS.
- B. Biel, X. Blase, F. Triozon and S. Roche, Phys. Rev. Lett., 2009, 102, 096803 CrossRef PubMed.
- A. Lherbier, B. Biel, Y. M. Niquet and S. Roche, Phys. Rev. Lett., 2008, 100, 036803 CrossRef PubMed.
- M. J. Allen, V. C. Tung and R. B. Kaner, Chem. Rev., 2010, 110(1), 132–145 CrossRef CAS PubMed.
- M. R. Rezapour, C. W. Myung, J. Yun, A. Ghassami, N. Li, S. U. Yu, A. Hajibabaei, Y. Park and K. S. Kim, ACS Appl. Mater. Interfaces, 2017, 9, 24393–24406 CrossRef CAS PubMed.
- A. C. Ferrari, et al. , Nanoscale, 2015, 7, 4598–4810 RSC.
- Z. H. Pan, N. Liu, L. Fu and Z. F. Liu, J. Am. Chem. Soc., 2011, 133, 17578–17581 CrossRef CAS PubMed.
- S. Thomas, A. C. Rajan, M. R. Rezapour and K. S. Kim, J. Phys. Chem. C, 2014, 118(20), 10855–10858 CrossRef CAS.
- G. T. Feliciano, C. Sanz-Navarro, M. D. Coutinho-Neto, P. Ordejón, R. H. Scheicher and A. R. Rocha, Phys. Rev. Appl., 2015, 3, 034003 CrossRef.
- F. Muñoz-Rojas, J. Fernández-Rossier and J. J. Palacios, Phys. Rev. Lett., 2009, 102, 136810 CrossRef PubMed.
- M. R. Rezapour, J. Yun, G. Lee and K. S. Kim, J. Phys. Chem. Lett., 2016, 7(24), 5049–5055 CrossRef CAS PubMed.
- N. Leconte, D. Soriano, S. Roche, P. Ordejón, J. C. Charlier and J. J. Palacios, ACS Nano, 2011, 5, 3987–3992 CrossRef CAS PubMed.
- X. Jiang, J. Nisar, B. Pathak, J. Zhao and R. Ahuja, J. Catal., 2013, 299, 204–209 CrossRef CAS.
- M. R. Rezapour, G. Lee and K. S. Kim, Carbon, 2019, 153, 525–530 CrossRef CAS.
- I. Choudhuri, N. Patra, A. Mahata, R. Ahuja and B. Pathak, J. Phys. Chem. C, 2015, 119, 24827–24836 CrossRef CAS.
- S. M. Avdoshenko, D. Nozaki, C. Gomes da Rocha, J. W. González, M. H. Lee, R. Gutierrez and G. Cuniberti, Nano Lett., 2013, 13, 1969–1976 CrossRef CAS PubMed.
- H. S. Kim and Y. H. Kim, Biosens. Bioelectron., 2015, 69, 186–198 CrossRef CAS PubMed.
- B. Biel, F. Triozon, X. Blase and S. Roche, Nano Lett., 2009, 9, 2725–2729 CrossRef CAS PubMed.
- M. R. Rezapour, G. Lee and K. S. Kim, Nanoscale Adv., 2020, 2, 5905–5911 RSC.
- T. Ahmed, S. Kilina, T. Das, J. T. Haraldsen, J. J. Rehr and A. V. Balatsky, Nano Lett., 2012, 12, 927–931 CrossRef CAS PubMed.
- J. H. Lee, Y. K. Choi, H. J. Kim, R. H. Scheicher and J. H. Cho, J. Phys. Chem. C, 2013, 117, 13435–13441 CrossRef CAS.
- Y. Kubota, K. Watanabe, O. Tsuda and T. Taniguchi, Science, 2007, 317, 932–934 CrossRef CAS PubMed.
- L. Song, L. Ci, H. Lu, P. B. Sorokin, C. Jin, J. Ni, A. G. Kvashnin, D. G. Kvashnin, J. Lou, B. I. Yakobson and P. M. Ajayan, Nano Lett., 2010, 10, 3209–3215 CrossRef CAS PubMed.
- B. Aufray, A. Kara, Ś. Vizzini, H. Oughaddou, C. Ĺandri, B. Ealet and G. L. Lay, Appl. Phys. Lett., 2010, 96, 183102 CrossRef.
- P. Vogt, P. De Padova, C. Quaresima, J. Avila, E. Frantzeskakis, M. C. Asensio, A. Resta, B. Ealet and G. L. Lay, Phys. Rev. Lett., 2012, 108, 155501 CrossRef PubMed.
- M. E. Dávila, L. Xian, S. Cahangirov, A. Rubio and G. Le Lay, New J. Phys., 2014, 16, 095002 CrossRef.
- F. Zhu, W. Chen, Y. Xu, C. Gao, D. Guan and C. Liu, Nat. Mater., 2015, 14, 1020–1025 CrossRef CAS PubMed.
- S. Das, M. Demarteau and A. Roelofs, ACS Nano, 2014, 8, 11730–11738 CrossRef CAS PubMed.
- L. Li, Y. Yu, G. J. Ye, Q. Ge, X. Ou, H. Wu, D. Feng and X. H. Chen, Nat. Nanotechnol., 2014, 9, 372–377 CrossRef CAS PubMed.
- A. K. Geim and I. V. Grigorieva, Nature, 2013, 499, 419–425 CrossRef CAS PubMed.
- Q. H. Wang, K. Kalantar-Zadeh, A. Kis, J. N. Coleman and M. S. Strano, Nat. Nanotechnol., 2012, 7, 699–712 CrossRef CAS PubMed.
- A. Molle, C. Grazianetti, L. Tao, D. Taneja, M. HasibulAlam and D. Akinwande, Chem. Soc. Rev., 2018, 47, 6370–6387 RSC.
- L. Chen, C. C. Liu, B. Feng, X. He, P. Cheng, Z. Ding, S. Meng, Y. Yao and K. Wu, Phys. Rev. Lett., 2012, 109, 056804 CrossRef PubMed.
- L. Tao, E. Cinquanta, D. Chiappe, C. Grazianetti, M. Fanciulli, M. Dubey, A. Molle and D. Akinwande, Nat. Nanotechnol., 2015, 10, 227–231 CrossRef CAS PubMed.
- M. E. Dávila and G. L. Lay, Sci. Rep., 2016, 6, 20714 CrossRef PubMed.
- L. Zhang, P. Bampoulis, A. N. Rudenko, Q. Yao, A. van Houselt, B. Poelsema, M. I. Katsnelson and H.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) J. W. Zandvliet, Phys. Rev. Lett., 2016, 116, 256804 CrossRef CAS PubMed.
J. W. Zandvliet, Phys. Rev. Lett., 2016, 116, 256804 CrossRef CAS PubMed. - A. Acun, L. Zhang, P. Bampoulis, M. Farmanbar, A. van Houselt, A. N. Rudenko, M. Lingenfelder, G. Brocks, B. Poelsema, M. I. Katsnelson and H. J. W. Zandvliet, J. Phys.: Condens. Matter, 2015, 27, 443002 CrossRef CAS PubMed.
- S. Balendhran, S. Walia, H. Nili, S. Sriram and M. Bhaskaran, Small, 2015, 11, 640–652 CrossRef CAS PubMed.
- E. Bianco, S. Butler, S. Jiang, O. D. Restrepo, W. Windl and J. E. Goldberger, ACS Nano, 2013, 7, 4414–4421 CrossRef CAS PubMed.
- Q. Chen, L. Liang, G. Potsi, P. Wan, J. Lu, T. Giousis, E. Thomou, D. Gournis, P. Rudolf and J. Ye, Nano Lett., 2019, 19, 1520–1526 CrossRef CAS.
- Q. Pang, Y. Zhang, J. M. Zhang, V. Ji and K. W. Xu, Nanoscale, 2011, 3, 4330–4338 RSC.
- M. M. Monshi, S. M. Aghaei and I. Calizo, RSC Adv., 2017, 7, 18900 RSC.
- A. Nijamudheen, R. Bhattacharjee, S. Choudhury and A. Datta, J. Phys. Chem. C, 2015, 119, 3802–3809 CrossRef CAS.
- L. C. Loaiza, L. Monconduit and V. Seznec, Small, 2020, 16, 1905260 CrossRef CAS PubMed.
- N. Liu, G. Bo, Y. Liu, X. Xu, Y. Du and S. X. Dou, Small, 2019, 15, 1805147 CrossRef PubMed.
- T. Hussain, T. Kaewmaraya, S. Chakraborty, H. Vovusha, V. Amornkitbamrung and R. Ahuja, ACS Sens., 2018, 3(4), 867–874 CrossRef CAS PubMed.
- J. Zhao, H. Liu, Z. Yu, R. Quhe, S. Zhou, Y. Wang, C. C. Liu, H. Zhong, N. Han, J. Lu, Y. Yao and K. Wu, Prog. Mater. Sci., 2016, 83, 24–151 CrossRef CAS.
- Q. Pang, L. Li, D. L. Gao, R. P. Chai, C. L. Zhang and Y. L. Song, Phys. E, 2017, 88, 237–242 CrossRef CAS.
- J. Prasongkit, R. G. Amorim, S. Chakraborty, R. Ahuja, R. H. Scheicher and V. Amornkitbamrung, J. Phys. Chem. C, 2015, 119, 16934–16940 CrossRef CAS.
- S. J. Heerema and C. Dekker, Nat. Nanotechnol., 2016, 11, 127–136 CrossRef CAS PubMed.
- M. M. Monshi, S. M. Aghaei and I. Clizo, Surf. Sci., 2017, 665, 96–102 CrossRef CAS.
- V. Nagarajan and R. Chandiramouli, Superlattices Microstruct., 2017, 101, 160–171 CrossRef CAS.
- V. Nagarajan and R. Chandiramouli, J. Mol. Liq., 2017, 234, 355–363 CrossRef CAS.
- R. G. Amorim and R. H. Scheicher, Nanotechnology, 2015, 26, 154002 CrossRef PubMed.
- T. Hussain, H. Vovusha, T. Kaewmaraya, V. Aamornkitbamrung and R. Ahuja, Sens. Actuators, B, 2018, 255, 2713–2720 CrossRef CAS.
- A. C. Rajan, M. R. Rezapour, J. Yun, Y. Cho, W. J. Cho, S. K. Min, G. Lee and K. S. Kim, ACS Nano, 2014, 8, 1827–1833 CrossRef CAS PubMed.
- A. E. Miroshnichenko, S. Flach and Y. S. Kivshar, Rev. Mod. Phys., 2010, 82, 2257 CrossRef CAS.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758 CrossRef CAS.
- S. D. Chakarova-Käck, E. Schröder, B. I. Lundqvist and D. C. Langreth, Phys. Rev. Lett., 2006, 96, 146107 CrossRef PubMed.
- J. Björk, F. Hanke, C. A. Palma, P. Samori, M. Cecchini and M. Persson, J. Phys. Chem. Lett., 2010, 1, 3407–3412 CrossRef.
- A. Tkatchenko and M. Scheffler, Phys. Rev. Lett., 2009, 102, 073005 CrossRef PubMed.
- T. Bučko, S. Lebègue, J. Hafner and J. G. Ángyán, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 064110 CrossRef.
- J. Taylor, H. Guo and J. Wang, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 245407 CrossRef.
- S. Datta, Electronic Transport in Mesoscopic Systems, Cambridge University Press, Cambridge, 1997 Search PubMed.
- M. Brandbyge, J. L. Mozos, P. Ordejón, J. Taylor and K. Stokbro, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 165401 CrossRef.
- S. Cahangirov, M. Topsakal and S. Ciraci, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 195120 CrossRef.
- S. Cahangirov, M. Topsakal, E. Aktürk, H. Şahin and S. Ciraci, Phys. Rev. Lett., 2009, 102, 236804 CrossRef CAS PubMed.
- H. K. Gürel and B. Salmankurt, Biosensors, 2021, 11, 59–72 CrossRef PubMed.
- G. Liu, W. W. Luo, X. Wang, X. L. Lei, B. Xu, C. Y. Ouyang and S. B. Liu, J. Mater. Chem. C, 2018, 6, 5937–5948 RSC.
- I. S. S. de Oliveira and R. H. Miwa, J. Chem. Phys., 2015, 142, 044301 CrossRef CAS PubMed.
- S. S. Raya, A. S. Ansari and B. Shong, Surf. Interfaces, 2021, 24, 101054 CrossRef CAS.
- S. Gowtham, R. H. Scheicher, R. Ahuja, R. Pandey and S. P. Karna, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 033401 CrossRef.
- M. R. Rezapour, A. C. Rajan and K. S. Kim, J. Comput. Chem., 2014, 35, 1916–1920 CrossRef CAS PubMed.
- W. Xia, W. Hu, Z. Li and J. Yang, Phys. Chem. Chem. Phys., 2014, 16, 22495–22498 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1nr07336b |
| This journal is © The Royal Society of Chemistry 2022 |