Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
When ring makes the difference: coordination properties of Cu2+/Cu+ complexes with sulfur-pendant polyazamacrocycles for radiopharmaceutical applications†
Marianna
Tosato
 a,
Matteo
Pelosato
a,
Matteo
Pelosato
 a,
Sara
Franchi
a,
Abdirisak Ahmed
Isse
a,
Sara
Franchi
a,
Abdirisak Ahmed
Isse
 a,
Nóra Veronica
May
b,
Giordano
Zanoni
a,
Nóra Veronica
May
b,
Giordano
Zanoni
 a,
Fabrizio
Mancin
a,
Fabrizio
Mancin
 a,
Paolo
Pastore
a,
Paolo
Pastore
 a,
Denis
Badocco
a,
Mattia
Asti
a,
Denis
Badocco
a,
Mattia
Asti
 c and
Valerio
Di Marco
c and
Valerio
Di Marco
 *a
*a
aDepartment of Chemical Sciences, University of Padova, 35131 Padova, Italy. E-mail: valerio.dimarco@unipd.it
bCentre for Structural Science, Research Centre for Natural Sciences, 1117 Budapest, Hungary
cRadiopharmaceutical Chemistry Section, Nuclear Medicine Unit, AUSL-IRCCS di Reggio Emilia, 42122 Reggio Emilia, Italy
First published on 22nd April 2022
Abstract
Three polyazamacrocyclic ligands, i.e. 1,5,9-tris[2-(methylsulfanyl)ethyl]-1,5,9-triazacyclododecane (TACD3S), 1,4,7,10-tetrakis[2-(methylsulfanyl)ethyl]-1,4,7,10-tetrazacyclotridecane (TRI4S) and 1,4,8,11-tetrakis[2-(methylsulfanyl)ethyl]-1,4,8,11-tetrazacyclotetradecane (TE4S), were considered as potential chelators for the medically relevant copper radioisotopes. The ligands have been synthesized through facile, single-step reactions, and their acidity constants have been measured in aqueous solution at 25 °C. The kinetic, thermodynamic, electrochemical and structural properties of their Cu2+ and Cu+ complexes were investigated in aqueous solution at 25 °C using spectroscopic (UV-Visible, EPR, NMR) and electrochemical techniques (pH-potentiometric titrations, cyclic voltammetry and electrolysis). TACD3S was demonstrated to be unable to stabilize Cu2+, whereas for TRI4S and TE4S the formation of stable monocupric (CuL2+) and monocuprous (CuL+) complexes was detected. TRI4S coordinates Cu2+via a [4N] and a [4N]S array of donor atoms while with TE4S only the latter geometry exists. The thermodynamic stability and the kinetic inertness of the copper complexes formed by TACD3S, TRI4S and TE4S were compared with those previously reported for 1,4,7,10-tetrakis-[2-(methylsulfanyl)ethyl]-1,4,7,10-tetrazacyclododecane (DO4S) to unravel the influence of the ring size and the nitrogen donor array on the copper chelation properties of these sulfur-rich macrocycles. The copresence of four nitrogen atoms is an essential feature to allow effective copper coordination when a 12-member ring is employed, as the Cu2+–DO4S complexes were far more stable than those of Cu2+–TACD3S. Furthermore, the larger ring size of TRI4S and TE4S, when compared to DO4S, progressively increases the rate of the Cu2+ complexation reactions but decreases the thermodynamic stability of the Cu2+ complexes. Despite this, the ability of TRI4S and TE4S to stably accommodate both copper oxidation states makes them very attractive for application in nuclear medicine as they could avoid the demetallation after the biologically triggered Cu2+/Cu+ reduction.
Introduction
A great deal of progress toward patient-specific treatment has been made in recent years sparked by the theranostic approach, in which a radioactive drug is used to diagnose and subsequently treat cancer.1 Among the medically useful candidate radiometals, copper perfectly matches this philosophy as it possesses a unique combination of isotopes capable of both imaging and therapy. Copper-64 (64Cu, t1/2 12.7 h) decays through a combination of electron capture (IEC 43%), β− (Iβ− 39%, Eβ−,max 573 keV) and β+ (Iβ+ 18%, Eβ+,max 655 keV) emission, which makes it suitable for both positron emission tomography (PET) imaging and radiotherapy.2–5 On the other hand, copper-67 (67Cu, t1/2 61.9 h) is a promising candidate for therapy as well as for single photon emission computed tomography (SPECT) imaging due to its β− (Iβ− 100%, Eβ−,max 141 keV) and γ-rays emission (Eγ 93 keV, Iγ 16%; Eγ 185 keV, Iγ 49%).6–8The stable complexation through a bifunctional chelator (BFC) covalently tethered to a targeting biomolecule is imperative to securely deliver [64/67Cu]Cu2+ to tumour cells.9–11 The Cu2+-BFC complex must possess high thermodynamic stability and kinetic inertness to avoid the release of the radiometal in vivo. Particularly, the biologically-induced reduction of Cu2+ to Cu+ is a potential pathway for the demetallation of [64/67Cu]Cu2+ radioconjugates, as unstable and labile cuprous species can trigger the dissociation of the complexes.2,12–15 As a result, the unbound radiometal can spread through the body leading to a loss of selectivity for the target to be imaged or treated.15
Despite the huge number of BFCs that have been evaluated for [64/67Cu]Cu2+ complexation, chelating ligands able to simultaneously coordinate both 2+ and 1+ copper oxidation states have received markedly less attention.16–18 Given the borderline character of Cu2+ according to Pearson's Hard–Soft Acid–Base theory (HSAB), the investigated BFCs for its chelation are mostly confined to polyazamacrocyclic scaffolds with pendant carboxylate arms such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA), 1,4,8,11-tetraazacyclotetradecane-1,4,8,11-tetraacetic acid (TETA) and their derivatives (Fig. 1). These ligands form thermodynamically stable complexes with Cu2+ but suffer from in vivo demetalation.13,19–21 Variation of the ring structure and/or the substituents afforded more stable and inert cross-bridged systems like 1,4,8,11-tetraazabicyclo[6.6.2]-hexadecane-4,11-diacetic acid (CB-TE2A) and 1,4,7,10-tetraazabicyclo[5.5.2]tetradecane-4,10-diacetic acid (CB-DO2A) (Fig. 1), but their sluggish radiolabeling kinetics necessitated the use of harsh protocols, hampering their use for thermally-sensitive molecule labelling.21–24 Generally promising results have been obtained with 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA) and its 1-glutaric acid derivative (NODAGA).5 However, the development of [64/67Cu]Cu2+ chelators combining high in vivo stability and kinetic inertness toward transmetallation, transchelation and reduction to Cu+ remains a challenge.13,25,26
 | ||
| Fig. 1 (A) Representative state-of-the-art ligands for copper radioisotopes chelation. The (B) “first” and (C) “second generation” series of sulfur-bearing ligands developed by our group.27 | ||
We have recently reported the evaluation of a series of sulfur-containing cyclen derivatives as chelators for [64/67Cu]Cu2+/+ which exhibited very high thermodynamic stability for both copper oxidation states.27 The design of this ligand series was based on the idea that the presence of S, N, and O donors would allow the coordination of both Cu2+ and Cu+. This “first generation” series consisted of 1,4,7,10-tetrakis[2-(methylsulfanyl)ethyl]-1,4,7,10-tetraazacyclododecane (DO4S), 1,4,7-tris[2-(methylsulfanyl)ethyl]-1,4,7,10-tetraazacyclododecane (DO3S), 1,4,7-tris[2-(methylsulfanyl)ethyl]-10-acetamido-1,4,7,10-tetraazacyclododecane (DO3SAm) and 1,7-bis[2-(methylsulfanyl)ethyl]-4,10,diacetic acid-1,4,7,10-tetraazacyclododecane (DO2A2S) (Fig. 1).27,28
Based on this promising strategy, we decided to expand the family of sulfur-based polyazamacrocyclic ligands to evaluate the impact of a larger macrocyclic ring and a different number of N/S donor atoms on the thermodynamic, kinetic, and redox properties of their cupric and cuprous complexes. For this purpose, we have considered a “second generation” series of polyazamacrocycles incorporating sulfanyl pendant arms. 1,5,9-Tris[2-(methylsulfanyl)ethyl]-1,5,9-triazacyclododecane (TACD3S) possesses the same number of atoms in the ring as DO4S but fewer overall donors (3N3S vs. 4N4S), while 1,4,7,10-tetrakis[2-(methylsulfanyl)ethyl]-1,4,7,10-tetrazacyclotridecane (TRI4S) and 1,4,8,11-tetrakis[2-(methylsulfanyl)ethyl]-1,4,8,11-tetrazacyclotetradecane (TE4S) have the same number of nitrogen and sulfanyl pendants as DO4S but a progressively larger ring size (Fig. 1). To the best of our knowledge, TACD3S and TRI4S are novel ligands, whereas TE4S has been considered by Schmid et al.,29 who however only investigated the solid-state structure and not the aqueous solution chemistry of its Cu2+ complexes. It is worth mentioning that the complex formation of Cu2+ or of other metal ions was investigated very rarely with polyazamacrocycles bearing sulfur-containing pendant arms, and when this was done, the investigated molecules contained only one sulfur side chain, or were based on different rings than cyclen, 1,4,7,10-tetrazacyclotridecane (TRI), and cyclam.30–33
The investigation of the acid–base and Cu2+/Cu+ complexation properties of TACD3S, TRI4S and TE4S in aqueous solution was performed herein using a combination of spectroscopic (UV-Vis, EPR and NMR) and electrochemical techniques (potentiometry, cyclic voltammetry and electrolysis). The final aim of investigating this series of sulfur-pendant polyazamacrocycles is to unravel the physicochemical properties of their copper complexes, in order to rationalize the alterations induced by the reported structural modifications, and thus guide the design of improved copper chelators for radiopharmaceutical applications.
Experimental
Materials
All reagents and solvents were obtained from commercial suppliers (Sigma Aldrich, VWR Chemicals) and were used as received. 1,5,9-Triazacyclododecane (TACD), 1,4,7,10-tetrazacyclotridecane (TRI), 1,4,7,10-tetrazacyclododecane (cyclen) and 1,4,8,11-tetrazacyclotetradecane (cyclam) were purchased from Chematech. All solutions were prepared with ultrapure water (18.2 MΩ cm−1) which was purified with a Purelab Chorus (Veolia) system.Ligand synthesis
All ligands were synthesized starting from the unsubstituted macrocyclic ring, potassium carbonate and acetonitrile (15 mL) mixed in a pressure tube flushed with nitrogen.2-Chloroethyl methyl sulfide was added and the pressure tube was heated to 60 °C under stirring for 24 h. The mixture was then evaporated under reduced pressure. All products were purified by flash column chromatography on silica (60 Å, 230–400 mesh, 40–63 μm, Sigma-Aldrich) using CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3OH 9
CH3OH 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 + 0.5% NH3(aq) 30% as the eluent and collected as a yellowish paste. NMR spectra were recorded using a Bruker AV III 500 spectrometer operating at 500 MHz for 1H and 125.8 MHz for 13C{1H}. Chemical shifts (δ) were reported as parts per million (ppm) relative to the residual solvent peak and coupling constants (J) in hertz (Hz). Multiplicity is given as follows: s = singlet, d = doublet, t = triplet, q = quartet, qn = quintet, m = multiplet, and br = broad peak. High-resolution mass spectra (HRMS, ESI) were recorded with an Agilent Technologies LC/MSD Trap SL mass spectrometer.
1 + 0.5% NH3(aq) 30% as the eluent and collected as a yellowish paste. NMR spectra were recorded using a Bruker AV III 500 spectrometer operating at 500 MHz for 1H and 125.8 MHz for 13C{1H}. Chemical shifts (δ) were reported as parts per million (ppm) relative to the residual solvent peak and coupling constants (J) in hertz (Hz). Multiplicity is given as follows: s = singlet, d = doublet, t = triplet, q = quartet, qn = quintet, m = multiplet, and br = broad peak. High-resolution mass spectra (HRMS, ESI) were recorded with an Agilent Technologies LC/MSD Trap SL mass spectrometer.
In the following, the reactant and product amounts are given for each compound together with the spectral data.
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH2N). 13C{1H} NMR (126 MHz, CDCl3): δ 54.98 (CH2), 54.58 (CH2), 52.46 (CH2), 52.35 (CH2), 51.63 (CH2), 50.95 (CH2), 31.89 (CH2SCH3), 31.65 (CH2SCH3), 23.35 (NCH2
2CH2N). 13C{1H} NMR (126 MHz, CDCl3): δ 54.98 (CH2), 54.58 (CH2), 52.46 (CH2), 52.35 (CH2), 51.63 (CH2), 50.95 (CH2), 31.89 (CH2SCH3), 31.65 (CH2SCH3), 23.35 (NCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH2N), 15.86 (SCH3). HRMS (ESI) m/z [M + H+]: 483.2784; calc. 483.2678.
2CH2N), 15.86 (SCH3). HRMS (ESI) m/z [M + H+]: 483.2784; calc. 483.2678.
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH2N). 13C{1H} NMR (126 MHz, CDCl3): δ 54.56 (CH2), 51.22 (CH2), 50.39 (CH2), 31.23 (CH2SCH3), 23.01 (NCH2
2CH2N). 13C{1H} NMR (126 MHz, CDCl3): δ 54.56 (CH2), 51.22 (CH2), 50.39 (CH2), 31.23 (CH2SCH3), 23.01 (NCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH2N), 14.46 (SCH3). HRMS (ESI) m/z [M + H+]: 497.2935; calc. 497.2835.
2CH2N), 14.46 (SCH3). HRMS (ESI) m/z [M + H+]: 497.2935; calc. 497.2835.
Formation kinetics
The formation kinetics of Cu2+ complexes with the investigated ligands was monitored as a function of pH at room temperature via UV-Vis spectroscopy, following the increasing intensity of the charge transfer and/or d–d bands of the complexes at the characteristic wavelengths. The electronic spectra were recorded in the 200–800 nm range at different time points using a Cary 60 UV-Visible spectrophotometer (Agilent) equipped with a 1 cm path length quartz cell. Complexation kinetics experiments were carried out by mixing equimolar amounts of metal and ligand solutions (final concentrations: CCu2+ = CL = 10−4 M) in a buffered medium at pH 2 (HCl 10−2 M), pH 3.7 (acetic/acetate buffer) and pH 7.5 (2-[4-(2-hydroxyethyl)piperazin-1-yl]ethane sulfonic acid – HEPES – buffer).Protonation and metal–ligand stability constants
0.1 M nitric acid stock solutions (Aristar – VWR Chemicals) were prepared from a concentrated one and standardised against sodium carbonate (Aldrich, 99.95–100.5%). 0.1 M sodium hydroxide solutions with a low carbonate content were prepared from commercial pellets (Fluka, 99% min) and their accurate concentrations were obtained by titration of the previously standardized HNO3 solutions. Stock solutions of the ligands and Cu2+ were freshly prepared by direct dissolution of the synthesized compounds (∼ 10−3 M) and from analytical-grade Cu(NO3)2·3H2O (Sigma Aldrich) (∼ 10−2 M), respectively. HNO3 (CH+ = 4.2·CL) was coadded to the ligand solution to avoid carbonatation and facilitate dissolution. The solubility of the ligands in water depends on pH, and in all cases minimal values occur at pH > 9 where the non-charged, totally deprotonated form L predominates. The limit solubility of L was estimated from the pH at which precipitation starts, knowing the pKa values of each ligand (see below) and the stoichiometric ligand concentration: the results for TACD3S, TRI4S and TE4S were 1.5 × 10−4 M, 1.9 × 10−4 M and 1.3 × 10−4 M, respectively.
The concentration of the ligands in the titration vessel ranged from 7 × 10−4 M to 2 × 10−3 M while the metal:ligand ratio varied from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The ligand solutions were acidified with a known volume of HNO3, followed by the addition of Cu2+ in the case of the metal–ligand titrations, and the titrations were then carried out by adding known volumes of NaOH. The purity of the synthesized chelators was demonstrated to be adequate (> 95%). The explored pH range was 2–12 except for Cu2+–TRI4S where the titrations were started at pH ∼ 4 to avoid too slow complexation kinetics. Each titration was performed independently at least in triplicate.
2. The ligand solutions were acidified with a known volume of HNO3, followed by the addition of Cu2+ in the case of the metal–ligand titrations, and the titrations were then carried out by adding known volumes of NaOH. The purity of the synthesized chelators was demonstrated to be adequate (> 95%). The explored pH range was 2–12 except for Cu2+–TRI4S where the titrations were started at pH ∼ 4 to avoid too slow complexation kinetics. Each titration was performed independently at least in triplicate.
Between pH 4 and 12, direct titrations were carried out. Typically, equimolar metal-to-ligand solutions were mixed (final concentrations: CCu2+ = CL = 10−4 M), the pH was adjusted to ∼ 4, and the titrations were carried out by adding a known volume of NaOH, similarly to the pH-potentiometric titrations. After each addition, the pH was allowed to equilibrate, an aliquot was transferred to the spectrophotometric cell and the spectrum was recorded. Then, the aliquot was transferred back to the titration vessel and a new addition was made. The maximum pH value was ∼ 12. Typical titrand and titrant volumes were in the range 10–25 mL and less than 100 μL, respectively. The same protocol was used for the determination of the ligand protonation constants but, in this case, no Cu2+ was added.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. After Cu2+ additions, the UV-Vis spectra were recorded, and the stoichiometry was determined by plotting the absorbance at the characteristic wavelengths as a function of the metal-to-ligand ratios.
1. After Cu2+ additions, the UV-Vis spectra were recorded, and the stoichiometry was determined by plotting the absorbance at the characteristic wavelengths as a function of the metal-to-ligand ratios.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βpqr = [MpLqHr]/[M]p[L]q[H]r) and are therefore referred to the overall equilibrium pMm+ + qH+ + rLl− ⇆ MpHqLrpm+q−rl, where the metal and ligand are designated as M and L, respectively.36 The refinements of the overall formation constants included, in each case, the previously determined ligand protonation constants and the metal hydrolysis products, whose equilibrium constants were fixed to the literature values.39 The errors quoted are the standard deviations calculated by the PITMAP program.36 The following parameters, which strongly correlated with each other, were calculated by the same program from acid–base titrations, and then kept constant in subsequent ligand and Cu2+–ligand titrations: water ionization constant (pKw), glass electrode alkaline error (given by the selectivity coefficient for Na+, pkNa), and carbonatation degree of the NaOH solution (%), which were 13.54, 12.0 and 0.5% on average, respectively.
βpqr = [MpLqHr]/[M]p[L]q[H]r) and are therefore referred to the overall equilibrium pMm+ + qH+ + rLl− ⇆ MpHqLrpm+q−rl, where the metal and ligand are designated as M and L, respectively.36 The refinements of the overall formation constants included, in each case, the previously determined ligand protonation constants and the metal hydrolysis products, whose equilibrium constants were fixed to the literature values.39 The errors quoted are the standard deviations calculated by the PITMAP program.36 The following parameters, which strongly correlated with each other, were calculated by the same program from acid–base titrations, and then kept constant in subsequent ligand and Cu2+–ligand titrations: water ionization constant (pKw), glass electrode alkaline error (given by the selectivity coefficient for Na+, pkNa), and carbonatation degree of the NaOH solution (%), which were 13.54, 12.0 and 0.5% on average, respectively.
EPR
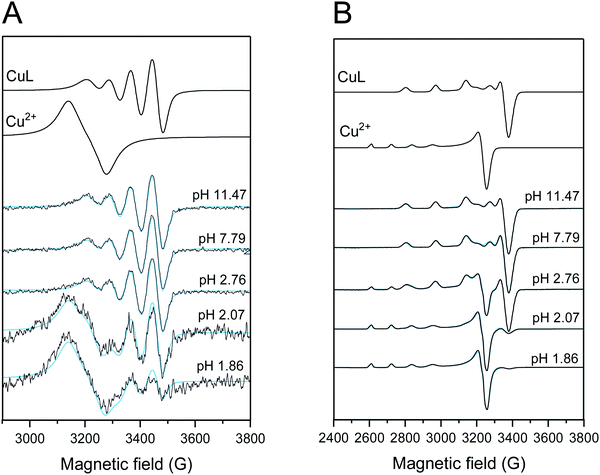
X-band CW-EPR spectra were recorded with a Bruker EleXsys E500 spectrometer (microwave frequency 9.4 GHz, microwave power 13 mW, modulation amplitude 5 G, modulation frequency 100 kHz).The Cu2+–TE4S sample was prepared the day before the measurement and stirred overnight at room temperature to take into account the slow kinetics of complex formation. pH-Dependent EPR spectra were measured in the pH range 1.86–11.47. NaOH or HCl were employed to adjust the pH.
Room temperature EPR spectra were recorded in capillaries using six scans. Room temperature spectra were corrected with the background spectrum of pure aqueous solution before the simulation. Frozen solution EPR spectra were measured in quartz EPR tubes placed into Dewar containing liquid nitrogen at 77 K. 0.2 mL of sample was placed in the tube and 0.05 mL methanol was added to avoid the crystallization of water. All CW-EPR spectra were simulated by the EPR software.40 Room temperature spectra were described by the isotropic EPR parameters go and AoCu copper hyperfine couplings, and the relaxation parameters α, β, and γ which define the linewidths in the equation σMI = α + βMI + γMI2, where MI denotes the magnetic quantum number of the copper nucleus. The super hyperfine coupling (aN0) of four equivalent nitrogen atoms significantly improved the fit. The anisotropic spectra were analysed with the help of axial g- and A-tensor values (g⊥, g‖, A⊥Cu, A‖Cu) and the orientation-dependent linewidth parameters. Since natural CuCl2 was used for the measurements, the spectra were calculated as the sum of the spectra of 63Cu and 65Cu weighted by their natural abundances. The copper and nitrogen coupling constants and the relaxation parameters were obtained in field units (Gauss = 10−4 T).
Stability/inertness with competitive metal cations
Different aliquots of Zn2+ and Ni2+ solutions were added to a solution of the preformed cupric complexes (CCuL2+ = 10−4 M) to obtain the following n(M2+)/n(CuL2+) ratios: 50 ≤ n(Zn2+)/n(CuL2+) ≤ 1000 and 50 ≤ n(Ni2+)/n(CuL2+) ≤ 250. The spectral variations induced by the addition of the competitor were monitored at room temperature using UV-Vis spectroscopy for 5 days.Acid-mediated decomplexation kinetics
Acid-mediated decomplexation studies of the Cu2+ complexes were performed under pseudo-first-order conditions in 0.1–1 M HCl at 25 °C (CCuL2+ = 10−4 M). The reactions were monitored by UV-Vis spectroscopy following the decrease in intensity of the charge transfer transitions of the Cu2+ complexes at the characteristic wavelength at specific time points. The dkobs values were calculated from the experimental data by using a single-exponential model A(t) = A(0)·e−dkobs·t. The corresponding half-life was obtained from the equation t1/2 = ln(2)/dkobs.Cyclic voltammetry
Cyclic voltammetry was carried out in aqueous solution at room temperature with an Autolab PGSTAT 302N potentiostat operated with NOVA 2.1 (Metrohm) data acquisition software. The experiments were performed with a six-neck glass cell using a glassy carbon (GC) working electrode (WE) fabricated from a 3 mm-diameter rod (Tokai GC-20), a saturated calomel reference electrode (SCE) and a platinum wire counter electrode (CE). The WE surface was routinely polished with 0.25 μm diamond paste, followed by ultrasonic rinsing in ethanol for 5 minutes before use.The sample solutions containing the copper complexes were degassed by bubbling Ar before all measurements and kept under an Ar blanket throughout the measurements. 0.15 M NaNO3 was used as a supporting electrolyte and the pH was adjusted with NaOH and HNO3 solutions.
Cyclic voltammograms with scan rates ranging from 0.01 to 0.1 V s−1 were recorded in the region from −0.5 to 0.5 V. In this potential range, the solvent, the background electrolyte, and the ligands were electrochemically inactive.
Electrolysis and NMR
Exhaustive electrolyses of the pre-formed Cu2+ complexes were carried out using large area glassy carbon WE in a two-compartment cell. The CE was a Pt gauze in 0.15 M NaNO3 solution, separated from the WE solution by two glass frits (G3). A saturated calomel electrode was used as a reference electrode. The electrolyses were performed at a fixed potential equal to E = −0.45 V and E = −0.40 V for Cu2+–TRI4S and Cu2+–TE4S, respectively. Linear scan voltammetry (LSV) on a rotating disc electrode was used to monitor the evolution of the species in solution. Each electrolysis was considered complete when the cathodic current reached ≲2% of the initial value.1H-NMR spectra of the in situ generated Cu+ complexes were collected at 25 °C using the same spectrometer and the same protocol described above.
Results and discussion
Ligand synthesis
TACD3S, TRI4S and TE4S were synthesized by direct complete alkylation of the parent macrocycles with an excess of 2-chloroethyl methyl sulfide in acetonitrile at 60 °C for 24 hours according to Scheme S1 (ESI†).Ligand protonation constants
Combined potentiometric, 1H-NMR and UV-Vis spectrophotometric titrations in aqueous solution were used to determine the protonation constants of TACD3S, TRI4S and TE4S at 25.0 °C with the ionic strength adjusted to 0.15 M NaNO3. The stepwise protonation constants (pKa) are outlined in Table 1 and compared with those of DO4S and structurally related ligands (tetramethyl-cyclen and tetramethyl-cyclam) or with the parent unsubstituted macrocycles (1,5,9-triazacyclododecane – TACD, 1,4,7,10-tetrazacyclododecane – cyclen, 1,4,7,10-tetrazacyclotridecane – TRI and 1,4,8,11-tetrazacyclotetradecane – cyclam). The protonation speciation diagrams for TACD3S, TRI4S and TE4S are presented in Fig. S1 (ESI†).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β values) of TACD3S, TRI4S and TE4S at T = 25 °C and I = 0.15 M NaNO3. The pKa values of the corresponding unsubstituted macrocycles and other structurally related compounds are reported for comparison purposes. Unless otherwise stated, the values were obtained by pH-potentiometry
β values) of TACD3S, TRI4S and TE4S at T = 25 °C and I = 0.15 M NaNO3. The pKa values of the corresponding unsubstituted macrocycles and other structurally related compounds are reported for comparison purposes. Unless otherwise stated, the values were obtained by pH-potentiometry
| Ligand | Equilibriuma | |||
|---|---|---|---|---|
| HL+ ⇌ L + H+ | H2L2+ ⇌ HL+ + H+ | H3L3+ ⇌ H2L2+ + H+ | L + 3H+ ⇌ H3L3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
|
a L represents the completely deprotonated form of each chelator. The reported uncertainty was obtained by the fitting procedure and represents one standard deviation unit.
b Obtained from 1H-NMR data, no ionic strength control.
c Obtained from UV-Vis data, no ionic strength control.
d The first pKa value in each cell was considered for the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β computation.
e From ref. 28, I = 0.15 M NaNO3, T = 25 °C.
f From ref. 27, no ionic strength control, T = 25 °C.
g From ref. 41, I = 0.15 M KNO3, T = 25 °C.
h From ref. 42, I = 0.2 M NaClO4, T = 25 °C.
i From ref. 43, I = 0.1 M NaNO3, T = 25 °C.
j From ref. 44, I = 0.1 M NaNO3, T = 25 °C. β computation.
e From ref. 28, I = 0.15 M NaNO3, T = 25 °C.
f From ref. 27, no ionic strength control, T = 25 °C.
g From ref. 41, I = 0.15 M KNO3, T = 25 °C.
h From ref. 42, I = 0.2 M NaClO4, T = 25 °C.
i From ref. 43, I = 0.1 M NaNO3, T = 25 °C.
j From ref. 44, I = 0.1 M NaNO3, T = 25 °C.
|
||||
| TACD3S | 9.60 ± 0.02; 9.6 ± 0.2b | 5.57 ± 0.05; 5.41 ± 0.06b; 5.29 ± 0.03c | 1.62 ± 0.07b | 16.79 |
| DO4Sef | 10.14e | 7.29e | 1.9f | 19.3 |
| TRI4S | 9.76 ± 0.03; 9.4 ± 0.1b | 6.69 ± 0.03; 6.0 ± 0.3b; 6.10 ± 0.05c | 1.5 ± 0.1b | 18.0 |
| TE4S | 10.60 ± 0.01 | 7.73 ± 0.05; 7.5 ± 0.2c | 1.7 ± 0.2b | 20.0 |
| TACDg | 12.6 | 7.57 | 2.41 | 22.6 |
| Cyclenef | 10.63e | 9.51e | 1.6f | 21.7 |
| Tetramethyl-cyclenh | 11.06 | 8.95 | — | 20.01 |
| TRIi | 11.02 | 9.96 | 1.96 | 22.94 |
| Cyclamj | 11.3 | 10.23 | 1.43 | 23.0 |
| Tetramethyl-cyclamj | 9.34 | 8.99 | 2.58 | 20.91 |
For all the investigated chelators, the constants for the deprotonation of HL+ and H2L2+ (Table 1) can be assigned to the deprotonation of two opposite or adjacent nitrogen atoms of the azamacrocyclic rings. The other deprotonation constants (i.e. those for H3L3+ and, if applicable, for H4L4+) were always found to be very high (Ka > 10−2, i.e. pKa < 2, Table 1), primarily due to the Coulombic repulsion between the positive charges resulting from the protonated amines that are forced into proximity by the cyclic nature of the ligands.
Comparing TRI4S and TE4S with DO4S, the slight differences in the pKa values are related to the different ring sizes and the relative position of the nitrogen atoms. For TE4S, the higher pKa values with respect to those of DO4S likely reflect the larger separation between the nitrogen atoms afforded by the larger backbone, which lowers the charge–charge repulsion, allowing better stabilization of the proton binding. On the contrary, the tertiary nitrogen atoms of TRI4S are less basic than those of DO4S and TE4S, indicating that the former molecule adopts conformers in which the ring protons are less stable than for the other two chelators. Simple charge–repulsion arguments do not explain this trend, which, as a tentative reason, might be ascribed to the asymmetry of TRI4S.
Although TACD3S and DO4S possess the same number of atoms in the macrocyclic scaffold, the former is significantly more acidic. In TACD3S the tertiary amines are separated by a larger distance with respect to DO4S, but the lower number of possible microstates (where protons are localized on different nitrogens) leads to lower stability of the protonated species for TACD3S, thus explaining the observed behaviour.
As previously found for DO4S and its derivatives,28 a decrease of pKa was also observed (especially for the deprotonation of H2L2+) with all the investigated sulfur-bearing ligands when compared to the bare macrocycles or the non-sulfanyl analogues. While the former effect can be explained by the consequence of the destabilization of the protonated species resulting from the decrease in water solvation after the N-alkylation, the latter was attributed to the presence of the sulfur atoms on the side arms.28
1H-NMR spectroscopy was employed to confirm some pKa values and to determine the most acidic ones (pKa ≪ 2) not accessible by potentiometric measurements. In addition, it was also used to gain insights into the solution structure and the dynamics of the protonation/deprotonation processes of the investigated ligands. The pKa values determined by NMR are also reported in Table 1. Some minor discrepancies between the protonation constants obtained by NMR and potentiometry can be attributed to the uncontrolled ionic strength during NMR experiments.
The 1H-NMR spectra of TACD3S, TRI4S and TE4S and the 1H chemical shift variations as a function of pH are shown in Fig. S2–S4 and S5–S7 (ESI†). The signal assignments, supported by the bidimensional spectra of Fig. S8–S11 (ESI†), are reported in Tables S1–S3 (ESI†). A thorough description of the spectra is given in the ESI.
According to the sharpness of the signals and the absence of multiple patterns, all the deprotonation processes (H3L3+ ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) H2L2+ + H+
H2L2+ + H+ ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) HL+ + H+) should be fast on the NMR timescale (Fig. S2–S4 and S12, ESI†). An exception is represented by the last deprotonation step of TACD3S, which appears markedly slower since both patterns of HL+ and L, together with a signal enlargement, can be observed (Fig. S2, ESI†). The estimated molar ratio between these two species obtained by the integration of NMR signals is in good agreement with the values calculated by potentiometry. Moreover, the sharpness of the signals also indicates that the conformational equilibria within a single species are fast on the NMR timescale (except for TACD3S in its totally deprotonated form).
HL+ + H+) should be fast on the NMR timescale (Fig. S2–S4 and S12, ESI†). An exception is represented by the last deprotonation step of TACD3S, which appears markedly slower since both patterns of HL+ and L, together with a signal enlargement, can be observed (Fig. S2, ESI†). The estimated molar ratio between these two species obtained by the integration of NMR signals is in good agreement with the values calculated by potentiometry. Moreover, the sharpness of the signals also indicates that the conformational equilibria within a single species are fast on the NMR timescale (except for TACD3S in its totally deprotonated form).
On the contrary, it is worth noting that in the cyclen-based analogue, i.e. DO4S, the multiplets were sharp only in its neutral form.28 The lower energetic barrier of the conformer interconversion, resulting from the CH2 spacer added in the ring of TACD3S, TRI4S and TE4S, could justify this difference.
As regards the UV-Vis analysis, the electronic spectra of TACD3S, TRI4S and TE4S display a strong absorption in the UV region (below 250 nm) which showed an absorbance increase close to the pKa values (Fig. S13, ESI†), similarly to DO4S and its derivatives.28 UV-Vis data were fitted to determine some pKa values (Table 1) which agree reasonably well with those obtained from potentiometric titrations and NMR.
Cu2+ complexes: formation kinetics
The formation kinetics of the Cu2+ complexes with TACD3S, TRI4S and TE4S were assessed as a function of pH by UV-Vis spectroscopy, following the absorbance changes observed at selected wavelengths over time. This qualitative investigation was necessary to determine the timing required for reaching equilibrium, thus allowing the subsequent thermodynamic investigations.For TRI4S and TE4S, reaction times from seconds to many hours were found (Fig. S14, S15 and Table S4, ESI†). The complex formation rate strongly decreased upon decreasing the pH, reflecting the dissimilar reactivity of the differently protonated ligand species predominating at different pH (Fig. S1, ESI†): higher protonation states correspond to the higher electrostatic repulsion between Cu2+ and the ring cavity where the donor atoms are located, as previously reported for DO4S and its derivatives.27
The increased ring size in TRI4S and TE4S significantly accelerates the formation of the Cu2+ complexes when compared with DO4S (Table S4, ESI†).27 This complexation rate enhancement could be explained by the lower electrostatic repulsion between the metal ion and the nitrogen donors driven by the larger ring. TACD3S was found not to be able to complex Cu2+ except at nearly neutral pH (Fig. S15, ESI†), where the reaction rate was comparable to that of the other ligands.
Cu2+ complexes: thermodynamics
UV-Vis spectrophotometric titrations at equilibrium conditions were performed to determine the formation constants (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β) of the Cu2+-complexes with TACD3S, TRI4S and TE4S. The slow equilibration timing evidenced at acidic pH forced the use of out-of-cell titrations at pH ≤ 4 together with the direct in-cell ones (pH > 4). The high binding Cu2+ affinity of TRI4S obviated the use of conventional pH-potentiometric techniques, while for TE4S pH-potentiometry was also employed.
β) of the Cu2+-complexes with TACD3S, TRI4S and TE4S. The slow equilibration timing evidenced at acidic pH forced the use of out-of-cell titrations at pH ≤ 4 together with the direct in-cell ones (pH > 4). The high binding Cu2+ affinity of TRI4S obviated the use of conventional pH-potentiometric techniques, while for TE4S pH-potentiometry was also employed.
As shown in Fig. S14 and S15 (ESI†), the Cu2+ addition to solutions containing any ligand causes the appearance of two absorption bands in the UV-Vis spectra, which are accountable for the metal complexation event. These bands change in intensity but not in shape with pH, so that the formation of the same Cu2+ complex in all the pH range was deduced. Fig. S16 (ESI†) shows the spectra at various pH; the fitting procedure indicated the presence of only one complex having stoichiometry CuL2+ (where L indicates the deprotonated ligand form). UV-Vis titrations at different metal-to-ligand molar ratios, which showed saturation at equimolar values (Fig. S17, ESI†), further confirmed this speciation model, whereas potentiometric titrations (at pH ≥ 2 for TE4S, at pH ≥ 4 for TRI4S) showed no protonation/deprotonation events occurring for the CuL2+ complexes in the investigated pH range. The overall stability constants are reported in Table 2 while the distribution diagrams are shown in Fig. 2 and Fig. S18 (ESI†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β) and pCu values of the Cu2+ and Cu+ complexes formed by TACD3S, TRI4S and TE4S at I = 0.15 M NaCl and T = 25 °C. Literature data for Cu2+/+–DO4S are reported for comparison purposes. Unless otherwise stated, log
β) and pCu values of the Cu2+ and Cu+ complexes formed by TACD3S, TRI4S and TE4S at I = 0.15 M NaCl and T = 25 °C. Literature data for Cu2+/+–DO4S are reported for comparison purposes. Unless otherwise stated, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β for Cu2+ were obtained by UV-Vis spectrophotometric titrations whereas those for Cu+ were computed from cyclic voltammetrya
β for Cu2+ were obtained by UV-Vis spectrophotometric titrations whereas those for Cu+ were computed from cyclic voltammetrya
| Ligand | TACD3S | DO4Sd | TRI4S | TE4S |
|---|---|---|---|---|
| a L denotes the ligand in its totally deprotonated form. The reported uncertainty was obtained by the fitting procedure and represents one standard deviation unit. b pCu2+/+ calculated at CCu2+/+ = 10−6 M and CL = 10−5 M at pH = 7.4 using the constants of Tables 1 and 2 or taken from ref. 27. c Obtained by pH-potentiometric titrations, I = 0.15 M NaNO3. d From ref. 27. | ||||
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β Cu2+ β Cu2+ |
6.6 ± 0.4 | 19.8 | 18.53 ± 0.04 | 17.24 ± 0.07; 17.01 ± 0.02c |
pCu2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
6.2 | 17.7 | 17.0 | 14.5 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β Cu+ β Cu+ |
— | 19.8 | 16.8 ± 0.1 | 16.3 ± 0.1 |
pCu+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
— | 17.2 | 15.3 | 13.6 |
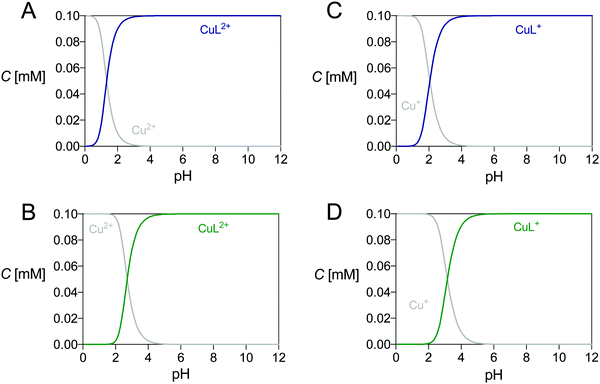 | ||
| Fig. 2 Distribution diagrams of (A) Cu2+–TRI4S, (B) Cu2+–TE4S, (C) Cu+–TRI4S and (D) Cu+–TE4S. The plots were calculated from the overall stability constants reported in Tables 1 and 2 at CCu = CL = 1.0 × 10−4 M. | ||
The pCu2+ values (pCu2+ = −log[Cu2+]free) were calculated at pH 7.4 to compare the chelating ability of the different ligands at physiological conditions: the higher the pCu2+, the higher the stability of the complexes under the specified conditions.45 The obtained values are listed in Table 2. As a comparison, the pCu2+ values for other DO4S derivatives and selected state-of-the-art [64/67Cu]Cu2+ chelators are given in Table S5 (ESI†).
Surprisingly, TACD3S gave a very low pCu2+ demonstrating it was not able to complex Cu2+ except at nearly neutral pH. It follows that the copresence of four nitrogen atoms is an essential feature in a 12-member macrocyclic structure to allow an effective copper coordination when the side chains contain S donors, as the removal of a nitrogen donor and a sulfur side chain have a huge impact on the Cu2+ coordination. This low Cu2+–complex stability hampered any further investigation with TACD3S, so that this ligand will no longer be considered in the following discussion.
The addition of a ring carbon atom in the DO4S scaffold, leading to TRI4S, only slightly affects the complex stability at physiological pH (pCu2+ 17.0 for TRI4S and 17.7 for DO4S, Table 2).27 Contrarily, the further increase of the ring size, leading to TE4S, is detrimental in terms of the stability of the resulting Cu2+ complexes, as the pCu2+ of TE4S is 2.5 orders of magnitude lower than that of TRI4S. These behaviours are likely related to the worst matching between the size of the metal cation and ring cavity, thus resulting in a stability drop.
Cu2+ complexes: electronic and EPR structural analysis
The analysis of the electronic spectra of the investigated Cu2+ complexes allowed us to evaluate the structural changes induced by the ring size increase. A comparison between the UV-Vis spectra of the Cu2+–DO4S, Cu2+–TRI4S and Cu2+–TE4S complexes is reported in Fig. 3. The involvement of the chloride anions in the Cu2+ coordination sphere is negligible for both Cu2+–TRI4S and Cu2+–TE4S as the spectra measured upon the addition of an excess of NaCl (I = 0.15 M) to aqueous solutions (I = 0 M) do not change (Fig. S19, ESI†). | ||
| Fig. 3 Comparison of the normalized electronic spectra of the cupric complexes with DO4S, TRI4S and TE4S (ε = absorbivity coefficient). The electronic spectrum of the Cu–DO4S system was taken from ref. 27. | ||
Cu2+–TRI4S displays a strongly intense UV transition at 313 nm accompanied by a less intense single broad band in the visible region at 598 nm (Table S6, ESI†). This band set is very similar to the one previously obtained for Cu2+–DO4S,27 so that the same structural features can be hypothesized in both cases. In particular, two different isomers having either [4N]Sax or [4N] coordination arrangements might exist in solution with TRI4S, as was found for the cupric complex of DO4S.27 When turning to Cu2+–TE4S, the maximum absorption is maintained (Table S6, ESI†), but a remarkable increase of the shoulder at 370 nm accompanied with a redshift of the d–d band (from 598 nm to 626 nm) can be observed, thus suggesting that TE4S binds Cu2+ through a different coordination mode.
To further investigate this result, EPR analysis was carried out for Cu2+–TE4S solutions. The EPR spectra recorded at room temperature showed the appearance of free copper ion only below pH 2 (Fig. 4). At higher pH, the CuL2+ complex spectra were measured, and no further spectral changes were detected, in agreement with the speciation model (Table 2, Fig. 2). In frozen solution, the complex becomes predominant at higher pH, and at pH 2.76, ∼ 30% free copper was detected. The component ratios are shown in Fig. S20 (ESI†).
The obtained EPR parameters are reported in Table 3. The parameters for Cu2+–TE4S are close to those of Cu2+–DO4S isomer (2), reported in our previous work, where the coordination of the macrocycle is driven by four nitrogen and complemented axially by a sulfide side chain (Table 3). However, for Cu2+–TE4S a significantly higher A⊥ was detected.27 This is probably due to the higher symmetrical arrangement of the nitrogen donor atoms in the equatorial sphere, which makes the copper centre in plane with the nitrogen atoms, resulting in higher copper hyperfine coupling values. The observed redshift in the UV-Vis spectra of Cu2+–TE4S, when compared to Cu2+–DO4S, could be therefore rationalized considering that the UV-Vis spectrum of the latter is a mixture of the spectra deriving from two components, [4N] and [4N]Sax,27 while Cu2+–TE4S contains only [4N]Sax. This result is in perfect agreement with the data obtained by Schmidt et al. in the solid-state, as their Cu2+–TE4S crystal, when investigated by X-ray, was also demonstrated to possess a [4N]Sax structure.29
| Isotropic parametersa | Anisotropic parametersb | Calculatedc | |||||
|---|---|---|---|---|---|---|---|
| g 0 | A 0 [ × 104cm−1] | g ⊥ | g ‖ | A ⊥ [ × 10−4cm−1] | A ‖ [ × 10−4cm−1] | g 0,calc | |
| a The experimental error was ± 0.001 for g0 and ± 1 × 10−4 cm−1 for A0. b The experimental error was ± 0.002 for g⊥ and ± 0.001 for g‖ and ± 1·10−4 cm−1 for A⊥ and A‖. c Calculated by the equation g0,calc = (2g⊥+ g‖)/3 on the basis of anisotropic values. d From ref. 27. | |||||||
| Cu(aq) | 2.194 | 34.1 | 2.084 | 2.423 | 4.9 | 126.1 | 2.197 |
| Cu2+–TE4S | 2.101 | 73.3 | 2.048 | 2.204 | 38.2 | 168.1 | 2.100 |
| Cu2+–DO4S (2)d | 2.103 | 63.6 | 2.048, 2.058 | 2.209 | 20.3, 23.5 | 171.2 | 2.105 |
The significantly distended Cu2+ cation in the cyclam backbone indicates a mismatch within the ligand metal-binding cleft which could be correlated to the lower thermodynamic stability when compared to DO4S (Table 2).
Cu2+ complexes: dissociation kinetics
Promising BFC candidates for radiopharmaceutical applications must exhibit high thermodynamic stability at physiological pH, but also high kinetic inertness toward dissociation.21The kinetic inertness of the Cu2+–TRI4S and Cu2+–TE4S complexes were at first evaluated in the presence of an excess of competitor cations, i.e. Ni2+ and Zn2+, by studying the species evolution with time after the competitor additions. Ni2+ and Zn2+ were chosen as they are common metallic impurities in radiocopper solutions, and Zn2+ also represents a biologically relevant cation.21 In all cases, only minor decomplexation was observed within 5 days, as indicated by the slight decrease of the absorption in the electronic spectra of the two cupric complexes (Fig. S21 and S22, ESI†): the percentages of intact complexes are reported in Tables S7 and S8 (ESI†) and they were always > 90%. These results can be related to an interplay between a marked kinetic inertness of the investigated Cu2+ complexes in the tested conditions and a not very high thermodynamic stability of the corresponding Zn2+ and Ni2+ complexes. The latter was qualitatively evaluated by 1H-NMR or UV-Vis spectra as briefly described in the captions of Fig. S23 and S24 (ESI†).
The inertness of the Cu2+–TRI4S and Cu2+–TE4S complexes was also investigated in harsher conditions than above, by evaluating the acid-assisted dissociation kinetics. Albeit this assay could not predict the in vivo integrity of the resulting complex, it is considered a convenient and popular gauge of relative kinetic inertness of copper–tetraamine complexes to Cu2+ decomplexation in aqueous media and as a first screening for monitoring the Cu2+–chelator stability.46–48 Also, the first generation ligands DO4S and DO2A2S were tested through these assays for comparison purposes.
Representative data are shown in Fig. S25–S28 (ESI†). The experimentally observed dissociation rate constants (dkobs) and the corresponding half-life (t1/2) at different HCl concentrations are compiled in Tables S9 and S10 (ESI†), respectively. For DO4S and DO2A2S, the dkobs values linearly change with the proton content (Fig. S29, ESI†), indicating that only one complex undergoes dissociation, and that the protonation constant of the pre-dissociation step is low.49,50 The second-order rate constant (dk) was thus obtained using dkobs = dk[H+] as the fitting equation. In the case of TRI4S, the dkobsvs. [H+] dependence was not apparently linear, and a negative intercept was obtained, so that the above-stated conditions do not apply. If two paths are assumed to coexist, involving the addition of one and of two protons, respectively, the equation dkobs = dk[H+] + dk2[H+]2 represents a better model.50 Therefore, we have used this equation (Fig. S29, ESI†), from which the constants given in Table S10 (ESI†) were obtained.
The dissociation kinetics of the Cu2+ complexes strongly depends on the ligand side arms: Cu2+–DO2A2S is the most inert, likely due to the presence of coordinated carboxylates in the ligand structure, either in protonated or unprotonated form. Likely, the sulfur arms do not provide a significant contribution to the kinetic inertness of the Cu2+ complexes: this can be deduced if the dk value of Cu2+–DO4S is compared with those of Cu2+–cyclen and Cu2+–DOTA given in the literature (dk = ∼ 5 × 10−4 and 6 × 10−6 s−1, respectively).51 Moreover, the obtained results demonstrated that the ring dimension makes the difference in the inertness of the Cu2+ complexes, because the Cu2+ complex formed by DO4S is kinetically much more inert than those formed by TRI4S and TE4S. The contrast between related cyclen- and cyclam-based complexes is dramatic since the Cu2+–TE4S complex dissociates within minutes (Table S10, ESI†) even at the lowest HCl concentrations (due to the few experimental points and the high speed of the dissociation, only a rough dk value of ∼ 10−2 M−1 s−1 can be given for this ligand). Thus, the increase in the ring size not only affects the thermodynamic stability but also the acid-assisted dissociation behaviour of these complexes. If the results of Table 2 and Table S10 (ESI†) are compared, it follows that the decomplexation rates of the cupric complex in highly acidic solution are strongly correlated with the complex stabilities. For example, this correlation is clearly visible in Fig. S30 (ESI†) where pCu2+ values at physiologic pH are plotted vs. log(t1/2) at pH 1.
Cu+ complexes: cyclic voltammetry, solution thermodynamics and structural analysis
A cyclic voltammetry (CV) study was undertaken in water using 0.15 M NaNO3 as a supporting electrolyte to evaluate the stabilities of the investigated complexes upon reduction to Cu+.The electrochemical behaviour of the unbound TRI4S and TE4S was firstly assessed. Both ligands were demonstrated to be electrochemically inactive in the potential range of the Cu2+/Cu+ pair, i.e. from +0.5 to −0.5 V vs. SCE (Fig. S31, ESI†). The CV of the unbound Cu2+ was acquired using the same experimental conditions: two poorly separated cathodic peaks, followed by an anodic stripping peak in the backward scan were observed (Fig. S32, ESI†). Stepwise reduction of Cu2+ to electrodeposited Cu0 occurs in the forward scan, while the latter is anodically stripped from the electrode upon scan reversal.
Representative cyclic voltammograms of the Cu complexes with TRI4S and TE4S acquired at physiological pH and different scan rates are shown in Fig. 5, while the electrochemical properties of their Cu complexes are listed in Table 4.
The copper complexes of both ligands displayed a redox process ascribed to a quasi-reversible one-electron reduction of Cu2+ to Cu+ (Fig. 5) at E1/2, Cu-TRI4S = −0.223 ± 0.003 V vs. SCE and E1/2, Cu-TE4S = −0.170 ± 0.003 vs. SCE. The peak separation (ΔEp) was higher than the canonical 60 mV for Nernstian electron transfer (ET) processes (Table 4), indicating that the ET are quasi-reversible. The voltammetric pattern was unchanged with multiple reduction/oxidation cycles and at the different scan rates (Fig. 5): in both cases, a linearity between the intensity of the cathodic peak current (ipc) and the square root of the scan rate (v1/2) was found (Fig. S33, ESI†), indicating that the electrode process is under diffusion control.
The complex formed between Cu+ and both ligands at pH 7 was assumed to be CuL+ because the “first-generation” ligands form this complex under the same conditions.27 This was further confirmed from cyclic voltammograms acquired at different pH which do not show any pH-dependent variation of their pattern (Fig. S34, ESI†). The stability constants of each CuL+ complex were determined as described in our previous work.27 Briefly, a thermodynamic cycle was used, which involved the stability constant of the Cu2+ complex (Cu2+L), and the standard potential (E0) for the Cu2+ + e− → Cu+ and Cu2+L + e− → Cu+L semi-reactions (assuming that the experimental E1/2 values approximate the E0). The corresponding free Gibbs energies were summed to obtain the unknown stability constant of Cu+L. The results are detailed in Table 2 and the corresponding distribution diagrams are shown in Fig. 2.
The calculated pCu+ values (pCu+ = −log[Cu+]free) reported in Table 2 indicate that the increase of the ring size from a 12-(DO4S) to a 13-(TRI4S) and then to a 14-member macrocycle (TE4S) corresponds to a progressive decrease of the Cu+ complexes stability, likewise to what was found for the +2 oxidation state (Table 2). It can also be observed that the pCu+ for DO4S is considerably higher than the pAg+ determined in our previous work28 (17.2 vs. 14.5), and preliminary data about silver complexation (in due course in our laboratories) indicate that a similar trend exists also for TRI4S and TE4S. It is known that polyamines coordinate Cu+ more strongly than Ag+: for example, Cu+ forms complexes in aqueous solution with both ethylenediamine and triethylenetetramine which are three orders of magnitude more stable than those formed by Ag+.52 This stability difference appears to be retained by our ligands, thus indicating that the thioethers in the coordination sphere do not show specific preference for either Cu+ or Ag+.
The reversibility of the CV process suggests that all electrogenerated cuprous complexes are stable and do not dissociate during the CV timescale. The long-term stability of the CuL+ complexes was then further confirmed by controlled-potential electrolysis of the Cu2+ complexes. Linear-scan voltammetry (LSV) was employed to monitor the reduction processes (Fig. S35, ESI†). It was evidenced that the CuL+ complexes formed by both ligands remained stable at least for some hours after their in situ formation by CuL2+ reduction. These results demonstrate the ability of TRI4S and TE4S to adapt to both the Cu2+ and Cu+ coordination requirements, analogously to what we have previously found with DO4S.27 A donor switching can be expected upon copper reduction, as reported for a number of similar polyazamacrocycles.53
The CV data also allow us to obtain information on the ability of the investigated complexes to resist the demetallation process that could be induced in vivo by the biologically triggered redox switching between Cu2+ and Cu+. The standard reduction potentials of the Cu2+ complexes were calculated as E1/2 = (Epc + Epa)/2 and assuming that E1/2 = E0. The reduction potentials for Cu2+–TRI4S and Cu2+–TE4S (Table 4) are higher than the estimated potential threshold for typical bioreductants (E0 = −0.64 V vs. SCE), which indicates that they would be vulnerable to in vivo reduction.27,54 Indeed, the short- and the long-term stability observed in the voltammetric and electrolytic measurements suggests that the resulting Cu+ complex would not undergo demetallation and so the copper would remain anchored to the radiopharmaceutical.
Thanks to their long-term stability, the Cu+–TRI4S and Cu+–TE4S solutions obtained after electrolysis at pH ∼ 7 and ∼ 8, respectively, were characterized by 1H-NMR measurements. 1H NMR spectra of the Cu+ complexes, compared with the spectra of the free ligands, are reported in Fig. 6. The signals assignment is presented in Table S11 (ESI†), supported by the TOCSY spectra in the case of Cu+–TE4S (Fig. S36, ESI†).
The significant changes in chemical shift and coupling pattern observed among the spectra of the free ligands and those of the Cu+ complexes undoubtedly confirm the complexation event. All the signals experience a downfield shift upon complexation (more pronounced in the case of Cu+–TRI4S) likely because of the electron density donation from the ligand to the metal ion, thereby suggesting that all the donors are interacting on average with Cu+.
For Cu+–TRI4S, the SCH3 protons of the side chains resonate as two narrow singlets with the same intensity at 2.50 and 2.55 ppm (Table S11, ESI†). This, combined with the observed downfield shift, is consistent with the involvement of the S donors in the coordination of Cu+. Their two-by-two equivalence likely reflects the intrinsic asymmetry of the ligand.
For Cu+–TE4S, the methyl groups of the side chains (SCH3) resonate as a singlet at 2.29 ppm, thus indicating that also in this case the S atoms are involved in the coordination sphere of the metal ion. For both ligands, the S donors can be simultaneously bound to the metal ions, or in rapid exchange with respect to the NMR timescale.
The signals of the SCH2 and NCH2 protons of the side chains and the ring resonate as non-resolved multiplets in both cases (Table S11, ESI†). The main difference between the NMR spectra of Cu+–TRI4S and Cu+–TE4S is that the latter has broader signals, suggesting that the Cu+–TE4S complex is more fluxional and/or its conformational equilibria are slower.
The multiplet attributed to the methylene protons of the propylenic chain of the ring is split into two broad signals of equal relative integral only when Cu+ is coordinated by TRI4S; these two signals have been attributed to the axial and equatorial protons of the same molecule, which for conformational constraint become non-magnetically equivalent.
Conclusions
The stabilization of coordinatively labile and redox-active copper ions in biological environments remains a challenge for the development of improved diagnostic and therapeutic strategies with [64/67Cu]Cu2+, as the in vivo integrity of these complexes could be thwarted by the bio-induced reduction of Cu2+ to Cu+ that may result in demetallation processes. The N-functionalized cyclen derivatives listed in Fig. 1, bearing borderline N and soft S donors, were considered in our previous work in an attempt to stabilize both oxidation states.27Subtle changes in the ligand structure and donor arms can cause drastic changes to the stability of their radiometal complexes, so that the effects induced by the modification of the azamacrocyclic ring on the physicochemical properties of the corresponding Cu2+ and Cu+ complexes were investigated herein. As a consequence of the larger ring size with respect to the previously developed 12-member sulfanyl analogue (DO4S), TRI4S and TE4S present a faster complexation process than DO4S, which is a feature of particular interest for radiopharmaceutical applications. The stability of the Cu2+ complexes demonstrated that the increase of one carbon in the azamacrocyclic ring has a negligible influence, as TRI4S and DO4S form complexes of comparable stability. However, a further increase in the ring size leading to TE4S results in a noticeable drop in the stability constants and the corresponding pCu2+ values. The same trend was observed as regards their inertness towards acid-mediated decomplexations and the stability of their Cu+ complexes. The number of nitrogen donors has a major influence on the stability of the Cu2+ complexes, at least for ring sizes larger than or equal to 12, as TACD3S was not able to strongly stabilize the metal cation.
On the other hand, the changing ring size did not affect the exceptional inertness of the copper complexes in reductive media as revealed by cyclic voltammetry and electrolysis experiments. While the E1/2 still make these Cu2+-complexes susceptible to in vivo reduction, no subsequent Cu+ loss should occur.
Radiolabelling, in vitro and in vivo experiments, although out of the scope of the present paper, will be necessary in order to validate the potential of these ligands for future radiopharmaceutical applications.
Author contributions
Marianna Tosato: conceptualization, formal analysis, investigation, methodology, writing – original draft, writing – review & editing. Matteo Pelosato: investigation (potentiometry, UV-Vis, NMR, cyclic voltammetry). Sara Franchi: investigation (potentiometry, UV-Vis). Abdirisak Ahmed Isse: investigation (cyclic voltammetry). Nóra Veronica May: investigation (EPR), funding acquisition. Giordano Zanoni: investigation (synthesis). Fabrizio Mancin: investigation (synthesis). Paolo Pastore: formal analysis (cyclic voltammetry), funding acquisition, resources. Denis Badocco: formal analysis (cyclic voltammetry). Mattia Asti: supervision, formal analysis. Valerio Di Marco: supervision, data curation, formal analysis, methodology, project administration, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research work was supported by the ISOLPHARM_EIRA project funded by the Legnaro National Laboratories of the Italian Institute of Nuclear Physics (Istituto Nazionale di Fisica Nucleare – Laboratori Nazionali di Legnaro, INFN-LNL, Italy) and by the National Research, Development and Innovation Office-NKFIA (Hungary) through project K124544.References
- A. Yordanova, E. Eppard, S. Kürpig, R. A. Bundschuh, S. Schönberger, M. Gonzalez-Carmona, G. Feldmann, H. Ahmadzadehfar and M. Essler, OncoTargets Ther., 2019, 10, 4821–4828 CrossRef PubMed.
- E. Boros, J. F. Cawthray, C. L. Ferreira, B. O. Patrick, M. J. Adam and C. Orvig, Inorg. Chem., 2012, 51, 6279–6284 CrossRef CAS PubMed.
- N. M. Di Bartolo, A. M. Sargeson, T. M. Donlevya and S. V. Smith, J. Chem. Soc., Dalton Trans., 2001, 2303–2309 RSC.
- M. Shokeen and C. J. Anderson, Acc. Chem. Res., 2009, 42, 832–841 CrossRef CAS PubMed.
- E. Boros and A. B. Packard, Chem. Rev., 2019, 119, 870–901 CrossRef CAS PubMed.
- F. Borgna, M. Ballan, C. Favaretto, M. Verona, M. Tosato, M. Caeran, S. Corradetti, A. Andrighetto, V. Di Marco, G. Marzaro and N. Realdon, Molecules, 2018, 23, 2437 CrossRef PubMed.
- G. Hao, T. Mastren, W. Silvers, G. Hassan, O. K. Öz and X. Sun, Sci. Rep., 2021, 11, 3622 CrossRef CAS PubMed.
- I. Carbo-Bague and C. F. Ramogida, in Encyclopedia of Inorganic and Bioinorganic Chemistry, R. A. Scott., 2021 Search PubMed.
- C. Ramogida and C. Orvig, Chem. Commun., 2013, 49, 4720–4739 RSC.
- T. I. Kostelnik and C. Orvig, Chem. Rev., 2019, 119, 902–956 CrossRef CAS PubMed.
- E. W. Price and C. Orvig, Inorg. Chem., 2014, 43, 260–290 CAS.
- K. S. Woodin, K. J. Heroux, C. A. Boswell, E. H. Wong, G. R. Weisman, W. Niu, S. A. Tomellini, C. J. Anderson, L. N. Zakharov and A. L. Rheingold, Eur. J. Inorg. Chem., 2005, 4829–4933 CrossRef CAS.
- A. Guillou, L. M. P. Lima, M. Roger, D. Esteban-Gomez, R. Delgado, C. Platas-Iglesias, V. Patinec and R. Tripier, Eur. J. Inorg. Chem., 2017, 2435–2443 CrossRef CAS.
- S. Bhattacharyya and M. Dixit, Dalton Trans., 2011, 40, 6112–6128 RSC.
- N. Camus, N. Le Bris, S. Nuryyeva, M. Chessé, D. Esteban-Gómez, C. Platas-Iglesias, R. Tripier and M. Elhabiri, Dalton Trans., 2017, 46, 11479–11490 RSC.
- M. Le Fur, M. Beyler, N. Le Poul, L. M. P. Lima, Y. Le Mest, R. Delgado, C. P. Iglesias, V. Patinec and R. Tripier, Dalton Trans., 2016, 45, 7406–7420 RSC.
- E. Bodio, M. Boujtita, K. Julienne, P. Le Saec, S. G. Gouin, J. Hamon, E. Renault and D. Deniaud, ChemPlusChem, 2014, 79, 1284–1293 CrossRef CAS.
- S. Shuvaev, E. A. Suturina, N. J. Rotile, C. A. Astashkin, C. J. Ziegler, A. W. Ross, T. L. Walker, P. Caravan and I. S. Taschner, Dalton Trans., 2020, 49, 14088–14098 RSC.
- M. S. Cooper, M. T. Ma, K. Sunassee, K. P. Shaw, J. D. Williams, R. L. Paul, P. S. Donnelly and P. J. Blower, Bioconjugate Chem., 2012, 23, 1029–1039 CrossRef CAS PubMed.
- L. M. Lima, D. Esteban-Gomez, R. Delgado, C. Platas-Iglesias and R. Tripier, Inorg. Chem., 2012, 51, 6916–6927 CrossRef CAS PubMed.
- R. Gillet, A. Roux, J. Brandel, S. Huclier-Markai, F. Camerel, O. Jeannin, A. M. Nonat and L. J. Charbonniere, Inorg. Chem., 2017, 56, 11738–11752 CrossRef CAS PubMed.
- E. Boros, E. Rybak-Akimova, J. P. Holland, T. Rietz, N. Rotile, F. Blasi, H. Day, R. Latifi and P. Caravan, Mol. Pharmaceutics, 2014, 11, 617–629 CrossRef CAS PubMed.
- T. J. Wadas, E. H. Wong, G. R. Weisman and C. J. Anderson, Curr. Pharm. Des., 2007, 13, 3–16 CrossRef CAS PubMed.
- D. J. Stigers, R. Ferdani, G. R. Weisman, E. H. Wong, C. J. Anderson, J. A. Golen, C. Moore and A. L. Rheingold, Dalton Trans., 2010, 39, 1699–1701 RSC.
- C. Gotzmann, F. Braun and M. D. Bartholomä, RSC Adv., 2016, 6, 119–131 RSC.
- R. Ševčík, J. Vaněk, R. Michalicová, P. Lubal, P. Hermann, I. C. Santos, I. Santos and M. P. C. Campello, Dalton Trans., 2016, 45, 12723–12733 RSC.
- M. Tosato, M. Dalla Tiezza, N. V. May, A. A. Isse, S. Nardella, L. Orian, M. Verona, C. Vaccarin, A. Alker, H. Mäcke, P. Pastore and V. Di Marco, Inorgan. Chem., 2021, 60, 11530–11547 CrossRef CAS PubMed.
- M. Tosato, M. Verona, R. Doro, M. Dalla Tiezza, L. Orian, A. Andrighetto, P. Pastore, G. Marzaro and V. Di Marco, New J. Chem., 2020, 44, 8337–8350 RSC.
- C. L. Schmid, M. Neuburger, M. Zehnder, T. A. Kaden, K. Bujno and R. Bilewicz, Helv. Chim. Acta, 1997, 80, 241–252 CrossRef CAS.
- S. Lacerda, M. P. Campello, I. C. Santos, I. Santos and R. Delgado, Polyhedron, 2007, 26, 3763–3773 CrossRef CAS.
- R. Ševčíková, P. Lubal, M. P. C. Campello and I. Santos, Polyhedron, 2013, 62, 268–273 CrossRef.
- S. Lacerda, M. P. Campello, F. Marques, L. Gano, V. Kubıček, P. Fousková, É. Tóth and I. Santos, Dalton Trans., 2009, 4509–4518 RSC.
- R. Ma, M. J. Welch, J. Reibenspies and A. E. Martell, Inorg. Chim. Acta, 1995, 236, 75–82 CrossRef CAS.
- M. Tosato, M. Asti, M. Dalla Tiezza, L. Orian, D. Häussinger, R. Vogel, U. Köster, M. Jensen, A. Andrighetto, P. Pastore and V. Di Marco, Inorg. Chem., 2020, 59, 10907–10919 CrossRef CAS PubMed.
- T. L. Hwang and A. J. Shaka, J. Magn. Reson., Ser. A, 1995, 112, 275–279 CrossRef CAS.
- V. Di Marco, PhD thesis, University of Padova, 1998.
- L. G. Sillen, Acta Chem. Scand., 1964, 18, 1085 CrossRef.
- W. H. Press, B. P. Flannery, S. A. Teukolsky and W. T. Vetterling, Numerical Recipes: The Art of Scientific Computing, Cambridge University Press, 1986 Search PubMed.
- C. F. Baes Jr. and R. E. Mesmer, The Hydrolysis of cations, New York, 1976 Search PubMed.
- A. Rockenbauer and L. Korecz, Appl. Magn. Reson., 1996, 10, 29–43 CrossRef CAS.
- L. J. Zompa, Inorg. Chem., 1978, 17, 2531–2536 CrossRef CAS.
- M. Kodama and E. Kimura, J. Chem. Soc., Dalton Trans., 1976, 1720–1724 RSC.
- A. Bianchi, M. Micheloni and P. Paoletti, Coord. Chem. Rev., 1991, 110, 17–113 CrossRef CAS.
- B. S. Nakani, J. J. B. Welsh and R. D. Hancock, Inorg. Chem., 1983, 22, 2956–2958 CrossRef CAS.
- M. Tosato and V. Di Marco, Biomolecules, 2019, 9, 269 CrossRef CAS PubMed.
- J. Kotek, P. Lubal, P. Hermann, I. Císarová, I. Lukes, T. Godula, I. Svobodová, P. Táborský and J. Havel, Chem. – Eur. J., 2003, 9, 233–248 CrossRef CAS PubMed.
- H. Aneetha, Y. H. Lai, S. C. Lin, K. Panneerselvam, T. H. Lu and C. S. Chung, J. Chem. Soc., Dalton Trans., 1999, 2885–2892 RSC.
- A. V. Dale, G. I. An, D. N. Pandya, Y. S. Ha, N. Bhatt, N. Soni, H. Lee, H. Ahn, S. Sarkar, W. Lee, P. T. Huynh, J. Y. Kim, M. R. Gwon, S. H. Kim, J. G. Park, Y. R. Yoon and J. Yoo, Inorg. Chem., 2015, 54, 8177–8186 CrossRef CAS PubMed.
- V. Kubíček, Z. Böhmová, R. Ševčíková, J. Vaněk, P. Lubal, Z. Poláková, R. Michalicová, J. Kotek and P. Hermann, Inorg. Chem., 2018, 57, 3061–3072 CrossRef PubMed.
- M. Paúrová, T. David, I. Císařová, P. Lubal, P. Hermann and J. Kotek, New J. Chem., 2018, 42, 11908–11929 RSC.
- I. Voráčová, J. Vaněk, J. Pasulka, Z. Střelcová, P. Lubal and P. Hermann, Polyhedron, 2013, 61, 99–104 CrossRef.
- P. D. Bernardo, A. Melchior, R. Portanova, M. Tolazzi and P. L. Zanonato, Coord. Chem. Rev., 2008, 252, 1270–1285 CrossRef.
- D. B. Rorabacher, Chem. Rev., 2004, 104, 651–698 CrossRef CAS PubMed.
- A. Rodríguez-Rodríguez, Z. Halime, L. M. P. Lima, M. Beyler, D. Deniaud, N. Le Poul, R. Delgado, C. Platas-Iglesias, V. Patinec and R. Tripier, Inorg. Chem., 2016, 55, 619–632 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2nj01032a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2022 |