Open Access Article
Open Access ArticleConstruction of a redox-responsive drug delivery system utilizing the volume of AS1411 spatial configuration gating mesoporous silica pores†
Lu
Zhou
 a,
Yajie
Zhang
a,
Yajie
Zhang
 b and
Yong
Ma
b and
Yong
Ma
 *a
*a
aDepartment of Chemistry, School of Forensic Medicine, China Medical University, Shenyang 110122, China. E-mail: yma10@cmu.edu.cn
bDepartment of Gastroenterology, Shengjing Hospital of China Medical University, Shenyang 110004, China
First published on 19th August 2022
Abstract
In recent years, diverse redox-responsive drug delivery systems have emerged to prevent premature drug release and reduce drug toxicity in the human body in cancer treatment. In this paper, we put forward a view of directly utilizing the spatial structure size of the AS1411 aptamer as the nano-gatekeeper on the pore openings of MCM-41 type mesoporous silica and thus constructed a redox-responsive drug delivery system named MCM-41-SS-AS1411. The particles obtained at each step were characterized by TEM, FTIR, SXRD, TGA and zeta potential measurement. The characterization data confirmed that the particles were successfully prepared. The binding amount of the aptamer was ca. 3.1 × 103 for each carrier particle averagely. The anticancer drug Dox was regarded as a drug model to investigate the redox-controlled drug release behavior by fluorescence measurements. The investigation results demonstrate that the spatial volume of aptamer AS1411 can block the mesopore, and this drug-carrier can realize controlled drug release by GSH. We hope this idea can play a prompt role in relevant research. Meanwhile, the preparation steps of this DDS are simplified.
1. Introduction
Premature drug release has been a challenging problem in drug delivery systems (DDSs) used to overcome cancer. If this problem can be prevented, the side effects would be reduced, and drug efficacy would be improved. Hence the development of stimulus-responsive DDSs has received extensive attention in recent years. Some materials can encapsulate the drug molecules in carriers before the DDS reaches the target sites, and drugs will not be released in advance during transportation. Moreover, these materials can be affected by changes in pH,1,2 redox,3,4 enzymes,5 temperature or light. Through such stimuli, drug molecules can be released. Redox is widely used as an endogenous stimulus. In redox stimulation, glutathione (GSH) which can break disulfide bonds (–S–S–) is an important reductant in biochemical reactions. The concentration of GSH was about 10 µM in extracellular fluid, while it was up to 1–10 mM in intracellular fluid.6 And the concentration of GSH in the cytoplasm of most tumor cells is at least 3 times higher than that of normal cells.7 The disulfide bonds can exist stably in extracellular fluid and be easily broken in intracellular fluid due to the significant difference in the concentration of GSH in intracellular and extracellular fluids. This feature can be used for stimulus-responsive DDSs.Mesoporous silica nanoparticles (MSNs) are regarded as an ideal carrier for stimulus-responsive DDSs,8 and MCM-41 is a commonly used type.9 The pores of MCM-41 present a one-dimensional hexagonal highly ordered arrangement with various pore diameters from 1.5 to 10 nm.9–11
Researchers have proposed targeted controlled-release DDSs so as to deliver effective doses of drugs to target tumor cells or tissues,12 reduce drug accumulations in healthy organs or tissues, and improve the biodistribution of drugs in the whole body. An aptamer is a kind of oligomeric single-stranded DNA (ssDNA) or RNA molecule with specific recognition function, which was selected through systematic evolution of ligands by exponential enrichment (SELEX) technology.13–15 It can fold into a unique three-dimensional conformation through intramolecular interactions14 and specifically bind to target molecules such as target polypeptides, protein receptors and intracellular biological macromolecules by its excellent affinity.16 It also provides attractive characteristics such as small size, easy synthesis and modification, and low toxicity and immunogenicity, as well as high thermal stability. Therefore, it has become an ideal molecular probe for diagnosis and therapeutic applications.17,18 The AS1411 aptamer is a kind of ssDNA with 26 bases (sequence: 5′-GGTGGTGGTGGT-TGTGGTGGTGGTGG-3′), which can selectively bind to nucleolin. Nucleolin is overexpressed on the surface of many cancer cells but not on normal ones. In recent years, there have been a few research studies on combining mesoporous silica and an aptamer. For example, Sa et al.19 impregnated pores of SBA-15 with an aptamer (functionalized for the tumor marker MUC-1) and demonstrated the ability of the complex to bind and internalize into the cells. Nejabat et al.20 coated the surface of doxorubicin-encapsulated HMSNs with acetylated carboxymethyl cellulose (Ac-CMC) and then covalently conjugated to the AS1411 aptamer for guided drug delivery to nucleolin. Li et al.21 reported anti-miR-155-loaded MSNs modified with polymerized dopamine (PDA) and the AS1411 aptamer (MSNs-anti-miR-155@PDA-Apt) for the targeted treatment of CRC. Nevertheless, we intended to construct a DDS that could fill drug molecules in the silica pores and has multiple functions, such as stimuli-responsiveness, pore-capping and targeting cancer cells or tissues, but only a single coating is enough. Therefore, we put forward an idea of directly utilizing the spatial structure size of the AS1411 aptamer to cap the pore-opening of Dox-loaded mesoporous silica to simplify the preparation of the multifunctional DDS.
In the present work, MCM-41 type MSN was used as the drug carrier, and its surface was modified with –SH. The AS1411 aptamer modified with –SH at one end of its chain was bound to the Dox-loaded MSN through one disulfide bond. AS1411 forms a structure of G-quadruplex due to its rich G content, and the diameter of its spatial structure is 2–3 nm (ref. 22) which was suitable for capping the pore of the MSN (pore size 3–5 nm) used in this study. The aptamers will be separated, and the pores can open and release Dox when –S–S– is broken by glutathione. For one thing, the spatial volume of AS1411 can cap the pores so as to prevent premature drug release. For another, the prepared DDS can respond to GSH so that cargo molecules can be released in a tumor cell microenvironment. Moreover, aptamers are well known for their targeting characteristics and has attracted extensive attention, and the targeting effect of AS1411 has been confirmed by many research studies.23–27 So it can be inferred that the concept of directly using AS1411 to cap the pores can provide many advantages. This DDS is expected to possess comprehensive features and functions simultaneously. But in this paper, we mainly focus on discussing the encapsulating effect of utilizing the spatial volume of AS1411 as well as the stimulus-responsive controlled release behavior of the DDS.
2. Results and discussion
2.1 TEM
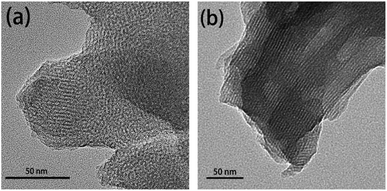
The morphology of mesopores of MCM-41 and MCM-41-SS-AS1411 were determined by transmission electron microscopy (TEM) (Fig. 1). As can be seen from the figure, the pore channel is well ordered, and its average diameter is 3–5 nm. The change of the pore structure is hardly observed before and after AS1411 was grafted. Fig. S1† shows the particle size and morphology of MCM-41 and MCM-41-SS-AS1411.2.2 FTIR
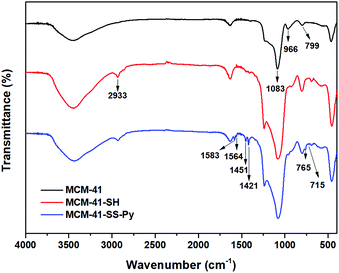
The FTIR spectra of MSNs are shown in Fig. 2. The spectrum of original MCM-41 is included for comparison. The band at around 1083 cm−1 is due to Si–O–Si stretching vibration, and the band at 799 cm−1 is due to Si–C. Compared with MCM-41, the weak new absorption peak at 2566 cm−1 is –SH28 ( red curve). And the new peak at 2933 cm−1 is caused by the asymmetric stretching vibration of –CH2– contained in the modified group which confirmed the formation of MCM-41-SH. Meanwhile, the weak band at around 966 cm−1 associated with Si–OH groups is present in the pure MCM-41 but disappears after modification with MPTMS. It also strongly reveals that –SH has been anchored on the surface of MCM-41 through the interaction with the surface silanol groups. These illustrate that the –SH groups have been successfully grafted on the surface of MCM-41 via covalent bonds. After DTDP reacted with MCM-41-SH, the spectrum of their product MCM-41-SS-Py is also shown in Fig. 2 as the blue curve. The characteristic absorption bands at around 1583, 1564, 1451 and 1421 cm−1 are attributed to the stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds in the pyridine structures, respectively. New characteristic absorption peaks at 765 and 715 cm−1 are due to the out-of-plane vibration of C–H on the pyridine ring, which also proves the successful synthesis of these particles.
N bonds in the pyridine structures, respectively. New characteristic absorption peaks at 765 and 715 cm−1 are due to the out-of-plane vibration of C–H on the pyridine ring, which also proves the successful synthesis of these particles.
2.3 SXRD
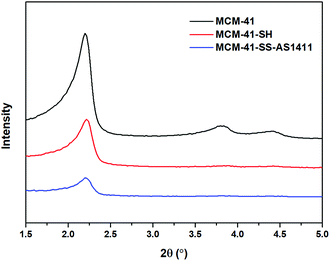
As presented in Fig. 3, the small-angle X-ray diffraction (SXRD) patterns of MCM-41 exhibit the (100), (110) and (200) diffraction peaks at 2θ = 2.2, 3.8 and 4.4°, respectively, which are typical features of a hexagonal unit cell, indicating that the raw material MCM-41 has a highly ordered hexagonal structure. After modification with –SH, the intensity of the (110) and (200) diffraction peaks decreases or even disappears compared with the original MCM-41, which decreases inevitably due to the order of channel structure after functionalization. With the further modification of SH-AS1411 aptamers, the intensity of the diffraction peak gets further decreased for the same reason. In addition, as can be seen from Fig. 3, the peak position of the (100) plane shifts slightly to the right with every modification, so the pore size of the carrier gets reduced slightly, indicating the successful modifications. The SXRD patterns prove that –SH and aptamers had been successfully modified on MCM-41. Meanwhile, the persistence of the (100) diffraction peak indicated that after functionalization of –SH and SH-AS1411, the particles still had a hexagonal structure.2.4 TGA
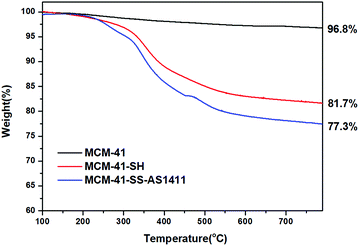
Fig. 4 shows the TGA curves of MCM-41, MCM-41-SH and MCM-41-SS-AS1411 recorded in a N2 flow at a heating rate of 10 °C min−1. The weight loss of MCM-41 was very low. For MCM-41-SH, the weight loss is due to the loss of grafted organic groups. MCM-41-SS-AS1411 has a further weight loss which is caused by both –S–S– and AS1411 loss. These changes indicated that the aptamer had been grafted on MCM-41, and there was about 4.4 wt% AS1411 aptamer bonded to the MCM-41 surface via one disulfide bond.2.5 Measurement of the zeta potential
Dynamic light scattering (DLS) was used to measure the zeta potential of unloaded empty MSNs. The zeta potentials of MCM-41, MCM-41-SH and MCM-41-SS-AS1411 are as shown in Table 1, which are (−42.20 ± 0.49) mV, (−45.37 ± 3.01) mV and (−53.10 ± 0.21) mV, respectively. The thiol group has a negative charge, so the zeta potentials of MCM-41-SH got lower after the negatively charged MCM-41 was modified with –SH. Similarly, the further reduced zeta potential of MCM-41-SS-AS1411 was attributed to the negatively charged DNA aptamer. The zeta potential results also show that the AS1411 aptamer conjugated with MSNs.| Sample | Zeta potential (mV) |
|---|---|
| MCM-41 | −42.20 ± 0.49 |
| MCM-41-SH | −45.37 ± 3.01 |
| MCM-41-SS-AS1411 | −53.10 ± 0.21 |
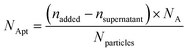
2.6 Determination of the aptamer binding amount
According to the fluorescence intensity of the supernatant after the reaction, it was calculated that each milligram of MCM-41-SS-Py could bind about 0.55 nmol of SH-AS1411-Cy5. Then calculated by using formulas (1) and (2), the number of AS1411 aptamers binding to each carrier particle is ca. 3.1 × 103 averagely (Table 2).| Carrier particle amount | Corresponding aptamer binding amount |
|---|---|
| 1 | ∼3.1 × 103 |
2.7 Loading amount of Dox
Table 3 shows the loading mass of Dox per milligram of MSNs calculated by using formula (3) according to the result of fluorescence detection. As illustrated in Table 3, the Dox amount loaded in AS1411-gated particles (MCM-41-SS-AS1411) was higher than that of bare MCM-41. AS1411 has a negative charge, and Dox has a positive charge in PBS solution.28 Dox was attracted to AS1411 due to electrostatic interaction. Therefore, AS1411 also loaded some Dox in addition to MCM-41.| Carrier | Loading amount of Dox (µg mg−1) |
|---|---|
| MCM-41 | 62.5 ± 17.1 |
| MCM-41-SS-AS1411 | 93.5 ± 12.8 |
2.8 Investigation of GSH-controlled drug release
The GSH-controlled drug release process is as shown in Scheme 1. Aptamers gate the pores of MSNs through the disulfide bonds. When GSH is present, the disulfide bond can be broken by GSH, which makes the aptamer separate from the mesopores, so that Dox in the MSN carrier can gradually diffuse out.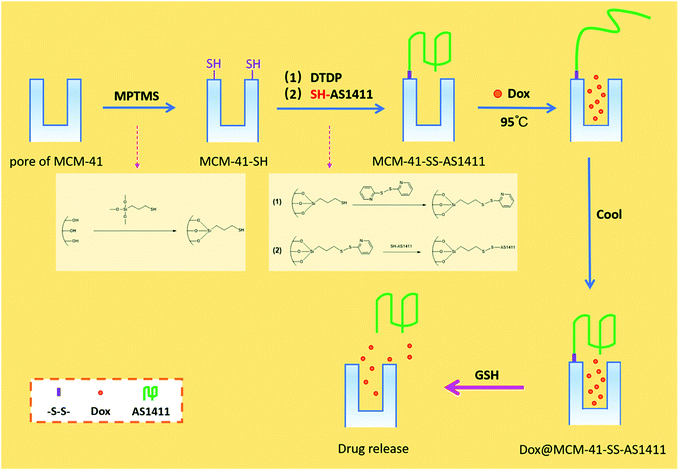 | ||
| Scheme 1 Schematic illustration of the AS1411 aptamer nano-gatekeeper grafted and open at mesoporous silica pores. | ||
As we all know, the microenvironment in tumors is generally more acidic than in normal tissues.29 Normal blood remains constant at pH 7.4. While in tumor cells, pH is 5.5–6.0 in endosomes and 4.5–5.0 in lysosomes.30,31 So in the research study, we use pH 7.4 PBS to simulate blood circulation and normal tissues, pH 5.0 PBS to simulate endosomes and lysosomes and 10 mM GSH solution to simulate GSH in intracellular fluid.
Fig. 5a shows the curve of Dox cumulative release from bare MCM-41 with time at pH = 7.4 or 5.0, with or without 10 mM GSH. As can be seen from the figure, Dox will release rapidly and massively when the MSN is not gated; there is little difference in the release of Dox in four different environments.
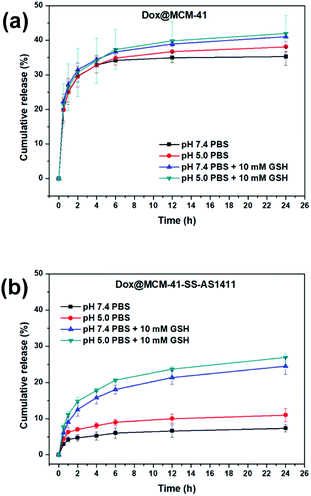 | ||
| Fig. 5 The cumulative release rates of Dox with time in different pH environments with or without 10 mM GSH. (a) Non-gated DDS: Dox@MCM-41; (b) AS1411-gated DDS: Dox@MCM-41-SS-AS1411. | ||
Fig. 5b shows the curve of Dox cumulative release from MCM-41-SS-AS1411 with time at pH = 7.4 or 5.0 with or without 10 mm GSH. As can be seen from the figure, there is a small amount of drug leakage in the absence of GSH. But the aptamer-blocking effect is significant enough compared with the results in Fig. 5a. This may be because only one end of AS1411 is fixed on the surface of the carrier, and the other end is still open. The configuration of G-quadruplex may vary with different pHs, resulting in an inevitable small amount of leakage. But in general, it still played the role of a gatekeeper. In the presence of GSH, Dox release increased significantly. This shows that GSH can open the aptamer to release the drug, which reflects that the DDS can respond to GSH. As can be seen from Fig. 5b, in the acidic environment with pH = 5.0, the Dox released amount in the presence of GSH was slightly higher than that in the environment with pH = 7.4, which could be attributed to the increasingly acidic conditions which weakened the electrostatic interaction between Dox and the negatively charged carrier, resulting in more release in the pH = 5.0 environment.28 This result suggested that the DDS could be beneficial for killing tumor cells and reducing damage to normal cells more or less.
As can be seen from Table 3 shown above, we found that MCM-SS-AS1411 had the higher drug loading capacity. However, comparing Fig. 5a and b, in the presence of GSH, MCM-SS-AS1411 displayed a lower amount of cumulative release rate than MCM-41 on the contrary. The reason was that AS1411 did not denature at 37 °C, which was the test temperature simulating the body temperature, and maintained its G-quadruplex configuration. So the Dox wrapped inside the spatial configuration of AS1411 was kept in it and difficult to release to the environmental media. Therefore, the cumulative drug release rates of MCM-SS-AS1411 were relatively lower than those of MCM-41 in the presence of GSH.
In summary, the MCM-41-SS-AS1411 drug delivery system can encapsulate the loaded drug molecules and release the drug in the presence of GSH.
3. Experimental
3.1 Synthetic procedures
 | (1) |
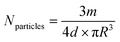 | (2) |
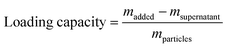 | (3) |
3.2 Materials
MCM-41 particles were purchased from Nanjing XFNANO Materials Tech Co., Ltd (Nanjing, China). Trimethoxysilylpropanethiol (MPTMS, 99%) was received from Sigma-Aldrich. Toluene (99%) was purchased from Beijing Chemical Reagent Company. Glutathione (GSH, 98%), 2,2′-dithiodipyridine (DTDP, 98%) and Dox (98%) were obtained from Aladdin Chemical Inc. (Shanghai, China). Phosphate buffered saline (PBS, pH 7.4) was purchased from Huibai Biotech Co., Ltd (Shenyang, China). Aptamers were purchased from Sangon Biotech Co., Ltd (Shanghai, China), and the sequences are shown in Table 4.| Name | Sequences (5′ → 3′) |
|---|---|
| SH-AS1411 | C6SH-GGTGGTGGTGGTTGTGGTGGTGGTGG |
| SH-AS1411-Cy5 | C6SH-GGTGGTGGTGGTTGTGGTGGTGGTGG-Cy5 |
All other chemicals were analytical grade and used without further purification. The buffer (PBS, pH 5.0) was prepared with ultrapure water produced from an ELGA PURELAB Classic water system (ELGA, UK).
3.3 Instrumentation
The TEM measurements were conducted using an FEI Tecnai G2 F30 transmission electron microscope (FEI, USA). Fourier transform infrared spectrophotometric (FTIR) spectra were recorded on a Cary 630 (Agilent, USA) spectrometer using the KBr pellet technique. Small-angle X-ray diffraction (SXRD) was performed on a SmartLab (RIGAKU, Japan). Thermogravimetric analysis (TGA) was performed on a TGA/DSC3+ instrument (METTLER TOLEDO, Switzerland) with a heating rate of 10 °C min−1 under a nitrogen flow. Zeta potentials were determined on a Nano-ZS90 laser particle size and zeta potential analyzer (Malvern, UK). The fluorescence spectra were examined with an F-7100 fluorescence spectrophotometer (Hitachi, Japan).4. Conclusions
A redox-responsive controlled release drug delivery system was prepared by using MCM-41 type mesoporous silica as the drug carrier, AS1411 aptamers as the gatekeeper and each AS1411 fixed on the pore outlet of MCM-41 through one disulfide bond. We mainly aimed to demonstrate that the spatial volume of the G-quadruplex configuration of the AS1411 aptamer can be utilized to cap the pore of the DDS. The results of FTIR, SXRD and TGA show that AS1411 aptamers were successfully grafted on MCM-41. Quantitative analysis of the binding capacity of the aptamer showed that there were about 3.1 × 103 aptamers bonded to each carrier particle averagely. Taking Dox as the drug model, this DDS was studied to release drug by controlling the aptamer gatekeeper in the presence or absence of GSH under pH 7.4 and 5.0 conditions, respectively. The fluorescence detection results confirmed that MCM-41-SS-AS1411 can encapsulate drug molecules and can open the AS1411 gatekeeper to release Dox under the stimulation of 10 mM GSH. We hope this point of view of utilizing the volume of G-quadruplex configuration of AS1411 to cap the pore of the DDS can play a prompt role in relevant research.Author contributions
Conceptualization, L. Z.; methodology, L. Z.; software, Y. Z.; investigation, L. Z.; validation, L. Z. and Y. Z.; writing, L. Z.; supervision, Y. M.; project administration, Y. M.; funding acquisition, Y. M. and L. Z. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the National Natural Science Foundation of China (Grant No. 21372262) and Basic Research Program of Higher Education of Liaoning Province of China (Grant No. LQNK201742). The Education Center for Medical Basic Experiment of China Medical University and China Medical University-The Queen's University of Belfast Joint College (CQC) are also gratefully acknowledged.References
- R. Guo, L. L. Li, W. H. Zhao, Y. X. Chen, X. Z. Wang, C. J. Fang, W. Feng, T. L. Zhang, X. Ma, M. Lu, S. Q. Peng and C. H. Yan, Nanoscale, 2012, 4, 3577–3583 RSC.
- C. H. Lee, S. H. Cheng, I. P. Huang, J. S. Souris, C. S. Yang, C. Y. Mou and L. W. Lo, Angew. Chem., Int. Ed. Engl., 2010, 49, 8214–8219 CrossRef CAS PubMed.
- J. Guo, Y. Wang, J. Wang, X. Zheng, D. Chang, S. Wang and T. Jiang, Asian J. Pharm. Sci., 2016, 11, 735–743 CrossRef.
- W. Chen, P. Zhong, F. Meng, R. Cheng, C. Deng, J. Feijen and Z. Zhong, J. Controlled Release, 2013, 169, 171–179 CrossRef CAS PubMed.
- C. Park, H. Kim, S. Kim and C. Kim, J. Am. Chem. Soc., 2009, 131, 16614–16615 CrossRef CAS PubMed.
- R. Cheng, F. Feng, F. Meng, C. Deng, J. Feijen and Z. Zhong, J. Controlled Release, 2011, 152, 2–12 CrossRef CAS.
- G. Saito, J. A. Swanson and K.-D. Lee, Adv. Drug Delivery Rev., 2003, 55, 199–215 CrossRef CAS.
- Y. Zhao, J. L. Vivero-Escoto, I. I. Slowing, B. G. Trewyn and V. S. Lin, Expert Opin., 2010, 7, 1013–1029 CAS.
- J. Wen, K. Yang, F. Liu, H. Li, Y. Xu and S. Sun, Chem. Soc. Rev., 2017, 46, 6024–6045 RSC.
- J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T.-W. Chu, D. H. Olson, E. W. Sheppard, S. B. McCullen, J. B. Higgins and J. L. Schlenker, J. Am. Chem. Soc., 1992, 114, 10834–10843 CrossRef CAS.
- B. L. Haruo Takahashi, T. Sasaki, C. Miyazaki, T. Kajino and S. Inagaki, Chem. Mater., 2000, 12, 3301–3305 CrossRef.
- E. Aznar, M. Oroval, L. Pascual, J. R. Murguia, R. Martinez-Manez and F. Sancenon, Chem. Rev., 2016, 116, 561–718 CrossRef CAS PubMed.
- M. R. Dunn, R. M. Jimenez and J. C. Chaput, Nat. Rev. Chem., 2017, 1, 0076 CrossRef CAS.
- A. D. Ellington and J. W. Szostak, Nature, 1990, 346, 818–822 CrossRef CAS.
- N. Zhang, B. Liu, X. Cui, Y. Li, J. Tang, H. Wang, D. Zhang and Z. Li, Talanta, 2021, 223, 121729 CrossRef CAS.
- R. R. White, B. A. Sullenger and C. P. Rusconi, J. Clin. Invest., 2000, 106, 929–934 CrossRef CAS PubMed.
- E. Levy-Nissenbaum, A. F. Radovic-Moreno, A. Z. Wang, R. Langer and O. C. Farokhzad, Trends Biotechnol., 2008, 26, 442–449 CrossRef CAS PubMed.
- K. W. Thiel and P. H. Giangrande, Oligonucleotides, 2009, 19, 209–222 CrossRef CAS PubMed.
- L. T. Sa, S. Simmons, S. Missailidis, M. I. da Silva and R. Santos-Oliveira, J. Drug Targeting, 2013, 21, 427–434 CrossRef CAS PubMed.
- M. Nejabat, M. Mohammadi, K. Abnous, S. M. Taghdisi, M. Ramezani and M. Alibolandi, Carbohydr. Polym., 2018, 197, 157–166 CrossRef CAS PubMed.
- Y. Li, Y. Duo, J. Bi, X. Zeng, L. Mei, S. Bao, L. He, A. Shan, Y. Zhang and X. Yu, Int. J. Nanomed., 2018, 13, 1241–1256 CrossRef CAS PubMed.
- Z. Peixian, Z. Shaoqiu, C. Ting and Z. Chunling, J. Funct. Mater., 2017, 48, 4049–4059 Search PubMed.
- P. J. Bates, D. A. Laber, D. M. Miller, S. D. Thomas and J. O. Trent, Exp. Mol. Pathol., 2009, 86, 151–164 CrossRef CAS.
- Y.-A. Shieh, S.-J. Yang, M.-F. Wei and M.-J. Shieh, ACS Nano, 2010, 4, 1433–1442 CrossRef CAS PubMed.
- E. M. Reyes-Reyes, Y. Teng and P. J. Bates, Cancer Res., 2010, 70, 8617–8629 CrossRef CAS PubMed.
- A. Miranda, T. Santos, E. Largy and C. Cruz, Pharmaceuticals, 2021, 14, 121 CrossRef CAS PubMed.
- Y. Zhang, J. Tan, L. Zhou, X. Shan, J. Liu and Y. Ma, ACS Omega, 2020, 5, 31227–31233 CrossRef CAS PubMed.
- Y. Yang, Y. Lin, D. Di, X. Zhang, D. Wang, Q. Zhao and S. Wang, J. Colloid Interface Sci., 2017, 508, 323–331 CrossRef CAS PubMed.
- I. F. Tannock and D. Rotin, Cancer Res., 1989, 49, 4373–4384 CAS.
- H. Wu, L. Zhu and V. P. Torchilin, Biomaterials, 2013, 34, 1213–1222 CrossRef CAS PubMed.
- H. Yin, E. S. Lee, D. Kim, K. H. Lee, K. T. Oh and Y. H. Bae, J. Controlled Release, 2008, 126, 130–138 CrossRef CAS PubMed.
- P. Sun, A. Leidner, S. Weigel, P. G. Weidler, S. Heissler, T. Scharnweber and C. M. Niemeyer, Small, 2019, 15, 1900083 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: More details of experimental procedures. See https://doi.org/10.1039/d2na00446a |
| This journal is © The Royal Society of Chemistry 2022 |