Hitchhiking probiotic vectors to deliver ultra-small hafnia nanoparticles for ‘Color’ gastrointestinal tract photon counting X-ray imaging†
Fatemeh
Ostadhossein‡
ab,
Parikshit
Moitra‡
c,
Nivetha
Gunaseelan
cd,
Michael
Nelappana
 a,
Chiara
Lowe
e,
Mahdieh
Moghiseh
ef,
Anthony
Butler
a,
Chiara
Lowe
e,
Mahdieh
Moghiseh
ef,
Anthony
Butler
 efghi,
Niels
de Ruiter
efgi,
Harish
Mandalika
fgi,
Indu
Tripathi
a,
Santosh K.
Misra
efghi,
Niels
de Ruiter
efgi,
Harish
Mandalika
fgi,
Indu
Tripathi
a,
Santosh K.
Misra
 a and
Dipanjan
Pan
a and
Dipanjan
Pan
 *abcdj
*abcdj
aDepartment of Bioengineering, University of Illinois at Urbana-Champaign, 611 West Park Street, Urbana, IL, USA. E-mail: dipanjan@som.umaryland.edu
bBeckman Institute of Advanced Science and Technology, 405 N. Mathews M/C 251, Urbana, IL 61801-2325, USA
cDepartments of Diagnostic Radiology and Nuclear Medicine and Pediatrics, University of Maryland Baltimore, 670 W Baltimore St., Baltimore, Maryland 21201, USA
dDepartment of Chemical, Biochemical and Environmental Engineering, University of Maryland Baltimore County, Baltimore, Maryland 21250, USA
eUniversity of Otago Christchurch, Christchurch, New Zealand
fMARS Bioimaging Limited, Christchurch, New Zealand
gUniversity of Canterbury, Christchurch, New Zealand
hEuropean Organization for Nuclear Research (CERN), Geneva, Switzerland
iHuman Interface Technology Laboratory New Zealand, University of Canterbury, Christchurch, New Zealand
jDepartment of Materials Science and Engineering, 201 Materials Science and Engineering Building, 1304 W. Green St. MC 246, Urbana, IL 61801, USA
First published on 14th March 2022
Abstract
Gastrointestinal (GI) tract is one of the hard-to-reach target tissues for the delivery of contrast agents and drugs mediated by nanoparticles due to its harsh environment. Herein, we overcame this barrier by designing orally ingestible probiotic vectors for ‘hitchhiking’ ultrasmall hafnia (HfO2) (∼1–2 nm) nanoparticles. The minute-made synthesis of these nanoparticles is accomplished through a simple reduction reaction. These nanoparticles were incubated with probiotic bacteria with potential health benefits and were non-specifically taken up due to their small size. Subsequently, the bacteria were lyophilized and packed into a capsule to be administered orally as the radiopaque contrast agents for delineating the GI features. These nano-bio-hybrid entities could successfully be utilized as contrast agents in vivo in the conventional and multispectral computed tomography (CT). We demonstrated in ‘color’ the accumulated nanoparticles using advanced detectors of the photon counting CT. The enhanced nano-bio-interfacing capability achieved here can circumvent traditional nanoparticle solubility and delivery problems while offering a patient friendly approach for GI imaging to replace the currently practiced barium meal.
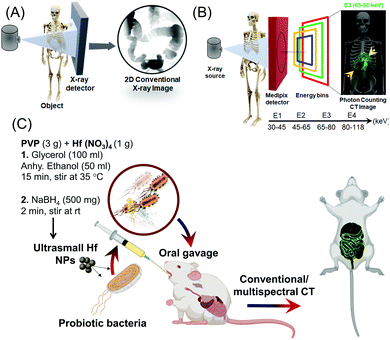
New conceptsThe gastrointestinal tract (GI) is one of the hard-to-reach areas for imaging and the clinical method for the interrogation of information is limited to Barium meal using CT imaging. However, this method has low patient compliance due to the unpleasant taste of the contrast agent taken orally. Herein, we introduced a biomimetic capsule-packed contrast agent material based on patient friendly probiotic bacteria for the color delineation of GI tract. The ultrasmall (1–2 nm) HfO2 nanoparticles (NPs) were taken up by bacteria via a passive route and subsequently the bacteria were freeze-dried. The NPs were fabricated on Gram-scale in a minute-made synthesis. We utilized these entities in photon counting spectral CT in vivo to see through the GI tract in color as being differentiated by the favorable specific K-edge binding energy of Hf. Since a photon-counting CT system employs a photon-counting detector (PCD) which registers the interactions of individual photons, the abundance of the contrast material, i.e., Hf is clearly delineated in the lining of the esophagus and differentiated from lipids, soft tissue, and the presence of other metals evidently present from the food intake. Overall, we demonstrated for the first time that tiny nanometric scale hafnium can be microencapsulated within a probiotic system and delivered successfully for K-edge weighted imaging. |
Introduction
For diagnostic probing of the stomach, traditionally, high-attenuation X-ray-based contrast agents have been used to enhance and distend the gastrointestinal (GI) tract.1,2 Intravenously administered positive contrast agents have an X-ray attenuation greater than that of water.3 Although these agents are generally well-tolerated and effective in producing good gastric distention, the delineation of the GI tract and stomach is not optimal. Contrast agents administered by the oral route is the most convenient due to high patient compliance, less sterility limitations and the ease of scale up.4–7 Contrast agents that are oil-dispersible produce satisfactory image contrast of the stomach wall but are not very pleasant when given orally. On the other hand, the poor bioavailability of drug administered through an oral route is a major drawback of the process.8–12Engineered nanoparticles (NPs) delivered via biological entities can combine the benefit of precise control over synthetic procedures while leveraging the natural cell functions.13–15 This approach can lay the foundation of ‘nano-bio-hybrid materials’ which can mimic the biological processes and enhance the bio-interfacing ability of the NPs.16–18 A number of diseases are known to be originated from the dysregulation of innate immune system consisting of physical barriers (mucus layers and epithelia covering gastrointestinal and respiratory tracts) and molecules released by phagocytic cells, e.g. chemokines and cytokines.19 Numerous studies have already been conducted to demonstrate the interaction of nanoparticles on the immune system influenced by the physicochemical properties of the nanoparticles such as solubility, size, shape and surface charge.20–25 It was shown that neutrophils induced ROS production followed by the activation of NADPH oxidase after the exposure with different nanoparticles, especially inorganic nanoparticles (e.g. SiO2, TiO2, AgNPs, AuNPs, ZnO NPs etc.).26 Several studies also demonstrated that small and cationic nanoparticles induced more inflammatory response than larger and anionic/neutral ones due to the formation of protein-corona on the nanoparticle surface mediated by the electrostatic interaction of the nanoparticles with negatively charged proteins.27 This introduced release of various cytokines followed by different kinds of immune reactions under in vivo conditions. In some of the cases, the uptake of inorganic nanoparticles triggered a calcium flux inside cells and ROS production that induced NLRP3 inflammasome activation followed by stimulation of IL-1β maturation and liver inflammation.28 Multiple studies further showed the ability of inorganic nanoparticles to induce DNA damage, increase oxidative stress, and initiate apoptosis under in vitro conditions.29 The in vivo toxicity of the nanoparticles was also investigated on rodent models.30 With this background, we believed that the as-prepared Hf nanoparticles might face difficulties in meeting in vivo biocompatibility criteria if gavage by its own. Hence, we conceived of introducing these hafnia nanoparticles hitchhiking probiotics which may be a reasonable alternative.
Nano-bio-hybrid carriers can therefore potentially be exploited in the GI tract imaging to circumvent the problems associated with the bare NPs exposure. Current GI tract imaging approaches relying on either the invasive endoscopic methods or the non-invasive imaging methods are not patient friendly.31 For instance, in the omnipresent clinical method known as barium meal, the patient must ingest 100 ml of barium sulphate after 6–8 h of fasting while there are reports about unknown median lethal dose for this compound and potential false positive results.3,32
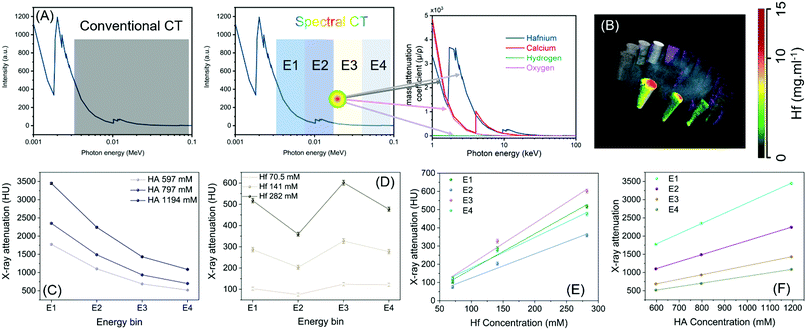
Spectral CT is an emerging technique where information is derived from photon-counting detectors that distinguish photon energies of individual photons.33–35 The detected X-rays are divided into several energy windows to discriminate multiple materials based on their unique k-edge energies (Fig. 1A and B).36 Photon counting CT (PCCT) imaging-based techniques are expected to offer lower radiation doses than conventional X-ray based techniques.37–39 Researchers have used iodinated polymer nanoparticles for ‘blood pool’ contrast agent,40 anti-plasma membrane Heat shock protein 70 (Hsp70) antibody functionalized gold nanoparticles for tumor-specific multimodal imaging,41 multimodal nanocarrier probes for optical and nuclear imaging of macrophage cells,42 hafnia nanodots for multi-color delineation of bone microdamage,43 rhenium sulfide nanoparticles for gastrointestinal tract imaging in vivo,44 gold nanoparticles to observe their biodistribution in vivo45 and bi-diethylene triamine pentaacetate acid (Bi-DTPA) complex for in vivo high-performance CT and spectral CT imaging.46
Contrast agents for conventional CT employ elements that have much higher Z values than those found in the body, such as iodine, barium, gold, bismuth and so on. An intrinsic limitation of CT is its low sensitivity to contrast (limit of detection 10−3 M) compared with MRI (gadolinium chelate, 10−5 M) or nuclear based (10−9 M) techniques.47,48 Photon counting CT has been proposed to encounter these issues and bring much needed improvement in sensitivity for CT imaging.49–52 Elements with K-shell electron energies within the bandwidth of the incident X-ray spectrum can serve as K-edge contrast agents, and this range is generally considered to extend between iodine and bismuth on the periodic table of elements. Hafnium has K-edge discontinuity well inside the energy regime and may become most relevant for medical X-ray imaging. Iodine (33.2 keV) and barium (37.4 keV), which are on the lower edge of the X-ray bandwidth, will be effective in rodents, but the effects of photon starvation and scattering occurring in patients may severely limit the utility of current clinically approved contrast agents for K-edge imaging applications. Only recently, dextran-coated cerium oxide nanoparticles has been used for noninvasive gastrointestinal tract imaging for inflammatory bowel disease.53 These nanoparticles produced strong CT contrast in the colitis area of large intestine and gets cleared from the body within 24 h of administration.
Fig. S1 (ESI†) shows cross sectional CT images of serially diluted iodine-based contrast agents, suspended BaSO4, and HfO2 nanoparticles under similar experimental conditions and concentration (0.01 mmol ml−1). As evident from the results, hafnium-based agents exhibited stronger contrast than iodine and barium. However, the benefit of using Hf will not be clear from conventional CT scanning as the test was performed at 80 keV. At this energy K-edge of iodine and barium would strongly influence their attenuation and the greater attenuation advantage of hafnium from its higher Z-value will be somewhat erased, with approximately equal attenuation from the two elements observed in practice.54,55 The advantage of hafnium is truly translation in nature, where the contrast agents based on this metal will not be compromised by the photon starvation and scattering as is the case for contrast agents with low K-edge energy, i.e., iodine and barium.
Herein, we report the rapid synthesis of ultra-small hafnia (HfO2 NPs) whose delivery has been mediated by nonvirulent probiotic strains. The engineering of this nano-bio-hybrid was inspired by the gut-friendly probiotics.56,57 This provided a biological basis for our attempt to deliver metallic contrast probes by shuttling or ‘hitchhiking’ the particles on probiotics. This reasoning led us to develop a prototype version of nano-bio-hybrid comprising probiotic-hitchhiking and metallic NPs. The hypothesized mechanism is that when probiotics are compressed through the GI tract, they transfer the NPs to the lining of the GI tract. We anticipate that this strategy will remarkably improve, generalize and may extend this early concept into producing a highly effective nanomedicine platform technology for GI diseases.
The synthesis of ultra-small hafnia (HfO2 NPs) is based on nitrate reduction of Hf containing precursor (Hf nitrate) at room temperature which resulted in large scale minute-made fabrication of ∼2 nm HfO2 nanodots stabilized by polyvinylpyrrolidone (PVP) (P-HfO2 NPs) without utilizing any toxic organic solvents. The internalization of these NPs in probiotics ensued by simple incubation to offer a nano-bio-hybrid entity (bio-HfO2) which was subsequently employed for the GI tract imaging using conventional and multispectral photon counting X-ray based imaging.33,34,58
Probiotics have the ability to colonize in the human GI tract and have been shown to confer therapeutic effects in several diseases such as the inflammatory bowel conditions and enteric infection.59,60 In this context, we utilized two probiotic strains namely Escherichia coli (E. coli) Nissle 1917 and Lactococcus lactis (L. lactis) which can internalize and concentrate the HfO2 NPs while they can withstand the rough GI condition to protect the cargo from leaching and degradation (Fig. 1C).
Hf has a strong X-ray attenuation coefficient and a well-positioned K-edge which makes it favorable as contrast agent in computed tomography (CT) and, in particular, in more advanced spectral CT technology.61 Its high biocompatibility and X-ray sensitizing are exploited in 50 nm NBTXR3 HfO2 NPs which is under clinical trials in Europe as radiosensitizer for the treatment of cancer.62 Previously, we have accomplished the synthesis of sub 5 nm HfO2 NPs via the sol–gel chemistry in nanoemulsion method to utilize for the specific differentiation and imaging of carious bacteria in complex dental biofilm.61 while we exploited a modified version of these NPs for the sensitive detection of bone microdamages.63 Others have concurrently reported the sol–gel chemistry followed by annealing, direct precipitation and microwave assisted hafnia NPs with sizes bigger than 15 nm.64–66 All these methods entail the utilization of toxic reagents while they are tedious and time consuming. In this work, we present a large-scale, rapid synthesis of ultra-small, well-dispersed HfO2 NPs.
The HfO2 NPs were obtained based on reduction and stabilization method. In a typical synthesis, 3 g of polyvinyl pyrrolidine (PVP) and 1 g of Hf(NO3)4 were dissolved in 100 ml of glycerol and 50 ml of anhydrous ethanol (EtOH) with a magnetic stir bar at ambient temperature. Subsequently, 500 mg of NaBH4 was added quickly to this solution and was allowed 2 min to form the P-HfO2 NPs. Finally, the NPs were centrifuged and were washed several times with ethanol and water in turn and were dried under vacuum. The NPs were easily dispersed in water by brief tip sonication.
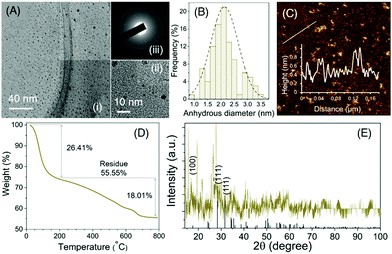
Transmission electron microscopy (TEM) indicated the formation of well-dispersed spherical NPs with the size distribution shown in Fig. 2Ai and ii. the average anhydrous diameter was calculated to be 2.13 ± 0.52 nm (Fig. 2B). The selected area diffraction pattern (SAED) is also shown in Fig. 2Aiii, where several diffraction spots and diffraction rings were identified implying the semi-crystalline nature of these ultra-small NPs. On the other hand, the height of P-HfO2 NPs was measured with atomic force microscopy (AFM) and the average height was determined to be 0.9 ± 0.1 nm (Fig. 2C).
The crystal structure of the material was further investigated with powder X-ray diffraction and the peak pattern can be assigned to (ICDD Card No. 00-034-0104) associated with HfO2 in monoclinic phase (Fig. 2F). Inductively coupled plasma–optical emission spectroscopy (ICP-OES) revealed that Hf percentage was 50.4 wt% while CHN analysis determined the C, H, N to be 4.05, 2.2, and 0.34 wt%, respectively. We characterized the amount of PVP coating with the aid of thermal gravimetric analysis (TGA) (Fig. 2E) where we observed a thermal event associated with the bound water in the sample. A second thermal event was starting from 200 °C attributed to PVP decomposition and the weight percentage was calculated to be 18.01% and afterwards the graph reached a plateau.
The UV-Vis absorbance spectrum is shown in Fig. 3A where a strong broad absorbance ranging from UV to NIR range can be noticed (Fig. 3A). In addition, the absorbance vs. concentration plot was constructed, and it could be seen that there is a linear relationship suggesting that the absorbance is not originating from scattering but rather from the innate absorbance from the NPs. To elucidate the chemical composition, we carried out Fourier transform infrared spectroscopy (FTIR) spectroscopy (Fig. 3B). For PVP, the peaks observed at 1019 cm−1 and 1281 cm−1 were attributed to C–N vibration, peak observed at 1435, 1655, 2953 cm−1 were related to CH2 bending vibration, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration in carbonyl, and asymmetric stretching vibration in CH2, respectively. Moreover, the peak at 3420 cm−1 was attributed to OH stretching vibration while 899 cm−1 was related to breathing vibration of the pyrrolidone ring. In the spectrum of P-HfO2 NPs, these peaks were preserved while the peak at 1655 cm−1 was shifted to 1637 cm−1 possibly due to the coordination of Hf ion and carbonyl oxygen.
O stretching vibration in carbonyl, and asymmetric stretching vibration in CH2, respectively. Moreover, the peak at 3420 cm−1 was attributed to OH stretching vibration while 899 cm−1 was related to breathing vibration of the pyrrolidone ring. In the spectrum of P-HfO2 NPs, these peaks were preserved while the peak at 1655 cm−1 was shifted to 1637 cm−1 possibly due to the coordination of Hf ion and carbonyl oxygen.
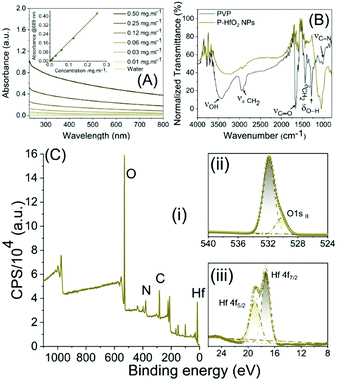 | ||
| Fig. 3 (A) UV-Vis absorbance spectra of NPs at various concentrations, (B) FTIR spectra; (C) XPS spectra in (i) survey mode, (ii) O1s region and (iii) Hf 4f region. | ||
We further analyzed the samples using X-ray photoelectron spectroscopy (XPS) which provides information on the surface functional groups. At first, the peak assignment for PVP was made (Fig. S2–S6, ESI†) where the peaks observed at 284.8, 285.80, and 287.49 eV in C1s (Fig. S5, ESI†) region could be correlated to C–C, C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively. On the other hand, the deconvolution of the peaks in the N1s region revealed the peak at 339.5 eV related to tertiary N (Fig. S4, ESI†) and finally the peaks of O1s at 531.0 eV was attributed to C
O, respectively. On the other hand, the deconvolution of the peaks in the N1s region revealed the peak at 339.5 eV related to tertiary N (Fig. S4, ESI†) and finally the peaks of O1s at 531.0 eV was attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in carboxyl (Fig. S3, ESI†). For P-HfO2 NPs, we inspected Hf 4f region to identify Hf 4f7/2 and Hf 4f5/2 observed at 17.3 and 18.9 eV related to Hf in valence number of 4+ observed in HfO2 (Fig. 3Ciii). The O1s peak at 530.2 eV can be correlated to OII in (Fig. 3Cii) and peak at 531.9 eV could be related to organic C
O in carboxyl (Fig. S3, ESI†). For P-HfO2 NPs, we inspected Hf 4f region to identify Hf 4f7/2 and Hf 4f5/2 observed at 17.3 and 18.9 eV related to Hf in valence number of 4+ observed in HfO2 (Fig. 3Ciii). The O1s peak at 530.2 eV can be correlated to OII in (Fig. 3Cii) and peak at 531.9 eV could be related to organic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.
O.
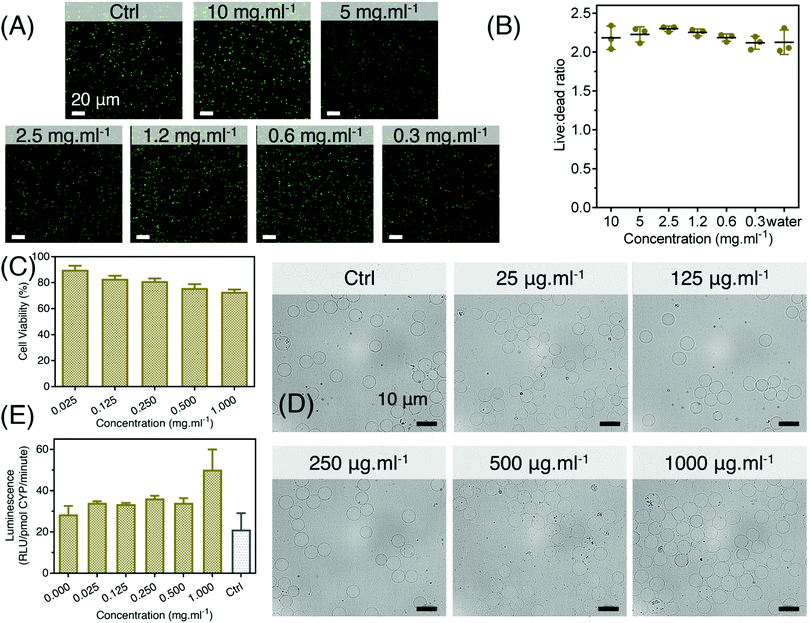
Having successfully synthesized and characterized these small NPs, we first investigated the dispersibility and stability of nanoparticles over time. It was observed that the time dependent increase in hydrodynamic diameter was quite insignificant at least up to six days and there was only minimal increase in size on the seventh day (Fig. S7, ESI†). It may be noted that the hydrodynamic diameter for the hafnia nanoparticles might be indicative of average size of the agglomerated particles which is found to be quite stable over time. Next, we examined their feasibility for the bio-interfacing application. We initially tested the cell compatibility of P-HfO2 NPs with the bacteria which can act as the vectors for their transport. We, therefore, incubated E. coli Nissle with NPs to concentrations as high as 10 mg ml−1. We subsequently carried out live/dead assay using BacLight™ bacterial viability kit which consists of two dyes; namely, SYTO9 and propidium iodide (PI) which determine the live and dead cells, respectively. The kit works based on the displacement of SYTO9 by PI when the bacterial cell membrane is damaged. The confocal images (Fig. 4A) revealed the comparable level of live bacteria identified by green compared to water treatment group without the damage to cell components. As shown in Fig. 4B, upon quantification, the ratio of live: dead cells are unchanged when increasing the concentration and there is no statistically significant difference among the groups. Interestingly, this ratio is maintained up to 10 mg ml−1 which is extremely high for conducting cell viability studies. This experiment has implication for CT imaging where the sensitivity of CT entails the application of contrast agents in mM range.
We further exposed the intestinal cells of human origin (Hs 1.Int cells (ATCC® CRL-7820™)) to determine the biocompatibility of HfO2 NPs using metabolic MTT assay. Intestinal cells were adopted for this experiment due to the oral route of administration for GI tract imaging. It was observed that the ultrasmall hafnia nanoparticles are biocompatible in nature, even at very high concentration of 1 mg ml−1 of the particles (Fig. 4C). This indicated optimum biosafety of the synthesized NPs.
In addition, the direct exposure of these NPs to blood is unlikely due to our administration method, it still presents a possible scenario in the case of GI bleeding, including hemorrhoids, peptic ulcers, and tearing or inflammation in the esophagus. With this mind, we further expanded our studies for the evaluation of hemocompatibility of P-HfO2 NPs. Briefly, we incubated the NPs with freshly acquired rabbit blood and then carried out ‘blood smear’ assay to account for their morphological deformation. No obvious clumping or morphological abnormalities in blood cells were perceived in fresh rabbit blood treated with various concentrations of ultrasmall HfO2 nanoparticles as compared with the control (untreated blood) as shown in Fig. 4D.
Ultimately, we evaluated the biosafety of the synthesized NPs by CYP3A4 assay. It is known that CYP3A4 are the most prevalent family of cytochrome P450 enzymes present in the liver and small intestine. The reduced activity of CYP3A4 enzyme is known to indicate slower metabolism of CYP3A4 substrates leading to an increase in substrate's in vivo concentration and thereby increased toxicity.67 On the contrary, we observed insignificant change in CYP3A4 activity with the increase in P-HfO2 NPs concentration. Moreover, there is a little positive cooperativity of the ultrasmall NPs with CYP substrates (Fig. 4E). This indicated that the ultrasmall HfO2 NPs will introduce insignificant metabolic toxicity and thus will be safe under in vivo conditions.
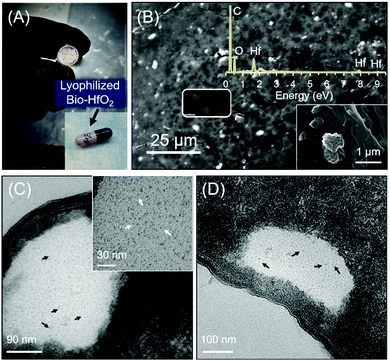
Having established their cytocompatibility, we reasoned that P-HfO2 NPs encapsulated in the probiotics (bio-HfO2) can provide a safer and patient-friendly means for GI tract imaging. To this end, we incubated P-HfO2 NPs with two probiotic types namely L. lactis and E. coli Nissle. As shown in the TEM images (Fig. 5C and D), the NPs are internalized by both strains of bacteria possibly due to their small size. No significant aggregation was noticed upon internalization by the bacteria (Fig. 5C inset). L. lactis is a Gram-positive bacterium while E. coli Nissle is a Gram-negative strain. Importantly, we noticed that after the incubation of NPs with these bacteria, the cell wall structure is well-preserved as shown in TEM images.
Furthermore, to make this method feasible for potential human application, we devised a strategy for their storage. We incubated E. coli Nissle with 10 mg ml−1 P-HfO2 NPs as mentioned previously and then washed multiple times to remove the excess of NPs. Subsequently, the samples were freeze dried for further application using 10 wt% sucrose as lyoprotective media. As could be inferred from the EDS of the lyophilized bacteria, the binding energy of Hf is evident, hence confirming the internalization of P-HfO2 NPs in the bacterial cells. Moreover, we revived the stored cells in the following days in the media and the cells indicated normal growth rate and OD600 reached to 2.09 after 24 h growth (Fig. S8, ESI†). This strategy can be useful for actual clinical setup, where the freeze-dried probiotic can be packed in a capsule for the facile oral administration by the patient. As a proof of concept, we packed the lyophilized bacteria in the gelatin capsule as shown in Fig. 5A which shows the potential means for the application of these NPs in the clinical settings.
Hf is a high atomic number material with a well-placed K-edge (65.5 keV) within the diagnostic X-ray energy range. Using Hf with spectral photon-counting CT generates high CT contrast due to a reasonable number of photons below and above its K-edge. Such spectrally effective imaging properties of Hf show its potential as a suitable element as contrast agents. MARS scanners use spectral photon counting detectors to divide conventional broad X-ray spectrum into multiple separate bins. The spectral information enables the identification and quantification of multiple materials simultaneously.68
A MARS scanner was used to assess the Hf spectral response. The scanner provides spectral and material images at high spatial resolution (∼90 μm cubic voxel size). We used four energy counters in the charge summing mode which provides high energy resolution (between 3 to 5 keV FWHM).69,70 A multi-material calibration phantom containing P-HfO2 NPs, bone-like hydroxyapatite (HA), and lipid were scanned. The calibration data was used to produce the material images for both the phantom and the upcoming in vivo studies.71,72
The calibration phantom is shown in Fig. S9 (ESI†) and the corresponding material channels are shown in Fig. 6B; all four materials are identified correctly in their calibration vials using the energy information. Examples of one CT slice from each energy bin are shown in Fig. S10 (ESI†). The spectral response for Hf and HA was graphically evaluated (Fig. 6C and D) and the linear correlation between attenuation and concentration across all four energy bins (Fig. 6E and F) was assessed. Fig. 6C shows the rise in attenuation in the third energy bin (65–80 keV) which corresponds to Hf's K-edge energy range, whereas no such response is observed for HA in Fig. 6B. Linear regression generated R2 values very close to 1 for all lines suggesting a good fit. The accuracy of material identification and quantification is influenced by the linearity established for each energy bin, using the calibration vials.
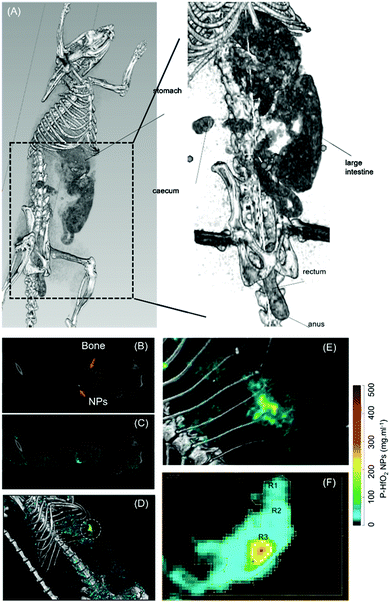
Having established the CT attenuation properties of P-HfO2 as well as their potential for application in spectral CT, we sought the application of bio-HfO2 NPs as a contrast agent in conventional CT in vivo. For this experiment, the OD600 for E. coli Nissle was adjusted to 0.6 and then it was incubated with 54 mg of NPs for 4 h. The amount of Hf internalized by the probiotics was measured by Inductively coupled plasma – optical emission spectrometry (ICP-OES), calculated from the ICP standard curve generated for Hf (Fig. S11, ESI†) and tabulated in the Table S1 (ESI†). It was observed that internalized Hf concentration in the probiotics was 4.582 ppm. Separately, the incubated probiotic sample was lyophilized as detailed above. The formulation was resuspended in 1 ml of PBS and was then administered via oral gavage to Sprague Dawley rats using a flexible feeding tube. The rats were then imaged after 30 min GI tract are lighting up in the presence of the bio-HfO2 (Fig. 7A). The internal organs namely intestine, caecum and small bowel diverticula were also observed. Interestingly, the contrast materials were detected in the rectum and anus (lower section) of the GI tract suggesting their fast clearance within 30 min. This experiment also indicated that the contrast agent can favorably withstand the various pH changes and harsh condition of the GI tract while avoiding the systemic absorption.
Fig. 7B and C show the one charge summing mode (CSM) energy bin of the rat without and with Hf material channel overlay, respectively. Fig. 7D shows the material images in 3D view and gives a clear indication of where P-HfO2 NPs has accumulated i.e., in the stomach/gut. Material decomposition showed Hf could be distinguished from bone (Fig. 7C) whereas, in the greyscale image, the bio-HfO2 NPs appear like calcium (Fig. 7B).
In addition, we quantified the concentration of bio-HfO2 (with reference to P-HfO2 NPs) within the region of interest circled in Fig. 7D. These concentration values provide information to assess the NPs delivery. This dense region of Hf material within the GI tract was selected for quantification. By selecting the region of interest within several slides, Hf was quantified (Fig. S14, ESI†). The quantification of Hf in the stomach revealed that the concentration ranged between 2.79 and 447.0 mg ml−1. The region was split into low, mid, and high concentrations. Several slices were selected and contoured according to min and max concentration (Fig. 7F). The resulting measurements are displayed in Table 1. The overall measurement (N = 6683) of Hf concentration was thus found to be a multi-modal distribution. Fig. S14a (ESI†) shows the distribution from one of these regions, which measured min, max, median, and mean. This experiment revealed the potential of bio-HfO2 for quantitative assessments using spectral CT.
| N | Min (mg ml−1) | Max (mg ml−1) | Mean (mg ml−1) | |
|---|---|---|---|---|
| R1 (low) | 3670 | 15 | 7 | 7 |
| R2 (mid) | 2479 | 78 | 30 | 30 |
| R3 (high) | 535 | 447 | 221 | 232 |
| Overall | 6683 | 3 | 11 | 35 |
Thus, we have developed a novel microencapsulation technique where probiotics were proposed as an effective ‘hitchhiker’ to improve the survival, resistance, and targeted release of nanoparticle contrast materials in the GI tract. The inherent sensitivity of CT is low and therefore, high concentration of contrast materials must be used for in vivo bioimaging. The microencapsulation technique developed here would allow us for effective entrapment of high quantities of metallic nanoparticles within a biocompatible microenvironment that protects the particles from exposure to exterior factors (such as low gastric pH) during digestion, and subsequently to reduce cell injury or cell death before their release at the target site.
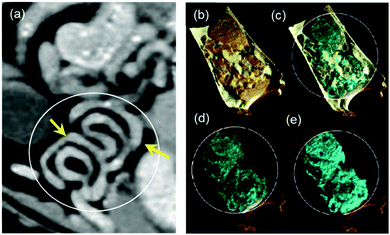
Proof-of-concept k-edge imaging study was also performed in an elaborate manner to compare conventional CT and spectral CT and the delineation of energy bins for 4 elements in phantoms. For in vivo study, material-weighted photon counting CT images of the GI tract was imaged and demonstrated to show unprecedented delineation of soft tissue, lipid, HA (calcium), which was absent in the conventional CT of the GI tract of a rat administered with iodine contrast agent (Fig. 7a and 8a). To demonstrate the transfer of the particles in the GI tract, we have also acquired multiple CT images to confirm the presence of hafnia nanoparticles. We have dissected the rat and excised its GI tract for imaging purposes. To demonstrate the clinical applicability of this technology, images without fasting before taking the probiotic-Hf capsule were acquired for the rats. Accordingly, the rats were fed before the gavage which means that there was food in the digestive organs, including the GI tract. It is known that most lab food provided to rats are calcium-rich,73 then we can expect to detect food (calcium) in the HA channel. In this scenario, we used 4 basis vectors to run the Materials Decomposition (MD) algorithms in the spectral CT: Hf, HA (to represent Ca), lipid, and water. It was understood that from the calibration vials, there is a chance that low HfO2 nanoparticle concentration can be misidentified as HA. But we have used quite a high amount of Hf during the experiment, i.e., 54 mg of hafnia nanoparticle in 1 ml of PBS which was then administered via oral gavage to Sprague Dawley rats using a flexible feeding tube. Therefore, the majority of the voxels contain a high concentration of Hf, which means the chance of misidentification is significantly low. It also indicated the high transfer of nanoparticles to the lining of GI tract when probiotics were compressed through the GI tract. Thus, where conventional CT cannot make the distinction between Hf and calcium/mineral material in food, the spectral CT images can efficiently discriminate Hf signal from food signal. The data related to this study has been provided in (Fig. 8). We further evaluated in vivo toxicity of the synthesized nanoparticle on different clearance organs. As the nanoparticles were gavaged, the toxicity of the nanoparticles was evaluated on small intestine, colon, rectum, liver and kidney. However, no change on hematoxylin and eosin profile was found for all the tested organs indicating insignificant in vivo toxicity of the nanoparticles (Fig. S15, ESI†).
In conclusion, we designed ultra-small (∼2 nm) P-HfO2 NPs which were obtained via an ultrafast facile synthetic procedure. Subsequently, in a proof-of-concept study, we utilized the interface of nanomaterials and biological entities (probiotics) to pack and deliver GI contrast agents for the patient-friendly oral delivery. The NPs showed immense cytocompatibility in concentrations as high as 10 mg ml−1. The CT imaging of the animal administered with these NPs promisingly indicated the details of GI tract. Interestingly, we could observe distinct contrast for P-HfO2 NPs under in vivo conditions even in the fed state post 30 min of administration. Hence, we will take this opportunity to investigate the effect on contrast post P-HfO2 NP administration in vivo at different time points through a separate full-blown study. In that future study, we will also monitor the time-dependent contrast and clearance of P-HfO2 NPs under in vivo setting. The strategy indicated here can potentially be applied for a gamut of diseases such as cancer and cardiovascular diseases in the future. The scope of this present work is, however, to propose for the first time hitchhiking a probiotic vector to deliver high density hafnium nanodots for multicolor delineation of intestinal wall using photon counting CT. The translation of nanoparticle-based contrast probes consists of several stages from idea conceptualization, in vitro testing, preclinical testing in a small cohort of animals to final large-scale clinical trials. This current study majorly emphasizes the idea conceptualization more than any other stage and is a proof-of-concept study and for the full translation of this technology more preclinical evaluation will be warranted in the future. The complete translation of this technology does indeed require more in-depth studies which will be warranted in the follow up future studies.
Author contributions
D. P. conceived the idea. F. O. and D. P. designed the study. F. O., M. N., I. T. and S. K. M. performed the initial study and developed the materials suitable for spectral C. T. C. L., M. M., A. B., N. R. and H. M. acquired the spectral C. T. scans. P. M. and N. G. contributed towards the revision of this manuscript by performing additional experiments to support the hypothesis. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. F. O. and P. M. contributed equally to this manuscript.Funding sources
This work was financially supported by UIUC and NIH. FO gratefully acknowledge partial support from American heart association grant (#18pre34080003/2018) and Beckman institute postdoctoral fellowship.Conflicts of interest
Prof. Pan is the founder or co-founder of three University based start ups. None of these entities however, supported this work. All the other authors declare no conflicts of interests.Acknowledgements
Zeta potential, FTIR, TEM, XRD and XPS measurements were conducted at the Frederick Seitz Materials Research Laboratory, UIUC. The authors thank Dr Richard Haasch for help with the analysis and experiment for XPS studies. The authors also greatly thank help from veterinary medicine CT suit staff (Susan Hartman). We appreciate help from Dr Mauro Sardela for carrying our XRD experiment.References
- J. Keller, G. Bassotti, J. Clarke, P. Dinning, M. Fox, M. Grover, P. M. Hellström, M. Ke, P. Layer and C. Malagelada, Nat. Rev. Gastroenterol. Hepatol., 2018, 15, 291 CrossRef PubMed.
- J. Burggraaf, I. M. Kamerling, P. B. Gordon, L. Schrier, M. L. De Kam, A. J. Kales, R. Bendiksen, B. Indrevoll, R. M. Bjerke and S. A. Moestue, Nat. Med., 2015, 21, 955 CrossRef CAS PubMed.
- X. Yang, J. F. Lovell and Y. Zhang, ChemBioChem, 2019, 20, 462–473 CrossRef CAS PubMed.
- Q. Cheng, H. Shi, H. Huang, Z. Cao, J. Wang and Y. Liu, Chem. Commun., 2015, 51, 17536–17539 RSC.
- M. W. Tibbitt, J. E. Dahlman and R. Langer, J. Am. Chem. Soc., 2016, 138, 704–717 CrossRef CAS PubMed.
- D. Pan, M. Pramanik, S. A. Wickline, L. V. Wang and G. M. Lanza, Contrast Media Mol. Imaging, 2011, 6, 378–388 CrossRef CAS PubMed.
- P. Mukherjee, S. K. Misra, M. C. Gryka, H.-H. Chang, S. Tiwari, W. L. Wilson, J. W. Scott, R. Bhargava and D. Pan, Small, 2015, 11, 4691–4703 CrossRef CAS PubMed.
- E. M. Pridgen, F. Alexis, T. T. Kuo, E. Levy-Nissenbaum, R. Karnik, R. S. Blumberg, R. Langer and O. C. Farokhzad, Sci. Transl. Med., 2013, 5, 213ra167 Search PubMed.
- S. Mitragotri, P. A. Burke and R. Langer, Nat. Rev. Drug Discovery, 2014, 13, 655–672 CrossRef CAS PubMed.
- R. Wang, L. Zhou, W. Wang, X. Li and F. Zhang, Nat. Commun., 2017, 8, 14702 CrossRef PubMed.
- C. T. N. Pham, D. G. Thomas, J. Beiser, L. M. Mitchell, J. L. Huang, A. Senpan, G. Hu, M. Gordon, N. A. Baker, D. Pan, G. M. Lanza and D. E. Hourcade, Nanomedicine, 2014, 10, 651–660 CrossRef CAS PubMed.
- D. Pan, M. Pramanik, A. Senpan, S. A. Wickline, L. V. Wang and G. M. Lanza, J. Nanosci. Nanotechnol., 2010, 10, 8118–8123 CrossRef CAS PubMed.
- D. Akin, J. Sturgis, K. Ragheb, D. Sherman, K. Burkholder, J. P. Robinson, A. K. Bhunia, S. Mohammed and R. Bashir, Nat. Nanotechnol., 2007, 2, 441 CrossRef CAS PubMed.
- M. H. Ross, A. K. Esser, G. C. Fox, A. H. Schmieder, X. Yang, G. Hu, D. Pan, X. Su, Y. Xu, D. V. Novack, T. Walsh, G. A. Colditz, G. H. Lukaszewicz, E. Cordell, J. Novack, J. A. J. Fitzpatrick, D. L. Waning, K. S. Mohammad, T. A. Guise, G. M. Lanza and K. N. Weilbaecher, Cancer Res., 2017, 77, 6299–6312 CrossRef CAS PubMed.
- M. S. Khan, S. K. Misra, K. Dighe, Z. Wang, A. S. Schwartz-Duval, D. Sar and D. Pan, Biosens. Bioelectron., 2018, 110, 132–140 CrossRef CAS PubMed.
- R. H. Fang, A. V. Kroll, W. Gao and L. Zhang, Adv. Mater., 2018, 30, 1706759 CrossRef PubMed.
- D. Pan, A. H. Schmieder, K. Wang, X. Yang, A. Senpan, G. Cui, K. Killgore, B. Kim, J. S. Allen, H. Zhang, S. D. Caruthers, B. Shen, S. A. Wickline and G. M. Lanza, Theranostics, 2014, 4, 565–578 CrossRef CAS PubMed.
- F. Ostadhossein and D. Pan, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2017, 9, e1436 Search PubMed.
- O. Felfoul, M. Mohammadi, S. Taherkhani, D. De Lanauze, Y. Z. Xu, D. Loghin, S. Essa, S. Jancik, D. Houle and M. Lafleur, Nat. Nanotechnol., 2016, 11, 941 CrossRef CAS PubMed.
- M. Mahmoudi, N. Bertrand, H. Zope and O. C. Farokhzad, Nano Today, 2016, 11, 817–832 CrossRef CAS.
- I. Srivastava, S. K. Misra, F. Ostadhossein, E. Daza, J. Singh and D. Pan, Nano Res., 2017, 10, 3269–3284 CrossRef CAS.
- M. S. Khan, K. Dighe, Z. Wang, I. Srivastava, E. Daza, A. S. Schwartz-Dual, J. Ghannam, S. K. Misra and D. Pan, The Analyst, 2018, 143, 1094–1103 RSC.
- S. K. Misra, H.-H. Chang, P. Mukherjee, S. Tiwari, A. Ohoka and D. Pan, Sci. Rep., 2015, 5, 14986 CrossRef CAS PubMed.
- D. Pan, Mol. Pharmaceutics, 2013, 10, 781–782 CrossRef CAS PubMed.
- S. K. Misra, F. Ostadhossein, E. Daza, E. V. Johnson and D. Pan, Adv. Funct. Mater., 2016, 26, 8031–8041 CrossRef CAS.
- M. A. Dobrovolskaia and S. E. McNeil, Nat. Nanotechnol., 2007, 2, 469 CrossRef CAS PubMed.
- B. Illes, P. Hirschle, S. Barnert, V. Cauda, S. Wuttke and H. Engelke, Chem. Mater., 2017, 29, 8042–8046 CrossRef CAS.
- M. Goldberg and I. Gomez-Orellana, Nat. Rev. Drug Discovery, 2003, 2, 289 CrossRef CAS PubMed.
- G. Traverso and R. Langer, Nature, 2015, 519, S19 CrossRef CAS PubMed.
- Z. Wu, L. Li, Y. Yang, P. Hu, Y. Li, S.-Y. Yang, L. V. Wang and W. Gao, Sci. Robotics, 2019, 4, eaax0613 CrossRef PubMed.
- G. Iddan, G. Meron, A. Glukhovsky and P. Swain, Nature, 2000, 405, 417 CrossRef CAS PubMed.
- Z. Liu, X. Ran, J. Liu, Y. Du, J. Ren and X. Qu, Biomaterials, 2016, 100, 17–26 CrossRef CAS PubMed.
- D. Pan, E. Roessl, J. P. Schlomka, S. D. Caruthers, A. Senpan, M. J. Scott, J. S. Allen, H. Zhang, G. Hu and P. J. Gaffney, Angew. Chem., Int. Ed., 2010, 49, 9635–9639 CrossRef CAS PubMed.
- D. Pan, C. O. Schirra, A. Senpan, A. H. Schmieder, A. J. Stacy, E. Roessl, A. Thran, S. A. Wickline, R. Proska and G. M. Lanza, ACS Nano, 2012, 6, 3364–3370 CrossRef CAS PubMed.
- D. P. Cormode, S. Si-Mohamed, D. Bar-Ness, M. Sigovan, P. C. Naha, J. Balegamire, F. Lavenne, P. Coulon, E. Roessl, M. Bartels, M. Rokni, I. Blevis, L. Boussel and P. Douek, Sci. Rep., 2017, 7, 4784 CrossRef PubMed.
- R. K. Panta, A. P. Butler, P. H. Butler, N. J. de Ruiter, S. T. Bell, M. F. Walsh, R. M. Doesburg, A. I. Chernoglazov, B. P. Goulter and P. Carbonez, IEEE Nuclear Science Symposium and Medical Imaging Conference Proceedings (NSS/MIC), 2018, p 1 Search PubMed.
- K. Taguchi and J. S. Iwanczyk, Med. Phys., 2013, 40, 100901 CrossRef PubMed.
- S. A. Si-Mohamed, J. Miailhes, P.-A. Rodesch, S. Boccalini, H. Lacombe, V. Leitman, V. Cottin, L. Boussel and P. Douek, J. Clin. Med., 2021, 10, 5757 CrossRef PubMed.
- M. J. Willemink, M. Persson, A. Pourmorteza, N. J. Pelc and D. Fleischmann, Radiology, 2018, 289, 293–312 CrossRef PubMed.
- J. Balegamire, M. Vandamme, E. Chereul, S. Si-Mohamed, S. A. Maache, E. Almouazen, L. Ettouati, H. Fessi, L. Boussel and P. Douek, Biomater. Sci., 2020, 8, 5715–5728 RSC.
- M. A. Kimm, M. Shevtsov, C. Werner, W. Sievert, W. Zhiyuan, O. Schoppe, B. H. Menze, E. J. Rummeny, R. Proksa and O. Bystrova, Cancers, 2020, 12, 1331 CrossRef CAS PubMed.
- X. Meng, Y. Wu and W. Bu, Adv. Healthcare Mater., 2020, 2000912 Search PubMed.
- F. Ostadhossein, I. Tripathi, L. Benig, D. LoBato, M. Moghiseh, C. Lowe, A. Raja, A. Butler, R. Panta and M. Anjomrouz, Adv. Funct. Mater., 2020, 30, 1904936 CrossRef CAS.
- X. Wang, J. Wang, J. Pan, F. Zhao, D. Kan, R. Cheng, X. Zhang and S.-K. Sun, ACS Appl. Mater. Interfaces, 2019, 11, 33650–33658 CrossRef CAS PubMed.
- S. Si-Mohamed, D. P. Cormode, D. Bar-Ness, M. Sigovan, P. C. Naha, J.-B. Langlois, L. Chalabreysse, P. Coulon, I. Blevis, E. Roessl, K. Erhard, L. Bousselab and P. Douek, Nanoscale, 2017, 9, 18246–18257 RSC.
- W. Liao, P. Lei, J. Pan, C. Zhang, X. Sun, X. Zhang, C. Yu and S.-K. Sun, Biomaterials, 2019, 203, 1–11 CrossRef CAS PubMed.
- M. W. Galper, M. T. Saung, V. Fuster, E. Roessl, A. Thran, R. Proska, Z. A. Fayad and D. P. Cormode, Invest Radiol., 2012, 47, 475–481 CrossRef CAS PubMed.
- A. Saraste, S. G. Nekolla and M. Schwaiger, Cardiovasc. Res., 2009, 83, 643–652 CrossRef CAS PubMed.
- L. Ren, N. Huber, K. Rajendran, J. G. Fletcher, C. H. McCollough and L. Yu, Invest. Radiol., 2022, 57, 122–129 CrossRef CAS PubMed.
- M. Moghiseh, C. Lowe, J. G. Lewis, D. Kumar, A. Butler, N. Anderson and A. Raja, Contrast Media Mol. Imaging, 2018, 2136840 Search PubMed.
- M. Getzin, J. J. Garfield, D. S. Rundle, U. Kruger, A. P. H. Butler, M. Gkikas and G. Wang, J. X-Ray Sci. Technol., 2018, 26, 707–726 CAS.
- S. A. Si-Mohamed, M. Sigovan, J. C. Hsu, V. Tatard-Leitman, L. Chalabreysse, P. C. Naha, T. Garrivier, R. Dessouky, M. Carnaru, L. Boussel, D. P. Cormode and P. C. Douek, Radiology, 2021, 300, 98–107 CrossRef PubMed.
- P. C. Naha, J. C. Hsu, J. Kim, S. Shah, M. Bouché, S. Si-Mohamed, D. N. Rosario-Berrios, P. Douek, M. Hajfathalian, P. Yasini, S. Singh, M. A. Rosen, M. A. Morgan and D. P. Cormode, ACS Nano, 2020, 14, 10187–10197 CrossRef CAS PubMed.
- C. Kramer, Circulation, 2008, 117, 1333–1339 CrossRef PubMed.
- P. A. Jackson, W. N. W. A. Rahman, C. J. Wong, T. Ackerly and M. Geso, Eur. J. Radiol, 2010, 75, 104–109 CrossRef PubMed.
- S. Zhang, R. Langer and G. Traverso, Nano Today, 2017, 16, 82–96 CrossRef CAS PubMed.
- Z. Zhou, X. Chen, H. Sheng, X. Shen, X. Sun, Y. Yan, J. Wang and Q. Yuan, Microbial Cell Factories, 2020, 19, 56 CrossRef PubMed.
- D. Pan, A. H. Schmieder, A. SenPan, X. Yang, S. A. Wickline, E. Roessl, R. Proksa, C. O. Schirra and G. M. Lanza, Design and Applications of Nanoparticles in Biomedical Imaging, Springer, 2017, pp. 385–402 Search PubMed.
- T. Danino, A. Prindle, G. A. Kwong, M. Skalak, H. Li, K. Allen, J. Hasty and S. N. Bhatia, Sci. Transl. Med., 2015, 7, 289ra84 Search PubMed.
- J. Suez, N. Zmora, E. Segal and E. Elinav, Nat. Med., 2019, 25, 716–729 CrossRef CAS PubMed.
- F. Ostadhossein, S. K. Misra, I. Tripathi, V. Kravchuk, G. Vulugundam, D. LoBato, L. E. Selmic and D. Pan, Biomaterials, 2018, 181, 252–267 CrossRef CAS PubMed.
- S. Bonvalot, P. L. Rutkowski, J. Thariat, S. Carrère, A. Ducassou, M.-P. Sunyach, P. Agoston, A. Hong, A. Mervoyer and M. Rastrelli, The Lancet Oncology, 2019, 20, 1148–1159 CrossRef CAS PubMed.
- F. Ostadhossein, I. Tripathi, L. Benig, D. LoBato, M. Moghiseh, C. Lowe, A. Raja, A. Butler, R. Panta and M. Anjomrouz, Adv. Funct. Mater., 2020, 30, 1904936 CrossRef CAS.
- T. L. McGinnity, O. Dominguez, T. E. Curtis, P. D. Nallathamby, A. J. Hoffman and R. K. Roeder, Nanoscale, 2016, 8, 13627–13637 RSC.
- L. R. Gerken, K. Keevend, Y. Zhang, F. H. Starsich, C. Eberhardt, G. Panzarasa, M. T. Matter, A. Wichser, A. Boss and A. Neels, ACS Appl. Mater. interfaces, 2018, 11, 437–448 CrossRef PubMed.
- O. Dominguez, T. L. McGinnity, R. K. Roeder and A. J. Hoffman, Appl. Phys. Lett., 2017, 111, 011101 CrossRef.
- S.-J. Choi and J.-S. Choi, Biomol. Ther., 2010, 18, 469–476 CrossRef CAS.
- N. G. Anderson and A. P. Butler, Contrast Media Mol. Imaging, 2014, 9, 3–12 CrossRef CAS PubMed.
- R. Ballabriga, M. Campbell, E. Heijne, X. Llopart and L. Tlustos, IEEE Trans. Nuclear Sci., 2007, 54, 1824–1829 Search PubMed.
- R. Ballabriga, J. Alozy, G. Blaj, M. Campbell, M. Fiederle, E. Frojdh, E. Heijne, X. Llopart, M. Pichotka and S. Procz, J. Instrumentation, 2013, 8, C02016 CrossRef.
- C. Bateman, Methods for Material Discrimination in MARS Multi-energy CT, PhD thesis, University of Otago, Christchurch, New Zealand, 2015 Search PubMed.
- C. Bateman, D. Knight, B. Brandwacht, J. Mc Mahon, J. Healy, R. Panta, R. Aamir, K. Rajendran, M. Moghiseh and M. Ramyar, J. Instrumentation, 2018, 13, P05020 CrossRef.
- S. M. Lewis, D. E. Ullrey, D. E. Barnard and J. J. Knapka, American College of Laboratory Animal Medicine, The Laboratory Rat, 2nd edn, 2006, pp. 219–301 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental section as well as XPS characterizations and more spectral CT data. See DOI: 10.1039/d1nh00626f |
| ‡ The authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |