Preparation and characterization of the Li1.12K0.05Mn0.57Ni0.24Nb0.02O2 cathode material with highly improved rate cycling performance for lithium ion batteries†
Cong
Liu
a,
Shuang
Zhang
a,
Yuanyuan
Feng
a,
Xiaowei
Miao
a,
Gang
Yang
 *a and
Jie
Li
*b
*a and
Jie
Li
*b
aSuzhou Key Laboratory of Functional Ceramic Materials, Changshu Institute of Technology, Changshu, 215500, P.R. China. E-mail: gyang@cslg.edu.cn
bDepartment of Energy, Politecnico di Milano, Campus Bovisa - Via Lambruschini 4a, Milan, 20156, Italy. E-mail: jie1.li@polimi.it
First published on 30th November 2021
Abstract
In this work, Li1.12K0.05Mn0.57Ni0.24Nb0.02O2 (LMN–K/Nb) as a novel and high energy density cathode material is successfully synthesized and applied in lithium ion batteries. By combining interlayer exchange and elemental analysis, it can be confirmed that K+ and Nb5+ substitution is respectively in the lithium layer and transition metal (TM) layer since H+ replaces the cations that remain in the lithium layer rather than those in the TM layer. The effect of K+ and Nb5+ co-substitution on the kinetic behavior of insertion/extraction of Li+ is evaluated by electrochemical impedance spectroscopy (EIS), the galvanostatic intermittent titration technique (GITT) and galvanostatic charge/discharge (GCD). LMN–K/Nb delivers an initial capacity of 145 mA h g−1 at 5C rate and 112 mA h g−1 at 10C rate, and maintains 83.1% after 400 cycles at 5C rate and 82.5% at 10C rate. By post-mortem analysis of long-term cycled LMN–K/Nb, K+ and Nb5+ are recognized to play a role in suppressing the irreversible side reactions in LLOs during cycling. This work demonstrates that dual elemental substitution into the lithium layer and TM layer is a feasible strategy to enhance the performance of LLO cathode materials.
1. Introduction
Mn-Based Li-rich layered oxides (LLOs) have been considered as one of the most promising cathode materials due to their higher specific capacities (>250 mA h g−1) and energy densities, and low cost in comparison with the commercial LiCoO2, LiFePO4 and ternary Ni–Co–Mn cathode materials.1,2 For practical application in lithium ion batteries (LIBs), LLOs meet the problems of low initial coulombic efficiency, fast voltage fading, and inferior rate cycling stability,3,4 mainly due to the reasons including dissolution of transition metal ions into the electrolyte, low conductivity and irreversible formation of the spinel phase arising from the transition metal ions migrating from the transition metal (TM) layer to the lithium layer during long charging/discharging cycles.5,6In the past few years, researchers have been found that elemental substitution/doping into the LLO host structure is an effective method to improve structural stability and electrochemical performance.7–11 Two types of substitution/doping have been discussed in the literature, i.e. substitution of lithium ions in the lithium layer by alkali metals and replacing transition metal elements (i.e. Ni, Co and Mn) in the TM layer by hetero metal elements. Qing et al. reported that sodium substitution at lithium sites could improve the electrochemical performance of the 0.5Li2MnO3·0.5LiNi1/3Co1/3Mn1/3O2 material.9 Another work by Zheng et al. confirmed that potassium substitution at lithium sites of Li1.232Mn0.615Ni0.154O2 effectively enhanced the structural stability and electrochemical performance.10 In our previous study on Li1.167−xKxMn0.583Ni0.25O2 (x = 0, 0.025, 0.05 and 0.075) materials, we also proved that K+ substitution results in small perturbations in the host structure and improved the lithium diffusion ability and hindered the detrimental interphase growth of the spinel phase during cycling.11 The substitution by K+/Na+ with a larger radius is efficient in significantly improving the cycling performance of LLOs, but the specific capacity improvement is still limited.
Besides the heteroatomic substitution into the lithium layer, some studies have shown the substitution into the TM layer by alien ions, such as Al3+, Fe3+, Ca2+, Ru4+, Nb5+, etc.12–16 Passerini's group studied Li1.2Mn0.585Ni0.185Fe0.03O2 in which iron substitution effectively mitigated the voltage decay and capacity degradation.13 Dong et al. synthesized Li1.2(Ni0.13Co0.13Mn0.54)1−xNbxO2 (x = 0, 0.01, 0.02, 0.04) in which appropriate Nb substitution accelerated the lithium-ion diffusion ability and stabilized the crystal structure.14 The substitution in the TM layer has significantly improved the specific capacity of LLOs, but an improvement in the long-term cycling performance still needs more investigation.
Since substitution in the lithium layer or TM layer brings different benefits to the host material, co-substitution with the aim of utilizing synergistic effects from two or more substituted elements would be a promising strategy to improve the material performance.17–19 For example, Na and Al co-substitution has been reported to be an effective way to reduce the voltage decay of LLOs and inhibit the side reaction between the cathode materials and electrolyte during cycling.17 The co-substitution via Mg and La respectively in the lithium layer or TM layer has been proved to improve the structural stability and electrochemical performance of Li(Li0.2Mn0.54Co0.13Ni0.13)O2.18 To the best of our knowledge, there is no research on the co-substitution of LLOs with K+ and Nb5+, the structural stability mechanism and the improved properties of LLOs from the synergistic effect of K+ and Nb5+ co-substitution is unknown.
In this work, K/Nb co-substituted Li1.12K0.05Mn0.51Ni0.24Nb0.02O2 is successfully synthesized, and its structural stability and electrochemical performance are compared with those of Li1.17Mn0.52Ni0.25O2. The synergistic effect of K/Nb co-substitution on structural stability is revealed through inductively coupled plasma-atomic emission spectrometry (ICP-AES), X-ray powder diffraction (XRD), scanning and transmission electron microscopy (SEM and TEM), X-ray photoelectron spectroscopy (XPS), electrochemical impedance spectroscopy (EIS), galvanostatic charge/discharge (GCD), etc. At 2C rate, LMN–K/Nb delivers 164 mA h g−1 at the 1st cycle and retains 98% of its initial capacity after 200 cycles, while LMN delivers only 123 mA h g−1 at the 1st cycle and retains only 65% of its initial capacity at the 200th cycle. This work reports an approach for dual-ion substitution and provides it as an effective way to develop LLO cathode materials with stable cycling performance for energy storage applications.
2. Experimental section
2.1. Materials preparation
In this work, Li2CO3, MnO2, NiCO3 and absolute ethanol were bought from Sinopharm Chemical Reagent Co., Ltd and used without any pretreatment. Li1.17Mn0.58Ni0.25O2 and Li1.12K0.05Mn0.57Ni0.24Nb0.02O2 were prepared through a wet ball mill and high-temperature treatment. The parameters of ball milling are as follows: absolute ethanol used as a dispersant for the wet ball mill, NiCO3, MnO2 and Li2CO3 reagents in stoichiometric amounts mixed using a planetary ball mill (QM-1SP4-CL, Nanjing, China). Herein, a 5 wt% extra amount of lithium source besides its stoichiometric amount is used, compensating for lithium loss during high temperature treatment. The weight ratio of the milling ball with reactants, and the milling time and speed are 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10 h and 360 rpm, respectively. The precursor after ball milling was heated at 450 °C for 3 h and then at 900 °C for 12 h under an air atmosphere. The as-produced Li1.17Mn0.58Ni0.25O2 was simply named LMN. By using K2CO3 and Nb2O5 as the potassium source and niobium source, Li1.12K0.05Mn0.57Ni0.24Nb0.02O2 was synthesized by the same route and was named LMN–K/Nb.
1, 10 h and 360 rpm, respectively. The precursor after ball milling was heated at 450 °C for 3 h and then at 900 °C for 12 h under an air atmosphere. The as-produced Li1.17Mn0.58Ni0.25O2 was simply named LMN. By using K2CO3 and Nb2O5 as the potassium source and niobium source, Li1.12K0.05Mn0.57Ni0.24Nb0.02O2 was synthesized by the same route and was named LMN–K/Nb.
2.2. Characterization
Crystallographic structures of LMN and LMN–K/Nb were obtained by powder X-ray diffraction (XRD, Dmax-2200, Cu-Kα radiation) in the angle range of 10°–80° at 0.02° per scanning step and 4s counting time at each step. The compositions of Li, Ni, Mn, K and Nb were obtained by inductively coupled plasma atomic emission spectrometry (ICP-AES, Plasma-400, PerkinElmer). The surface morphologies and microstructures of the samples were characterized by scanning electron microscopy (SEM, ZEISS Sigma) and transmission electron microscopy (TEM, JEM-2100). The surface elemental valences of samples were analyzed by X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific, Escalab250Xi).2.3. Electrochemical measurements
The electrochemical properties of the samples were measured by both types of half-cell (CR2016-type coin cell) and full cell (pouch-type cell). The positive electrode was composed of active materials, polyvinylidene fluoride (PVdF) and carbon black in a weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and N-methyl-2-pyrrolidone (NMP) as a solvent. The mixture was stirred for 24 h, and the slurry was coated on Al foil. The electrode was dried at 100 °C for 12 h under vacuum. In a half-cell, the area and thickness of the positive electrode are 1.21 cm2 and 25 μm, respectively. The mass loading of the electrode is 1.8 mg cm−2. By using metallic lithium foil as a negative electrode, Celgard 2500 as a separator, 1 M LiPF6 dissolved in dimethyl carbonate (DMC), ethyl methyl carbonate (EMC) and ethylene carbonate (EC) (1
1 and N-methyl-2-pyrrolidone (NMP) as a solvent. The mixture was stirred for 24 h, and the slurry was coated on Al foil. The electrode was dried at 100 °C for 12 h under vacuum. In a half-cell, the area and thickness of the positive electrode are 1.21 cm2 and 25 μm, respectively. The mass loading of the electrode is 1.8 mg cm−2. By using metallic lithium foil as a negative electrode, Celgard 2500 as a separator, 1 M LiPF6 dissolved in dimethyl carbonate (DMC), ethyl methyl carbonate (EMC) and ethylene carbonate (EC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume) as an electrolyte, the coin cells were assembled in an argon-filled glove box where both water and oxygen concentrations are less than 0.1 ppm. Electrochemical impedance spectroscopy (EIS) was performed on an electrochemical workstation (PARSTAT 2273, Princeton, USA), and the frequency range was from 105 Hz to 10−2 Hz. Using the galvanostatic intermittent titration technique (GITT), the curves of the cathode material during the 3rd cycle were recorded at a time interval of 30 min by using a LAND CT2001A battery testing system.
1 in volume) as an electrolyte, the coin cells were assembled in an argon-filled glove box where both water and oxygen concentrations are less than 0.1 ppm. Electrochemical impedance spectroscopy (EIS) was performed on an electrochemical workstation (PARSTAT 2273, Princeton, USA), and the frequency range was from 105 Hz to 10−2 Hz. Using the galvanostatic intermittent titration technique (GITT), the curves of the cathode material during the 3rd cycle were recorded at a time interval of 30 min by using a LAND CT2001A battery testing system.
Considering the practical application, we have assembled a full cell (pouch type cell) to evaluate the electrochemical properties of LMN–K/Nb. In this work, commercial graphite (Sinopharm Chemical Reagent Co., Ltd) was used as a negative electrode in a pouch-type cell. The positive and negative electrodes were cut in squares, and the side lengths of the squares for the negative and positive electrodes are 35 and 40 mm, respectively. The designed capacity of the negative electrode was 1.1 times that of the positive electrode. The slurry of the negative electrode was prepared by mixing 80 wt% of commercial graphite, 10 wt% of PVdF and 10 wt% of carbon black. Generally, secondary sealing is necessary for lithium-rich cathode materials in a pouch-type full cell, because the gas accumulated after several charge/discharge cycles swells the bag and must be removed. In this work, secondary sealing for the pouch-type full cell was applied after 5 charging/discharging cycles to remove the gas accumulated during the redox reaction. A LAND CT2001A battery testing system (Wuhan LANHE, P.R. China) was used to record the galvanostatic charge/discharge profiles at 25 °C.
3. Results and discussion
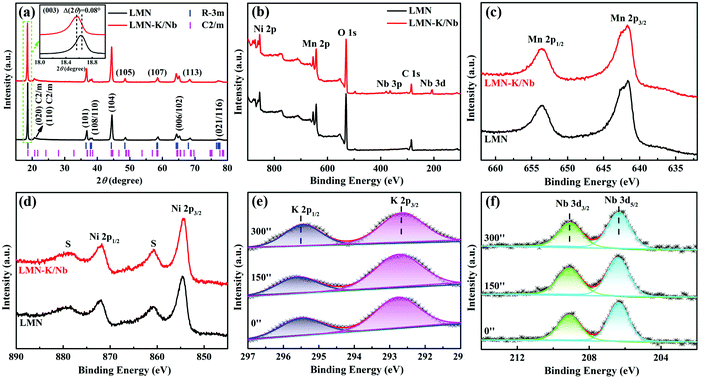
Fig. 1a shows the XRD patterns of LMN and LMN–K/Nb. Each sample presents clear and sharp reflections, indicating that both samples are high crystallized. Most reflections can be indexed to the hexagonal NaFeO2 structure (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m), besides the superlattice diffraction peaks between 20 and 25° that correspond to the Li2MnO3 phase (C2/m).20,21 No impurity peaks are found in LMN–K/Nb, indicating that K+ and Nb5+ are successfully substituted into the crystal lattice without affecting the host structure of LLOs. As shown in the inset image in Fig. 1a, the (003) peak of LMN–K/Nb shifts by a few degrees lower than that of LMN, indicating that the interlayer spacing of LMN–K/Nb has a slightly bigger value than that of LMN. This may be due to the fact that the intercalated K+ expands the interlayer spacing of LMN–K/Nb because K+ (1.38 Å) has a much larger ionic radius than Li+ (0.76 Å). The expanded interlayer spacing can increase the diffusion ability of lithium ions.22
m), besides the superlattice diffraction peaks between 20 and 25° that correspond to the Li2MnO3 phase (C2/m).20,21 No impurity peaks are found in LMN–K/Nb, indicating that K+ and Nb5+ are successfully substituted into the crystal lattice without affecting the host structure of LLOs. As shown in the inset image in Fig. 1a, the (003) peak of LMN–K/Nb shifts by a few degrees lower than that of LMN, indicating that the interlayer spacing of LMN–K/Nb has a slightly bigger value than that of LMN. This may be due to the fact that the intercalated K+ expands the interlayer spacing of LMN–K/Nb because K+ (1.38 Å) has a much larger ionic radius than Li+ (0.76 Å). The expanded interlayer spacing can increase the diffusion ability of lithium ions.22
In order to further study the structure of LMN and LMN–K/Nb, the XRD patterns were refined based on the two-phase mode of LiNi0.5Mn0.5O2 (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) and the Li2MnO3 monoclinic phase (C2/m) by using the General Structure Analysis System (GSAS). The values of the Rwp factor (less than 10%) confirm the reasonable refinement.20 The intensity ratios I(003)/I(104), as summarized in Table S1,† are greater than 1.2, indicating that both samples have an ordered layered structure with low cation mixing.22 The smaller I(003)/I(104) value of LMN–K/Nb than that of LMN again indicates the substitution of K+ and Nb5+ into the lattice structure of LLOs, which reduces the ordering layered structure to some extent. As detailed in the inset in Fig. S1,† the well-splitting reflections of (006)/(102) and (108)/(110) show that both samples have a typical layered structure.20 In addition, the lattice parameter c of LMN–K/Nb (14.2754 Å, R
m) and the Li2MnO3 monoclinic phase (C2/m) by using the General Structure Analysis System (GSAS). The values of the Rwp factor (less than 10%) confirm the reasonable refinement.20 The intensity ratios I(003)/I(104), as summarized in Table S1,† are greater than 1.2, indicating that both samples have an ordered layered structure with low cation mixing.22 The smaller I(003)/I(104) value of LMN–K/Nb than that of LMN again indicates the substitution of K+ and Nb5+ into the lattice structure of LLOs, which reduces the ordering layered structure to some extent. As detailed in the inset in Fig. S1,† the well-splitting reflections of (006)/(102) and (108)/(110) show that both samples have a typical layered structure.20 In addition, the lattice parameter c of LMN–K/Nb (14.2754 Å, R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase) is higher than that of LMN (14.2608 Å), which corresponds to the (003) peak shift of LMN–K/Nb in Fig. 1a. The large interplanar spacing of LMN–K/Nb is conducive to the rapid diffusion of Li+, thereby showing excellent rate performance.
m phase) is higher than that of LMN (14.2608 Å), which corresponds to the (003) peak shift of LMN–K/Nb in Fig. 1a. The large interplanar spacing of LMN–K/Nb is conducive to the rapid diffusion of Li+, thereby showing excellent rate performance.
The Li![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K
K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn
Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni
Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Nb atomic ratios for LMN and LMN–K/Nb were measured by ICP. The values are respectively Li1.17Mn0.59Ni0.25 and Li1.11K0.05Mn0.58Ni0.24Nb0.02 (the value after each metal element is the calculated atomic ratio), and they are very close to the theoretical values of Li1.17Mn0.58Ni0.25 and Li1.12K0.05Mn0.57Ni0.24Nb0.02 for LMN and LMN–K/Nb, respectively. Until now, it was a challenge to directly prove the substitution of heteroelements into the lithium layer or TM layer of LLOs. The cations that remain in lithium layers could be exchanged with H+ by soaking the samples in acidic solution, instead of those in TM layers.23 In this work, the substituted site of K+ and Nb5+ in the lithium layer and TM layer is studied by using this method. To do so, 500 mg of the sample was dispersed in 500 mL of HCl solution (1 mol L−1) under magnetic stirring for 72 h, and the HCl solution was renewed every 24 h. Then, the sample was centrifuged, washed with deionized water and dried at 80 °C. Before and after H+ exchange, the atomic ratios of metal elements were measured by ICP. The calculated atomic ratios for LMN and LMN–K/Nb after H+ exchange are Li0.36Mn0.57Ni0.25 and Li0.30K0.01Mn0.58Ni0.24Nb0.02, respectively. Lithium atomic ratios in both LMN and LMN–K/Nb dramatically reduced to about 0.3, and the K atomic ratio in LMN–K/Nb significantly decreased from 0.05 to 0.01 after H+ exchange. However, the Nb atomic ratio in LMN–K/Nb shows no change before and after H+ exchange. Since H+ replaces the cations that remain in the lithium layer rather than those in the TM layer, the aforementioned results may strongly confirm that K+ and Nb5+ substitution is respectively in the lithium layer and TM layer.
Nb atomic ratios for LMN and LMN–K/Nb were measured by ICP. The values are respectively Li1.17Mn0.59Ni0.25 and Li1.11K0.05Mn0.58Ni0.24Nb0.02 (the value after each metal element is the calculated atomic ratio), and they are very close to the theoretical values of Li1.17Mn0.58Ni0.25 and Li1.12K0.05Mn0.57Ni0.24Nb0.02 for LMN and LMN–K/Nb, respectively. Until now, it was a challenge to directly prove the substitution of heteroelements into the lithium layer or TM layer of LLOs. The cations that remain in lithium layers could be exchanged with H+ by soaking the samples in acidic solution, instead of those in TM layers.23 In this work, the substituted site of K+ and Nb5+ in the lithium layer and TM layer is studied by using this method. To do so, 500 mg of the sample was dispersed in 500 mL of HCl solution (1 mol L−1) under magnetic stirring for 72 h, and the HCl solution was renewed every 24 h. Then, the sample was centrifuged, washed with deionized water and dried at 80 °C. Before and after H+ exchange, the atomic ratios of metal elements were measured by ICP. The calculated atomic ratios for LMN and LMN–K/Nb after H+ exchange are Li0.36Mn0.57Ni0.25 and Li0.30K0.01Mn0.58Ni0.24Nb0.02, respectively. Lithium atomic ratios in both LMN and LMN–K/Nb dramatically reduced to about 0.3, and the K atomic ratio in LMN–K/Nb significantly decreased from 0.05 to 0.01 after H+ exchange. However, the Nb atomic ratio in LMN–K/Nb shows no change before and after H+ exchange. Since H+ replaces the cations that remain in the lithium layer rather than those in the TM layer, the aforementioned results may strongly confirm that K+ and Nb5+ substitution is respectively in the lithium layer and TM layer.
The chemical valences of elements at the sample surface and near-surface are evaluated by XPS coupled with Ar+ etching. The survey spectra of LMN and LMN–K/Nb as shown in Fig. 1b present the relative metal elements without any impurities. The Mn 2p spectra (Fig. 1c) of LMN and LMN–K/Nb show the same characteristic peaks respectively corresponding to Mn 2p1/2 at 653.0 eV and Mn 2p3/2 at 641.5 eV. The Ni 2p spectra (Fig. 1d) of LMN and LMN–K/Nb show the same characteristic peaks at 855.0 and 872.5 eV, which correspond to Ni 2p3/2 and Ni 2p1/2, respectively. The peak positions of Mn 2p and Ni 2p in LMN and LMN–K/Nb corresponding to the presence of Mn4+ and Ni2+ are very close to the previous report,24 and the results indicate that the substitution has no effect on the valence state of Mn and Ni.
For LMN–K/Nb, the peaks at 292.4 eV and 295.5 eV in K 2p spectra (Fig. 1e) assigned to K 2p3/2 and K 2p1/2,25 and the peaks at 206.3 eV and 209.1 eV in Nb 3d spectra (Fig. 1f) are respectively assigned to Nb 3d5/2 and Nb 3d3/2.26 In addition, Fig. 1e and f show the K 2p and Nb 3d peaks of LMN–K/Nb at different Ar+ etching times of 0, 150 and 300 s (the relative etching depth in the range of 0–150 nm). Because of the same quantity of K and Nb elements detected at different depths of the sample crystal, there is strong evidence that K and Nb elements are uniformly incorporated into the structure but do not simply reside on the sample surface.
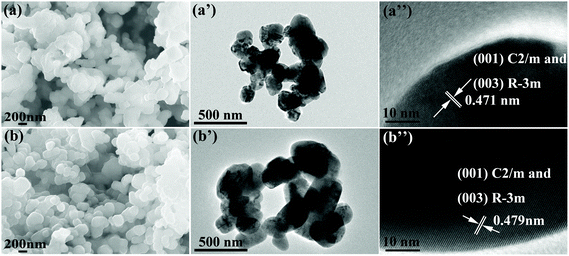
The crystal morphology of LMN and LMN–K/Nb are characterized by SEM, TEM and HRTEM (Fig. 2). The particles of both samples are homogeneous with similar crystal shapes and the sizes in the range of 200–400 nm (Fig. 2a, b, a′ and b′). The results show that the K+ and Nb5+ substitution plays a small role in crystal morphology. As shown in the HRTEM images in Fig. 2a′′ and b′′, both samples show clear lattice fringes. The lattice distances for LMN and LMN–K/Nb are 0.471 and 0.479 nm corresponding to the (003) crystal plane.2 A minor bigger lattice distance of (003) in LMN–K/Nb than that in LMN may be due to K+ with a bigger ionic radius than Li+ in lithium layers.
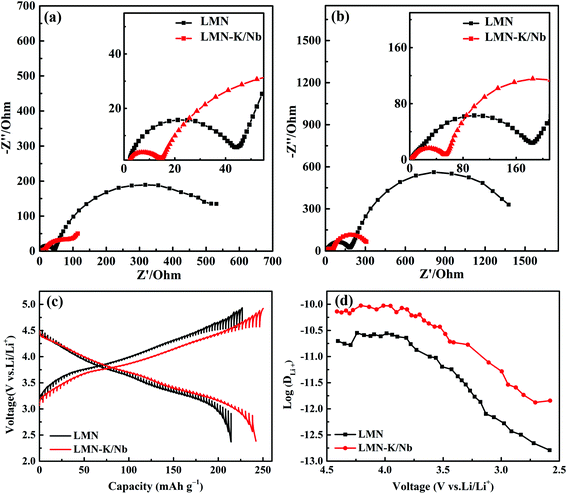
The effects of K+ and Nb5+ co-substitution on the electrochemical properties of LMN–K/Nb are studied in comparison with LMN. Fig. 3a and b show the Nyquist plots of half cells with LMN and LMN–K/Nb as positive electrodes, and the cells have been discharged to 3.5 V at the 3rd cycle and 50th cycle, respectively. The Nyquist plots are well refined by an equivalent circuit in Fig. S2,† and the exact values of Re (ohmic resistance), Rsf (resistance of SEI film) and Rct (charge transfer resistance) are summarized in Table S2.† It is noted that both samples show no obvious change in Re values during cycling. However, the Rsf and Rct values of both LMN and LMN–K/Nb at the 50th cycle are increased two times those at the 3rd cycle, arising from their phase change from the layered to the spinel structure and irreversible side reactions during cycling.11,27 For example, the Rct values of LMN and LMN–K/Nb are increased from 505.71 and 90.63 Ω at the 3rd cycle to 1353.32 and 266.16 Ω at the 50th cycle. At the 3rd and 50th cycles, LMN–K/Nb shows much smaller Rsf and Rct values than LMN, which might be due to the fact that the synergistic effect of K+ and Nb5+ co-substitution effectively increases the structural stability and lithium diffusion ability.
The effect of K+ and Nb5+ co-substitution on the kinetic behavior of insertion/extraction of Li+ is further evaluated by GITT. Each current pulse (charge or discharge) of 10 min at a current density of 0.1C followed by keeping at a 30 min open circuit was repeatedly employed until the charge/discharge voltages reach 4.9 V/2.5 V, respectively. Based on the GITT curves of each sample at the 3rd cycle (Fig. 3c), the lithium diffusion coefficient of Li+ (DLi+) is calculated according to the following equation:11,28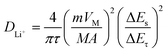
wherein m and M respectively represent mass (g) and molecular mass (g mol−1) of the active materials, VM is the molar volume deduced from the crystallographic data (cm3 mol−1), A is the surface area (cm2) of the electrode, ΔEs is the change in the steady-state voltage and ΔEτ is the voltage change in a single step GITT experiment. The DLi+ values of LMN and LMN–K/Nb at various states of discharge are plotted in Fig. 3d. It can be seen that the DLi+ values of both samples are slowly decreased from ∼1.2 × 10−11 to ∼1.5 × 10−12 cm2 s−1 during the discharging process, and LMN–K/Nb shows a higher DLi+ value than LMN. According to numerous research results,22,27,28 it can be concluded that the extended interlayer spacing can improve the Li+ diffusion capability. Since the radius of K+ (1.38 Å) is larger than that of Li+ (0.76 Å), the LMN–K/Nb with an extended interlayer spacing exhibits a higher diffusion coefficient of Li+, which helps it to exhibit excellent rate performance.
To further ascertain the cause for improved cycling and rate performance by K+ and Nb5+ co-substitution, galvanostatic charging and discharging of LMN and LMN–K/Nb were performed. The cells are cycled in the voltage range of 2.5–4.9 V at various current rates. The initial charge–discharge curves at 0.1C (1C = 200 mA g−1) of both samples present two charging steps (Fig. 4a), i.e. the charging below 4.5 V corresponds to the reaction of Ni2+/Ni4+ in the LiNi0.5Mn0.5O2 layered phase, and the other voltage plateau of ∼4.6 V is attributed to the extraction of Li+ and O evolution from the lithium rich Li2MnO3 phase.29 The latter often shows poor reversibility and it leads to a large irreversible capacity in the first cycle. The initial specific discharge capacities of LMN and LMN–K/Nb are 211 and 244 mA h g−1, and the coulombic efficiencies (CEs) are 59.6 and 77.2%, respectively. Since Nb could offer stronger metal oxygen bonds, which is beneficial for suppressing the O evolution of LLOs.14
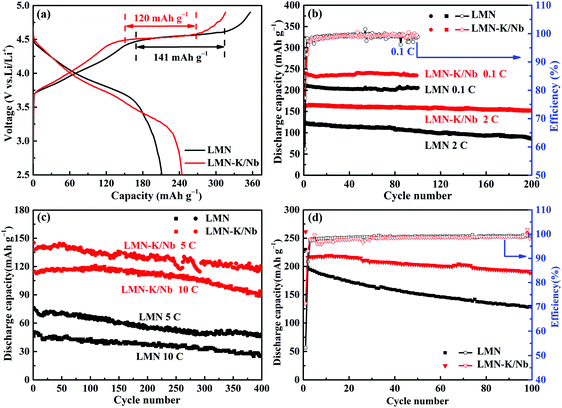 | ||
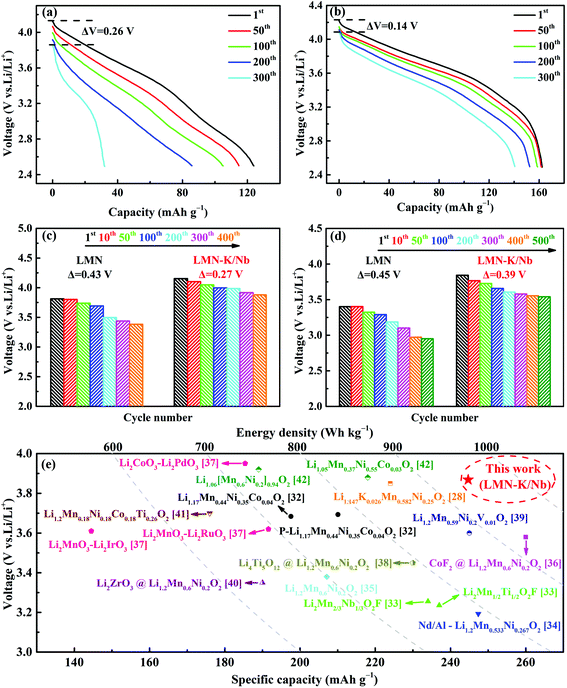
| Fig. 4 The 1st charge/discharge curves (a), the cycling performance at 0.1C and 2C (b), and 5C and 10C (c), and the cycling performance of pouch-type full cells at 0.1C (d). | ||
Fig. 4b and c show a comparison of the cycling performance and coulombic efficiency (CE) of LMN and LMN–K/Nb at various current rates. LMN–K/Nb presents much higher specific capacity than LMN at each rate. At 0.1C, LMN–K/Nb delivers 246 mA h g−1 at the initial cycle and 231 mA h g−1 at the 100th cycle, while LMN only delivers 209 mA h g−1 at the 1st cycle and 203 mA h g−1 at the 100th cycle. At 2C rate, LMN–K/Nb delivers 164 mA h g−1 at the 1st cycle and retains 98% of its initial capacity after 200 cycles, while LMN delivers only 123 mA h g−1 at the 1st cycle and retains only 65% of its initial capacity at the 200th cycle (Fig. 4b).
At higher current rates of 5C and 10C, LMN–K/Nb delivers two times the specific capacity than LMN through 400 cycles (as shown in Fig. 4c). At 5C, LMN–K/Nb delivers 145 mA h g−1 at the 1st cycle and retains 83.1% of its initial capacity at the 400th cycle, while LMN delivers only 75 mA h g−1 at the 1st cycle and retains only 60% of its initial capacity at the 400th cycle (Fig. 4c). At 10C rate, the specific capacity of LMN decreases to 48 mA h g−1 and 28 mA h g−1 at the 1st and 400th cycles, respectively, while LMN–K/Nb still delivers a high capacity of 112 mA h g−1 at the 1st cycle and retains 92 mA h g−1 at the 400th cycle. The much better rate cycling performance of LMN–K/Nb than LMN should be attributed to the success of K+ and Nb5+ co-substitution.
To evaluate the practical application of the synthesized cathode materials, full cells (pouch type cell) with the prepared materials as a positive electrode and commercial graphite as a negative electrode are assembled. A formation cycle at a current rate of 0.01C is expected as the solid electrolyte interphase (SEI) layer is grown.30 The cycling performance of full cells is measured between 2.5 and 4.9 V at 0.1C rate, and the cycling performance is presented in Fig. 4d. LMN–K/Nb shows much higher capacity than LMN, especially, the capacity retention of LMN–K/Nb is 88.3% after 100 cycles which is much higher than that of LMN of only 64.8%. As shown in Fig. S3,† the rate performance of the LMN and LMN–K/Nb samples is evaluated at 0.1C, 0.2C, 0.4C, 0.5C, 0.8C and 1C. By increasing the current density from 0.1 to 1C, the discharge specific capacity of the samples is relatively reduced. For example, the discharge capacities of LMN are 196.6, 171.1, 149.0, 134.2, 111.9, 94.3 and 166.2 mA h g−1, but LKMNO delivers higher values of 216.6, 194.5, 162.5, 147.8, 130.3, 117.5 and 205.8 mA h g−1 at 0.1, 0.2, 0.4, 0.5, 0.8, 1 and 0.1C, respectively. The full cell performance further confirms that K+ and Nb5+ co-substitution efficiently improves the capacity contribution and cycling stability of LLO cathode materials.
It is well known that IR drop is an important criterion to evaluate the electrochemical performance of electrode materials during redox reactions.13,31 As shown in Fig. 5a and b, the IR drop values of LMN and LMN–K/Nb are respectively 0.26 and 0.14 V from the 1st to 200th cycles at 2C rate. Compared with the discharge curves for hundreds of cycles, the discharge voltages of LMN and LMN–K/Nb have all decreased along with an increase in charging/discharging cycles. The starting discharge voltages of LMN and LMN–K/Nb decrease from the 1st cycle to the 400th cycle at 5C rate and the decay values are respectively 0.43 and 0.27 V (Fig. 5c). A similar trend of the sample at 10C rate can be found in Fig. 5d. LMN–K/Nb presents a smaller IR drop than LMN, indicating that K/Nb co-substitution effectively mitigates the voltage decay and capacity degradation. In addition, Fig. 5e shows a comparison of the specific capacity and energy density of LMN–K/Nb with the reported LLO cathode materials in detail. Herein, more cobalt-free LLOs are deliberately selected and different modification methods are selected as examples, such as substitution by V, F and Nd/Al and coating by CoF2, Li2ZrO3 and Li4Ti5O12.32–42 It is evident that LMN–K/Nb exhibits higher specific capacity and energy density than most of those previously reported. Therefore, the results show that K+ and Nb5+ co-substituted LLO cathode materials have huge advantages in practical applications, especially in devices that require high energy densities.
In order to understand the effect of K+ and Nb5+ co-substitution on enhancing the structural stability of LLOs during cycling, the differential capacity curves (vs. voltage dQ/dV) of LMN and LMN–K/Nb at various cycles (1st, 5th, 10th, 20th, 30th and 50th cycles) are plotted in Fig. 6. The peaks denoted as P1 and P2 were observed at 3.8 and 4.6 V during the first charging process. P1 is attributed to the redox reaction of Ni2+/Ni4+ in the LiNi0.5Mn0.5O2 phase, and P2 corresponds to an irreversible process of Li extraction and O2 release from the Li2MnO3 structure,43 which disappeared after the first cycle. From the 2nd cycle, a new peak at 3.35 V (denoted as P3) is observed in each sample, which is attributed to the redox reaction of Mn3+/Mn4+.29,44 During discharge, three peaks are observed at 4.31, 3.79 and 3.25 V (respectively denoted as P4, P5 and P6). P4 is related to Li occupation at the tetrahedral sites, and P5 is ascribed to Li occupation at the octahedral sites accompanied by the Ni4+/3+/2+ and Mn4+/Mn3+ redox reaction.22 P6 not only corresponds to the redox reaction of Mn3+/Mn2+ but also can be used to evaluate the phase transition from the layered structure to the spinel structure.45 The intensity and displacement of the P6 peak are directly related to the degree of phase transition.
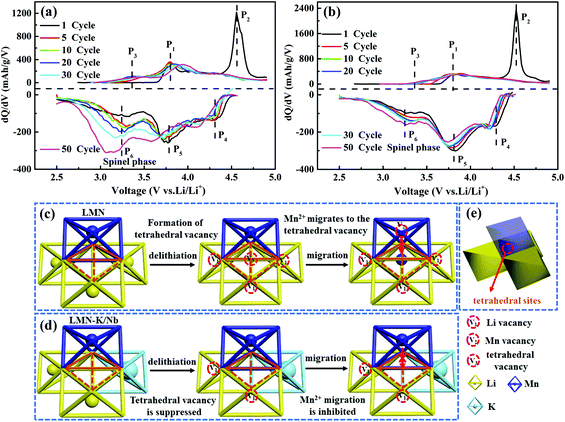 | ||
| Fig. 6 The dQ/dV profiles of LMN (a) and LMN–K/Nb (b) at 0.1C; schematic diagrams of the possible phase evolution routes for LMN (c) and LMN–K/Nb (d), and tetrahedral sites in the Li layer (e). | ||
The appearance of Mn3+ in LLOs during discharge along cycling leads to the disproportionation reaction and the formation of Mn4+ and Mn2+.28 The as-produced Mn2+ easily migrates into the lithium layer to form the spinel phase during the delithiation process. From the 1st cycle to the 50th cycle, LMN–K/Nb presents a weaker peak intensity and smaller deviation of the P6 peak than LMN (Fig. 6a and b), indicating the better structural stability of LMN–K/Nb. The hindering effect of K+ substitution on the formation of the spinel phase is explained in Fig. 6c–e. For LMN, during the charging (delithiation) process, the removal of lithium ions results in the formation of trivacancies, and adjacent Mn ions tend to migrate into the tetrahedral vacancies surrounded by these trivacancies. As a result, the spinel phase is irreversibly formed (Fig. 6c and d).25 Because K+ has a larger ion radius than Li+, K+ substitution into the Li layer plays a pillar role in preventing the migration of Mn2+ from the TM layer to the lithium layer (Fig. 6d). This effectively inhibits the formation of the spinel phase and improves the cycling performance of LMN–K/Nb.
The structural changes of the cycled LMN and LMN–K/Nb electrodes are evidenced by ex situ XRD. Fig. 7 shows the XRD patterns and enlarged characteristic peaks of both electrodes at the 1st, 10th and 50th cycles. The layer-spinel phase transition can be clearly evaluated by the (003) and (104) peak shifts.46 After the 1st cycle, the (003) peak of LMN observed at 18.3° (2θ) still represents a layered structure; after 10 cycles it shifted to 18.1° (2θ), representing the mixed structure of the layer and spinel; and after 50 cycles it shifted to 17.9° (2θ) arising from the major spinel structure (Fig. 7a′). A similar trend of structural transition with cycling is also found by the (104) peak shifting (Fig. 7a′′). The characteristic layer structure of LMN–K/Nb was excellently maintained after dozens of cycles at the unchanged (003) and (104) peak positions (Fig. 7b′ and b′′). This clearly reveals that K+ and Nb5+ co-substitution can effectively suppress the irreversible spinel growth in the LLO cathodes during cycling. After dozens of cycles, the grown spinel phase in LMN and the unchanged layer structure in LMN–K/Nb are in accordance with the aforementioned P6 in their dQ/dV results (Fig. 6a and b). The aforementioned results indicate that LMN has a large amount of Mn2+ dissolution, accompanied by phase transition from the layer to the spinel structure, and finally results in structural disruption and rapid capacity fading. For K+ and Nb5+ co-substitution, the structural stability and electrochemical properties of LMN–K/Nb are significantly improved, in which K+ substitution in the lithium layer effectively inhibits the phase transition and the Nb–O bonds arising from Nb5+ substitution effectively inhibits oxygen evolution and reduces the dissolution of Mn cations during cycling.
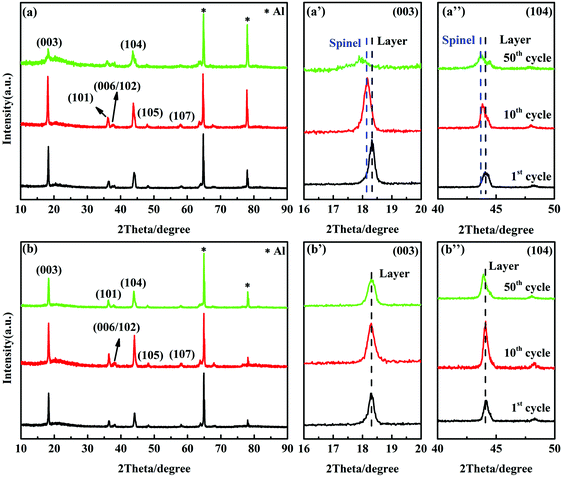 | ||
| Fig. 7 XRD patterns and the enlarged characteristic peaks of LMN (a, a′ and a′′) and LMN–K/Nb (b, b′ and b′′) after different cycles, and both samples are discharged to 2.5 V. | ||
Taking the above results into account, the significant improvement in the electrochemical performance of LMN–K/Nb can be attributed to the following synergistic effects: (1) K+ substitution in the lithium layer expands the interlayer spacing of the lithium layer, thereby showing excellent rate performance. (2) K+ can also inhibit the migration of transition metal ions, thus inhibiting the irreversible phase transition and improving the cycling stability. (3) The binding energy of Nb–O is higher than Ni–O and Mn–O, so Nb5+ substitution into the TM layer can inhibit the O evolution and stabilize the host material structure. Furthermore, K+ and Nb5+ are also recognized to play a role in suppressing the irreversible side reactions during cycling.
4. Conclusion
In this work, K+ and Nb5+ are successfully substituted into the lithium layer and TM layer of Li-rich cathode materials, and LMN–K/Nb shows improved structural stability and electrochemical properties in comparison with the pristine sample. It delivers initial capacities of 145 mA h g−1 and 112 mA h g−1 at current rates of 5C and 10C, respectively, and shows capacity retentions of 83.1% after 400 cycles at 5C. K+ substitution in the lithium layer expands the interlayer spacing of the lithium layer and inhibits irreversible phase transition, thus leading to improved rate and cycling stability. The binding energy of Nb–O is higher than those of Ni–O and Mn–O, so Nb5+ substitution into the TM layer can inhibit the O evolution and stabilize the material structure. Furthermore, K+ and Nb5+ are also recognized to play a role in suppressing the irreversible side reactions during cycling.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors greatly appreciate the financial support from the National Natural Science Foundation of China (grant number 51802030) and the Natural Science Foundation of Jiangsu Province, China (Grant No. BK20161267).References
- J. Xie and Y. C. Lu, A retrospective on lithium-ion batteries, Nat. Commun., 2020, 11, 2499 CrossRef CAS PubMed.
- F. Wu, J. Maier and Y. Yu, Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries, Chem. Soc. Rev., 2020, 49, 1569–1614 RSC.
- H. Xie, J. Cui, Z. Yao, X. Ding, Z. Zhang, D. Luo and Z. Lin, Revealing the role of spinel phase on Li-rich layered oxides: A review, Chem. Eng. J., 2022, 427, 131978 CrossRef CAS.
- Y. Zhou, C. H. Wang, W. Lu and L. Dai, Recent advances in fiber-shaped supercapacitors and lithium-ion batteries, Adv. Mater., 2020, 32, 1902779 CrossRef CAS PubMed.
- S. Li, H. Li, H. Zhang, S. Zhang, Y. Lai and Z. Zhang, Constructing stable surface structures enabling fast charging for Li-rich layered oxide cathodes, Chem. Eng. J., 2022, 427, 132036 CrossRef CAS.
- P. Liu, H. Zhang, W. He, T. Xiong, Y. Cheng, Q. Xie, Y. Ma, H. Zheng, L. Wang, Z. Z. Zhu, Y. Peng, L. Mai and D. L. Peng, Lithium deficiencies engineering in Li-rich layered oxide Li1.098Mn0.533Ni0.113Co0.138O2 for high-stability cathode, J. Am. Chem. Soc., 2019, 141, 10876–10882 CrossRef CAS PubMed.
- W. Zuo, M. Luo, X. Liu, J. Wu, H. Liu, J. Li, M. Winter, R. Fu, W. Yang and Y. Yang, Li-rich cathodes for rechargeable Li-based batteries: reaction mechanisms and advanced characterization techniques, Energy Environ. Sci., 2020, 13, 4450–4497 RSC.
- J. Liu, J. Wang, Y. Ni, K. Zhang, F. Cheng and J. Chen, Recent breakthroughs and perspectives of high-energy layered oxide cathode materials for lithium ion batteries, Mater. Today, 2021, 43, 132–165 CrossRef CAS.
- R. P. Qing, J. L. Shi, D. D. Xiao, X. D. Zhang, Y. X. Yin, Y. B. Zhai, L. Gu and Y. G. Guo, Enhancing the kinetics of Li-rich cathode materials through the pinning effects of gradient surface Na+ doping, Adv. Energy Mater., 2016, 6, 1501914 CrossRef.
- Z. Zheng, X. D. Guo, Y. J. Zhong, W. B. Hua, C. H. Shen, S. L. Chou and X. S. Yang, Host structural stabilization of Li1.232Mn0.615Ni0.154O2 through K-doping attempt: toward superior electrochemical performances, Electrochim. Acta, 2016, 188, 336–343 CrossRef CAS.
- C. Liu, M. M. Wu, Z. X. Guo, X. K. Luo, H. M. Ji, G. Yang and W. H. Hou, Preparation and characterization of Li1.167−xKxMn0.583Ni0.25O2 (x = 0, 0.025, 0.05 and 0.075) as cathode materials for highly reversible lithium-ion batteries, Electrochim. Acta, 2020, 341, 136014 CrossRef CAS.
- W. T. Lo, C. Yu, E. G. Leggesse, S. Nachimuthu and J. C. Jiang, Understanding the role of dopant metal atoms on the structural and electronic properties of lithium-rich Li1.2Ni0.2Mn0.6O2 cathode material for lithium-ion batteries, J. Phys. Chem. Lett., 2019, 10, 4842–4850 CrossRef CAS PubMed.
- F. L. Wu, G. T. Kim, M. Kuenzel, H. Zhang, J. Asenbauer, D. Geiger, U. Kaiser and S. Passerini, Elucidating the effect of iron doping on the electrochemical performance of cobalt-free lithium-rich layered cathode materials, Adv. Energy Mater., 2019, 9, 1902445 CrossRef CAS.
- S. D. Dong, Y. Zhou, C. X. Hai, J. B. Zeng, Y. X. Sun, Y. Shen, X. Li, X. F. Ren, C. Sun, G. T. Zhang and Z. W. Wu, Understanding electrochemical performance improvement with Nb doping in lithium-rich manganese-based cathode materials, J. Power Sources, 2020, 462, 228185 CrossRef CAS.
- M. Zubair, G. Y. Li, B. Y. Wang, L. Wang and H. J. Yu, Electrochemical kinetics and cycle stability improvement with Nb doping for lithium-rich layered oxides, ACS Appl. Energy Mater., 2018, 2, 503–512 CrossRef.
- J. C. Knight, P. Nandakumar, W. H. Kan and A. Manthiram, Effect of Ru substitution on the first charge-discharge cycle of lithium-rich layered oxides, J. Mater. Chem. A, 2015, 3, 2006–2011 RSC.
- J. C. Yang, Y. X. Chen, Y. J. Li, X. M. Xi, J. C. Zheng, Y. L. Zhu, Y. K. Xiong and S. W. Liu, Encouraging voltage stability upon long cycling of Li-rich Mn-based cathode materials by Ta-Mo dual doping, ACS Appl. Mater. Interfaces, 2021, 13, 25981–25992 CrossRef CAS PubMed.
- M. Li, H. Y. Wang, L. M. Zhao, Y. Zhou, F. Zhang and D. N. He, Improving the electrochemical performance of lithium-rich oxide layer material with Mg and La co-doping, J. Alloys Compd., 2019, 782, 451–460 CrossRef CAS.
- X. Q. Tian, S. Z. Liu, X. Q. Jiang, F. Ye and R. Cai, A new lithium–rich layer–structured cathode material with improved electrochemical performance and voltage maintenance, Int. J. Energy Res., 2019, 43, 7547–7556 CAS.
- C. Huang, Z. Q. Fang, Z. J. Wang, J. W. Zhao, S. X. Zhao and L. J. Ci, Accelerating the activation of Li2MnO3 in Li-rich high-Mn cathodes to improve its electrochemical performance, Nanoscale, 2021, 13, 4921–4930 RSC.
- L. Ku, Y. Cai, Y. Ma, H. Zheng, P. Liu, Z. Qiao, Q. Xie, L. Wang and D. L. Peng, Enhanced electrochemical performances of layered-spinel heterostructured lithium-rich Li1.2Ni0.13Co0.13Mn0.54O2 cathode materials, Chem. Eng. J., 2019, 370, 499–507 CrossRef.
- Q. Li, G. S. Li, C. C. Fu, D. Luo, J. M. Fan and L. P. Li, K+-doped Li1.2Mn0.54Co0.13Ni0.13O2: a novel cathode material with an enhanced cycling stability for lithium-ion batteries, ACS Appl. Mater. Interfaces, 2014, 6, 10330–10341 CrossRef CAS PubMed.
- Z. M. Hu, M. Chen, H. Zhang, L. Huang, K. S. Liu, Y. S. Ling, H. Zhou, Z. Jiang, G. Feng and J. Zhou, Stabilization of layered manganese oxide by substitutional cation doping, J. Mater. Chem. A, 2019, 7, 7118–7127 RSC.
- J. H. Wang, Y. Wang, Y. Z. Guo, C. W. Liu and L. L. Dan, Electrochemical characterization of AlPO4 coated LiNi1/3Co1/3Mn1/3O2 cathode materials for high temperature lithium battery application, Rare Met., 2021, 40, 78–83 CrossRef.
- Y. X. Sun, L. J. Zhang, Y. Zhou, Y. Shen, C. X. Hai, X. Li, J. B. Zeng, X. F. Ren, L. X. Ma, X. X. Zhang, S. D. Dong and G. C. Qi, Study on potassium doped modification of Li1.2Ni0.13Co0.13Mn0.54O2 materials synthesized by novel method for lithium ion battery, J. Electrochem. Soc., 2018, 165, A333–A338 CrossRef CAS.
- R. X. Sun, Y. Yue, X. F. Cheng, K. Zhang, S. Y. Jin, G. Y. Liu, Y. X. Fan, Y. Bao and X. D. Liu, Ionic liquid-induced ultrathin and uniform N-doped carbon-wrapped T-Nb2O5 microsphere anode for high-performance lithium-ion battery, Rare Met., 2021, 40, 3205–3214 CrossRef CAS.
- T. T. Xu, C. Liu, Z. X. Guo, W. L. Li, Y. H. Li and G. Yang, Improved rate and cyclic performance of potassium doped nickel-rich ternary cathode material for lithium ion Batteries, J. Mater. Sci., 2021, 56, 2399–2411 CrossRef CAS.
- C. Liu, M. M. Wu, S. J. Hang, T. T. Xu, G. Yang, H. M. Ji and Y. Yang, Synthesis of Li1.147K0.026Mn0.582Ni0.25O2 cathode material with high rate cyclic performance and the application to lithium-ion full cells, J. Alloys Compd., 2019, 787, 700–710 CrossRef CAS.
- K. Redel, A. Kulka, K. Walczak, A. Plewa, E. Hanc, M. Marzec, L. Lu and J. Molenda, Origin of extra capacity in advanced Li–rich cathode materials for rechargeable Li–ion batteries, Chem. Eng. J., 2021, 424, 130293 CrossRef CAS.
- M. Lu, H. Cheng and Y. Yang, A comparison of solid electrolyte interphase (SEI) on the artificial graphite anode of the aged and cycled commercial lithium ion cells, Electrochim. Acta, 2008, 53, 3539–3546 CrossRef CAS.
- H. P. Yang, H. H. Wu, M. Y. Ge, L. J. Li, Y. F. Yuan, Q. Yao, J. Chen, L. F. Xia, J. M. Zheng, Z. Y. Chen, J. F. Duan, K. Kisslinger, X. C. Zeng, W. K. Lee, Q. B. Zhang and J. Lu, Simultaneously dual modification of Ni-rich layered oxide cathode for high-energy lithium-ion batteries, Adv. Funct. Mater., 2019, 29, 1808825 CrossRef.
- G. Sun, C. Zhao, F. D. Yu, R. Yu, J. Wang, J. Zhou, G. Shao, X. Sun and Z. B. Wang, In situ surface chemical and structural self-reconstruction strategy enables high performance of Li-rich cathode, Nano Energy, 2021, 79, 105459 CrossRef CAS.
- J. Lee, D. A. Kitchaev, D. H. Kwon, C. W. Lee, J. K. Papp, Y. S. Liu, Z. Lun, R. J. Clement, T. Shi, B. D. McCloskey, J. Guo, M. Balasubramanian and G. Ceder, Reversible Mn2+/Mn4+ double redox in lithium-excess cathode materials, Nature, 2018, 556, 185–190 CrossRef CAS PubMed.
- W. Jiang, C. Zhang, Y. Feng, B. Wei, L. Chen, R. Zhang, D. G. Ivey, P. Wang and W. Wei, Achieving high structure and voltage stability in cobalt-free Li-rich layered oxide cathodes via selective dual-cation doping, Energy Storage Mater., 2020, 32, 37–45 CrossRef.
- P. Hou, F. Li, H. Zhang and H. Huang, Stabilizing the cationic/anionic redox chemistry of Li-rich layered cathodes by tuning the upper cut-off voltage for high energy-density lithium-ion batteries, J. Mater. Chem. A, 2020, 8, 14214–14222 RSC.
- S. Chong, Y. Chen, W. Yan, S. Guo, Q. Tan, Y. Wu, T. Jiang and Y. Liu, Suppressing capacity fading and voltage decay of Li-rich layered cathode material by a surface nano-protective layer of CoF2 for lithium-ion batteries, J. Power Sources, 2016, 332, 230–239 CrossRef CAS.
- S. Kim, M. Aykol, V. I. Hegde, Z. Lu, S. Kirklin, J. R. Croy, M. M. Thackeray and C. Wolverton, Material design of high-capacity Li-rich layered-oxide electrodes: Li2MnO3 and beyond, Energy Environ. Sci., 2017, 10, 2201–2211 RSC.
- Y. Liu, Q. Wang, Z. Zhang, A. Dou, J. Pan and M. Su, Investigation the electrochemical performance of layered cathode material Li1.2Ni0.2Mn0.6O2 coated with Li4Ti5O12, Adv. Powder Technol., 2016, 27, 1481–1487 CrossRef CAS.
- Y. Zang, X. Sun, Z. F. Tang, H. F. Xiang and C. H. Chen, Vanadium-doped lithium-rich layered-structured cathode material Li1.2Ni0.2Mn0.6O2 with a high specific capacity and improved rate performance, RSC Adv., 2016, 6, 30194–30198 RSC.
- J. Zhang, H. Zhang, R. Gao, Z. Li, Z. Hu and X. Liu, New insights into the modification mechanism of Li-rich Li1.2Mn0.6Ni0.2O2 coated by Li2ZrO3, Phys. Chem. Chem. Phys., 2016, 18, 13322–13331 RSC.
- S. Liu, Z. Liu, X. Shen, X. Wang, S. C. Liao, R. Yu, Z. Wang, Z. Hu, C. T. Chen, X. Yu, X. Yang and L. Chen, Li-Ti cation mixing enhanced structural and performance stability of Li-rich layered oxide, Adv. Energy Mater., 2019, 9, 1901530 CrossRef.
- J. Li, J. Camardese, R. Shunmugasundaram, S. Glazier, Z. Lu and J. R. Dahn, Synthesis and characterization of the lithium-rich core-shell cathodes with low irreversible capacity and mitigated voltage fade, Chem. Mater., 2015, 27, 3366–3377 CrossRef CAS.
- C. P. Laisa, R. N. Ramesha and K. Ramesha, Enhanced electrochemical performance of lithium rich layered cathode materials by Ca2+ substitution, Electrochim. Acta, 2017, 256, 10–18 CrossRef CAS.
- R. N. Ramesha, C. P. Laisa and K. Ramesha, Improving electrochemical stability by transition metal cation doping for manganese in lithium-rich layered cathode, Li1.2Ni0.13Co0.13Mn0.54−xMxO2 (M = Co, Cr and Fe), Electrochim. Acta, 2017, 249, 377–386 CrossRef CAS.
- H. L. Li, X. Wei, P. H. Yang, Y. B. Ren, S. B. Wang, Y. L. Xing and S. C. Zhang, Uniform Li1.2Ni0.13Co0.13Mn0.54O2 hollow microspheres with improved electrochemical performance by a facile solvothermal method for lithium ion batteries, Electrochim. Acta, 2018, 261, 86–95 CrossRef CAS.
- S. Zhang, H. Gu, H. Pan, S. Yang, W. Du, X. Li, M. Gao, Y. Liu, M. Zhu, L. Ouyang, D. Jian and F. Pan, A novel strategy to suppress capacity and voltage fading of Li- and Mn-rich layered oxide cathode material for lithium-ion batteries, Adv. Energy Mater., 2016, 7, 1601066 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1nr06824e |
| This journal is © The Royal Society of Chemistry 2022 |