Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A laser-driven optical atomizer: photothermal generation and transport of zeptoliter-droplets along a carbon nanotube deposited hollow optical fiber†
Hyeonwoo
Lee
 a,
Mikko
Partanen
a,
Mikko
Partanen
 ab,
Mingyu
Lee
a,
Sunghoon
Jeong
a,
Hyeung Joo
Lee
a,
Kwanpyo
Kim
cd,
Wonhyoung
Ryu
ab,
Mingyu
Lee
a,
Sunghoon
Jeong
a,
Hyeung Joo
Lee
a,
Kwanpyo
Kim
cd,
Wonhyoung
Ryu
 *e,
Kishan
Dholakia
*af and
Kyunghwan
Oh
*e,
Kishan
Dholakia
*af and
Kyunghwan
Oh
 *a
*a
aPhotonic Device Physics Laboratory, Department of Physics, Yonsei University, 50 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea. E-mail: koh@yonsei.ac.kr
bPhotonics Group, Department of Electronics and Nanoengineering, Aalto University, P.O. Box 13500, 00076 Aalto, Finland
cDepartment of Physics, Yonsei University, Seoul 03722, Korea
dCenter for Nanomedicine, Institute for Basic Science (IBS), Seoul 03722, Korea
eBiomedical and Energy System Laboratory, Department of Mechanical Engineering, Yonsei University, 50 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea. E-mail: whryu@yonsei.ac.kr
fSUPA, School of Physics and Astronomy, University of St Andrews, KY16 9SS, UK. E-mail: kd1@st-andrews.ac.uk
First published on 18th March 2022
Abstract
From mechanical syringes to electric field-assisted injection devices, precise control of liquid droplet generation has been sought after, and the present state-of-the-art technologies have provided droplets ranging from nanoliter to subpicoliter volume sizes. In this study, we present a new laser-driven method to generate liquid droplets with a zeptoliter volume, breaking the fundamental limits of previous studies. We guided an infrared laser beam through a hollow optical fiber (HOF) with a ring core whose end facet was coated with single-walled carbon nanotubes. The laser light was absorbed by this nanotube film and efficiently generated a highly localized microring heat source. This evaporated the liquid inside the HOF, which rapidly recondensed into zeptoliter droplets in the surrounding air at room temperature. We spectroscopically confirmed the chemical structures of the liquid precursor maintained in the droplets by atomizing dye-dissolved glycerol. Moreover, we explain the fundamental physical principles as well as functionalities of the optical atomizer and perform a detailed characterization of the droplets. Our approach has strong prospects for nanoscale delivery of biochemical substances in minuscule zeptoliter volumes.
Introduction
Controlling the diameter and volume of liquid droplets is a fundamental and critical issue in various areas, such as fluid mechanics, biochemistry, and environmental science.1–6 In particular, the physical properties of liquid droplets with nanometer-scale maximize their potential to create academic breakthroughs. The rapid development of nanoscience has recently realized this potential, and inducing novel magnetism and specimen preparation for transmitting electronic microscopy are their impactful precedents.7–10 Furthermore, current airborne plagues have created demands to emulate droplet generation from viscous bioliquids to investigate how the airborne droplets behave.11–13 Systematic delivery of nano-micro viscous droplets in ambient air can also benefit many interdisciplinary research fields, such as three-dimensional nano printing, advanced molecular synthesis, and drug delivery.14–18In response to these demands, various liquid jetting systems have been developed, where liquid droplets have been ejected and delivered through a nozzle to a target area with droplet diameter/volume distributions artificially controlled by physical impulses.19–21 These previous approaches commonly require three driving mechanisms: (1) ejection of the liquid, (2) formation of liquid droplets, and (3) delivery of the droplets to the outer environment. Meanwhile, several driving mechanisms, such as ultrasound,22,23 flow focusing,24,25 and electrohydrodynamic jetting,26,27 have been investigated. Nevertheless, they have failed to reach the single liquid droplet volume in the zeptoliter regime, hindering the development of high-resolution nanofluidics. These previous studies have fundamental limits in the relatively large form factor in the nozzle and the requirements of high-energy physical impulses from external sources.28–30 As an alternative driving mechanism, we have recently investigated the feasibility of controlling the liquid flow using continuous-wave (CW) milliwatt laser light in fiber optic geometry,31 which generated nanodroplets with a zeptoliter volume scale.
In this study, we propose a novel method called a laser-driven optical atomizer, providing a consistent and repeatable means for droplet generation in a compact all-fiber platform, droplet delivery through the air, and its deposition on a target. We successfully generated liquid droplets whose diameter is in the nanometer range with a single liquid volume scale of zeptoliters. This is the first time such a small volume has been realized on a spray platform. It is well-known that single-walled carbon nanotubes (SWCNTs) work as a perfect light absorber in a wide spectral range from ultraviolet to infrared.32,33 In this study, we optically deposited SWCNTs at the end of a hollow optical fiber (HOF) with a ring core as a film. We launched a CW infrared laser beam (of milliwatts power) through the HOF. The optical energy of over 90% was absorbed by this carbon nanotube film, efficiently generating a highly localized microring heat source. After launching the laser, the liquid inside the HOF was heated and evaporated, which rapidly recondensed into zeptoliter droplets at room temperature in the air.34,35 The droplets were delivered over a spherical surface in a well-defined conical geometry over a few hundred micrometers from the end of the HOF. The diameter of the area covered by zeptoliter droplets on a target Si–SiOx wafer was of the order of a few hundred micrometers, which can be further varied by the distance between the HOF end facet and the wafer, as well as the laser power. Additionally, we confirmed that the chemical composition of the droplets was identical to the bulk liquid precursor by applying spectroscopic analyses, such as fluorescent measurement and Raman spectroscopy. This indicates that the proposed method has excellent prospects for liquid chemical delivery at the nanoscale. The proposed method provides new insight into liquid control physics and droplet manipulation technology. Additionally, the simple all-fiber geometry may enable in situ real-time applications in microscopic in vivo environments in a minimally invasive manner.36,37 In the following sections, we describe the physical principles of operation of this all-fiber laser-driven atomizer and discuss the droplet generation and transport characteristics.
Results and discussion
Structure and physical mechanism of the optical atomizer
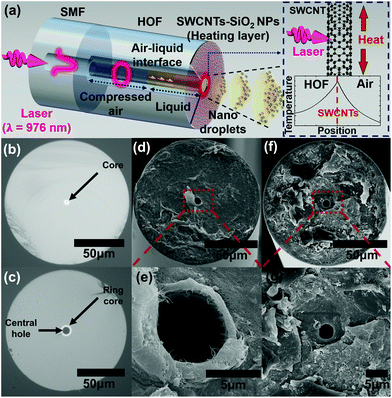
The laser-driven optical atomizer's structure consists of three parts: (1) HOF with a central air hole serving as a liquid reservoir and a high refractive index ring core as a laser waveguide,38 (2) SWCNTs are deposited on the end facet of HOF serving as a film acting as a microring heater by absorbing laser light, and (3) a fiber-coupled laser diode (LD) emitting at λ = 976 nm with a power ranging up to tens of milliwatts. This structure is schematically shown in Fig. 1(a). HOF is fusion spliced to a single-mode fiber (SMF) to adiabatically convert the fundamental mode of SMF to a ring mode of HOF with a low loss.38 The laser from LD propagates from SMF to HOF, and it is absorbed by carbon nanotube thin film to make a ring-shaped micro-heater. See the right inset of Fig. 1(a).The liquid glycerol was filled in the central hole of HOF by the capillary force and expelled by a combination of the vapor pressure of the photothermally heated liquid and the air pressure remnant inside the HOF air hole. The droplets from the end facet and air–liquid interface inside HOF were monitored using separate optical microscopes to investigate the droplet transport and liquid dispensation, respectively. The droplets were targeted to a Si–SiOx wafer at room temperature and collected to investigate the spatial distribution of droplet sizes.
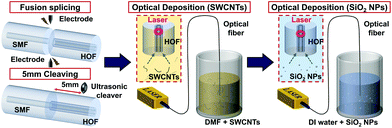
Fabrication of the all-fiber optical atomizer
Scheme 1 shows that one end of HOF was adiabatically fusion spliced with SMF (Corning HI1060), as presented in Fig. 1(b). The HOF used in this study has a central air hole and ring core outer diameters of 8 and 11 μm, respectively as illustrated in Fig. 1(c). The SMF was connected to LD with a very low loss of less than 1 dB. The other end of HOF was cleaved vertically using an ultrasound cleaver, and the total HOF length in the atomizer was approximately 5 mm. The cleaved end was immersed in an SWCNT colloidal suspension (Sigma-Aldrich, Cat. No. 805033). The concentration was 0.17 g L−1 in dimethylformamide (Sigma-Aldrich), and the ultrasonic homogenizer dispersed SWCNTs. Approximately 100 mW laser was launched into the core of the optical fibers for an hour while keeping the HOF inside the solution. SWCNT flakes were deposited near the ring core at the end facet by the optical gradient force, consistent with the previously reported optical deposition of SWCNTs on an optical fiber.39,40Fig. 1(d, e) show the scanning electron microscope (SEM) images of the prepared fiber facet. Over the SWCNT thin film, we subsequently deposited SiO2 nanoparticles (SiO2 NPs, PlasmaChem GmbH) with an average diameter of 20 nm to reduce the hydrophobicity of the SWCNT deposited surface.41–44 We immersed the prepared fiber facet into SiO2 nanoparticle colloidal suspension. SiO2 particles were loaded approximately 17 mg L−1 in deionized water. We also launched a laser of approximately 500 μW to HOF for 5 s. This process is necessary to facilitate the liquid filling into HOF since hydrophobic SWCNT film competes with the capillary force, repelling the liquid from the HOF end face.41–44Fig. 1(f and g) show the SEM image of the optical atomizer after SiO2 NPs deposition. The laser light absorption A through the prepared SWCNT deposited HOF was approximately 93%, providing an efficient photothermal conversion. The thickness of SWCNT thin film zSWCNTs was estimated as 60 nm using the reported SWCNT absorption coefficient α of approximately 4.2 × 105 cm−1 and eqn (1), which is derived from the Beer–Lambert law.33,45–47| A = (1 − exp(−αzSWCNTs)). | (1) |
We filled the prepared HOF tip using the capillary force by dipping HOF into the liquid reservoir. The volume of a liquid filled inside the fiber is only a few hundred picoliters. A liquid with a high vapor pressure of more than 0.004 kPa at 25 °C, such as water or alcohol, rapidly evaporates within a few seconds at a normal humidity of approximately 40%.48,49 Appropriate chemicals may be added to decrease the vapor pressure of these liquids. We successfully atomized low vapor pressure viscous liquids, such as oleic acid and triethylene glycol. Details of these studies will be described in a separate paper.50,51 In this paper, we specifically chose glycerol with a high viscosity, causing technical challenges in previous atomizers despite its various potential applications in biochemical technologies.52–54 Note that HOF was not completely filled by glycerol, but air with a volume of approximately 238 pl remained inside, providing approximately 5.5 kPa outward pressure to the liquid. This pressure is attributed to one of the main driving forces to eject and deliver the droplets.
Liquid droplet generation and transport in the all-fiber optical atomizer
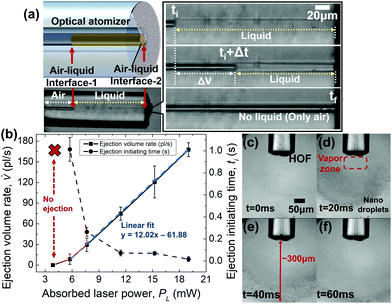
We discovered that two air–liquid interfaces were created inside the HOF at the inner part (called interface-1) and at the end facet of the fiber (called interface-2). These served as good functional indicators of our optical atomizer. Fig. 2(a) shows that the two interfaces behaved predictably for an absorbed laser power of less than 20 mW. To examine the capability of the optical atomizer, we used a bright-field optical microscope to monitor interface-1. The image sensor was synchronized with the LD driver to start the video recording simultaneously with the laser irradiation. We varied the absorbed laser power from 0 to 19 mW. We used another optical microscope near the end facet to observe interface-2 and droplet generation from HOF and its transport in air.Consequently, we obtained that the proposed optical atomizer required a certain amount of laser power for the operation to heat the glycerol above its evaporation temperature. Specifically, to eject the liquid inside the HOF, the SWCNT heater must induce a vapor pressure Pvapor exceeding the pressure Pcapillary of the air–liquid interface-2. We define such a moment as the ejection initiating time ti. Here the Laplace–Young equation can express Pcapillary, which is a function of the hole radius Rhole of HOF, surface tension of the liquid σ, and contact angle of liquid θ inside the capillary, as shown in eqn (2).55
Pcapillary = 2σcos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/Rhole < Pvapor(ti). θ/Rhole < Pvapor(ti). | (2) |
Theoretical analysis with solutions of the heat equation is provided in ESI.† Experimentally, there existed a temporal delay, which was the liquid ejection initiating time, between the liquid atomization and laser onset. By carefully analyzing the frames taken by the computational metal–oxide semiconductor (CMOS) camera on the optical microscope and monitoring the air–liquid interface-1, we measured the liquid ejection initiating time and the ejection volume rate as a function of the absorbed laser power at the SWCNT film on the HOF end facet. Fig. 2(b) summarizes the details.
Moreover, we performed laser irradiation for 0.1–2.5 s and observed that interface-1 did not shift until the absorbed laser power reached 5.76 mW. The liquid ejection time required in the optical atomizer was measured for various absorbed laser powers. The results are summarized as solid circles in Fig. 2(b). The liquid ejection initiating time rapidly decreased from 1 second to less than 300 ms as the laser power increased above 7 mW.
For an absorbed laser power ranging from 5.76 to 19.0 mW, we consistently observed that interface-1 shifted toward the HOF end facet (Fig. 2(a)). The initial liquid-air interface at t = ti is shown above, and the interface at t = tf when the laser is turned off. Here, the darker area denotes the air (refractive index n = 1), and glycerol (n = 1.46) was not distinguishable from the silica (n = 1.45) of HOF using the bright-field optical microscope.
In this absorbed laser power range, interface-1 shifted toward the end facet on the right-hand side, whereas interface-2 remained at its initial position. Thus, the shift of the interface-1 directly represents the volume of the liquid ejected out of the HOF in this laser power range. The liquid ejection volume rate (![[V with combining dot above]](https://www.rsc.org/images/entities/i_char_0056_0307.gif) ), defined as the volume difference of glycerol between the ejection initiating time (ti) and the end of the liquid atomization (tf), was experimentally measured for various laser power. Fig. 2(b) summarizes the details. We obtained that
), defined as the volume difference of glycerol between the ejection initiating time (ti) and the end of the liquid atomization (tf), was experimentally measured for various laser power. Fig. 2(b) summarizes the details. We obtained that ![[V with combining dot above]](https://www.rsc.org/images/entities/i_char_0056_0307.gif) and the absorbed laser power were linearly correlated with regression parameters of 12.0 pl (s mW)−1. Based on these observations, the liquid ejection volume (V) during an arbitrary time t(ti < t < tf), at the given absorbed laser power (PL), ti, and tf, is well-approximated as follows:
and the absorbed laser power were linearly correlated with regression parameters of 12.0 pl (s mW)−1. Based on these observations, the liquid ejection volume (V) during an arbitrary time t(ti < t < tf), at the given absorbed laser power (PL), ti, and tf, is well-approximated as follows:
V = ![[V with combining dot above]](https://www.rsc.org/images/entities/i_char_0056_0307.gif) (PL,ti,tf)(t − ti). (PL,ti,tf)(t − ti). | (3) |
Note that ![[V with combining dot above]](https://www.rsc.org/images/entities/i_char_0056_0307.gif) can be flexibly controlled from the minimum value of 8.61 pl s−1 for the laser power of 5.76 mW to the maximum value of 168 pl s−1 for the absorbed laser power of 19.0 mW. Note that ti and tf varied with the absorbed laser power. Table 1 summarizes the details. From the experimental observation in Fig. 2(b), we estimated the mass flow of 211 ng s−1 using the proposed optical atomizer by multiplying the density of glycerol of 1.26 g ml−1, which is several orders of magnitude lower than previous atomizers,56–58 to numerically analyze the behavior of the droplets, as shown in Fig. S1 (ESI).†
can be flexibly controlled from the minimum value of 8.61 pl s−1 for the laser power of 5.76 mW to the maximum value of 168 pl s−1 for the absorbed laser power of 19.0 mW. Note that ti and tf varied with the absorbed laser power. Table 1 summarizes the details. From the experimental observation in Fig. 2(b), we estimated the mass flow of 211 ng s−1 using the proposed optical atomizer by multiplying the density of glycerol of 1.26 g ml−1, which is several orders of magnitude lower than previous atomizers,56–58 to numerically analyze the behavior of the droplets, as shown in Fig. S1 (ESI).†
| Absorbed laser power (mW) | 5.76 | 7.65 | 11.4 | 15.2 | 19.0 |
|---|---|---|---|---|---|
| t i (s) | 1.01 × 100 | 2.63 × 10−1 | 7.23 × 10−2 | 6.93 × 10−2 | 1.66 × 10−2 |
| t f (s) | 2.33 × 100 | 6.50 × 10−1 | 2.24 × 10−1 | 1.63 × 10−1 | 8.40 × 10−2 |
We used another optical microscope to observe the droplet generation and its transport in the air near the film deposited end facet of HOF.
In Fig. 2(c–f), the frames are shown sequentially for the absorbed laser power of 19 mW. The droplet recondensation in the ambient air was insufficient to be observed by the microscope at lower power. In Fig. 2(c), we observed the air–liquid interface near the SWCNT deposited end facet at t = 0 ms. Meanwhile, at t = 20 ms, which is close to ti, we observed observe nanodroplets with a zeptoliter volume scale exhumed from HOF. It is worth noting that the droplets with different sizes were formed at a certain distance away from the HOF end facet. We also observed a region where liquid droplets were not observed, and only vapor existed. See the “vapor zone” in Fig. 2(d). The “vapor zone” extended to 60 μm from the end facet. Beyond this zone, glycerol started recondensation to form nanodroplets with a zeptoliter volume scale. In the subsequent frame at t = 40 ms, we observed the typical transport pattern of the optical atomizer such that all droplets were distributed on a spherical surface in the air, whose lateral boundary had a conical shape. Its maximum radius was approximately 300 μm, and the conical angle was approximately 29°.
This unique trajectory of the droplet is because of the convective flow of the ambient air induced by the nanotube film heater. It is numerically demonstrated in Movie S1–3 and Fig. S1 (ESI).† Additionally, we investigated the droplet size/volume dependence on their longitudinal distance using a finite element method package, COMSOL. The details of the corresponding results are summarized in Fig. S2 of ESI.† Furthermore, droplets propagated toward a target wafer, and their size distribution was statistically analyzed as described in the following sections.
Droplet size distribution measured by cryogenic SEM
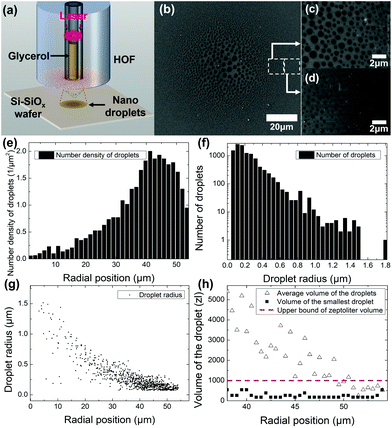
To characterize droplets from the proposed optical atomizer, we collected them on a Si–SiOx wafer, as shown in Fig. 3(a). Here, the atomizer was vertically placed 40 μm away from the wafer, and the absorbed laser power was 9 mW with an irradiation time of approximately 100 ms. We used a cryogenic SEM (cryo-SEM)59 freezing the droplets at liquid nitrogen temperature (under 77 K) immediately after they were deposited on the wafer. The frozen glycerol droplets prevented evaporation in a high vacuum experimental condition in the SEM chamber. The frost undesirably formed on the sample was removed via sublimation, adjusting the temperature and atmospheric pressure inside the cryo-SEM chamber.Images taken from the cryo-SEM are shown in Fig. 3(b–d), illustrating a unique spatial distribution. In the vicinity of the optical atomizer axis, we observed significantly larger droplets at the center of Fig. 3(b). This is attributed to the coalescence of multiple droplets.60,61 As the liquid inside HOF is atomized, its gas molecules diffuse in the air and condense into droplets as they reach the substrate. In terms of statistical mechanics, only a few gas molecules can reach far from the fiber axis, requiring a high radial velocity and long diffusion distance.62 For this reason, several nanodroplets with a zeptoliter volume scale are separately deposited at the edge. However, since most gas molecules exist in a high concentration near the central axis, several nanodroplets are generated and are in contact with one another.62,63 As shown in a cryo-SEM image, their contact coalesces into large microdroplets near the center.63,64 Consequently, the diameter of the droplets decreased as its position moved radially outward where droplets had rarely coalesced. We magnified the image of droplets in two regions whose radial positions were 40 and 50 μm, as shown in Fig. 3(c and d), respectively. These figures show that the proposed atomizer generated nanodroplets with a zeptoliter volume scale. We used an image recognition routine in MATLAB to collect information on the spatial distribution of droplet size along the horizontal axis of the cryo-SEM image.
Fig. 3(e) shows the number density of droplets per unit area as a function of the radial position referenced from the center. Here, we obtained that an order of larger number of droplets 1.5–2.0 μm−2 was deposited in the peripheral (radial position in the range of 40–50 μm) than 0.1–0.3 μm−2 near the center. In Fig. 3(f), we obtained that nanodroplets with a zeptoliter volume scale outnumbered micron diameter droplets with a femtoliter volume scale by several orders of magnitude, strongly indicating that the proposed optical atomizer generated nanodroplets with a zeptoliter volume scale. Glycerol droplets with a radius of approximately 74 nm showed more than 1000 counts in the figure, and their volume corresponded to 156 zl. In this volume calculation, we assumed that they have spherical cap shapes. The contact angle of the bulk glycerol and Si–SiOx wafer was experimentally evaluated as 51° using a conventional method based on a digital camera.65,66 Thus, to investigate the volume of relatively less coalesced droplets, we calculated the average and minimum volume as a function of a radial position with 38–54 μm domain, as shown in Fig. 3(h). The average volume of the droplets increases monotonically as the radial position gets closer to the center since the number of coalesced droplets increases. Nevertheless, the minimum volume of the droplet was consistent in the zeptoliter scale, demonstrating that most of the large droplets imaged by cryo-SEM are because of coalescing of the nanodroplets with a zeptoliter volume scale. To the best of our knowledge, this volume is the smallest observed in liquid atomizing technology. Fig. 3(g) shows the droplet size distribution as a function of the radial position, where the droplet radius monotonically decreased from a few micrometers to tens of nanometers as the radial position increased.67,68
Comparison of chemical structures of bulk liquid and atomized liquid nanodroplets
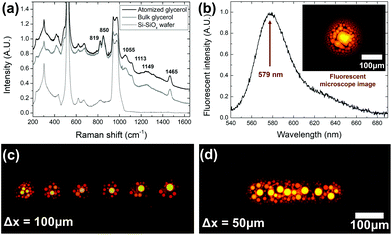
It would be highly beneficial if the chemical composition of the liquid could be maintained through the laser-driven atomization process. This means that the physical and chemical properties of the bulk liquid can be transferred to the nanodroplets, which can open up a new avenue for nanoscopic chemical delivery applications. In this study, we applied micro-Raman spectroscopy (LabRAM ARAMIS, HORIBA Jobin Yvon Raman Division) and fluorescence measurements (HR4000, Ocean Optics) to investigate the chemical structure of the liquid droplets sprayed using the proposed optical atomizer and compared the data with that of the original bulk liquid.First, we used glycerol without any chemical additives and set the optical atomizer similarly to those of Fig. 3(a). To obtain a sufficiently high Raman scattering signal from the droplets, we extended the irradiation time to 1 s, increasing the central drop diameter to approximately 100 μm. Note that the large central droplet was formed by coalescing individual nanodroplets with a zeptoliter volume scale, and it can represent their chemical properties. A bulk glycerol droplet was also formed on the same wafer using the micropipette with a diameter of approximately 1 mm. Fig. 4(a) summarizes the Raman spectra of the atomized droplet, bulk glycerol, and wafer. Unique Raman signatures of glycerol liquid69 were clearly observed for both liquid samples to confirm that the chemical structures were maintained through the proposed optical atomization process.
Moreover, we investigated the chemical delivery capability of the proposed optical atomizer by dissolving a fluorescent dye in the glycerol. We prepared a glycerol solution with a rhodamine B concentration of 3 g L−1. Consequently, droplets were generated by the proposed optical atomizer with an absorbed laser power of 15 mW and a laser irradiation time of 200 ms. A pump laser at λ = 532 nm was focused near the central droplet, and the fluorescence from the dye-doped glycerol droplet was observed through a fluorescent microscope (IX71, Olympus), as shown in the inset of Fig. 4(b). The emission from the central droplet was collected, and its spectrum is shown in Fig. 4(b). A peak at 579 nm with a full width at half maximum of 40 nm was observed, which is highly consistent with the emission characteristics of rhodamine B.70
We also demonstrated that the optical atomizer can highly repeatable multiple ejections with glycerol-rhodamine B solution. Based on the experimental setup similar to Fig. 3(a), where the HOF was placed 30 μm above the wafer, absorbed laser power was 19 mW, and irradiation time was 27.5 ms. After single atomization, the optical atomizer was translated by a constant interval Δx. This operation was repeated six times. Fig. 4(c) shows that the distinguishable and uniform marks of six times of atomization were observed through a fluorescent microscope for Δx = 100 μm. This result shows that multiple atomization processes can be consistently conducted using the dye-dissolved glycerol. Furthermore, once the interval was decreased to Δx = 50 μm, the microscope confirmed a line was formed on the wafer where the individual shots were not distinguishable, as shown in Fig. 4(d).
The experimental confirmation in Fig. 4 strongly indicates that the proposed optical atomizer can effectively deliver the chemical and physical properties of a bulk liquid to nanodroplets with a zeptoliter volume scale. Additionally, transporting dye-dissolved solutions is highly expectable and repeatable, which can initiate new metrology for micro–nanoscopic drug delivery.
Discussions
The proposed all-fiber optical atomizer was compared with previous technologies (see Table 2) in terms of the operation principle, driving source, liquid properties, liquid volume, droplet volume, and physical size. In this comparison, the all-fiber optical atomizer uses the least amount of energy (<20 mJ), <20 mW CW laser irradiation for <1 s. These represent more moderate experimental conditions than previous technologies that require a few hundred-kilowatts pulsed laser and kilovolt scale electric potential. This results in a moderate speed of droplets of a few centimeters per second, whereas several other techniques produce higher velocities at the meter-per-second order.| Principle | Driving source | Liquid | Liquid viscosity | Liquid volume per shot | Single droplet volume | Hole/outer diameter of nozzle | Speed of droplets |
|---|---|---|---|---|---|---|---|
| Photothermal effect (this work) | <20 mW CW laser diode | Glycerol | 1412 mPa·s | 9 pl | ∼156 zl | 8 μm/125 μm | ∼1 cm s−1 |
| Laser induced cavitation (ref. 71) | ∼300 kW Nd-YAG nanosecond laser | Glycerol-water mixture | 100 mPa·s | 10 nl | N.A. | 150 μm/3 mm | ∼100 m s−1 |
| coulombic force (ref. 56) | ∼1.6 kV electric potential | Water | 0.89 mPa·s | 40 nl | ∼1 fl | 42 μm/85 μm | N.A. |
| Air blast (ref. 57) | Multiphase flow | Glycerol-water mixture | ∼6 mPa·s | 5 ml | N.A. | 2 mm/4 mm | ∼5 m s−1 |
| Air blast (ref. 72) | Multiphase flow | Glycerol-water mixture | 259 mPa·s | N.A. | ∼5 pl | 0.41 mm/∼1.3 cm | N.A. |
| Ultrasound atomization (ref. 73) | ∼3 W acoustic wave | Water | 0.89 mPa·s | ∼10 μl | ∼1 pl | 130 μm/1.24 mm | ∼19 mm s−1 |
| Pressure from a Piezo actuator (ref. 58) | Piezoelectric disk | Water | 0.89 mPa·s | 5 μl | N.A. | 8 μm/10 mm | N.A. |
This low energy requirement enables the proposed optical atomizer to easily deliver viscous liquids to fragile targets, such as soft tissue, biological samples, and flexible organic substrates. Furthermore, the volume control of the ejected liquid from the atomizer (a few picoliters per shot) and the single droplet volume of a few hundred zeptoliters are the smallest to date. The proposed device consists of only a single strand of fiber with a cladding and hole diameters of 125 and 8 μm, respectively, providing the smallest form factor and enabling real-time, in situ liquid ejections in a microscopic environment, which was not possible in previous technologies. This enables optical atomizers to deposit liquids in a highly localized area, as shown in Fig. 2(c–f) and 3(b), in contrast to traditional spray platforms that only target macroscopic objects over 1 mm. In terms of chemical capability, previous studies of liquid atomization (see Table 2) demonstrated the delivery of various materials dissolved in the liquid. For example, some previous studies implemented human tissue mimicry by depositing polymer materials,20,74 precision printing using colored inks,75,76 and drug delivery with therapeutic molecules.17,18 In this study, we also confirmed that an optical atomizer could dissolve and eject a fluorescent dye, rhodamine B, at a much smaller scale than other technologies. This suggests that the proposed optical atomizer technology can be used for other various chemical applications based on these previous studies. Furthermore, multiple ejections of the dye-dissolved glycerol can be achieved using this device for a unique spray patterning process, indicating a high potential to be a strong alternative to the current spray printing equipment and conventional single droplet inkjet technologies.77–80
Conclusion
In this study, we have successfully explored a new application of SWCNTs integrated with a HOF to convert bulk liquid into nanodroplets with a zeptoliter volume scale using a monolithic optical fiber solution. By inducing the photothermal generation on the carbon nanotube thin film with tens of milliwatt CW laser, the glycerol filled inside the central hole of HOF was evaporated and recondensed to be transported approximately 300 μm longitudinal distance. The nanoscopic morphology of the nanodroplets with a zeptoliter volume scale was confirmed by employing cryo-SEM. We confirmed that its chemical structure was preserved through Raman spectroscopy and fluorescent measurement. As a potential application, we could deliver dye-dissolved liquid so that glycerol-rhodamine B solution preserved its unique fluorescent characteristic after the atomization. The proposed device was demonstrated to be used for the patterning process of dye-dissolved liquid. Hence, this technology uses fiber optic components to form zeptoliter viscous droplets, as a powerful solution for highly localized targets, beyond the spatial limit of the conventional liquid spray methodologies. With a microscopic feature, low energy requirement, and high volumetric precision, the approach will enable advanced in vivo applications and future patterning technologies.Author contributions
H. Lee conceived the idea and designed and performed the experiments and simulations. H. Lee wrote the manuscript. M. Partanen figured out the principle of this device and analyzed the cryo-SEM data. M. Lee, S. Jeong, and H. J. Lee conducted helpful discussions and arranged the references. K. Kim advised about the nanodroplets characterization. W. Ryu, K. Dholakia, and K. Oh acquired funding, supervised this study, and critically reviewed/edited the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant by the Korea government (MSIT) (No. 2019R1A2C2011293) and University of Sydney – Yonsei University Partnership Collaboration Awards. M. P. acknowledges European Union's Horizon 2020 Marie Sklodowska-Curie Actions (MSCA) individual fellowship under Contract No. 846218. KD thanks the UK Engineering and Physical Sciences Research Council for funding (grant EP/P030017/1). H. Lee thanks Dr Yangjin Lee, Mr Jun-Yeong Yoon, and Mr Joong-Eon Jung for helpful discussion.References
- M. L. Kovarik and S. C. Jacobson, Anal. Chem., 2007, 79, 1655–1660 CrossRef CAS PubMed.
- E. Ko, J. S. Lee, H. Kim, S. Y. Yang, D. Yang, K. Yang, J. Lee, J. Shin, H. S. Yang, W. Ryu and S. W. Cho, ACS Appl. Mater. Interfaces, 2018, 10, 7614–7625 CrossRef CAS PubMed.
- P. Cadinu, B. P. Nadappuram, D. J. Lee, J. Y. Y. Sze, G. Campolo, Y. Zhang, A. Shevchuk, S. Ladame, T. Albrecht, Y. Korchev, A. P. Ivanov and J. B. Edel, Nano Lett., 2017, 17, 6376–6384 CrossRef CAS PubMed.
- B. K. Kim, J. Kim and A. J. Bard, J. Am. Chem. Soc., 2015, 137, 2343–2349 CrossRef CAS PubMed.
- S. I. Kim, Y. J. Kim, H. Hong, J. H. Yun and W. H. Ryu, ACS Appl. Mater. Interfaces, 2020, 12, 54683–54693 CrossRef CAS PubMed.
- S. H. Eom, S. Senthilarasu, P. Uthirakumar, S. C. Yoon, J. Lim, C. Lee, H. S. Lim, J. Lee and S. H. Lee, Org. Electron., 2009, 10, 536–542 CrossRef CAS.
- S. Chung, Q. T. Le, M. Ahlberg, A. A. Awad, M. Weigand, I. Bykova, R. Khymyn, M. Dvornik, H. Mazraati, A. Houshang, S. Jiang, T. N. A. Nguyen, E. Goering, G. Schutz, J. Grafe and J. Akerman, Phys. Rev. Lett., 2018, 120, 217204 CrossRef CAS PubMed.
- Z. Lecz and A. Andreev, Phys. Rev. Res., 2020, 2, 023088 CrossRef CAS.
- J. M. Yuk, J. Park, P. Ercius, K. Kim, D. J. Hellebusch, M. F. Crommie, J. Y. Lee, A. Zettl and A. P. Alivisatos, Science, 2012, 336, 61–64 CrossRef CAS PubMed.
- Q. Chen, J. M. Smith, J. Park, K. Kim, D. Ho, H. I. Rasool, A. Zettl and A. P. Alivisatos, Nano Lett., 2013, 13, 4556–4561 CrossRef CAS PubMed.
- M. P. Atkinson and L. M. Wein, Bull. Math. Biol., 2008, 70, 820–867 CrossRef PubMed.
- G. Buonanno, L. Stabile and L. Morawska, Environ. Int., 2020, 141, 105794 CrossRef CAS PubMed.
- W. G. Lindsley, T. A. Pearce, J. B. Hudnall, K. A. Davis, S. M. Davis, M. A. Fisher, R. Khakoo, J. E. Palmer, K. E. Clark, I. Celik, C. C. Coffey, F. M. Blachere and D. H. Beezhold, J. Occup. Environ. Hyg., 2012, 9, 443–449 CrossRef PubMed.
- Y. W. Han and J. Y. Dong, J. Micro Nano-Manuf., 2018, 6, 040802 CrossRef.
- S. Aphinyan, E. Y. M. Ang, J. J. Yeo, T. Y. Ng, R. M. Lin, Z. S. Liu and K. R. Geethalakshmi, Modell. Simul. Mater. Sci. Eng., 2019, 27, 055005 CrossRef CAS.
- H. G. Nie, Z. W. Wei, L. Q. Qiu, X. S. Chen, D. T. Holden and R. G. Cooks, Chem. Sci., 2020, 11, 2356–2361 RSC.
- Y. Cao, Y. L. Chen, T. Yu, Y. Guo, F. Q. Liu, Y. Z. Yao, P. Li, D. Wang, Z. G. Wang, Y. Chen and H. T. Ran, Theranostics, 2018, 8, 1327–1339 CrossRef CAS PubMed.
- N. Rapoport, Adv. Exp. Med. Biol., 2016, 880, 221–241 CrossRef CAS PubMed.
- O. J. Jaber, A. Kourmatzis and A. R. Masri, Energy Fuels, 2021, 35, 7144–7155 CrossRef CAS.
- S. Y. Yang, T. H. Hwang, L. Che, J. S. Oh, Y. Ha and W. Ryu, Biomed. Mater., 2015, 10, 035011 CrossRef PubMed.
- M. Cloupeau and B. Prunetfoch, J. Aerosol Sci., 1994, 25, 1021–1036 CrossRef CAS.
- B. Avvaru, M. N. Patil, P. R. Gogate and A. B. Pandit, Ultrasonics, 2006, 44, 146–158 CrossRef CAS PubMed.
- A. Dalmoro, A. A. Barba, G. Lamberti and M. d'Amore, Eur. J. Pharm. Biopharm., 2012, 80, 471–477 CrossRef CAS PubMed.
- A. M. Ganan-Calvo, Appl. Phys. Lett., 2005, 86, 214101 CrossRef.
- J. M. Parrilla-Gutierrez, S. Tsuda, J. Grizou, J. Taylor, A. Henson and L. Cronin, Nat. Commun., 2017, 8, 1–9 CrossRef CAS PubMed.
- S. P. Zhang, J. Lata, C. Y. Chen, J. Mai, F. Guo, Z. H. Tian, L. Q. Ren, Z. M. Mao, P. H. Huang, P. Li, S. J. Yang and T. J. Huang, Nat. Commun., 2018, 9, 1–11 CrossRef PubMed.
- M. S. Onses, C. Song, L. Williamson, E. Sutanto, P. M. Ferreira, A. G. Alleyne, P. F. Nealey, H. Ahn and J. A. Rogers, Nat. Nanotechnol., 2013, 8, 667–675 CrossRef CAS PubMed.
- B. Bessaire, M. Mathieu, V. Salles, T. Yeghoyan, C. Celle, J. P. Simonato and A. Brioude, ACS Appl. Mater. Interfaces, 2017, 9, 950–957 CrossRef CAS PubMed.
- L. Rodriguez, N. Passerini, C. Cavallari, M. Cini, P. Sancin and A. Fini, Int. J. Pharm., 1999, 183, 133–143 CrossRef CAS.
- J. Rosell-Llompart and A. M. Ganan-Calvo, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2008, 77, 036321 CrossRef PubMed.
- H. Choi, M. Park, D. S. Elliott and K. Oh, Phys. Rev. A, 2017, 95, 053817 CrossRef.
- C. J. Chen, Y. J. Li, J. W. Song, Z. Yang, Y. Kuang, E. Hitz, C. Jia, A. Gong, F. Jiang, J. Y. Zhu, B. Yang, J. Xie and L. B. Hu, Adv. Mater., 2017, 29, 1701756 CrossRef PubMed.
- Z. Yin, H. M. Wang, M. Q. Jian, Y. S. Li, K. L. Xia, M. C. Zhang, C. Y. Wang, Q. Wang, M. Ma, Q. S. Zheng and Y. Y. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 28596–28603 CrossRef CAS PubMed.
- H. Zhao and D. Beysens, Langmuir, 1995, 11, 627–634 CrossRef CAS.
- R. Marek and J. Straub, Int. J. Heat Mass Transfer, 2001, 44, 39–53 CrossRef CAS.
- J. A. Guggenheim, J. Li, T. J. Allen, R. J. Colchester, S. Noimark, O. Ogunlade, I. P. Parkin, I. Papakonstantinou, A. E. Desjardins, E. Z. Zhang and P. C. Beard, Nat. Photonics, 2017, 11, 714–719 CrossRef CAS.
- I. T. Leite, S. Turtaev, X. Jiang, M. Siler, A. Cuschieri, P. S. Russell and T. Cizmar, Nat. Photonics, 2018, 12, 33–39 CrossRef CAS.
- K. W. Oh, S. Choi, Y. M. Jung and J. W. Lee, J. Lightwave Technol., 2005, 23, 524–532 CrossRef.
- A. Martinez, K. Fuse, B. Xu and S. Yamashita, Opt. Express, 2010, 18, 23054–23061 CrossRef CAS PubMed.
- J. W. Nicholson, R. S. Windeler and D. J. DiGiovanni, Opt. Express, 2007, 15, 9176–9183 CrossRef CAS PubMed.
- F. F. Chen, Y. Jia, Q. G. Wang, X. B. Cao, Y. H. Li, Y. Lin, X. Cui and J. Q. Wei, Carbon, 2018, 137, 88–92 CrossRef CAS.
- Y. J. Mao, C. L. Chen and Y. W. Zhang, Appl. Phys. A: Mater. Sci. Process., 2013, 111, 747–754 CrossRef CAS.
- S. Iglauer, A. Salamah, M. Sarmadivaleh, K. Y. Liu and C. Phan, Int. J. Greenhouse Gas Control, 2014, 22, 325–328 CrossRef CAS.
- U. B. Singh, R. P. Yadav, R. K. Pandey, D. C. Agarwal, C. Pannu and A. K. Mittal, J. Phys. Chem. C, 2016, 120, 5755–5763 CrossRef CAS.
- F. De Nicola, C. Pintossi, F. Nanni, I. Cacciotti, M. Scarselli, G. Drera, S. Pagliara, L. Sangaletti, M. De Crescenzi and P. Castrucci, Carbon, 2015, 95, 28–33 CrossRef CAS.
- D. F. Swinhart, J. Chem. Educ., 1962, 39, 333–335 CrossRef.
- T. G. Mayerhöfer, H. Mutschke and J. Popp, ChemPhysChem, 2016, 17, 1948–1955 CrossRef PubMed.
- V. Nedela, E. Tihlarikova and J. Hrib, Microsc. Res. Tech., 2015, 78, 13–21 CrossRef PubMed.
- B. Tabah, I. N. Pulidindi, V. R. Chitturi, L. M. R. Arava, A. Varvak, E. Foran and A. Gedanken, J. Mater. Chem. A, 2017, 5, 15486–15506 RSC.
- F. Gironi, M. Maschietti and V. Piemonte, Energy Sources, Part A, 2010, 32, 1861–1868 CrossRef CAS.
- F. Meyer, R. Eggers, K. Oehlke, B. Harbaum-Piayda, K. Schwarz and M. A. Siddiqi, Eur. J. Lipid Sci. Technol., 2011, 113, 1363–1374 CrossRef CAS.
- D. F. Pisani, C. D. K. Bottema, C. Butori, C. Dani and C. A. Dechesne, Biochem. Biophys. Res. Commun., 2010, 396, 767–773 CrossRef CAS PubMed.
- R. Ciriminna and M. Pagliaro, Adv. Synth. Catal., 2003, 345, 383–388 CrossRef CAS.
- A. D. Hocking and J. I. Pitt, Appl. Environ. Microbiol., 1980, 39, 488–492 CrossRef CAS PubMed.
- D. B. Asay, M. P. de Boer and S. H. Kim, J. Adhes. Sci. Technol., 2010, 24, 2363–2382 CrossRef CAS.
- J. H. Jeong, H. Choi, K. Park, H. Kim, J. Choi, I. Park and S. S. Lee, Polymer, 2020, 194, 122405 CrossRef CAS.
- Z. Feichi, Z. Thorsten, M. Thomas, W. Simon, J. Tobias, H. Peter, Z. Nikolaos, T. Dimosthenis and K. Thomas, Renewable Sustainable Energy Rev., 2020, 134, 110411 CrossRef CAS.
- Y. Y. Feng, Z. Y. Zhou, J. H. Zhu and G. B. Du, Acta Mech. Sin., 2007, 23, 163–172 CrossRef.
- H. J. Ensikat, A. J. Schulte, K. Koch and W. Barthlott, Langmuir, 2009, 25, 13077–13083 CrossRef CAS PubMed.
- G. F. Christopher, J. Bergstein, N. B. End, M. Poon, C. Nguyen and S. L. Anna, Lab Chip, 2009, 9, 1102–1109 RSC.
- F. Shen, Y. Li, Z. M. Liu, R. T. Cao and G. R. Wang, Chin. J. Anal. Chem., 2015, 43, 1942–1954 CAS.
- S. J. Blundell and K. M. Blundell, Concepts in thermal physics, Oxford University Press on Demand, 2010 Search PubMed.
- J. E. Castillo and J. A. Weibel, Int. J. Heat Mass Transfer, 2018, 118, 708–719 CrossRef.
- J. R. Castrejon-Pita, E. S. Betton, K. J. Kubiak, M. C. T. Wilson and I. M. Hutchings, Biomicrofluidics, 2011, 5, 014112 CrossRef CAS PubMed.
- Z. A. Zhou, H. Hussein, Z. H. Xu, J. Czarnecki and J. H. Masliyah, J. Colloid Interface Sci., 1998, 204, 342–349 CrossRef CAS PubMed.
- V. Fernandez and M. Khayet, Front. Plant Sci., 2015, 6, 510 Search PubMed.
- L. A. Alvarez-Trujillo, V. Lazic, J. Moros and J. J. Laserna, Appl. Opt., 2017, 56, 3773–3782 CrossRef CAS PubMed.
- N. Sharma, W. D. Bachalo and A. K. Agarwal, Phys. Fluids, 2020, 32, 023304 CrossRef CAS.
- C. M. Gryniewicz-Ruzicka, S. Arzhantsev, L. N. Pelster, B. J. Westenberger, L. F. Buhse and J. F. Kauffman, Appl. Spectrosc., 2011, 65, 334–341 CrossRef CAS PubMed.
- M. Stobiecka and M. Hepel, Phys. Chem. Chem. Phys., 2011, 13, 1131–1139 RSC.
- P. Rohilla and J. Marston, Int. J. Pharm., 2020, 589, 119714 CrossRef CAS PubMed.
- S. Mandato, E. Rondet, G. Delaplace, A. Barkouti, L. Galet, P. Accart, T. Ruiz and B. Cuq, Powder Technol., 2012, 224, 323–330 CrossRef CAS.
- J. Kohling and V. Wagner, Int. J. Heat Mass Transfer, 2021, 169, 120939 CrossRef.
- H. J. Jin, J. S. Chen, V. Karageorgiou, G. H. Altman and D. L. Kaplan, Biomaterials, 2004, 25, 1039–1047 CrossRef CAS PubMed.
- B. L. Huang, T. L. Guo, S. Xu, Y. Ye, E. G. Chen and Z. X. Lin, IEEE Photonics J., 2019, 11, 1–9 Search PubMed.
- Z. Y. Hu, N. P. Bradshaw, B. Vanthournout, C. Forman, K. Gnanasekaran, M. P. Thompson, P. Smeets, A. Dhinojwala, M. D. Shawkey, M. C. Hersam and N. C. Gianneschi, Chem. Mater., 2021, 33, 6433–6442 CrossRef CAS.
- S. H. Lee, A. Mahadevegowda, C. Huang, J. D. Evans and P. S. Grant, J. Mater. Chem. A, 2018, 6, 13133–13141 RSC.
- B. H. Zhao, Y. B. Wang, S. Sinha, C. J. Chen, D. P. Liu, A. Dasgupta, L. B. Hu and S. Das, Nanoscale, 2019, 11, 23402–23415 RSC.
- A. Friederich, J. R. Binder and W. Bauer, J. Am. Ceram. Soc., 2013, 96, 2093–2099 CrossRef CAS.
- Y. Jang, Y. D. Park, J. A. Lim, H. S. Lee, W. H. Lee and K. Cho, Appl. Phys. Lett., 2006, 89, 183501 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1nr06211e |
| This journal is © The Royal Society of Chemistry 2022 |