Ugi and Passerini reactions enable the incorporation of ΔAA into N-alkylated peptides and depsipeptides†
Abstract
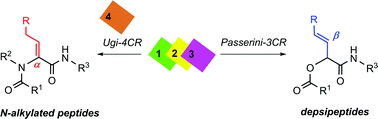
This work renders operational simplicity under mild conditions to synthesize some N-alkylated peptides and depsipeptides containing olefins. A sequential one-pot protocol merging a multicomponent reaction with oxidative elimination of phenylselenoxide allows the formation of α,β and β,γ olefin containing peptides and depsipeptides. Consequently, the regioselective construction of α,β-dehydrobutyrine and α,β-dehydro-homophenylalanine was possible using the Ugi four-component reaction; meanwhile, the Passerini three-component reaction produces the β-enamide preferably.
- This article is part of the themed collection: 50th anniversary of ICCST: celebrating ICCST at its 15th Edition


 Please wait while we load your content...
Please wait while we load your content...