Rapid mass production of iron nickel oxalate nanorods for efficient oxygen evolution reaction catalysis†
Huixia
Hu‡
 ,
Xiang
Lei‡
,
Xiang
Lei‡
 *,
Shumei
Li
*,
Shumei
Li
 ,
Ruzhen
Peng
and
Jinliang
Wang
*
,
Ruzhen
Peng
and
Jinliang
Wang
*
Faculty of Materials Metallurgy and Chemistry, Jiangxi University of Science and Technology, Gan Zhou 341000, P. R. China. E-mail: xianglei108@sina.com
First published on 24th November 2021
Abstract
The NiFe layered-double-hydroxide (NiFe-LDH) and the NiFe metal–organic framework (NiFe-MOF) demonstrate the best catalytic activity among NiFe-based materials for the oxygen evolution reaction (OER), which is important for efficient hydrogen production. However, the preparation processes of these materials are usually cumbersome and have a low yield, which eliminates their most critical advantage of being low cost. We propose a method for rapidly and efficiently preparing porous (Ni0.5Fe0.5)C2O4 nanorods with an excellent OER catalytic performance. The overpotential of (Ni0.5Fe0.5)C2O4 is 266 mV at 20 mA cm−2 under alkaline conditions, and the Tafel slope is 54.39 mV dec−1. Furthermore, analysis of the changes in the surface properties of the material before and after catalysis determined that the real active material is (Ni0.5Fe0.5)(OH)x(C2O4)1−x. Using a simple scaled-up experiment, (Ni0.5Fe0.5)C2O4 is mass-produced (40 g) via direct synthesis in 5 min. The composition and performance of the mass-produced sample are analysed under the same conditions, and (Ni0.5Fe0.5)C2O4 still has a good catalytic performance and its composition has not changed. The efficient synthesis of (Ni0.5Fe0.5)C2O4 nanorods with a porous structure provides a new option for the development of commercial catalysts using non-precious metals.
1. Introduction
Hydrogen is supposed to be a potential replacement for traditional energy sources.1,2 However, 95% of the hydrogen industrially produced originates from fossil fuels, and the dependence on traditional energy sources has not been resolved.3,4 Currently, the cleanest method for producing hydrogen is via the electrolysis of water.5–7 The electrolysis of water comprises the oxygen evolution reaction (OER) and the hydrogen evolution reaction (HER).8,9 The OER involves four electrons and has a slow reaction rate, which slows down the overall kinetics of the electrolysis of water.10,11 The best catalysts for the OER are currently noble metal-based oxides such as RuO2 and IrO2.12,13 However, the high cost of the required precious metals limits the large-scale application of these catalysts.14 Thus, the development of a low-cost and efficient OER catalyst is a crucial task.An enormous amount of research in the field of OER catalysis has focused on NiFe-based materials owing to their low cost and excellent catalytic activity in alkaline environments.15–19 Among them, NiFe layered-double-hydroxide (NiFe-LDH) has been intensively studied for more than 30 years, and it represents a class of promising electrocatalysts for the OER.20–23 However, many studies on NiFe-LDH catalysts still use traditional preparation methods, such as solvothermal and electrodeposition methods.24 In recent years, many studies have started considering the NiFe metal–organic framework (NiFe-MOF) for OER catalysis.25,26 Unfortunately, most NiFe-MOF catalysts are produced under high-temperature and -pressure conditions with a low yield.27–29 Therefore, developing a simple, efficient and scalable preparation process for NiFe-based OER catalysts is crucial.
We developed a rapid and simple method for synthesising a new type of mixed NiFe oxalate nanorods (NRs). Experiments were performed to demonstrate the OER catalytic activity of the synthesised nanorods and the effectiveness of the proposed method even when increased to the mass-production scale This study presents a theoretical and experimental basis for effectively solving the bottleneck problem of the low synthesis efficiency for NiFe-based OER catalysts.
2. Experiment
2.1 Reagents
The chemical reagents used in the experiment were not further purified, and included N,N-dimethylacetamide (DMAC) (analytical reagent), oxalic acid (analytical reagent), ferrous chloride tetrahydrate (FeCl2·4H2O) (analytical reagent), nickel chloride hexahydrate (NiCl2·6H2O) (analytical reagent) and ruthenium oxide (RuO2) (Macklin). The water used in the experiment was deionized water.2.2 Synthesis of FeC2O4 (NiC2O4) NRs
Oxalic acid (10 mmol) was dissolved in DMAC as a solution A. FeCl2·4H2O (NiCl2·6H2O) (2 mmol) was dissolved in 25 mL deionized water as solution B. Solution B was slowly added to solution A with stirring for 5 min. The above solution was centrifuged with ethanol three times. The precipitate was dried in an oven at 60 °C and ground to obtain FeC2O4 (NiC2O4) nanorods (NRs).2.3 Synthesis of (Fe0.5Ni0.5)C2O4 NRs
Oxalic acid (10 mmol) was dissolved in DMAC as solution A. FeCl2·4H2O (1 mmol) and NiCl2·6H2O (1 mmol) were dissolved in 25 mL deionized water as solution B. Solution B was slowly added to solution A with stirring for 5 min. The above solution was centrifuged with ethanol three times. The precipitate was dried in an oven at 60 °C and ground to obtain (Fe0.5Ni0.5)C2O4 NRs.2.4 Synthesis of G-(Ni0.5Fe0.5)C2O4
Take 1 g of the prepared NiC2O4 and 1 g of the prepared FeC2O4 into a clean agate mortar, grind for 10 minutes, and name the sample G-(Ni0.5Fe0.5)C2O4.2.5 Synthesis of 40-(Ni0.5Fe0.5)C2O4
Oxalic acid (0.2 mol) was dissolved in DMAC as solution A. FeCl2·4H2O (20 mmol) and NiCl2·6H2O (20 mmol) were dissolved in 0.5 L deionized water as solution B. Solution B was slowly added to solution A with stirring for 5 min. The above solution was centrifuged with ethanol three times. The precipitate was dried in an oven at 60 °C and ground to obtain 40-(Fe0.5Ni0.5)C2O4 NRs.2.6 Electrochemical test
The catalytic activity of oxalate was studied using a CH Instruments Electrochemical Analyzer (CHI 760E). The classic three-electrode system was adopted in which a silver chloride electrode, a platinum wire and a foamed nickel (NF 2 mm × 2 mm see supporting documents for processing) were used as the reference electrode, the auxiliary electrode and the working electrode, respectively. The overpotential calculation formula was as follows: ERHE = EAg/AgCl + 0.197 + 0.0592pH. Linear sweep voltammograms (LSVs) were measured in 1 mol L−1 KOH saturated oxygen solution at a sweep speed of 5 mV s−1. The LSV measurements were iR compensated at 95%. Cyclic voltammetry (CV) was used to study the electrochemically active surface area (ECSA), and was carried out at different sweep speeds (20, 40, 60, 80, 100 and 120 mV s−1) when the voltage was 1.223–1.323 V (vs. RHE) in 1 mol L−1 KOH saturated oxygen solution. Electrochemical impedance spectroscopy (EIS) was recorded under 1. 6 V (vs. RHE) from 0.1 Hz to 100 kHz at the amplitude of the sinusoidal voltage of 5 mV. See supporting documents for all other detection methods.3. Results and discussion
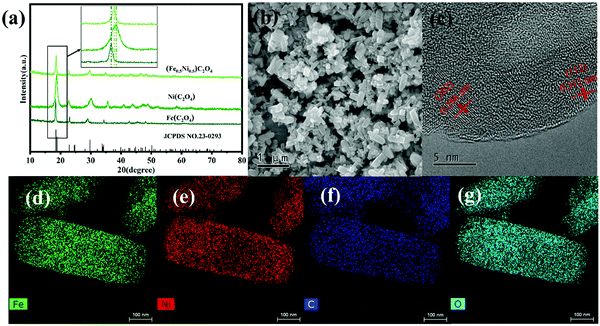
X-Ray diffraction (XRD) was used to analyse the composition of the oxalate. As shown in Fig. 1a, the diffraction peaks at 18.4°, 23.0° and 34.2° of the prepared FeC2O4 NRs corresponded well to the crystal plane facets (110), (−202) and (−121) of FeC2O4·H2O (JCPDS Card, No. 23-0293). Compared with the standard spectrum of FeC2O4·H2O, the diffraction peaks of the prepared NiC2O4 NRs were shifted to the right. This was attributed to the differences in the radii of Fe and Ni. Furthermore, the diffraction peaks of the (Fe0.5Ni0.5)C2O4 NRs at 18.5°, 29.5° and 34.8° were clearly located between the diffraction peaks of the FeC2O4 and NiC2O4 NRs. This result confirms that Fe and Ni existed in a certain proportion in the prepared binary metal oxalate NRs.Scanning electron microscopy (SEM) was used to analyse the morphology of the prepared samples. As shown in Fig. S2a, b (ESI†) and Fig. 1b, the prepared NiC2O4, FeC2O4 and (Fe0.5Ni0.5)C2O4 samples all exhibited a rod-like morphology. This was attributed to the small ionisation energy of metal ions in DMAC, which made the oxalate grow laterally.30 However, the samples had fairly different sizes. The length of the prepared NiC2O4 NRs was 100 nm, which is relatively short. Conversely, the length of the prepared FeC2O4 NRs was 5 μm. The prepared (Fe0.5Ni0.5)C2O4 NRs had a length between 5 and 100 μm. The microstructure and composition of the (Fe0.5Ni0.5)C2O4 NRs were studied via high-magnification transmission electron microscopy (TEM). Fig. S2d–f (ESI†) clearly show many pores on the surface of the (Fe0.5Ni0.5)C2O4 NRs. In addition, lattice fringes are clearly visible in Fig. 1c. The measured lattice spacings were 0.286 and 0.312 nm, which are similar to the lattice spacings of the FeC2O4 (202) and (112) crystal planes. The elemental distribution of the (Fe0.5Ni0.5)C2O4 NRs was confirmed through elemental mapping from energy-dispersive X-ray spectroscopy. As shown in Fig. 1d–g, Fe, Ni, C and O were uniformly distributed in the NRs. FeC2O4, NiC2O4 and (Fe0.5Ni0.5)C2O4 were analysed via their nitrogen adsorption method–desorption isotherm plots. From Fig. 2a–c, it can be clearly seen that the Brunauer–Emmett–Teller surface area and the pore volume of FeC2O4, NiC2O4 and (Fe0.5Ni0.5)C2O4 were 6.6581 m2 g−1, 63.0562 m2 g−1 and 15.2492 m2 g−1, and 0.015696 cm3 g−1, 0.193547 cm3 g−1 and 0.067713 cm3 g−1, respectively. These results reveal that NiC2O4 has the largest specific surface area, which is consistent with the conclusion obtained by SEM. This, in addition with the above XRD and TEM results, confirms that the (Fe0.5Ni0.5)C2O4 NRs exist as a single pure phase rather than two separate phases.
 | ||
| Fig. 2 (a–c) Adsorption–desorption isotherms of the FeC2O4, NiC2O4 and (Fe0.5Ni0.5)C2O4 samples, respectively. | ||
The catalytic activity of the sample was explored using the classic three-electrode system in 1 M KOH as the oxygen-saturated electrolyte. The linear sweep voltammetry (LSV) curves in Fig. 3a and Fig. S3 (ESI†) show that, at 50 mA cm−2, the overpotentials of (Fe0.75Ni0.25)C2O4, (Fe0.25Ni0.75)C2O4 (see ESI† for the preparation process), (Fe0.5Ni0.5)C2O4, NiC2O4, FeC2O4, and the blank nickel foam (NF) were 329, 315, 284, 365, 312, and 382 mV, respectively. Results when the ratio of Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni was 1
Ni was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 showed that the prepared samples had the best OER catalytic activity. (Fe0.5Ni0.5)C2O4, NiC2O4, FeC2O4 and NF had Tafel slopes of 54.39, 71.61, 61.42, and 71.36 mV dec−1, as presented in Fig. 3b. The (Fe0.5Ni0.5)C2O4 NRs had the smallest Tafel slope, which indicates that they can obtain a higher catalytic current density at the same overpotential. The electrochemically active surface area (ECSA) was determined via cyclic voltammetry (CV), as shown in Fig. 3c and Fig. S6a, b (ESI†). Fig. 3d shows that the double-layer capacitance values of (Fe0.5Ni0.5)C2O4, NiC2O4 and FeC2O4 were 13.88, 23.3 and 13.69 mF cm−2, respectively. The NiC2O4 NRs had the largest ECSA, because they had the smallest size. However, this result is inconsistent with the LSV curve, which indicates that the ECSA is not the primary cause for the good OER catalytic activity of (Fe0.5Ni0.5)C2O4. Electrochemical impedance spectroscopy (EIS) was used to measure the charge-transfer process, and the fitted data are shown in Fig. 3e. (Fe0.5Ni0.5)C2O4 had a much smaller radius than NiC2O4, which indicates that the (Fe0.5Ni0.5)C2O4 NRs had the least resistance to charge transfer. Therefore, promotion of the charge transfer is the main factor for the improved reaction kinetics of the OER. Stability is essential for catalyst materials. Thus, CV measurements were repeated to compare the catalytic activity after different cycles in 1 M KOH. As shown in Fig. 3f, minor attenuation occurred for (Ni0.5Fe0.5)C2O4 after 1000 cycles. The potential changes after 2000 and 3000 cycles were negligible. These results illustrate that the catalyst exhibited good stability after a long period of time without any significant change in current.
1 showed that the prepared samples had the best OER catalytic activity. (Fe0.5Ni0.5)C2O4, NiC2O4, FeC2O4 and NF had Tafel slopes of 54.39, 71.61, 61.42, and 71.36 mV dec−1, as presented in Fig. 3b. The (Fe0.5Ni0.5)C2O4 NRs had the smallest Tafel slope, which indicates that they can obtain a higher catalytic current density at the same overpotential. The electrochemically active surface area (ECSA) was determined via cyclic voltammetry (CV), as shown in Fig. 3c and Fig. S6a, b (ESI†). Fig. 3d shows that the double-layer capacitance values of (Fe0.5Ni0.5)C2O4, NiC2O4 and FeC2O4 were 13.88, 23.3 and 13.69 mF cm−2, respectively. The NiC2O4 NRs had the largest ECSA, because they had the smallest size. However, this result is inconsistent with the LSV curve, which indicates that the ECSA is not the primary cause for the good OER catalytic activity of (Fe0.5Ni0.5)C2O4. Electrochemical impedance spectroscopy (EIS) was used to measure the charge-transfer process, and the fitted data are shown in Fig. 3e. (Fe0.5Ni0.5)C2O4 had a much smaller radius than NiC2O4, which indicates that the (Fe0.5Ni0.5)C2O4 NRs had the least resistance to charge transfer. Therefore, promotion of the charge transfer is the main factor for the improved reaction kinetics of the OER. Stability is essential for catalyst materials. Thus, CV measurements were repeated to compare the catalytic activity after different cycles in 1 M KOH. As shown in Fig. 3f, minor attenuation occurred for (Ni0.5Fe0.5)C2O4 after 1000 cycles. The potential changes after 2000 and 3000 cycles were negligible. These results illustrate that the catalyst exhibited good stability after a long period of time without any significant change in current.
To clarify the catalytic mechanism and specific active substances, we used XPS to analyse the chemical composition and state of the (Ni0.5Fe0.5)C2O4 sample before and after OER catalytic process. The XPS spectrum of C1s (Fig. 4a) indicated that the peak intensity of C2O42− (288.7 eV) in the sample greatly weakened after catalysis but did not disappear completely.32,33 The XPS spectrum of O1s (Fig. 4b) showed only one O1s peak belonging to C2O42− (532.1 eV) before the OER measurements. However, after the OER measurements, the C2O42− peak was obviously weakened and the strongest peak belonged to O–H (531.1 eV).31–33 The Fe2p spectrum (Fig. 4c) showed that the main peak of Fe2p3/2 shifted by 1.2 eV after the catalytic process. Before the OER measurements, the peaks at 710.4 and 714.8 eV corresponded to Fe2p3/2. After the OER measurements, the peak at 711.6 eV and 716.6 eV corresponded to Fe2p3/2 of Fe(OH)2 and the peak 724.9 eV and 733.07 eV corresponded to Fe2p1/2 of Fe(OH)234,35 The spectrum of Ni2p (Fig. 4d) showed a prepeak (853.7 eV) and a main peak (856.7 eV) of Ni2+ in Ni2p3/2 before the OER measurements. After the OER measurements, the main peak of Ni2p shifted to the right by 1.1 eV. The peaks at 855.6 eV and 861.6 eV corresponded to Ni2p3/2 of Ni(OH)2. The peaks at 873.3 eV and 880.2 eV corresponded to Ni2p1/2 of Ni(OH)2.36–38 The prepeak disappeared because Ni changed from oxalate to hydroxide. The above results indicate that most of the (Ni0.5Fe0.5)C2O4 NRs immersed in alkaline solution were transformed into the corresponding hydroxide. To further study the phase transformation process, the G-(Ni0.5Fe0.5)C2O4 catalytic performance and phase transition before and after catalysis were studied. The LSV curve of G-(Ni0.5Fe0.5)C2O4 is shown in Fig. S7a (ESI†). The overpotential was 291 mV at 50 mA cm−2, and the Tafel slope was 59.62 mV dec−1. This is comparable to the catalytic performance of the prepared (Ni0.5Fe0.5)C2O4. Significantly, the XPS spectrum of G-(Ni0.5Fe0.5)C2O4 after the OER measurements (Fig. S4, ESI†) was completely consistent with that of the prepared (Ni0.5Fe0.5)C2O4. Furthermore, the results indicated that the real active substance was (Ni0.5Fe0.5)(OH)x(C2O4)1−x.
Therefore, we believe that the surface of the (Ni0.5Fe0.5)C2O4 NRs first dissolves in the KOH solution and is then deposited to form the active substance of (Ni0.5Fe0.5)(OH)x(C2O4)1−x. The structural transformation is shown in Fig. 4e. The reaction formulae are as follows:
| ½NiC2O4 + ½FeC2O4 + xOH + 2xe− → (Ni0.5Fe0.5)(OH)x(C2O4)1−x + xC2O42− (Ni0.5Fe0.5)C2O4 + xOH + 2xe− → (Ni0.5Fe0.5)(OH)x(C2O4)1−x + xC2O42− |
As shown in Fig. S8 (ESI†), we conducted a mass production experiment and obtained 40 g of (Ni0.5Fe0.5)C2O4. The composition, morphology and catalytic activity were analysed, and the XRD, SEM and XPS results are shown in Fig. S1a, S2c and S5 (ESI†), respectively. The mass production experiment had no effect on the morphology, composition and surface properties of the catalyst. Fig. S6c–e and S7a–c (ESI†) show the LSV curve and EIS measurement results. The overpotential of the sample was 278 mV at 50 mA cm−2, and the Tafel slope was 51.92 mV dec−1. As shown in Fig. S5d and e (ESI†), the double-layer capacitance was 12.55 mF cm−2. The catalytic performance also did not change significantly. Therefore, the proposed method is suitable for the rapid mass production of (Ni0.5Fe0.5)C2O4 as an efficient OER catalyst. In addition, as shown in Fig. S8 (ESI†), we compared the OER catalytic activity of (Ni0.5Fe0.5)C2O4 with the recently reported FeNi-based catalyst. It can be concluded that the preparation method of (Ni0.5Fe0.5)C2O4 is not only simple, but also has the same performance as other FeNi-based catalysts.39–43
4. Conclusion
We developed a simple and rapid co-precipitation process to prepare (Ni0.5Fe0.5)(C2O4) with a uniform morphology. Electrochemical tests confirmed that the (Ni0.5Fe0.5)(C2O4) NRs exhibited an excellent OER catalytic performance and stability.44,45 We analysed the phase transition before and after the catalytic process and confirmed that the real active material is (Ni0.5Fe0.5)(C2O4)1−x(OH)x. We conducted mass production experiments and analysed the morphology, composition and catalytic activity of the products. Results showed that the mass production experiment does not affect the performance of the catalyst. The developed method and catalyst should be helpful for realising the large-scale production and application of NiFe-based OER catalysts.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Educational Commission of Jiangxi Province of China (GJJ180470 and GJJ190431) and PhD Research Startup Funding of Jiangxi University of Science and Technology (jxxjbs18062). Jiangxi Provincial Key Laboratory of Flash Green Development and Recycling (20193BCD40019).References
- M. F. Lagadec and A. Grimaud, Nat. Mater., 2020, 19, 1140–1150 CrossRef CAS PubMed.
- K. P. Brooks, S. J. Sprik, D. A. Tamburello and M. J. Thornton, Int. J. Hydrogen Energy, 2020, 45, 24917–24927 CrossRef CAS.
- M. Kroschel, A. Bonakdarpour, J. T. H. Kwan, P. Strasser and D. P. Wilkinson, Electrochim. Acta, 2019, 317, 722–736 CrossRef CAS.
- S. E. Hosseini and M. A. Wahid, Renewable Sustainable Energy Rev., 2016, 57, 850–866 CrossRef CAS.
- F. Dionigi, Z. Zeng, I. Sinev, T. Merzdorf, S. Deshpande, M. B. Lopez, S. Kunze, I. Zegkinoglou, H. Sarodnik and D. Fan, Nat. Commun., 2020, 11, 1–10 Search PubMed.
- M. Zha, C. Pei, Q. Wang, G. Hu and L. Feng, J. Energy Chem., 2020, 47, 166–171 CrossRef.
- Y. Sun, H. Liao, J. Wang, B. Chen, S. Sun, S. J. H. Xi, S. Ong, C. Diao, Y. Du and J. Wang, Nat. Catal., 2020, 3, 554–563 CrossRef CAS.
- H. Liu, Z. Yan, X. Chen, J. Li, L. Zhang, F. Liu, G. Fan and F. Cheng, Research, 2020, 2020, 1–11 Search PubMed.
- S. Li, W. Chen, H. Pan, Y. Cao, Z. Jiang, X. Tian, X. Hao, T. Maiyalagan and Z. Jiang, ACS Sustainable Chem. Eng., 2019, 7, 8530–8541 CrossRef CAS.
- L. Wang, D. Kong, T. Xin, X. Shu, K. Zheng, L. Xiao, X. Sha, Y. Lu, Z. Zhang and X. Han, Appl. Phys. Lett., 2016, 108, 151903 CrossRef.
- X. Zhu, T. Jin, C. Tian, C. Lu, X. Liu, M. Zeng, X. Zhuang, S. Yang, L. He and H. Liu, Adv. Mater., 2017, 29, 1704091 CrossRef PubMed.
- X. Liu, F. Liu, J. Yu, G. Xiong, L. Zhao, Y. Sang, S. Zuo, J. Zhang, H. Liu and W. Zhou, Adv. Sci., 2020, 7, 2001526 CrossRef CAS PubMed.
- Y. Qu, B. Chen, Z. Li, X. Duan, L. Wang, Y. Lin, T. Yuan, F. Zhou, Y. Hu and Z. Yang, J. Am. Chem. Soc., 2019, 141, 4505–4509 CrossRef CAS PubMed.
- S. Ghosh and R. N. Basu, Nanoscale, 2018, 10, 11241–11280 RSC.
- H. Liu, J. Guan, S. Yang, Y. Yu, R. Shao, Z. Zhang, M. Dou, F. Wang and Q. Xu, Adv. Mater., 2020, 32, 2003649 CrossRef CAS PubMed.
- M. L. Lindstrom, R. Gakhar, K. Raja and D. Chidambaram, J. Electrochem. Soc., 2020, 167, 46507 CrossRef CAS.
- L. Wu, G. Wan, N. Hu, Z. He, S. Shi, Y. Suo, K. Wang, X. Xu, Y. Tang and G. Wang, Nanomaterials, 2018, 8, 451 CrossRef PubMed.
- S. Zhao, Y. Wang, J. Dong, C. He, H. Yin, P. An, K. Zhao, X. Zhang, C. Gao and L. Zhang, Nat. Energy, 2016, 1, 1–10 Search PubMed.
- M. Luo, C. Zhao, C. Wang, Y. Bi, Q. Li, Y. Hao, L. Li, Y. Kuang, Y. Li and X. Lei, Nano Res., 2017, 10, 1732–1739 CrossRef CAS.
- Z. Cai, X. Bu, P. Wang, W. Su, R. Wei, J. C. Ho, J. Yang and X. Wang, J. Mater. Chem. A, 2019, 7, 21722–21729 RSC.
- W. Zhang, J. Qi, K. Liu and R. Cao, Adv. Energy Mater., 2016, 6, 1502489 CrossRef.
- S. Dutta, A. Indra, Y. Feng, T. Song and U. Paik, ACS Appl. Mater. Interfaces, 2017, 9, 33766–33774 CrossRef CAS PubMed.
- Y. Wang, S. Tao, H. Lin, G. Wang, K. Zhao, R. Cai, K. Tao, C. Zhang, M. Sun and J. Hu, Nano Energy, 2021, 81, 105606 CrossRef CAS.
- H. H. Sun, A. Dolocan, J. A. Weeks, A. Heller and C. B. Mullins, ACS Nano, 2020, 14, 17142–17150 CrossRef CAS PubMed.
- S. Xu, J. Du, J. Li, L. Sun and F. Li, J. Mater. Chem. A, 2020, 8, 16908–16912 RSC.
- J. Du, S. Xu, L. Sun and F. Li, Chem. Commun., 2019, 55, 14773–14776 RSC.
- H. Jia, Y. Yao, J. Zhao, Y. Gao, Z. Luo and P. Du, J. Mater. Chem. A, 2018, 6, 1188–1195 RSC.
- L. Gan, J. Fang, K. Zhang, Y. Lai and J. Li, Int. J. Hydrog. Energy, 2019, 44, 2841–2847 CrossRef CAS.
- H. Sun, Y. Lian, C. Yang, L. Xiong, P. Qi, Q. Mu, X. Zhao, J. Guo, Z. Deng and Y. Peng, Energy Environ. Sci., 2018, 11, 2363–2371 RSC.
- W. A. Ang, N. Gupta, R. Prasanth and S. Madhavi, ACS Appl. Mater. Interfaces, 2012, 4, 7011–7019 CrossRef CAS PubMed.
- X. Gao, D. Chen, J. Qi, F. Li, Y. Song, W. Zhang and R. Cao, Small, 2019, 15, 1904579 CrossRef CAS PubMed.
- A. M. Salvi, F. Langerame, A. E. Pace, M. E. E. Carbone and R. Ciriello, Surf. Sci. Spectra, 2015, 22, 21–31 CrossRef CAS.
- V. Hasija, P. Raizada, A. Hosseini-Bandegharaei, P. Singh and V. Nguyen, Top. Catal., 2020, 63, 1030–1045 CrossRef CAS.
- B. M. Hunter, W. Hieringer, J. R. Winkler, H. B. Gray and A. M. Müller, Energy Environ. Sci., 2016, 9, 1734–1743 RSC.
- Z. Zhang, B. He, L. Chen, H. Wang, R. Wang, L. Zhao and Y. Gong, ACS Appl. Mater. Interfaces, 2018, 10, 38032–38041 CrossRef CAS PubMed.
- W. Zhang, J. Qi, K. Liu and R. Cao, Adv. Energy Mater., 2016, 6, 1502489 CrossRef.
- Y. Zhou and N. López, ACS Catal., 2020, 10, 6254–6261 CrossRef CAS.
- M. C. Biesinger, B. P. Payne, L. W. M. Lau, A. Gerson and R. S. C. Smart, Surf. Interface Anal., 2009, 41, 324–332 CrossRef CAS.
- J. W. Zhang, H. Zhang, T. Z. Ren, Z. Y. Yuan and T. J. Bandosz, Front. Chem. Sci. Eng., 2020, 15, 3 Search PubMed.
- X. Xie, C. Cao, W. Wei, S. Zhou and Q. L. Zhu, Nanoscale, 2020, 12, 10 Search PubMed.
- L. Cao, Z. Li, K. Su, M. Zhang and B. Cheng, J. Energy Chem., 2020, 54, 143 Search PubMed.
- X. Gu, Z. Liu, M. Li, J. Tian and L. Feng, Appl. Catal., B, 2021, 297, 120462 CrossRef CAS.
- Y. Liu, G. Jia, Q. Wu, D. Zhang and X. Cui, Mater. Lett., 2021, 291, 129564 CrossRef CAS.
- X. Jia, Y. Zhao, G. Chen, L. Shang, R. Shi, X. Kang, G. I. N. Waterhouse, L. Z. Wu, C. H. Tung and T. Zhang, Adv. Energy Mater., 2016, 6, 1502585 CrossRef.
- Q. Wang, L. Shang, R. Shi, X. Zhang, Y. Zhao, G. I. N. Waterhouse, L. Z. Wu, C. H. Tung and T. Zhang, Adv. Energy Mater., 2017, 7, 1700461 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1nj04668c |
| ‡ These two authors are contributed equally to this work. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2022 |