A novel rapid synthesis of highly stable silver nanoparticle/carbon quantum dot nanocomposites derived from low-grade coal feedstock†
Monikankana
Saikia
ab,
Tonkeswar
Das
a and
Binoy K
Saikia
 *ab
*ab
aCoal & Energy Group, Materials Science and Technology Division, CSIR-North East Institute of Science & Technology, Jorhat 785006, India. E-mail: bksaikia@neist.res.in; bksaikia@gmail.com
bAcademy of Scientific & Innovative Research (AcSIR), Ghaziabad 201002, India
First published on 16th November 2021
Abstract
Silver nanoparticles/nanocomposites are gaining popularity for their unique features and wide-range of applications including bactericidal behaviour, sensing, and photocatalysis. In this report, a novel, single-step, sustainable, and low-cost fabrication of silver carbon quantum dot (Ag/CQD) nanocomposites by using coal-derived carbon quantum dots (CQDs) as a reductant and stabilizer having great potential for simple and quick synthesis of silver nanocomposites has been documented for the first time in this paper. The HRTEM image of the Ag/CQD nanocomposite depicts the crystalline nature with two distinct d-spacings of 0.22 and 0.24 nm attributed to the (100) and (110) in-plane of the graphitic core or aromatic carbon and d-spacings of 0.24 and 0.27 nm were also observed which are attributed to the (111) and (122) lattice planes of Ag. The size distribution histogram derived from the TEM image indicates the particles to be within 2–12 nm in diameter, complying with the Gaussian distribution. SAED patterns of the Ag/CQD nanocomposite showed concentric diffraction rings with the (111), (200), (220), and (311) lattice planes of the fcc-structured silver metal, which indicates the composition of silver nanoparticles. From the XPS deconvoluted Ag 3d spectrum of the nanocomposites, the binding energies of Ag 3d5/2 and Ag 3d3/2 at 367 and 373 eV can be observed, which indicates that the silver is present in the metallic zero valent [Ag(0)] oxidation state in the nanocomposite. Moreover, UV-vis spectra show a major absorption peak at 422.47 nm that contributes to surface plasmon resonance of the Ag/CQD nanocomposite. The nanocomposite shows a zeta potential of −9.91 mV, signifying their high stability. The effect of base and concentrations of the CQDs in the synthesis of Ag/CQD nanocomposites has also been studied. Oxygen possessing functional groups present on the CQD surface were observed to play a significant role in the reduction of Ag/CQD nanocomposites. In addition, the nanocomposite has promising bactericidal behaviour for both Gram-positive and -negative bacterial strains for application as an antibacterial material. The Ag/CQD nanocomposite shows a maximum inhibition against bacterial strain Rhodococcus soli with an inhibition zone diameter of 17 mm as compared to the other strains.
Introduction
Metallic nanoparticles/nanocomposites are intriguing as they exhibit significant properties that have made them gain much interest in various fields of applications like antibacterial, antimicrobial, catalysts, bio-sensors, opto-electronics, and improvements in medical sciences.1,2 The physical features of metal nanoparticles in the nanometre-scale differ from those of the particle and the bulk material. This may be on account of the larger surface to volume ratio of the nanoparticles showing both fascinating and unpredictable features.3 Owing to their unique features, there is an increasing interest regarding the development of novel techniques for simple and single-step synthesis of nanoparticles/nanocomposites.Silver nanoparticles (AgNPs) have been extensively investigated due to their exceptional physical, chemical, and biological features. Their nanostructures are intensely impacted by their size, structure, and nature.4 AgNPs have a wide variety of applications in optical sensors, in bio-labelling, as catalysts in chemical reactions, as drug carriers, as antibacterial and anticancer agents, as coating for trapping solar energy, as optical acceptors in electrical cells, as diagnostics in healthcare-products, as coating in medical devices, in bio-medical devices, and in water treatment plants.5,6 The antimicrobial activity of Ag is much better than that of other metals like Cu, Cr, Hg, Pb, and Sn.7 To meet the demand of AgNPs, several chemical and physical techniques have been developed such as electrochemical reduction,8 chemical reduction,9,10 photochemical reduction,11,12 and heat evaporation.13 AgNPs are commonly synthesized by reducing Ag+ ions using appropriate reducing agents like NaBH4, NaNH2, hydrazine hydrate, ascorbic acid, glucose, dextrose, sodium citrate, ethylene glycol, N-dimethylformamide (DMF), chitosan and several other plant extracts.2,14–16 Pyatenko et al.13 fabricated monodispersed and homogeneous silver colloids by utilizing an excess of a powerful oxidising agent such as sodium borohydride to attain control over the structure and morphology. As a result, the procedure becomes complex and toxic; thus it should be fascinating to create CQD/AgNP nanocomposites using a simple and environmentally acceptable method. On the other hand, the nanoparticles get quickly agglomerated if no or poor stabilising agents are utilised, consequently worsening their antibacterial properties. Therefore, some unique stabilizers and reductants such as carbon quantum dots are necessary to fix this issue. Jin et al.17 utilised carbon nanodots as a stabilizing and reducing agent for the fabrication of red emitting AgNCs at a temperature of 80 °C in ethanol. Liu et al.18 synthesized silver hybrid nanoclusters supported on carbon dots that show notable electrocatalytic behaviour regarding oxygen reduction. Moreover, Ju et al.,19 prepared Ag NPs–N–graphene quantum dot nanacomposites through a photocatalytic strategy which behave as a colorimetric sensor for the detection of glutathione. Besides silver nanocomposites, Pd–C hybrid nanocomposites were also fabricated using carbon nanodots that provide active surfaces for the oxidation of organic molecular fuels.20 In this perspective, Ag-nanocomposites (AgNCs) are synthesised by using coal-derived carbon quantum dots as a reductant and stabilizer.
Carbon quantum dots (CQDs/C-Dots) are a novel group of carbon nano-materials with excellent fluorescence properties that can be utilized for applications in several areas like bio-sensing, bio-imaging,21,22 catalysis,23 drug delivery,21 and opto-electronics.24 The development, synthesis, and utilization of new C-Dot based nanocomposites and doped and hybrid nano-resources have thus become increasingly attractive as advanced materials for preparing electrocatalysts and sensors.25,26 Recently, Gao et al.27 reviewed on the synthesis and properties of carbon quantum dots, with detailed analysis on metal ion sensing and the challenges faced regarding their fabrication. In another study, hydrothermal synthesis of N doped graphene quantum dots was reported by Ju et al.,28 which behave as a selective and sensitive sensor for the detection of Fe3+ ions. Surface-modification versatility, in addition to large number of functional groups, in particular –OH, –COOH, etc., on the CQD surfaces provides ideal sites for synthesising several CD-based nanomaterials. Moreover, it has been reported that C-Dots are also excellent electron donor species.29–31 C-Dots with high electron contributing potential enable metal ions to be reduced in the associated metal nanoparticles on C-Dot surfaces to create hybrids and composites of various metals and metallic oxides.32,33 Various forms of CQDs were used as reductant to synthesize AgNPs.34,35 Jin et al.34 reported a single-step reduction method for the fabrication of AgNPs by using sulphur doped C-Dots as a reductant and stabilizing agent. They prepared C-Dots by using sucrose as the starting material via the carbonization process and used ammonia as the base for the fabrication of AgNPs. The antibacterial properties of C-Dots capped with AgNPs were reported by Xu et al.36 Moreover, Gao et al.27 reported on the synthesis of luminescent carbon dots that were used for the detection of Ag+ ions based on the Ag+-induced improvement of fluorescence. Nevertheless, coal-derived CQDs have not been previously reported for the fabrication of silver nanoparticle/carbon quantum dot (Ag/CQDs) nanocomposite via a single step and a facile process.
Thus, in this study, an easy, sustainable, and cost-efficient approach for synthesizing silver nanoparticle/carbon quantum dot (Ag/CQD) nanocomposite has been designed and discussed extensively along with the wet-chemical ultrasonic synthesis of CQDs from high-sulfur, low-grade, subbituminous coals of North-East India. Here, fluorescent carbon quantum dots were first synthesized without the use of any harmful chemicals which was more environmentally friendly than those reported previously. The advantage of using coal for nanomaterial production is that the CQDs synthesized from coal undergo self-functionalisation with oxygen-possessing functional groups like carboxylic, carbonyl and phenolic groups. Then, using a standard procedure, silver nitrate solution was added to the CQD solution without any extra reducing agents, and Ag/CQD nanocomposite was generated and bonded to CQDs nearly at room temperature. The CQDs provide a large surface area in which the AgNPs are extensively distributed and stabilised. The originality derives from the fact that the current technique overcomes the disadvantages described previously, such as reaction time and ecological friendliness, and does not involve complex and toxic processes. The novelty of this investigation is that the nanocomposites were fabricated by an easy and relatively fast reduction method. Although this reduction method has been mentioned by Jin et al.,17,34 the present study does not utilize ammonia as the base for the fabrication of silver nanocomposites and here the CQDs were synthesized from coal rather than using other chemical sources. Moreover, this process does not require the use of any reducing agents like NaBH4, ascorbic acid, NaNH2, and sodium citrate, etc. For the first time, coal-derived carbon quantum dots were used as the reducing agent for the fabrication of Ag/CQD nanocomposite with high stability. Besides, the as-synthesized coal-based Ag/CQD nanocomposites have shown potential antibacterial activities against five different bacterial strains.
Experimental sections
Materials
The representative coal sample (subbituminous rank and Cenozoic age) was collected from the Changki coalfield of Nagaland (North East India) from different locations within a selected area of mining. The sample was properly labelled and kept inside a plastic sample bag. The samples were first crushed and ground to sizes below 0.211 mm via a standard process (ASTM 2010) and stored in polyethylene airtight containers with proper labelling. H2O2 (30%; Merck), NH3 (25% v/v), and silver nitrate (Sigma Aldrich) were used for subsequent experiments. Polytetrafluoroethylene hollow fibres with 1 kDa membrane and ultrafiltration system (KrosFlo TFF system; SYR2-U10-A; Spectrum) were used for the filtration and purification of the required CQDs.Synthesis of carbon quantum dots (CQDs) and Ag/CQD nanocomposite
The CQDs were synthesized from the subbituminous-rank coal sample as per the procedure described previously.37–39 For fabrication of Ag/CQD nanocomposites, 200 μL of the synthesised CQDs (10.79 mg mL−1) was put into 100 mL of hot boiled distilled water (∼100 °C) and the mixture was constantly stirred at a room temperature for 15 minutes. Consecutively, 5 mL of AgNO3 solution (3.42 mg mL−1) was added to the reaction mixture and the stirring process was continued for another 3 hours after processing the reduction reaction (45 minutes) for stabilization. The mixture was ultra-filtered so as to eliminate the remaining silver nitrate and finally the fabricated Ag/carbon quantum dot (Ag/CQD) nano-composite was attained. The schematic diagram representing the fabrication of Ag/CQD nanocomposite is depicted in Fig. S1 (ESI†).Characterization studies of CQDs and Ag/CQD nanocomposites
The proximate and ultimate data were evaluated by using a thermogravimetric analyzer (Leco, TGA-701) and Truspec CHN Macro Determinator (Leco, 630-100-300). Using a high resolution-transmittance electron microscope (HRTEM; Joel JEM-2100), the morphology as well as the microstructure of the CQDs and the fabricated Ag/CQD nanocomposites were analysed including energy dispersive X-ray spectroscopic (EDS) and selected area electron diffraction (SAED) analyses. By using “Image J” software, the TEM images were further modified and investigated. A Fourier transform infrared spectrophotometer (FTIR) (Model: PerkinElmer, Spectrum Two) was used to determine the functional groups by recording the FTIR spectra of the samples. X-ray photoelectron spectroscopy (XPS) (model: ESCALAB Xi+) was used to evaluate the carbon bonding environment in the CQDs and Ag/CQDs. The X-ray diffraction patterns were obtained using an X-ray diffractometer (Rigako, Ultima IV) by using an X-ray source of Cu Kα radiation (λ = 0.15418 nm). The dynamic light scattering (DLS) studies of the nanocomposites were carried out by using a ZETASIZER instrument (Model-Nano ZS, Malvern, UK). The UV-visible and fluorescence (FL) spectra of the synthesised CQDs and Ag/CQD nano-composites were recorded by using a UV-visible spectrophotometer (LABINDIA 1000+) and a fluorescence spectrophotometer (Horiba, Fluorlolog-3), respectively, in order to study their photo-physical properties.Antibacterial activity test
Five different bacterial strains (see Table 1) were selected to assess the anti-bacterial activity of the synthesized CQDs and fabricated Ag–CQD nanocomposite. The agar well diffusion method was used to determine the anti-bacterial properties.40,41 100 μL of each bacterial species solution were inoculated by spreading on nutrient agar plates. In each plate, three 6 mm wells were made using a sterilized cork-borer. After this, 20 μL of each test sample was inoculated into the hole. The inoculated plates were then incubated at 35 °C for 24 h, subsequently in order to assess the anti-bacterial activity of the synthesized nanocomposites, the diameter of the rings were measured.| Sl. no. | Strain ID | Strains | Gram |
|---|---|---|---|
| 1 | N43 | Bacillus pseudomycoides | Positive |
| 2 | N47 | Glutamibacternicotianae | Positive |
| 3 | N62 | Phytobacterursingii | Negative |
| 4 | N63 | Bacillus wiedmannii | Positive |
| 5 | N112 | Rhodococcus soli | Positive |
Results and discussions
Chemical characteristics of the coal feedstock
Table S1 (ESI†) summarises the physico-chemical characteristics of the coal sample (Coal–CC) used in this study. The result indicates the presence of carbon (78.46%), hydrogen (6.02%), nitrogen (0.96%), total sulphur (4.09%), and low-ash content (about 6.75%) in the raw coal samples. The FTIR spectra of the raw coal (see Fig. S2, ESI†) demonstrate the presence of aromatic as well as aliphatic functional groups in its structure including the absorption bands at 1019 and 1612 cm−1 attributed to the C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C
C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching. Aliphatic CH2/CH3 groups are also present as indicated by the presence of bands at 1446 cm−1 in the FTIR spectrum. Due to presence of bands at 2845 and 2928 cm−1, the CH3, CH2, and CH functional groups are shown to exist in the coal structure. At 3376 cm−1, a band due to NH stretching vibrations is identified while a band at 3617 cm−1 is identified due to O–H stretching. The existence of C
O stretching. Aliphatic CH2/CH3 groups are also present as indicated by the presence of bands at 1446 cm−1 in the FTIR spectrum. Due to presence of bands at 2845 and 2928 cm−1, the CH3, CH2, and CH functional groups are shown to exist in the coal structure. At 3376 cm−1, a band due to NH stretching vibrations is identified while a band at 3617 cm−1 is identified due to O–H stretching. The existence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–O, C
C, C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, N–H, and O–H surface functional groups in the coal from North-east India has also been reported by Saikia et al.42 The XPS survey spectra of the coal sample (see Fig. S3a, ESI†) also depict the existence of C, N, O, and S as per the peaks noticed at 283 eV, 399 eV, 530 eV, and 165 eV, respectively. After deconvolution, C 1s (Fig. S3b, ESI†) spectra show the presence of two major peaks at 283.3 eV (sp2 C–C), and 288.33 eV (C
O, N–H, and O–H surface functional groups in the coal from North-east India has also been reported by Saikia et al.42 The XPS survey spectra of the coal sample (see Fig. S3a, ESI†) also depict the existence of C, N, O, and S as per the peaks noticed at 283 eV, 399 eV, 530 eV, and 165 eV, respectively. After deconvolution, C 1s (Fig. S3b, ESI†) spectra show the presence of two major peaks at 283.3 eV (sp2 C–C), and 288.33 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) associated with the nature of carbon bonding in the coal structure.
O) associated with the nature of carbon bonding in the coal structure.
Chemical and structural properties of coal-based CQDs and Ag/CQD nanocomposite
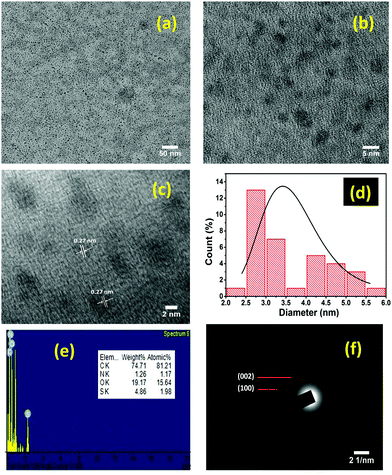
The TEM images (Fig. 2a and b) of the as-fabricated AgNP/CQD nanocomposites (after the reduction process) demonstrate them to be spherical and well-distributed with no agglomeration revealing their high stability. The HRTEM image (Fig. 2c) depicts the crystalline nature of the nanocomposites, showing two distinct d-spacings of 0.22 and 0.24 nm attributed to the (100) and (110) in-plane of the graphitic core or aromatic carbon.44,45 Moreover, the crystalline region of the HRTEM image also shows two well-defined d-spacings of 0.24 and 0.27 nm attributed to the (111) and (122) lattice planes of Ag.46,47 The amorphous region observed in the high-resolution TEM images of the nanocomposites indicated the formation of CQDs. Size distribution histogram (Fig. 2e) was determined, indicating that the particles are within 2–12 nm with average particles being 4–6 nm in diameter, complying with the Gaussian distribution. However, the diameters of the fabricated coal-derived Ag/CQD nanocomposites were found to be significantly smaller than those reported by other researchers using C-Dots derived from different sources as the reducing agents for the fabrication of Ag/CQD nanocomposites.34,36,48 SAED patterns of the Ag/CQD nanocomposite showed concentric diffraction rings with the (111), (200), (220), and (311) lattice planes of the fcc-structured silver metal, indicating the composition of silver nanoparticles (Fig. 2e). These planes (111), (200), (220), and (311) have d-spacings of 0.23, 0.19, 0.14, and 0.114 nm, respectively. In addition, SAED patterns specify that the fabricated nanocomposites are polycrystalline in nature. The existence of C, O, N, S, and Ag in the fabricated Ag/CQD nanocomposite are well-illustrated by TEM-EDS (Fig. 2f) and elemental mapping (Fig. 2g–k) analyses. It is further observed that the particle sizes of Ag/CQD nanocomposites were larger than those of CQDs, indicating the effectiveness of this novel synthesis of Ag/CQD nanocomposites from coal.
X-ray diffraction (XRD) analysis
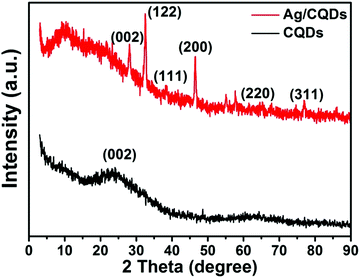
X-ray diffraction study was performed to evaluate the crystalline environment of the as-synthesised CQDs and Ag/CQD nanocomposite. As displayed in Fig. 3, the XRD pattern of CQDs possesses a wide peak with 2θ = 24° having a d-spacing of 0.37 nm ascribed to the (002) graphitic plane that reveals the formation of CQDs. This is similar to the result observed in the SAED patterns of CQDs indicating the (002) plane and the amorphous nature of the CQDs (Fig. 1f). The observed peak is near the graphitic peak of 2θ = 26.4° that may be altered from the original peak as a result of the existence of oxygen-bearing functional groups resembling amorphous carbon phases.49,50 After reduction, the XRD peak observed at 2θ = 28° having a lattice fringe of 0.32 nm for Ag/CQD nanocomposite corresponds to the (002) graphitic plane, which may be due to the interaction of CQDs with the silver nanoparticles. In addition, the XRD patterns of Ag/CQD nanocomposites present prominent peaks at 32.57°, 38.32°, 46.50°, 64.40°, and 77° which are attributed to the (112),(111), (200), (220), and (311) crystallographic planes of the fcc structure of silver crystals.1,35,48 The (112), (111), (200), (220), and (311) planes have lattice spacings of 0.27, 0.23, 0.20, 0.14, and 0.12 nm, respectively. The obtained lattice spacings in XRD analysis are similar to those obtained from SAED analysis (see Fig. 2e) and are also relevant with the lattice fringes obtained from the high resolution TEM image (Fig. 2c). Thus, the XRD results, as with those obtained from HRTEM and SAED analyses, reveal the existence of CQDs and silver nanoparticles in the Ag/CQD nanocomposite.X-ray photoelectron spectroscopy (XPS) analysis
X-ray photoelectron spectroscopy was conducted to examine the functional groups present on the surface, chemical bonding, and elemental components of the CQDs before and after the reduction process. The XPS survey spectra (Fig. 4a) of the synthesized CQDs show major peaks at 284.2, 400, 531.8, and 168 eV referring to C 1s, N 1s, O 1s, and S 2p, respectively. The deconvoluted carbon XPS spectra (Fig. 4b) of CQDs have major peaks at 283.4 eV and 287 eV referring to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–O groups, respectively. The peak at 531.3 eV in the deconvoluted oxygen spectrum of CQDs (Fig. 4c) suggested the existence of the organic C–O group. After the reduction process, the survey spectra of Ag/CQD nanocomposite (Fig. 4a) also show two apparent peaks centred at 283.7 and 530.2 eV attributed to C 1s and O 1s, respectively. The deconvoluted XPS spectrum (Fig. 4d) of C 1s of the Ag/CQDs refers to sp2 (C
C and C–O groups, respectively. The peak at 531.3 eV in the deconvoluted oxygen spectrum of CQDs (Fig. 4c) suggested the existence of the organic C–O group. After the reduction process, the survey spectra of Ag/CQD nanocomposite (Fig. 4a) also show two apparent peaks centred at 283.7 and 530.2 eV attributed to C 1s and O 1s, respectively. The deconvoluted XPS spectrum (Fig. 4d) of C 1s of the Ag/CQDs refers to sp2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) at 283.7, O–C
C) at 283.7, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 287 and C
O at 287 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 291.8 eV. At the same time, different peaks situated within 368–374 (Ag 3d) and 573–603 eV (Ag 3p) indicate the presence of Ag (see Fig. 4a). Moreover, the deconvoluted Ag (3d) spectrum shows two peaks; these are associated with the spin–orbit coupled energy states J = 5/2 and 3/2. The deconvoluted Ag 3d spectrum of the nanocomposites represents the binding energies of Ag 3d5/2 and Ag 3d3/2 at 367 and 373 eV, respectively, separated by 6.0 eV (Fig. 4e). This indicates that silver is present in the metallic zerovalent [Ag(0)] oxidation state in the composite.36,48,51 Table S2 (ESI†) shows the existence of elements along with their atomic percentages in the Ag/CQD nanocomposites, which also confirms the formation of Ag/CQD nanocomposite.
O at 291.8 eV. At the same time, different peaks situated within 368–374 (Ag 3d) and 573–603 eV (Ag 3p) indicate the presence of Ag (see Fig. 4a). Moreover, the deconvoluted Ag (3d) spectrum shows two peaks; these are associated with the spin–orbit coupled energy states J = 5/2 and 3/2. The deconvoluted Ag 3d spectrum of the nanocomposites represents the binding energies of Ag 3d5/2 and Ag 3d3/2 at 367 and 373 eV, respectively, separated by 6.0 eV (Fig. 4e). This indicates that silver is present in the metallic zerovalent [Ag(0)] oxidation state in the composite.36,48,51 Table S2 (ESI†) shows the existence of elements along with their atomic percentages in the Ag/CQD nanocomposites, which also confirms the formation of Ag/CQD nanocomposite.
FTIR analysis of functional groups in CQDs
FTIR analysis was performed in order to understand the chemical components and functional groups of the synthesised CQDs and Ag/CQD nanocomposite. It can be observed from Fig. 5 that the synthesized CQDs possess broad absorption bands at around 3434 and 3155 cm−1 attributed to the existence of C–OH and –NH2 surface functional groups. The bands at around 1690 cm−1 are assigned to the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C functional groups in the aromatic ring. The typical FTIR absorption bands at 1410 and 1390 cm−1 are attributed to the phenolic O–H functionalities and bands at 1102, 1115, and 1264 cm−1 are a result of the C–O groups. Moreover, sharp bands at 609, 617, and 799 cm−1 are attributed to the aromatic C–H out-of-plane bending vibrations. Similar functional groups are observed in the Ag/CQD nanocomposite structure signifying the stabilization of the nanocomposites with coal-derived CQDs. In Ag/CQD nanocomposite, the absorption band at 1640 cm−1 is due to quinone or conjugated C
C functional groups in the aromatic ring. The typical FTIR absorption bands at 1410 and 1390 cm−1 are attributed to the phenolic O–H functionalities and bands at 1102, 1115, and 1264 cm−1 are a result of the C–O groups. Moreover, sharp bands at 609, 617, and 799 cm−1 are attributed to the aromatic C–H out-of-plane bending vibrations. Similar functional groups are observed in the Ag/CQD nanocomposite structure signifying the stabilization of the nanocomposites with coal-derived CQDs. In Ag/CQD nanocomposite, the absorption band at 1640 cm−1 is due to quinone or conjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups, revealing the structural change of CQDs through the reduction process. Similar results on the structural changes in CQDs are also observed in the XPS analysis of Ag/CQD nanocomposites. There is a drop in the intensity of FTIR absorbance at around 3434 and 3155 cm−1 after the reduction procedure which is due to the utilization of –NH2 and C–OH functional groups. These findings established that these coal-derived CQDs are made of an aromatic framework whose surface possesses numerous amino and phenol hydroxyl functional groups, collectively acting as electron donors for reducing Ag ions (Ag+) to the elemental silver.46,52
O functional groups, revealing the structural change of CQDs through the reduction process. Similar results on the structural changes in CQDs are also observed in the XPS analysis of Ag/CQD nanocomposites. There is a drop in the intensity of FTIR absorbance at around 3434 and 3155 cm−1 after the reduction procedure which is due to the utilization of –NH2 and C–OH functional groups. These findings established that these coal-derived CQDs are made of an aromatic framework whose surface possesses numerous amino and phenol hydroxyl functional groups, collectively acting as electron donors for reducing Ag ions (Ag+) to the elemental silver.46,52
Photo-physical properties of CQDs and Ag/CQDs
Many of the prior findings reported utilization of bases such as ammonia, NaOH, etc., to be necessary for the production of Ag/CQD nanocomposites; however, in this study it is found that the Ag/CQD nanocomposite can be fabricated without the use of any such base.17,34,36 The parameters influencing the formation of Ag/CQD nanocomposites were also examined. Fig. 6b shows that Ag/CQD nanocomposites fabricated by using CQDs as the reducing agent exhibit good SPR absorption spectra without the use of a base. At the same time, when nanocomposite was synthesized using CQDs along with NH4OH as the base, no SPR absorption was observed, revealing that Ag/CQD nanocomposite can be fabricated without the addition of a base with coal-derived CQDs. The UV-vis spectra (Fig. 6c) also summarise the effect of the concentration of CQDs in the fabrication of Ag/CQD nanocomposite. A slight red-shifted absorption maxima accompanied by a steady decrease in absorption intensity is found when there is an increase in the concentration of CQDs.46 When 5 mL of silver nitrate solution was added to 200 μL coal-derived CQD solution to fabricate Ag/CQD nanocomposite at room temperature for 45 minutes, the reaction mixture turned brownish in colour, suggesting the formation of Ag/CQD nanocomposites as shown in Fig. 6h. The Ag/CQD nanocomposites show no signs of colour change or aggregation over a long period of storage, for about 60 days in this study. The coal-derived CQDs can act as a good reductant and stabilizer, preventing the metal NPs from aggregation, thereby demonstrating a strong affinity between Ag nanoparticles and CQDs.
The FL emission spectrum depicts the excitation dependent emission characteristics of CQDs as displayed in Fig. 6d. The maximum emission intensity of the FL spectrum was observed to be around 430–440 nm in the blue region of the visible spectrum. However, with increase in the excitation wavelength, the maximum emission of the FL spectra is slightly red shifted with decreasing FL intensity. This characteristic outcome is due to the availability of fluorophore or chromophore groups combined with aromatic systems in CQDs. The transitions from nN2p–σ* to nO2P–π* associated with the amino and protonated carboxyl groups and the π–π* transition related with the sp2 domain of the aromatic system are attributed to the blue-fluorescence based on the FL characteristics.55 The Ag/CQD nanocomposite, on the other hand, no longer has a fluorescence emission peak when excited at 320 nm. Perhaps this could be the result of the interaction of Ag+ ions with the functionalities present on the surface of CQDs, causing the transfer of electrons between CQDs and silver nanoparticles.
Time-resolved single-photon counting spectroscopy was also performed to measure the average fluorescence (FL) lifetimes of the nanomaterials. The average FL lifetimes of the fabricated CQDs and Ag/CQD nanocomposites at neutral pH were found to be 80.157 and 80.078 ns, respectively, as pointed out in Fig. 6e. The FL lifetime study showed an approximately steady average lifetime of 80.157–80.078 ns before and after the addition of Ag+ ions to the nanocomposite. Here the observed FL lifetimes of CQDs had not changed much; however, the UV-vis absorption spectra change after the addition of Ag+ ions due to the formation of the non-fluorescent Ag/CQD nano-complex in the ground state. These consequences may be referred to as static quenching.56,57 The obtained lifetimes recommend the fabricated CQDs and Ag/CQD nanocomposites for possible applications in the field of biological sciences.
Observation from zeta potential analysis: stability of Ag/CQDs
To identify the stability and surface charge of the fabricated CQDs and Ag/CQD nanocomposites, the zeta potential analysis was performed by using the DLS technique. The larger negative value can cause repulsion amongst Ag/CQD nanocomposites, leading to the stability of the fabricated nanocomposites.58 As displayed in Fig. 7, CQDs have a zeta potential value of −4.91 mV, while that of Ag/CQD nanocomposites is −9.91 mV, signifying the higher stability of Ag/CQD nanocomposites/nanoparticles for a longer time.Mechanism for the synthesis of Ag/CQD nanocomposites
Generally, the formation of Ag/CQD nanocomposites is assumed to possess two essential features regarding their interaction. First is the existence of oxygen-possessing functionalities on the surface of the carbon nano-structures, such as carboxyl, carbonyl, and phenolic groups that allow metal nano particles to be attached to them. Another assumption is that even in the deficit of oxygen possessing group, there is an attraction between the pristine carbon nano-structures and metal nanoparticles.30,59 As a result, the generation of highly stable Ag/CQD nanocomposites is due to the correlation between the p/π-orbitals of carbon and the d-orbitals of Ag in AgNPs, as well as the interaction of oxygen possessing functionalities on CQDs with AgNPs. Thus, the fabricated CQDs are observed to be functionalized with oxygen-possessing functionalities such as phenolic, carboxyl, and carbonyl groups that remain associated with the surface of CQDs as revealed via the above FTIR and XPS analyses. As a result of the existence of these functionalities, the surface of CQDs is negatively charged, so cationic Ag+ ions are liable to coordinate with these functional groups and get themselves reduced to Ag(0).38,46 Consequently, AgNPs are generated on the surface of CQDs. The marginal charges surrounding the CQDs aid them to sustain the AgNPs in aqueous systems and prohibit them from getting aggregated.Antibacterial activities
The antibacterial behaviour of Ag/CQD nanocomposite against Gram-negative (N62) and Gram-positive (N43, N47, N63, N112) bacteria was examined by the inhibition zone test. As displayed in Fig. S4 (ESI†), it is noticed that CQDs (20 μL) could not significantly inhibit the growth of bacteria indicating that CQDs alone do not possess any antibacterial properties. On the other hand, the Ag/CQD nanocomposite shows good antibacterial effect as it inhibits the growth of both Gram positive and Gram negative bacteria as shown in Fig. 8(a–e). Fig. 8f shows the inhibition zone diameters of the Ag/CQD nanocomposite against the bacterial strains and the strain Rhodococcus soli (N112) has a maximum inhibition zone diameter of 17 mm as compared to the other strains. The antibacterial activities indicate that the Gram-positive strains are more sensitive than the Gram-negative bacterial strains to Ag/CQD nanocomposite and the obtained results are in agreement with previously reported results.48,60 Thus, Ag/CQD nanocomposite has a far better antibacterial impact than CQDs alone. The impact of the Ag/CQD nanocomposite on the antibacterial activity was clearly superior to that of CQDs at a very lower concentration. The Ag/CQD nanocomposite shows a slightly better or similar inhibition to that of the results reported on other nanomaterials that has been summarized in Table S2 (ESI†).Moreover, the negative surface charge of Ag/CQD nanocomposite, −9.91 mV as evaluated by zeta potential, indicates that the negative charge allows more efficient electrostatic interaction with the positive charges of the bacterial cell wall as in contrast the positive charge is more capable for electrostatic interaction with the negative charges of the bacterial cell wall.61 This experimental finding highlights a key advantage of the fabricated Ag/CQD nanocomposites regarding safety in mammalian cells and tissues, considering that positively charged nanoparticles are more cytotoxic than neutral or negatively surface charged nanoparticles. As a result, the coal-derived Ag/CQD nanocomposite could be utilized as a plausible antibacterial agent.
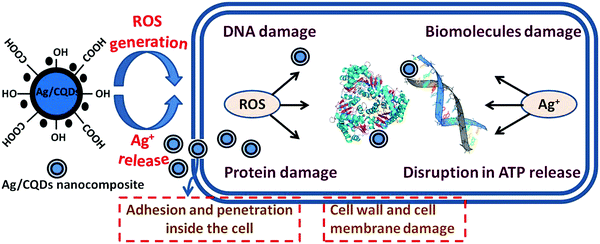
Mechanism for antibacterial activity of Ag/CQD nanocomposite
The exact mechanism through which silver nanocomposites exhibit antibacterial or anti-toxicological properties is still unknown. Silver nanocomposites can continuously discharge silver (Ag+) ions, which could be intended as the pathway for killing micro-organisms, leading to the antibacterial activity of Ag/CQD nanocomposites. To sustain its antibacterial properties, silver (Ag) should be basically in its ionized state which plays the main role in exhibiting the toxic properties of Ag. Moreover, Ag+ ions have been found to form complexes with nucleic acids, and unlike phosphate groups, they selectively bind with nucleosides of nucleic acids. Likewise, Ag+ ions bind to the cell wall and cytoplasm due to their affinity for sulfur (thiol group) proteins, resulting in increased permeability and disrupting bacterial casings.62 The respiratory enzymes are inhibited immediately after the Ag+ ions are taken up by the cells, following the generation of reactive oxygen species (ROS) that lead to disruption in adenosine triphosphate (ATP) release.63,64 When free radicals come into contact with bacteria, they have the ability to create pores in the cell wall leading to cell death.65 Moreover, the Ag+ ions bind to the surface of the bacterial cell wall and penetrates it, causing structural changes in the membrane or increasing its permeability, all of which cause the cells to die. Ag+ ions are thus produced by all types of silver or silver-containing composites with antibacterial properties. Fig. 9 represents the probable mechanism of action of Ag/CQD nanocomposites via the production of ROS that causes the damage of the bacterial strain.In addition, the generation of Ag+ ions is affected by the size and shape of silver nanocomposites. Nanocomposites possessing smaller size with spherical shape are more prone to the discharge of Ag+ ions on account of their larger surface area.66 According to Zhang et al.,67 smaller AgNPs cause higher toxicity to bacteria and have a stronger bactericidal impact than larger particles because of their larger surface area. Agnihotri et al.68 noted that AgNPs with sizes less than 10 nm have a higher antibacterial effect, but those with size of 5 nm show the best bactericidal effect among all other sizes of silver nanoparticles. Thus, the coal-derived Ag/CQD nanocomposites comprising of sizes smaller than 10 nm are commonly thought to have the ability to readily modify cell penetrability, enter microbial cells, and initiate cell lysis.
Conclusion
For the first time, coal-derived CQDs are satisfactorily utilized as a reductant and stabilizer for the fabrication of Ag/CQD nanocomposites. The used coal-derived CQDs prove to be a promising agent for the reduction of Ag+ to elemental silver under moderate conditions. The HRTEM, SAED, XRD, and XPS analyses established the existence of CQDs and silver nanoparticles in the Ag/CQD nanocomposite. The Ag/CQD nanocomposites with a zeta potential of −9.91 mV signifies the nanocomposites to be highly stable for a longer time. The surface plasmon resonance of Ag/CQD nanocomposites indicates the successful formation of silver nanoparticles in the nanocomposites by a simple reduction process. Additionally, the coal-derived Ag/CQD nanocomposite synthesised using a quite simple, sustainable, and environmentally friendly technique is thought to have many possibilities for antibacterial and biological applications in the future.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors are grateful to the Director (CSIR-NEIST) for his consent to publish the results. The funding received from CSIR (OLP-2055) and MoES (GPP364) is thankfully acknowledged by the authors. Authors are thankful to Dr Jim Hower for his English editing of the draft. Authors express special thanks to Aparna Choudhury for her analytical assistance in the antibacterial study. The comments received from the anonymous reviewers to improve the revision are thankfully acknowledged.References
- P. Premasudha, M. Venkataramana, M. Abirami, P. Vanathi, K. Krishna and R. Rajendran, Biological synthesis and characterization of silver nanoparticles using Eclipta alba leaf extract and evaluation of its cytotoxic and antimicrobial potential, Bull. Mater. Sci., 2015, 38(4), 965–973 CrossRef CAS.
- A. Verma and M. S. Mehata, Controllable synthesis of silver nanoparticles using Neem leaves and their antimicrobial activity, J. Radiat. Res. Appl. Sci., 2016, 9(1), 109–115 CrossRef CAS.
- K. Anandalakshmi, J. Venugobal and V. Ramasamy, Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity, Appl. Nanosci., 2016, 6(3), 399–408 CrossRef CAS.
- A. Syafiuddin, S. Salmiati, M. Salim, A. Kueh and T. Hadibarata, A Review of Silver Nanoparticles: Research Trends, Global Consumption, Synthesis, Properties, and Future Challenges, J. Chin. Chem. Soc., 2017, 64, 732–756 CrossRef CAS.
- M. Zargar, K. Shameli, G. R. Najafi and F. Farahani, Plant mediated green biosynthesis of silver nanoparticles using Vitex negundo L. extract, J. Ind. Eng. Chem., 2014, 20(6), 4169–4175 CrossRef CAS.
- S. Chernousova and M. Epple, Silver as antibacterial agent: Ion, nanoparticles, and metal, Angew. Chem., Int. Ed., 2012, 52, 1636–1653 CrossRef PubMed.
- S. Chen, G. Wu and H. Zeng, Preparation of high antimicrobial activity thiourea chitosan–Ag+ complex, Carbohydr. Polym., 2005, 60(1), 33–38 CrossRef CAS.
- M. V. Roldan, N. Pellegri and O. De Sanctis, Electrochemical Method for Ag-PEG Nanoparticles Synthesis, J. Nanopart., 2013, 2013, 7 Search PubMed.
- H. Wang, X. Qiao, J. Chen and S. Ding, Preparation of silver nanoparticles by chemical reduction method, Colloids Surf., A, 2005, 256(2), 111–115 CrossRef CAS.
- Z. Khan, S. A. Al-Thabaiti, A. Y. Obaid and A. O. Al-Youbi, Preparation and characterization of silver nanoparticles by chemical reduction method, Colloids Surf., B, 2011, 82(2), 513–517 CrossRef CAS PubMed.
- H.-H. Park, X. Zhang, Y.-J. Choi, H.-H. Park and R. H. Hill, Synthesis of Ag Nanostructures by Photochemical Reduction Using Citrate-Capped Pt Seeds, J. Nanomater., 2011, 2011, 7 Search PubMed.
- Z. Shen, G. Chen, Z. Chen, X. Qu, Y. Chen and R. Liu, Spatially Selective Photochemical Reduction of Silver on Nanoembossed Ferroelectric PZT Nanowires, Langmuir, 2011, 27(9), 5167–5170 CrossRef CAS PubMed.
- A. Pyatenko, M. Yamaguchi and M. Suzuki, Synthesis of Spherical Silver Nanoparticles with Controllable Sizes in Aqueous Solutions, J. Phys. Chem. C, 2007, 111(22), 7910–7917 CrossRef CAS.
- W. Lin, K. Huang, Y. Li, Y. Qin, D. Xiong, J. Ling, G. Yi, Z. Tang, J. Lin, Y. Huang, C. Yang and J. Wang, Facile In Situ Preparation and In Vitro Antibacterial Activity of PDMAEMA-Based Silver-Bearing Copolymer Micelles, Nanoscale Res. Lett., 2019, 14(1), 256 CrossRef PubMed.
- S. Lee and B.-H. Jun, Silver Nanoparticles: Synthesis and Application for Nanomedicine, Int. J. Mol. Sci., 2019, 20, 865 CrossRef CAS PubMed.
- N. M. Zain, A. G. F. Stapley and G. Shama, Green synthesis of silver and copper nanoparticles using ascorbic acid and chitosan for antimicrobial applications, Carbohydr. Polym., 2014, 112, 195–202 CrossRef CAS PubMed.
- J.-C. Jin, Z.-Q. Xu, H.-F. Zou, Z.-Q. Zhou, Q.-Q. Yang, B.-B. Wang, F.-L. Jiang and Y. Liu, Carbon dots reduced and stabilized silver nanoclusters: Synthesis and formation mechanisms, RSC Adv., 2016, 6, 76989–76995 RSC.
- M. Liu and W. Chen, Green synthesis of silver nanoclusters supported on carbon nanodots: enhanced photoluminescence and high catalytic activity for oxygen reduction reaction, Nanoscale, 2013, 5, 12558–12564 RSC.
- J. Ju, R. Zhang and W. Chen, Photochemical deposition of surface-clean silver nanoparticles on nitrogen-doped graphene quantum dots for sensitive colorimetric detection of glutathione, Sens. Actuators, B, 2016, 228, 66–73 CrossRef CAS.
- W. Wei and W. Chen, Naked Pd nanoparticles supported on carbon nanodots as efficient anode catalysts for methanol oxidation in alkaline fuel cells, J. Power Sources, 2012, 204, 85–88 CrossRef CAS.
- X.-W. Hua, Y.-W. Bao and F.-G. Wu, Fluorescent Carbon Quantum Dots with Intrinsic Nucleolus-Targeting Capability for Nucleolus Imaging and Enhanced Cytosolic and Nuclear Drug Delivery, ACS Appl. Mater. Interfaces, 2018, 10(13), 10664–10677 CrossRef CAS PubMed.
- C. Huang, H. Dong, Y. Su, Y. Wu, R. Narron and Q. Yong, Synthesis of Carbon Quantum Dot Nanoparticles Derived from Byproducts in Bio-Refinery Process for Cell Imaging and In Vivo Bioimaging, Nanomaterials, 2019, 9(3), 387 CrossRef CAS PubMed.
- T. S. Atabaev, Doped Carbon Dots for Sensing and Bioimaging Applications: A Minireview, Nanomaterials, 2018, 8(5), 342 CrossRef PubMed.
- X. Li, M. Rui, J. Song, Z. Shen and H. Zeng, Carbon and Graphene Quantum Dots for Optoelectronic and Energy Devices: A Review, Adv. Funct. Mater., 2015, 25(31), 4929–4947 CrossRef CAS.
- T.-Z. Hong, Q. Xue, Z.-Y. Yang and Y.-P. Dong, Great-enhanced performance of Pt nanoparticles by the unique carbon quantum dot/reduced graphene oxide hybrid supports towards methanol electrochemical oxidation, J. Power Sources, 2016, 303, 109–117 CrossRef CAS.
- Y. Yang, J. Liu, S. Guo, Y. Liu and Z. Kang, A nickel nanoparticle/carbon quantum dot hybrid as an efficient electrocatalyst for hydrogen evolution under alkaline conditions, J. Mater. Chem. A, 2015, 3(36), 18598–18604 RSC.
- X. Gao, C. Du, Z. Zhuang and W. Chen, Carbon quantum dots-based nanoprobes for metal ions detection, J. Mater. Chem. C, 2016, 4, 6927–6945 RSC.
- J. Ju and W. Chen, Synthesis of highly fluorescent nitrogen-doped graphene quantum dots for sensitive, label-free detection of Fe (III) in aqueous media, Biosens. Bioelectron., 2014, 58, 219–225 CrossRef CAS PubMed.
- D. Yoo, Y. Park, B. Cheon and M.-H. Park, Carbon Dots as an Effective Fluorescent Sensing Platform for Metal Ion Detection, Nanoscale Res. Lett., 2019, 14(1), 272 CrossRef PubMed.
- X. Han, Y. Han, H. Huang, H. Zhang, X. Zhang, R. Liu, Y. Liu and Z. Kang, Synthesis of carbon quantum dots/SiO2 porous nanocomposites and their catalytic ability for photo-enhanced hydrocarbon selective oxidation, Dalton Trans., 2013, 42(29), 10380–10383 RSC.
- I. Srivastava, J. S. Khamo, S. Pandit, P. Fathi, X. Huang, A. Cao, R. T. Haasch, S. Nie, K. Zhang and D. Pan, Influence of Electron Acceptor and Electron Donor on the Photophysical Properties of Carbon Dots: A Comparative Investigation at the Bulk-State and Single-Particle Level, Adv. Funct. Mater., 2019, 29(37), 1902466 CrossRef.
- H. Choi, S.-J. Ko, Y. Choi, P. Joo, T. Kim, B. R. Lee, J.-W. Jung, H. J. Choi, M. Cha, J.-R. Jeong, I.-W. Hwang, M. H. Song, B.-S. Kim and J. Y. Kim, Versatile surface plasmon resonance of carbon-dot-supported silver nanoparticles in polymer optoelectronic devices, Nat. Photonics, 2013, 7(9), 732–738 CrossRef CAS.
- R. Long, D. Casanova, W.-H. Fang and O. V. Prezhdo, Donor–Acceptor Interaction Determines the Mechanism of Photoinduced Electron Injection from Graphene Quantum Dots into TiO2: π-Stacking Supersedes Covalent Bonding, J. Am. Chem. Soc., 2017, 139(7), 2619–2629 CrossRef CAS PubMed.
- J.-C. Jin, Z.-Q. Xu, P. Dong, L. Lai, J.-Y. Lan, F.-L. Jiang and Y. Liu, One-step synthesis of silver nanoparticles using carbon dots as reducing and stabilizing agents and their antibacterial mechanisms, Carbon, 2015, 94, 129–141 CrossRef CAS.
- M. Jahanbakhshi and B. Habibi, A novel and facile synthesis of carbon quantum dots via salep hydrothermal treatment as the silver nanoparticles support: Application to electroanalytical determination of H2O2 in fetal bovine serum, Biosens. Bioelectron., 2016, 81, 143–150 CrossRef CAS PubMed.
- Z. Xu, H. He, S. Zhang, B. Wang, J. Jin, C. Li, X. Chen, B. Jiang and Y. Liu, Mechanistic studies on the antibacterial behavior of Ag nanoparticles decorated with carbon dots having different oxidation degrees, Environ. Sci.: Nano, 2019, 6(4), 1168–1179 RSC.
- M. Saikia, J. C. Hower, T. Das, T. Dutta and B. K. Saikia, Feasibility study of preparation of carbon quantum dots from Pennsylvania anthracite and Kentucky bituminous coals, Fuel, 2019, 243, 433–440 CrossRef CAS.
- T. Das, B. K. Saikia, H. P. Dekaboruah, M. Bordoloi, D. Neog, J. J. Bora, J. Lahkar, B. Narzary, S. Roy and D. Ramaiah, Blue-fluorescent and biocompatible carbon dots derived from abundant low-quality coals, J. Photochem. Photobiol., B, 2019, 195, 1–11 CrossRef CAS PubMed.
- B. K. Saikia, D. Tonkeswar, S. Roy, B. Narzary, H. P. Dekaboruah, M. Bordoloi, J. Lahkar, N. Dipankar and D. Ramaiah, Process for the preparation of blue-flourescence emitting carbon dots (CDTS) from sub-bituminous Tertiary high sulfur Indian coals, US Pat. 10655061B2, 2020 Search PubMed.
- S. Hwan, H.-S. Lee, D.-S. Ryu, S.-J. Choi and D.-S. Lee, Antibacterial Activity of Silver-Nanoparticles Against Staphylococcus Aureus and Escherichia Coli, Korean J. Microbiol. Biotechnol., 2011, 39(1), 77–85 Search PubMed.
- J. S. Kim, E. Kuk, K. N. Yu, J.-H. Kim, S. J. Park, H. J. Lee, S. H. Kim, Y. K. Park, Y. H. Park, C.-Y. Hwang, Y.-K. Kim, Y.-S. Lee, D. H. Jeong and M.-H. Cho, Antimicrobial effects of silver nanoparticles, Nanomedicine, 2007, 3(1), 95–101 CrossRef CAS PubMed.
- B. K. Saikia, R. K. Boruah and P. K. Gogoi, X-ray (Radial Distribution Function, RDF) and FT-IR analysis of high sulphur Tirap (India) coal, J. Energy Inst., 2009, 82(2), 106–108 CrossRef CAS.
- M. Saikia and B. K. Saikia, Carbon Dots Derived from Natural Carbon Sources: Preparation, Chemical Functionalization, Characterization, and Applications, In All-carbon Composites and Hybrids, The Royal Society of Chemistry, 2021, pp. 142–172 Search PubMed.
- X. Sun, C. Brückner and Y. Lei, One-pot and ultrafast synthesis of nitrogen and phosphorus co-doped carbon dots possessing bright dual wavelength fluorescence emission, Nanoscale, 2015, 7(41), 17278–17282 RSC.
- K. Holá, M. Sudolská, S. Kalytchuk, D. Nachtigallová, A. L. Rogach, M. Otyepka and R. Zbořil, Graphitic Nitrogen Triggers Red Fluorescence in Carbon Dots, ACS Nano, 2017, 11(12), 12402–12410 CrossRef PubMed.
- L. Shen, M. Chen, L. Hu, X. Chen and J. Wang, Growth and Stabilization of Silver Nanoparticles on Carbon Dots and Sensing Application, Langmuir, 2013, 29(52), 16135–16140 CrossRef CAS PubMed.
- S. K. Bhunia, L. Zeiri, J. Manna, S. Nandi and R. Jelinek, Carbon-Dot/Silver-Nanoparticle Flexible SERS-Active Films, ACS Appl. Mater. Interfaces, 2016, 8(38), 25637–25643 CrossRef CAS PubMed.
- X. Wei, F. Cheng, Y. Yao, X. Yi, B. Wei, H. Li, Y. Wu and J. He, Facile synthesis of a carbon dots and silver nanoparticles (CDs/AgNPs) composite for antibacterial application, RSC Adv., 2021, 11(30), 18417–18422 RSC.
- B. De and N. Karak, A green and facile approach for the synthesis of water soluble fluorescent carbon dots from banana juice, RSC Adv., 2013, 3(22), 8286–8290 RSC.
- M. Saikia, T. Das, N. Dihingia, X. Fan, L. F. O. Silva and B. K. Saikia, Formation of carbon quantum dots and graphene nanosheets from different abundant carbonaceous materials, Diamond Relat. Mater., 2020, 106, 107813 CrossRef CAS.
- A. M. Ferraria, S. Boufi, N. Battaglini, A. M. Botelho do Rego and M. ReiVilar, Hybrid Systems of Silver Nanoparticles Generated on Cellulose Surfaces, Langmuir, 2010, 26(3), 1996–2001 CrossRef CAS PubMed.
- Q. Lu, J. Deng, Y. Hou, H. Wang, Y. Zhang and S. Yao, Hydroxyl-rich C-dots Synthesized with One-pot Method and Its Application in the Preparation of Nobel Metal Nanoparticles, Chem. Commun., 2015, 51, 7164–7167 RSC.
- P. Mulvaney, Surface Plasmon Spectroscopy of Nanosized Metal Particles, Langmuir, 1996, 12(3), 788–800 CrossRef CAS.
- M. A. Noginov, G. Zhu, M. Bahoura, J. Adegoke, C. Small, B. A. Ritzo, V. P. Drachev and V. M. Shalaev, The effect of gain and absorption on surface plasmons in metal nanoparticles, Appl. Phys. B: Lasers Opt., 2007, 86(3), 455–460 CrossRef CAS.
- H. Ding, S.-B. Yu, J.-S. Wei and H.-M. Xiong, Full-Color Light-Emitting Carbon Dots with a Surface-State-Controlled Luminescence Mechanism, ACS Nano, 2016, 10(1), 484–491 CrossRef CAS PubMed.
- P. Li and S. F. Y. Li, Recent advances in fluorescence probes based on carbon dots for sensing and speciation of heavy metals, Nanophotonics, 2021, 10(2), 877–908 CrossRef CAS.
- K. K. Chan, S. H. K. Yap and K.-T. Yong, Biogreen Synthesis of Carbon Dots for Biotechnology and Nanomedicine Applications, Nano-Micro Lett., 2018, 10(4), 72 CrossRef CAS PubMed.
- T. Dutta, S. K. Chowdhury, N. N. Ghosh, M. Das, A. Chattopadhyay and V. Mandal, Green Synthesis of Antimicrobial Silver Nanoparticles using Fruit Extract of Glycosmis Pentaphylla and its Theoretical Explanations, J. Mol. Struct., 2022, 1247, 131361 CrossRef CAS.
- K. T. Chan, J. B. Neaton and M. L. Cohen, First-principles study of metal adatom adsorption on graphene, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77(23), 235430 CrossRef.
- A. S. Bhosale, K. K. Abitkar, P. S. Sadalage, K. D. Pawar and K. M. Garadkar, Photocatalytic and antibacterial activities of ZnO nanoparticles synthesized by chemical method, J. Mater. Sci.: Mater. Electron., 2021, 32, 20510–20524 CrossRef CAS.
- W. R. Li, X. B. Xie, Q. S. Shi, S. S. Duan, Y. S. Ouyang and Y. B. Chen, Antibacterial effect of silver nanoparticles on Staphylococcus aureus, Biometals, 2011, 24(1), 135–141 CrossRef CAS PubMed.
- S. Khorrami, A. Zarrabi, M. Khaleghi, M. Danaei and M. Mozafari, Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties, Int. J. Nanomed., 2018, 13, 8013–8024 CrossRef CAS PubMed.
- S. S. Das, S. Alkahtani, P. Bharadwaj, M. T. Ansari, M. D. AL Kahtani, Z. Pang, M. S. Hasnain, A. K. Nayak and T. Aminabhavi, Molecular Insights and Novel Approaches For Targeting Tumor Metastasis, Int. J. Pharm., 2020, 585, 119556 CrossRef CAS PubMed.
- S.-H. Kim, H.-S. Lee, D.-S. Ryu, S.-J. Choi and D.-S. Lee, Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli, Korean J. Microbiol. Biotechnol., 2011, 39, 77–85 CAS.
- D. Chen, X. Qiao, X. Qiu and J. Chen, Synthesis and electrical properties of uniform silver nanoparticles for electronic applications, J. Mater. Sci., 2009, 44, 1076–1081 CrossRef CAS.
- R. Shanmuganathan, D. MubarakAli, D. Prabakar, H. Muthukumar, N. Thajuddin, S. S. Kumar and A. Pugazhendhi, An enhancement of antimicrobial efficacy of biogenic and ceftriaxone-conjugated silver nanoparticles: green approach, Environ. Sci. Pollut. Res., 2018, 25(11), 10362–10370 CrossRef CAS PubMed.
- X.-F. Zhang, Z.-G. Liu, W. Shen and S. Gurunathan, Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches, Int. J. Mol. Sci., 2016, 17(9), 1534 CrossRef PubMed.
- S. Agnihotri, S. Mukherji and S. Mukherji, Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy, RSC Adv., 2014, 4(8), 3974–3983 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1nj04039a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2022 |