Material strategies for function enhancement in plasmonic architectures
Melissa E.
King
,
Maria V.
Fonseca Guzman
and
Michael B.
Ross
 *
*
Department of Chemistry, University of Massachusetts, Lowell, Lowell, MA 01854, USA. E-mail: Michael_ross@uml.edu
First published on 5th January 2022
Abstract
Plasmonic materials are promising for applications in enhanced sensing, energy, and advanced optical communications. These applications, however, often require chemical and physical functionality that is suited and designed for the specific application. In particular, plasmonic materials need to access the wide spectral range from the ultraviolet to the mid-infrared in addition to having the requisite surface characteristics, temperature dependence, or structural features that are not intrinsic to or easily accessed by the noble metals. Herein, we describe current progress and identify promising strategies for further expanding the capabilities of plasmonic materials both across the electromagnetic spectrum and in functional areas that can enable new technology and opportunities.
Introduction
Plasmonic materials have been actively investigated at both the fundamental and applied level for decades. Early impacts were in the areas of sensing, biomedical applications, and spectroscopy, while current advancements are rapidly influencing diverse areas that include catalysis, distillation, communications, information processing, and heat transfer.1–7 Continued progress in these areas, however, requires enhancement of the plasmonic material functionality to be suited to the specific application. However, this is challenging because the most common plasmonic materials, noble metals, have a limited plasmonic range and narrow set of chemical properties when compared to the broader set of transition metals and soft materials. Meeting the growing demands for greater material function necessitates expansion of plasmonic functionality beyond the visible and near-infrared in addition to learning to integrate chemical or physical functionality not native to noble metals.Localized surface plasmon resonance (LSPR), refers to the collective oscillation of conduction electrons, which is the origin of efficient light absorption in plasmonic nanoparticles. Using the LSPR to access new material functionalities is dependent on the integration of new kinds of functional materials and motifs and the resultant cooperative interactions between nanoscale components. Traditionally, gold and silver nanoparticles were the primary focus for plasmonic materials due to their synthetic accessibility, robust absorption, and colloidal stability.8 Noble and coinage metal plasmonics, however, are sharply limited in range and application due to narrow absorption regions, a relatively weak affinity for most adsorbates, and high cost.6 These challenges have spurred an ongoing interest in identifying new plasmonic materials that have better absorbance and increased spectral range as well as highly active surface properties. To access these enhanced properties, which would diversify plasmonic application significantly, other metals and materials must be identified.
Compositional enhancement through the incorporation of non-archetypal metals or functional ligands affords finely tuned electronic structures, broader absorbance ranges, and increased stability. Combining fundamental knowledge from synergistic areas has led to an increased number of functional plasmonic materials and an expansion of the applicable wavelengths for well-known but limited plasmonic materials. Herein, we describe progress toward identifying non-archetypal plasmonic materials with enhanced spectral activity or functional motifs that can enable the next generation of light-enhanced functional materials.
Emerging materials that support high and low energy plasmon resonances
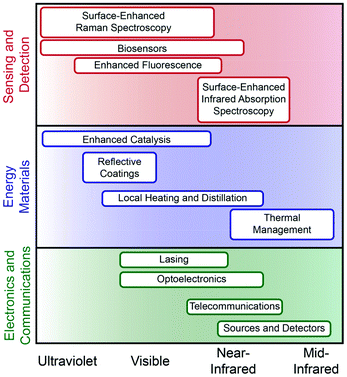
To widen the functional plasmonic electromagnetic range that is accessible the development and integration of new plasmonic components and functional motifs is necessary. Accessing higher and lower energy regions of the spectrum towards increased material functionality must include non-traditional components including semiconductors, transition metals, organic polymers, and two-dimensional (2D) materials. Both theoretical and experimental works have relied on using gold and silver as model systems, thus providing a comparative analysis that is thorough but limited to the visible.3,6 As such, the understanding of these materials, their dielectric properties, and their agreement with classical models is well understood. While not the focus of this review, for many applications it is essential to decrease the inherent loss of the LSPR for performance; we direct the reader to other reviews that discuss this in depth.1,6 For emerging materials, however, a better understanding of both the intrinsic material properties and their influence on LSPR is needed. This is particularly true for emerging metal nanoparticle platforms, such as Al and Bi, as well as a wide variety of potential alloys and intermetallics.6,9 Better understanding of the complex dielectric functions, surface oxidation, confinement, and interfacial effects will all provide guidance toward which compositions are best suited for a given application.Ultraviolet (UV) plasmonic materials are typically metals that do not have interfering interband transitions from approximately 200–400 nm (Fig. 1 and Table 1). Interest in UV plasmonic materials is primarily driven by the opportunity for taking advantage of higher energy resonances compared to the visible; the relatively higher energy of UV photons can promote enhanced sensing due to molecular resonance effects, as well as photocatalysis and enhanced photodegradation processes that use high-energy photons for bond breaking.10 Non-traditional plasmonic metals such as aluminum, rhodium, indium, and magnesium have consistently been shown to support plasmon resonances within the UV region of the spectrum.10–14 Aluminum has been investigated as a thin film,13 as nanoparticle antennas,15 and patterned in arrays16 for enhanced Raman and fluorescence spectroscopies. Rhodium nanoparticles with spherical and tripod morphologies have been successfully used for SERS and enhanced fluorescence via charge transfer and theoretical studies show significant promise for biosensing in the deep-UV (>6 eV).11,17 Indium nanoparticles fabricated in arrays have been used for refractive index sensing18 and to enhance blue light photodetectors.19 Colloidally synthesized indium particles have been used for deep-UV SERS,20 biomolecular imaging21 and anti-reflective coatings.22 Colloidally synthesized magnesium nanoparticles with a ∼6 nm self-limiting oxide layer exhibit size- and shape-dependent plasmon resonance in the UV.23 Dielectric function analysis suggests that bismuth, antimony, and gallium can have inducible plasmon resonances in the ultraviolet region that is mediated through interband transitions; this could create new tunability opportunities by manipulation of the metal band structure, rather than nanoparticle size or shape.24
| Material | Applications |
|---|---|
| Al | SERS, enhanced fluorescence (100–400 nm) |
| Rh | SERS, biosensing, enhanced fluorescence (100–400 nm) |
| In | SERS, biosensing, reflective coatings (100–400 nm) |
| Au | Biosensing, photothermal therapy, enhanced catalysis (520–1800 nm) |
| Ag | Enhanced catalysis, SERS, biosensing, enhanced fluorescence, reflective coatings (400–800 nm) |
| Cu | Enhanced catalysis, SERS (400–800 nm) |
| CuS/Se/Te | Optical communications (1100–1700 nm) |
| Graphene | Telecommunications, sources and detectors, optoelectronics (∼3000–8000 nm) |
| MoS2 | Telecommunications, sources and detectors, optoelectronics (∼3000–8000 nm) |
Traditional coinage metals have plasmon resonances that are found in the visible portion of the spectrum and have applications in sensing, photocatalysis, and photothermal therapy (Fig. 1 and Table 1). Gold nanoparticles can be tuned to support an LSPR from ∼520–1800 nm; these have been used extensively for biosensing and photothermal therapies25 as well as photocatalysis.26,27 Silver, like gold, has a similarly wide plasmonic range and generally supports stronger LSPRs; it has well-known applications in photocatalysis28 and SERS.29 Copper, while less well-studied than gold or silver, can be used for selective photocatalysis,30 SERS,31 and single-particle nanospectroscopy.32 Heavily-doped semiconductor materials are an emerging plasmonic colloidal platform, however, they face a significant number of challenges in reaching carrier concentrations sufficient for resonance in the visible range, which must be ∼1022 cm−3.33 Transition metal nitrides, such as titanium nitride (TiN), have higher carrier concentrations than their oxide counterparts, enabling them to support an LSPR in the visible.1,29
Doped semiconductors do, however, provide an emerging and remarkably tunable platform for plasmonics in the near-infrared (NIR (Fig. 1 and Table 1)).34 Transparent conducting oxides (TCO) such as aluminum-zinc-oxide and indium-tin-oxide can be doped to have resonances throughout the NIR.1 Similarly, sulfides and transition metal oxides have high electron mobility allowing them to have observable metallic properties in the NIR.1 Fluorine/indium co-doped cadmium oxide and fluorine/tin co-doped cadmium oxide are examples of cooperative effects that allow for increased charge carrier density in TCO's allowing for continuous tunability in the NIR.35 Cu–S/Se plasmonic materials can also be manipulated through dopant concentrations which alter the carrier density and have shown further tunability in the NIR based on size and shape.36 Such colloidal chalcogenide semiconductors composed of CuS or CuTe are self-doping through oxidation which allows for fine-tuning of the LSPR from 1100–1700 nm and are applicable for optical communication.37 Transition metal oxides such as RuO2, provide a functional branching point for plasmonic materials in the NIR due to their small negative permittivity and low losses making them ideal candidates for photonics applications.38
2D materials provide a unique platform for mid-infrared (MIR) plasmonics owing to their electrical conductivity, strong light–matter interactions, and mechanical flexibility (Fig. 1 and Table 1).39 Graphene displays metallic behavior and plasmon resonance in the MIR.40 Graphene's plasmon has been used in this region for thermal energy transfer, imaging, and sensing that are tunable via carrier density and surface geometry.41 MoS2 has lower carrier densities than graphene, however when layered its photoresponse increases from ∼7.5 mA, which is similar to graphene, to ∼100 mA, providing a comparable response to silicon of the same thickness thus making it a candidate for photoluminescent applications.39 Cerium-doped indium oxide nanocubes show better optical mobility than ITO nanocrystals because Ce doping minimizes ionized impurity scattering producing exceptionally narrow line widths. The result of this is that these nanocubes exhibit the highest quality factors observed for nanocrystals in this spectral region and can be used to enhance molecular vibration signals in the MIR.42 Black phosphorus is an electronically anisotropic plasmonic material that has a resonance in the MIR and is also classified as a hyperbolic material, meaning that it has potential applications in broadband thermal sources and propagation of directional surface plasmons.43
The extension from traditional plasmonic materials such as gold and silver, to non-noble metals, semiconductors, and 2D-materials expands the range of plasmonics from deep UV to the MIR. This broadening of the functional spectral window for plasmonics creates new opportunities for enhancing a wide variety of optical processes (Fig. 1). Furthermore, the increasing variety of material compositions that exhibit plasmonic functionality increases the environments and modalities through which these materials can be used. To fully utilize these novel plasmonic materials, fundamental research must characterize intrinsic properties as well as cooperative interactions in functional materials. Future discoveries in this area require continued synthetic advances for accessing new materials and improved dielectric and resonant understanding of transition metals, alloys, and semiconductors.
Designing materials for enhanced physical and chemical functionality
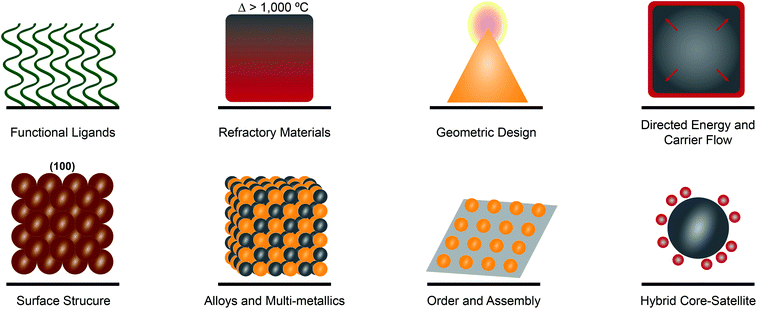
To realize many of the emerging applications of plasmonic materials, expansion of the spectral plasmonic window is not sufficient. That in itself only expands the functional electromagnetic range over which one can absorb and use photons. Incorporating functional motifs that can provide access to enhanced chemical and physical functionality remains essential. Functional motifs including ligands, geometries, surface structure, assembly, and judicious metallic-mixing are emerging as consistent strategies for enhancing for catalysis, sensing, and optoelectronic applications (Fig. 2).Plasmonic metal nanostructures have been shown to enhance efficiency in the conversion of solar energy to chemical energy. It has been shown that the integration of catalytically active materials with plasmonic materials can increase catalytic activity and selectivity (Fig. 2).5 These pairings result in hybrid plasmonic systems that direct energy into a catalytically relevant material that is not plasmonically active in that optical region.44 Core–shell cubic Ag@Pt nanoparticles have been studied for preferential CO oxidation and as a model system for distinguishing plasmonic and catalytic effects of the constituent materials. It was observed that there was directed plasmon energy transfer from the silver core to the platinum shell.44 Antenna/reactor particles also provide a secondary way to drive plasmon-enhanced photocatalysis whereby the ‘antenna’ drives hot electron generation in a neighboring catalytic metal or semiconductor.45 This proximity effect is based on the augmented field surrounding the plasmonic metal which is capable of driving a “forced” plasmon in the non-plasmonic metal. Such pairings include Aluminum/Palladium46 for H2 splitting and Aluminum/Iridium (Fig. 3e)47 for nitrous oxide decomposition. Incorporation of semiconducting materials allows for more effective separation of charge carriers in plasmonic systems, as evidenced by gold nanoparticle decorated p-type gallium nitride photocathodes48 and copper nanoparticle decorated p-type nickel oxide photocathodes49 exhibiting enhanced activity and selectivity for photoelectrochemical CO2 reduction. Moreover, the expanded understanding of plasmonic processes has enabled single metal systems where the incorporation of an ionic liquid medium in a photoelectrochemical cell opens an avenue towards the plasmonic photosynthesis of hydrocarbons.50
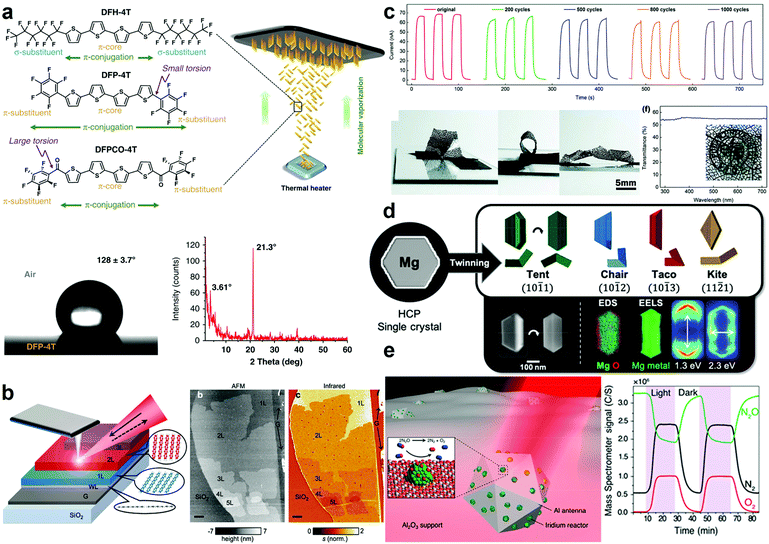 | ||
| Fig. 3 Recent demonstrations of function-enhanced plasmonic materials. (a) Schematic representation of the fabrication and characterization of fully organic nanostructured SERS films. Adapted from ref. 93 under creative commons attribution 4.0 http://creativecommons.org/licenses/by/4.0/. (b) Illustration and AFM topography of 2D graphene/pentacene heterostructured nanomaterials. Adapted from ref. 106 with permission from the American Chemical Society. (c) Chronoamperometry measurements under 350 nm illumination of bending cycles coupled with optical images of MXene UV photodetectors. Adapted from ref. 109 with permission from the Royal Society of Chemistry. (d) Cartoon representations, SEM images, and elemental analysis of twinned Mg nanoparticles. Adapted from ref. 73. (e) Schematic representation of Al-Ir nanoparticles and reactivity under irradiation measured via mass spectrometry. Adapted from ref. 47 with permission from the American Chemical Society. | ||
Sensing and spectroscopic enhancement is one of the most well-known and longest studied applications of plasmonic materials. Indeed, plasmonic materials have been used in surface-enhanced Raman spectroscopy (SERS), biosensing and diagnostics, plasmon-enhanced fluorescence (PEF), and surface-enhanced infrared spectroscopy (SEIRAS) for sensing from the UV to NIR.51,52 Gold and silver have historically been used for such applications due to their strong plasmonic absorbance and synthetic accessibility, however, the introduction of UV-active metals and doped semiconducting materials have expanded the functional optical range.13 Colloidal synthetic techniques have led to chiral magnesium nanoparticles that show enhanced chiral sensing in aqueous media.53 Furthermore, integration of distinct materials for plasmonic enhancement and analyte capture can further lower limits of detection (Fig. 2). Quantum dots composed of silver chalcogenides coupled with mercaptosuccinic acid have shown up to 10-fold enhancement for SEIRAS applications and are advantageous for potential applications in biological systems due to their low toxicity.54 The inclusion of soft materials through the deliberate incorporation of functional ligands allows for increased sensing specificity, especially in biological systems.55 Coupling peptides, which have been long used for self-assembly, with plasmonically active metallic nanoparticles produces stimuli responsive biosensing tools that have optical response that is identifiable as an SPR shift or observable color change that is enzyme specific (Fig. 2).56
Controlled phase change, either through modification of the crystal lattice, or transition from a solid to a liquid, falls into the area of ‘active plasmonics’ whereby a characteristic LSPR ‘signal’ can be modulated in real-time.57 Ge3Sb2Te6 and Ge2Sb2Te5 allow for a fast optical switch in response to thermal stimuli that originates from the pronounced changes in the dielectric properties that occur during the phase transition between amorphous and crystalline states.58 Single crystalline palladium nanocubes have also been shown to exhibit crystal structure phase changes from α to β as well as a redshift in the LSPR in H2 rich environments, providing avenues towards more sensitive and specific H2-sensing.59 Gallium and its alloys have garnered significant interest due to near-room temperature melting points that allow for ease of phase transition and the subsequent study of metallic plasmonic properties that are free from structural defects that lead to extrinsic losses.60,61 The incorporation of selectively fluorinated surface ligands (Fig. 2) allows for reversible, reconfigurable assemblies of Ag nanoparticles that achieve long-term switchability and a substantial plasmonic response originating from the reconfiguration process.62
Refractory materials with plasmonic properties are promising alternatives for high temperature electronic applications because of their stability at temperatures >1000 °C (Fig. 2).63 Interstitial transition metal nitrides with their excellent dopant capacities and tunable wavelengths, starting in the visible region, provide a practical solution to the problem of noble metal instability at elevated temperatures.29 TiN is a well-studied refractory material that has applications in biomedical sensing, cancer therapy, photocatalysis, and optoelectronics.29 The use of TiN nanoparticles embedded in Mg-based materials to transform photoexcitation to photothermal heating of a nanoparticle packed bed reactor has proved a promising alternative to the energy-intensive Haber–Bosch process for the production of ammonia.64
The incorporation of non-traditional materials with plasmonically-active ones along with the continued improvement in nanoscale design and structure, increases opportunities for plasmonic systems. A few of the emerging motifs that enhance plasmonic properties and functionality are highlighted in Fig. 2, however this is by no means exhaustive. Unlike traditional plasmonic metal nanomaterials, such as thin films or small nanoparticles, the use of functional materials can increase catalytic activity, augment sensitivity in plasmon-enhanced sensing techniques, decrease structural defects through ease of reconfiguration, and improve stability for high temperature applications. This increased functionality coupled with spectrally versatile components leads to emergent materials with greater spectral tunability and relevance in novel photonic applications.
Emerging platforms and materials
The development of new classes and applications of plasmonic materials is rapid. These emerging areas include entirely new compositions as well as new kinds of applications in diverse fields such as quantum materials. These areas continue to build upon the themes and motifs discussed above by integrating fundamental knowledge from distinct areas. These materials combine fundamental knowledge from previous plasmonic studies to push the boundaries of this field towards plasmonic composites with broad range absorbance and greater applicability in device fabrication. Here we highlight five areas: alloys and non-archetypal materials, quantum materials, hierarchical materials, soft-matter plasmonics, and 2D materials.Alloys and multi-metallic nanostructures have long been of interest for providing a tunable plasmonic optical response (Fig. 2).5,65–67 Additionally, the coupling of cooperative metals can provide access to enhanced electronic effects44 and tunable adsorption.68 Synthetic advances in both physical deposition and wet chemical methods have led to several new approaches. Layer-by-layer lithography and co-sputtering, for example, have led to the fabrication of supported ternary alloy arrays for hydrogen sensing69 and ultrathin Pd–Au alloys for low-temperature catalysis,70 respectively. Advanced synthetic techniques also provide an avenue towards efficient plasmonic materials including controlled galvanic replacement reactions for Au–Ag nanoparticles with tunable cavity size for enhanced photocatalytic hydrogen generation.71,72 While still novel, significant progress has been made on the optical properties and potential applications of magnesium nanoparticles as they have sustainable plasmon resonances from the UV to NIR (Fig. 3d).12,73 Gallium and gallium based alloys provide a unique opportunity to manipulate the plasmon resonances and regions actively and are tunable based on composition.74
Quantum materials combined with plasmonic ones could integrate the promise of new modes of information storage and manipulation with the intense optical enhancement provides by plasmonics.75,76 Incorporating plasmonic nanoparticles on quantum photonic platforms has lead faster operating speeds and functionality at room temperature.77 Additionally, the ability for plasmonic materials to confine light can be used to create switchable and enhanced emitters, as has been shown with Ag and WS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 78 and for metallised optical fibers.79 The quantum materials field is rapidly advancing, and the small mode volumes and rapid optical switchability of plasmonic materials will continue to provide unique enhancement opportunities.
78 and for metallised optical fibers.79 The quantum materials field is rapidly advancing, and the small mode volumes and rapid optical switchability of plasmonic materials will continue to provide unique enhancement opportunities.
Hierarchical materials, meanwhile, provide opportunities to arrange plasmonic components into two- and three-dimensions, such that the arrangement of multiple compositions and their interparticle coupling can be finely controlled (Fig. 2). A number of strategies exist for the arrangement of plasmonic nanoparticles, too many to detail here.8 2D gold nanoparticle superlattices provide an opportunity to better understand and manipulate nanolasing while providing insight into controllable multi-modal lasing capabilities through deliberate symmetry breaking.80 Meanwhile, 2D Cu–Pt nanoparticle lattices can significantly enhance hydrogen evolution reaction due to long-range coupling through the lattice.81 DNA-programmed crystalline superlattices can access a wide variety of colors when using Ag and Au nanoparticles, in addition to providing fine control over the collective refractive index of the composite material.82,83 Composite materials of gold nanoparticles and liquid organic crystals enable cooperative interactions that lead to tunable hierarchical structures with helical motifs and inherent optical chirality.84 More exotic techniques have also led to the coupling of gold and graphene for plasmon enhanced detection of bacteria,85 3D microreactors composed of aluminum with directed deposition of silver or gold particles decorating the interior of the cavity through plasmonic manipulation,86 and tubular nanomembrane arrays with engineered hotspots for ultrasensitive SERS.87 Thin films of cadmium oxide doped with n-type plasmonic oxide nanocrystals, comprised of indium, aluminum, and tin, exhibit tunable plasmon modes from the NIR to the MIR that are dictated by dopant concentration.88 Metamaterials fabricated through wide-area nanoimprinting with colloid Au nanocrystal building blocks afford superstructures with plasmon resonances from the visible to NIR.89 While the approaches to arrange plasmonic materials continue to grow, the applications that are accessible by multi-dimensional arrangement are still emerging.
Organic or “soft” materials provide an inexpensive and exceptionally tunable alternative or accompaniment to traditional plasmonic metals.90 The key to manipulating plasmonic activity in these structures lies in continuous conjugation such that metallic properties can emerge in these systems. For example, the incorporation of additional conjugated rings into polycyclic aromatic hydrocarbons induces a red-shift in the observed plasmon resonance.91 Advances in electrochromic devices for display technologies have been accomplished through the integration of graphene derivatives through stabilization in ion-gel membranes.92 Other π-conjugated systems, such as fluoroarene-modified oligothiophenes, have been shown to provide SERS enhancement factors >105 (Fig. 3a).93 Interestingly, conductive organic materials can behave as composites whereby a polymer matrix acts as the plasmonic material and as a redox-active, dynamically tunable nano-antenna.94 Fully organic plasmonic materials provide an avenue for flexible plasmonic devices such as wearable biosensors or flexible display technologies that do not require metal constituents, potentially making them more cost-effective and structurally deformable.
2D materials beyond graphene offer a unique opportunity for controlled volume plasmonic materials due to their tunable thickness and unique electronic properties that enable plasmonic response over a wide frequency range (Vis – THz).95 Indeed, coupling complementary 2D materials that harness phonon-polariton contributions to incident irradiation such as hBN, MoO3, and V2O5 provide access to greater optical tunability with lower loss potential.96–99 Graphene analogs, such as hexagonal boron nitride can be stacked to achieve heterostructures that have plasmon-phonon hybridization which can be controlled simultaneously with the total plasmon contribution.100–103 MoO3 and V2O5 have been recently shown to have directionally controllable phonon-polaritons along the axis of irradiation, making these materials ideally positioned for nanophotonic applications in communications and energy transmission.104,105 Interlayer electron transfer has been shown in similar heterostructures of pentacene and graphene through plasmon-driven tunneling-type electron transfer that is highly sensitive to molecular orientation thus, making it ideal for structural characterization of bionanoparticles (Fig. 3b).106 Photonic crystal cavity lasers, which can be applied to “on-chip” optical communications, have been realized for tungsten and molybdenum sulfides, selenides, and tellurides and are active in the visible region of the spectrum with precise control over the exciton emissions at the surface of the material.107 Transition metal carbides, such as InSe, are another plasmonic 2D material that have applications in electrodes and photodetectors (Fig. 3c).108,109 Finally, 2D transition-metal chalcogenides were recently shown to have significant SERS enhancement due to chemical effects, as opposed to the typically more significant electromagnetic effect.110
Outlook
The combination of new building blocks and synthetic techniques enables the creation of novel nanostructured plasmonic materials with optical properties ranging across the electromagnetic spectrum and enhanced chemical and physical functionality. The cooperative integration of functional motifs gives rise to advanced material properties, we have highlighted several relevant in fields including catalysis, detection, and communications.The materials highlighted herein could provide a basis for the improvement or enhancement of plasmonic materials and photonic devices, particularly in areas that include:64,68,71,85,87,91,95,100,106
• Lowering the energy barriers for catalytic reactions
• Electronic structure control for sensing enhancement and surface sensitivity
• Hybridization of plasmon-phonon interaction for next-generation communications devices
Meanwhile, the combination of refractory materials with non-archetypal metals, for example, has led to the light-driven production of ammonia.64 Moreover, the manipulation of active plasmonic materials provides new platforms for the real-time manipulation of dielectric properties58 and all -organic reversible photoswitches.94 2D materials provide access to terahertz communication frequencies at room temperature.78 Finally, material advances are changing our perception of SERS, a relatively mature application of plasmonics, where organic semiconductors93 and 2D molybdenum telluride110 can provide significant chemical enhancement effects that may enable highly specific detection platforms.
Future technologies that rely on plasmonic materials will continue to require materials innovation that provides high quality response across the electromagnetic spectrum while incorporating and increasing the diversity of functional motifs. Integrating materials, such as metals and oxides, continues to be important both for intrinsic performance and for complementary metal oxide semiconductor (CMOS)-compatibility. Continued effort should be made to investigate non-archetypal plasmonic materials, including multi-metallic structures, non-noble metals such as Ga, In, and Mg, and organic and 2D materials. A particular challenge in this area is the lack of established and consistent dielectric function information, due to variability in the electronic properties due to differences in processing conditions and synthesis. Because many applications of plasmonic materials require consistent performance in harsh conditions, such as catalysis, distillation, and thermal management, continued investigation of refractory materials and plasmonic response in extreme conditions is essential. Overall, novel functionality in plasmonic nanomaterials continues to be driven by nanoscale integration, robust characterization, and thoughtful combination of functional components. Continued breakthroughs in these areas will expand the spectral and functional versatility of plasmonic materials.
Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work relates to Department of Navy award N00014-20-1-2858 issued by the Office of Naval Research. The United States Government has a royalty-free license throughout the world in all copyrightable material contained herein. This material was also supported by the University of Massachusetts Lowell and the Commonwealth of Massachusetts. M. E. K. gratefully acknowledges support from the American Association of University Women American Postdoctoral fellowship. M. V. F. G. gratefully acknowledges support from the RIST Institute for Sustainability and Energy.References
- G. V. Naik, V. M. Shalaev and A. Boltasseva, Adv. Mater., 2013, 25, 3264–3294 CrossRef CAS PubMed.
- M. I. Stockman, K. Kneipp, S. I. Bozhevolnyi, S. Saha, A. Dutta, J. Ndukaife, N. Kinsey, H. Reddy, U. Guler, V. M. Shalaev, A. Boltasseva, B. Gholipour, H. N. S. Krishnamoorthy, K. F. MacDonald, C. Soci, N. I. Zheludev, V. Savinov, R. Singh, P. Groß, C. Lienau, M. Vadai, M. L. Solomon, D. R. Barton, M. Lawrence, J. A. Dionne, S. V. Boriskina, R. Esteban, J. Aizpurua, X. Zhang, S. Yang, D. Wang, W. Wang, T. W. Odom, N. Accanto, P. M. de Roque, I. M. Hancu, L. Piatkowski, N. F. van Hulst and M. F. Kling, J. Opt., 2018, 20, 043001 CrossRef.
- P. R. West, S. Ishii, G. V. Naik, N. K. Emani, V. M. Shalaev and A. Boltasseva, Laser Photonics Rev., 2010, 4, 795–808 CrossRef CAS.
- Y. Zhong, S. D. Malagari, T. Hamilton and D. Wasserman, J. Nanophotonics, 2015, 9, 093791 CrossRef CAS.
- S. Linic, S. Chavez and R. Elias, Nat. Mater., 2021, 20(7), 916–924 CrossRef CAS PubMed.
- M. G. Blaber, M. D. Arnold and M. J. Ford, J. Phys.: Condens. Matter, 2010, 22, 143201 CrossRef CAS PubMed.
- J. M. Luther, P. K. Jain, T. Ewers and A. P. Alivisatos, Nat. Mater., 2011, 10, 361–366 CrossRef CAS PubMed.
- M. R. Jones, K. D. Osberg, R. J. Macfarlane, M. R. Langille and C. A. Mirkin, Chem. Rev., 2011, 111, 3736–3827 CrossRef CAS PubMed.
- M. W. Knight, N. S. King, L. Liu, H. O. Everitt, P. Nordlander and N. J. Halas, ACS Nano, 2014, 8, 834–840 CrossRef CAS PubMed.
- J. M. McMahon, G. C. Schatz and S. K. Gray, Phys. Chem. Chem. Phys., 2013, 15, 5415–5423 RSC.
- A. Ahmadivand, R. Sinha, S. Kaya and N. Pala, Plasmonics, 2016, 11, 839–849 CrossRef CAS.
- E. Ringe, J. Phys. Chem. C, 2020, 124, 15665–15679 CrossRef CAS PubMed.
- B. Sharma, M. F. Cardinal, M. B. Ross, A. B. Zrimsek, S. V. Bykov, D. Punihaole, S. A. Asher, G. C. Schatz and R. P. Van Duyne, Nano Lett., 2016, 16, 7968–7973 CrossRef CAS PubMed.
- M. V. Fonseca Guzman and M. B. Ross, J. Phys. Chem. C, 2021, 125(35), 19428–19437 CrossRef.
- M. W. Knight, L. Liu, Y. Wang, L. Brown, S. Mukherjee, N. S. King, H. O. Everitt, P. Nordlander and N. J. Halas, Nano Lett., 2012, 12, 6000–6004 CrossRef CAS PubMed.
- G. Maidecchi, G. Gonella, R. Proietti Zaccaria, R. Moroni, L. Anghinolfi, A. Giglia, S. Nannarone, L. Mattera, H.-L. Dai, M. Canepa and F. Bisio, ACS Nano, 2013, 7, 5834–5841 CrossRef CAS PubMed.
- S. Kundu, Y. Chen, W. Dai, L. Ma, A. M. Sinyukov and H. Liang, J. Mater. Chem. C, 2017, 5, 2577–2590 RSC.
- X. Xu, X. Hu, X. Chen, Y. Kang, Z. Zhang, K. B. Parizi and H. S. P. Wong, ACS Appl. Mater. Interfaces, 2016, 8, 31871–31877 CrossRef CAS PubMed.
- Y. Wang, C.-W. Ge, Y.-F. Zou, R. Lu, K. Zheng, T.-F. Zhang, Y.-Q. Yu and L.-B. Luo, Adv. Opt. Mater., 2016, 4, 291–296 CrossRef CAS.
- Y. Kumamoto, A. Taguchi, M. Honda, K. Watanabe, Y. Saito and S. Kawata, ACS Photonics, 2014, 1, 598–603 CrossRef CAS.
- Y. Kumamoto, A. Taguchi and S. Kawata, Adv. Opt. Mater., 2019, 7, 1801099 CrossRef.
- W.-J. Ho, H.-Y. Yang, J.-J. Liu, P.-J. Lin and C.-H. Ho, Appl. Surf. Sci., 2020, 508, 145275 CrossRef CAS.
- J. S. Biggins, S. Yazdi and E. Ringe, Nano Lett., 2018, 18, 3752–3758 CrossRef CAS PubMed.
- J. Toudert and R. Serna, Opt. Mater. Express, 2016, 6, 2434–2447 CrossRef CAS.
- X. Huang, P. K. Jain, I. H. El-Sayed and M. A. El-Sayed, Lasers Med Sci., 2007, 23, 217 CrossRef PubMed.
- C. Wang, X.-G. Nie, Y. Shi, Y. Zhou, J.-J. Xu, X.-H. Xia and H.-Y. Chen, ACS Nano, 2017, 11, 5897–5905 CrossRef CAS PubMed.
- S. Mukherjee, F. Libisch, N. Large, O. Neumann, L. V. Brown, J. Cheng, J. B. Lassiter, E. A. Carter, P. Nordlander and N. J. Halas, Nano Lett., 2013, 13, 240–247 CrossRef CAS PubMed.
- P. Christopher, H. Xin and S. Linic, Nat. Chem., 2011, 3, 467–472 CrossRef CAS PubMed.
- U. Guler, V. M. Shalaev and A. Boltasseva, Mater. Today, 2015, 18, 227–237 CrossRef CAS.
- A. Marimuthu, J. Zhang and S. Linic, Science, 2013, 339, 1590–1593 CrossRef CAS PubMed.
- N. E. Markina, E. K. Volkova, A. M. Zakharevich, I. Y. Goryacheva and A. V. Markin, Microchim. Acta, 2018, 185, 481 CrossRef PubMed.
- Q.-C. Sun, Y. Ding, S. M. Goodman, H. H. Funke and P. Nagpal, Nanoscale, 2014, 6, 12450–12457 RSC.
- G. V. Naik, J. Kim and A. Boltasseva, Opt. Mater. Express, 2011, 1, 1090–1099 CrossRef CAS.
- A. Agrawal, R. W. Johns and D. J. Milliron, Annu. Rev. Mater. Res., 2017, 47, 1–31 CrossRef CAS.
- X. Ye, J. Fei, B. T. Diroll, T. Paik and C. B. Murray, J. Am. Chem. Soc., 2014, 136, 11680–11686 CrossRef CAS PubMed.
- S.-W. Hsu, W. Bryks and A. R. Tao, Chem. Mater., 2012, 24, 3765–3771 CrossRef CAS.
- I. Kriegel, C. Jiang, J. Rodríguez-Fernández, R. D. Schaller, D. V. Talapin, E. da Como and J. Feldmann, J. Am. Chem. Soc., 2012, 134, 1583–1590 CrossRef CAS PubMed.
- L. Wang, C. Clavero, K. Yang, E. Radue, M. T. Simons, I. Novikova and R. A. Lukaszew, Opt. Express, 2012, 20, 8618–8628 CrossRef CAS PubMed.
- Y. Li, Z. Li, C. Chi, H. Shan, L. Zheng and Z. Fang, Adv. Sci., 2017, 4, 1600430 CrossRef PubMed.
- F. J. García de Abajo, ACS Photonics, 2014, 1, 135–152 CrossRef.
- V. W. Brar, M. C. Sherrott, M. S. Jang, S. Kim, L. Kim, M. Choi, L. A. Sweatlock and H. A. Atwater, Nat. Commun., 2015, 6, 7032 CrossRef CAS PubMed.
- E. L. Runnerstrom, A. Bergerud, A. Agrawal, R. W. Johns, C. J. Dahlman, A. Singh, S. M. Selbach and D. J. Milliron, Nano Lett., 2016, 16, 3390–3398 CrossRef CAS PubMed.
- A. Nemilentsau, T. Low and G. Hanson, Phys. Rev. Lett., 2016, 116, 066804 CrossRef PubMed.
- U. Aslam, S. Chavez and S. Linic, Nat. Nanotechnol., 2017, 12, 1000–1005 CrossRef CAS PubMed.
- K. Li, N. J. Hogan, M. J. Kale, N. J. Halas, P. Nordlander and P. Christopher, Nano Lett., 2017, 17, 3710–3717 CrossRef CAS PubMed.
- H. Robatjazi, M. Lou, B. D. Clark, C. R. Jacobson, D. F. Swearer, P. Nordlander and N. J. Halas, Nano Lett., 2020, 20, 4550–4557 CrossRef CAS PubMed.
- D. F. Swearer, H. Robatjazi, J. M. P. Martirez, M. Zhang, L. Zhou, E. A. Carter, P. Nordlander and N. J. Halas, ACS Nano, 2019, 13, 8076–8086 CrossRef CAS PubMed.
- J. S. DuChene, G. Tagliabue, A. J. Welch, W.-H. Cheng and H. A. Atwater, Nano Lett., 2018, 18, 2545–2550 CrossRef CAS PubMed.
- J. S. DuChene, G. Tagliabue, A. J. Welch, X. Li, W.-H. Cheng and H. A. Atwater, Nano Lett., 2020, 20, 2348–2358 CrossRef CAS PubMed.
- S. Yu and P. K. Jain, ACS Energy Lett., 2019, 4, 2295–2300 CrossRef CAS.
- M. F. Cardinal, E. Vander Ende, R. A. Hackler, M. O. McAnally, P. C. Stair, G. C. Schatz and R. P. Van Duyne, Chem. Soc. Rev., 2017, 46, 3886–3903 RSC.
- J. Langer, D. Jimenez de Aberasturi, J. Aizpurua, R. A. Alvarez-Puebla, B. Auguié, J. J. Baumberg, G. C. Bazan, S. E. J. Bell, A. Boisen, A. G. Brolo, J. Choo, D. Cialla-May, V. Deckert, L. Fabris, K. Faulds, F. J. García de Abajo, R. Goodacre, D. Graham, A. J. Haes, C. L. Haynes, C. Huck, T. Itoh, M. Käll, J. Kneipp, N. A. Kotov, H. Kuang, E. C. Le Ru, H. K. Lee, J.-F. Li, X. Y. Ling, S. A. Maier, T. Mayerhöfer, M. Moskovits, K. Murakoshi, J.-M. Nam, S. Nie, Y. Ozaki, I. Pastoriza-Santos, J. Perez-Juste, J. Popp, A. Pucci, S. Reich, B. Ren, G. C. Schatz, T. Shegai, S. Schlücker, L.-L. Tay, K. G. Thomas, Z.-Q. Tian, R. P. Van Duyne, T. Vo-Dinh, Y. Wang, K. A. Willets, C. Xu, H. Xu, Y. Xu, Y. S. Yamamoto, B. Zhao and L. M. Liz-Marzán, ACS Nano, 2020, 14, 28–117 CrossRef CAS PubMed.
- H.-H. Jeong, A. G. Mark and P. Fischer, Chem. Commun., 2016, 52, 12179–12182 RSC.
- C. F. Pereira, I. M. A. Viegas, I. G. Souza Sobrinha, G. Pereira, G. A. L. Pereira, P. Krebs and B. Mizaikoff, J. Mater. Chem. C, 2020, 8, 10448–10455 RSC.
- P. D. Howes, R. Chandrawati and M. M. Stevens, Science, 2014, 346, 1247390 CrossRef PubMed.
- C. Pigliacelli, R. Sánchez-Fernández, M. D. García, C. Peinador and E. Pazos, Chem. Commun., 2020, 56, 8000–8014 RSC.
- K. F. MacDonald and N. I. Zheludev, Laser Photonics Rev., 2010, 4, 562–567 CrossRef CAS.
- N. Jiang, X. Zhuo and J. Wang, Chem. Rev., 2018, 118, 3054–3099 CrossRef CAS PubMed.
- A. Baldi, T. C. Narayan, A. L. Koh and J. A. Dionne, Nat. Mater., 2014, 13, 1143–1148 CrossRef CAS PubMed.
- S. R. C. Vivekchand, C. J. Engel, S. M. Lubin, M. G. Blaber, W. Zhou, J. Y. Suh, G. C. Schatz and T. W. Odom, Nano Lett., 2012, 12, 4324–4328 CrossRef CAS PubMed.
- M. G. Blaber, C. J. Engel, S. R. C. Vivekchand, S. M. Lubin, T. W. Odom and G. C. Schatz, Nano Lett., 2012, 12, 5275–5280 CrossRef CAS PubMed.
- M. Bagiński, E. Tomczyk, A. Vetter, R. N. S. Suryadharma, C. Rockstuhl and W. Lewandowski, Chem. Mater., 2018, 30, 8201–8210 CrossRef.
- U. Guler, A. Boltasseva and V. M. Shalaev, Science, 2014, 344, 263–264 CrossRef CAS PubMed.
- D. F. Swearer, N. R. Knowles, H. O. Everitt and N. J. Halas, ACS Energy Lett., 2019, 4, 1505–1512 CrossRef CAS.
- M. Rebello Sousa Dias and M. S. Leite, Acc. Chem. Res., 2019, 52, 2881–2891 CrossRef CAS PubMed.
- F. A. A. Nugroho, B. Iandolo, J. B. Wagner and C. Langhammer, ACS Nano, 2016, 10, 2871–2879 CrossRef CAS PubMed.
- W.-T. Koo, J. E. Millstone, P. S. Weiss and I.-D. Kim, ACS Nano, 2020, 14, 6407–6413 CrossRef CAS PubMed.
- U. Aslam, V. G. Rao, S. Chavez and S. Linic, Nat. Catal., 2018, 1, 656–665 CrossRef.
- I. Darmadi, F. A. A. Nugroho, S. Kadkhodazadeh, J. B. Wagner and C. Langhammer, ACS Sens., 2019, 4, 1424–1432 CrossRef CAS PubMed.
- J. P. McClure, J. Boltersdorf, D. R. Baker, T. G. Farinha, N. Dzuricky, C. E. P. Villegas, A. R. Rocha and M. S. Leite, ACS Appl. Mater. Interfaces, 2019, 11, 24919–24932 CrossRef CAS PubMed.
- X. Yue, J. Hou, H. Zhao, P. Wu, Y. Guo, Q. Shi, L. Chen, S. Peng, Z. Liu and G. Cao, J. Energy Chem., 2020, 49, 1–7 CrossRef.
- P.-C. Chen, X. Liu, J. L. Hedrick, Z. Xie, S. Wang, Q.-Y. Lin, M. C. Hersam, V. P. Dravid and C. A. Mirkin, Science, 2016, 352, 1565–1569 CrossRef CAS PubMed.
- J. Asselin, C. Boukouvala, E. R. Hopper, Q. M. Ramasse, J. S. Biggins and E. Ringe, ACS Nano, 2020, 14, 5968–5980 CrossRef CAS PubMed.
- P. Reineck, Y. Lin, B. C. Gibson, M. D. Dickey, A. D. Greentree and I. S. Maksymov, Sci. Rep., 2019, 9, 5345 CrossRef PubMed.
- M. S. Tame, K. R. McEnery, Ş. K. Özdemir, J. Lee, S. A. Maier and M. S. Kim, Nat. Phys., 2013, 9, 329–340 Search PubMed.
- Z. Jacob and V. M. Shalaev, Science, 2011, 334, 463–464 CrossRef CAS PubMed.
- S. I. Bogdanov, A. Boltasseva and V. M. Shalaev, Science, 2019, 364, 532–533 CrossRef CAS PubMed.
- C. Han and J. Ye, Nat. Commun., 2020, 11, 713 CrossRef CAS PubMed.
- S. Keramati, A. Passian, V. Khullar, J. Beck, C. Uiterwaal and H. Batelaan, New J. Phys., 2020, 22, 083069 CrossRef CAS.
- D. Wang, A. Yang, W. Wang, Y. Hua, R. D. Schaller, G. C. Schatz and T. W. Odom, Nat. Nanotechnol., 2017, 12, 889–894 CrossRef CAS PubMed.
- S. Deng, B. Zhang, P. Choo, P. J. M. Smeets and T. W. Odom, Nano Lett., 2021, 21, 1523–1529 CrossRef CAS PubMed.
- M. B. Ross, J. C. Ku, B. Lee, C. A. Mirkin and G. C. Schatz, Adv. Mater., 2016, 28, 2790–2794 CrossRef CAS PubMed.
- M. B. Ross, J. C. Ku, V. M. Vaccarezza, G. C. Schatz and C. A. Mirkin, Nat. Nanotechnol., 2015, 10, 453–458 CrossRef CAS PubMed.
- M. Bagiński, M. Tupikowska, G. González-Rubio, M. Wójcik and W. Lewandowski, Adv. Mater., 2020, 32, 1904581 CrossRef PubMed.
- R. Siavash Moakhar, T. AbdelFatah, A. Sanati, M. Jalali, S. E. Flynn, S. S. Mahshid and S. Mahshid, ACS Appl. Mater. Interfaces, 2020, 12, 23298–23310 CrossRef CAS PubMed.
- Z. Wang, B. Ai, Y. Wang, Y. Guan, H. Möhwald and G. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 35429–35437 CrossRef CAS PubMed.
- X. Fan, Q. Hao, M. Li, X. Zhang, X. Yang, Y. Mei and T. Qiu, ACS Appl. Mater. Interfaces, 2020, 12, 28783–28791 CrossRef CAS PubMed.
- B. T. Diroll, T. R. Gordon, E. A. Gaulding, D. R. Klein, T. Paik, H. J. Yun, E. D. Goodwin, D. Damodhar, C. R. Kagan and C. B. Murray, Chem. Mater., 2014, 26, 4579–4588 CrossRef CAS.
- A. T. Fafarman, S.-H. Hong, H. Caglayan, X. Ye, B. T. Diroll, T. Paik, N. Engheta, C. B. Murray and C. R. Kagan, Nano Lett., 2013, 13, 350–357 CrossRef CAS PubMed.
- A. J. Wilson and K. A. Willets, Annu. Rev. Anal. Chem., 2016, 9, 27–43 CrossRef PubMed.
- A. Lauchner, A. E. Schlather, A. Manjavacas, Y. Cui, M. J. McClain, G. J. Stec, F. J. García de Abajo, P. Nordlander and N. J. Halas, Nano Lett., 2015, 15, 6208–6214 CrossRef CAS PubMed.
- G. J. Stec, A. Lauchner, Y. Cui, P. Nordlander and N. J. Halas, ACS Nano, 2017, 11, 3254–3261 CrossRef CAS PubMed.
- G. Demirel, R. L. M. Gieseking, R. Ozdemir, S. Kahmann, M. A. Loi, G. C. Schatz, A. Facchetti and H. Usta, Nat. Commun., 2019, 10, 5502 CrossRef CAS PubMed.
- S. Chen, E. S. H. Kang, M. Shiran Chaharsoughi, V. Stanishev, P. Kühne, H. Sun, C. Wang, M. Fahlman, S. Fabiano, V. Darakchieva and M. P. Jonsson, Nat. Nanotechnol., 2020, 15, 35–40 CrossRef CAS PubMed.
- Z. Dai, G. Hu, Q. Ou, L. Zhang, F. Xia, F. J. Garcia-Vidal, C.-W. Qiu and Q. Bao, Chem. Rev., 2020, 120(13), 6197–6246 CrossRef CAS PubMed.
- N. Rivera, T. Christensen and P. Narang, Nano Lett., 2019, 19, 2653–2660 CrossRef CAS PubMed.
- S. Foteinopoulou, G. C. R. Devarapu, G. S. Subramania, S. Krishna and D. Wasserman, Nanophotonics, 2019, 8, 2129–2175 Search PubMed.
- S. Castilla, I. Vangelidis, V.-V. Pusapati, J. Goldstein, M. Autore, T. Slipchenko, K. Rajendran, S. Kim, K. Watanabe, T. Taniguchi, L. Martín-Moreno, D. Englund, K.-J. Tielrooij, R. Hillenbrand, E. Lidorikis and F. H. L. Koppens, Nat. Commun., 2020, 11, 4872 CrossRef CAS PubMed.
- Z. Tong, T. Dumitrică and T. Frauenheim, Nano Lett., 2021, 21, 4351–4356 CrossRef CAS PubMed.
- Y. Jia, H. Zhao, Q. Guo, X. Wang, H. Wang and F. Xia, ACS Photonics, 2015, 2, 907–912 CrossRef CAS.
- S. Dai, Q. Ma, M. K. Liu, T. Andersen, Z. Fei, M. D. Goldflam, M. Wagner, K. Watanabe, T. Taniguchi, M. Thiemens, F. Keilmann, G. C. A. M. Janssen, S. E. Zhu, P. Jarillo-Herrero, M. M. Fogler and D. N. Basov, Nat. Nanotechnol., 2015, 10, 682–686 CrossRef CAS PubMed.
- X. Yang, F. Zhai, H. Hu, D. Hu, R. Liu, S. Zhang, M. Sun, Z. Sun, J. Chen and Q. Dai, Adv. Mater., 2016, 28, 2931–2938 CrossRef CAS PubMed.
- A. Woessner, M. B. Lundeberg, Y. Gao, A. Principi, P. Alonso-González, M. Carrega, K. Watanabe, T. Taniguchi, G. Vignale, M. Polini, J. Hone, R. Hillenbrand and F. H. L. Koppens, Nat. Mater., 2015, 14, 421–425 CrossRef CAS PubMed.
- W. Dong, R. Qi, T. Liu, Y. Li, N. Li, Z. Hua, Z. Gao, S. Zhang, K. Liu, J. Guo and P. Gao, Adv. Mater., 2020, 32, 2002014 CrossRef CAS PubMed.
- J. Taboada-Gutiérrez, G. Álvarez-Pérez, J. Duan, W. Ma, K. Crowley, I. Prieto, A. Bylinkin, M. Autore, H. Volkova, K. Kimura, T. Kimura, M. H. Berger, S. Li, Q. Bao, X. P. A. Gao, I. Errea, A. Y. Nikitin, R. Hillenbrand, J. Martín-Sánchez and P. Alonso-González, Nat. Mater., 2020, 19, 964–968 CrossRef PubMed.
- F. Hu, M. Kim, Y. Zhang, Y. Luan, K. M. Ho, Y. Shi, C. Z. Wang, X. Wang and Z. Fei, Nano Lett., 2019, 19, 6058–6064 CrossRef CAS PubMed.
- S. Wu, S. Buckley, J. R. Schaibley, L. Feng, J. Yan, D. G. Mandrus, F. Hatami, W. Yao, J. Vučković, A. Majumdar and X. Xu, Nature, 2015, 520, 69–72 CrossRef CAS PubMed.
- Y. Yang, J. Jeon, J.-H. Park, M. S. Jeong, B. H. Lee, E. Hwang and S. Lee, ACS Nano, 2019, 13, 8804–8810 CrossRef CAS PubMed.
- J. Chen, Z. Li, F. Ni, W. Ouyang and X. Fang, Mater. Horiz., 2020, 7(7), 1828–1833 RSC.
- J. P. Fraser, P. Postnikov, E. Miliutina, Z. Kolska, R. Valiev, V. Švorčík, O. Lyutakov, A. Y. Ganin and O. Guselnikova, ACS Appl. Mater. Interfaces, 2020, 12, 47774–47783 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |