Synthesis of 2-acetal-1,3-enynes by Sonogashira reaction of bromovinyl acetals with alkynes: application to the formal synthesis of a glucagon antagonist†
Abstract
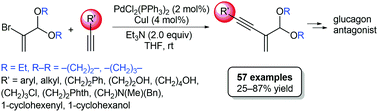
The preparation of functionalized 1,3-enynes bearing an acetal moiety at the 2-position has been studied through Sonogashira reaction of bromovinyl acetals with various alkyl- and aryl-substituted terminal alkynes. The palladium-catalyzed coupling reaction tolerated a wide substrate scope and a range of 2-acetal-1,3-enynes were obtained in up to 87% yield under mild conditions that could be transposed to multi-gram scale. This protocol has been applied to the preparation of a key intermediate in the synthesis of a glucagon antagonist.


 Please wait while we load your content...
Please wait while we load your content...