Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Polymorphic Ga2S3 nanowires: phase-controlled growth and crystal structure calculations†
Kidong
Park‡
a,
Doyeon
Kim‡
 a,
Tekalign Terfa
Debela‡
a,
Tekalign Terfa
Debela‡
 b,
Mourad
Boujnah
b,
Getasew Mulualem
Zewdie
b,
Jaemin
Seo
b,
Mourad
Boujnah
b,
Getasew Mulualem
Zewdie
b,
Jaemin
Seo
 a,
Ik Seon
Kwon
a,
Ik Seon
Kwon
 a,
In Hye
Kwak
a,
In Hye
Kwak
 a,
Minkyung
Jung
a,
Minkyung
Jung
 c,
Jeunghee
Park
c,
Jeunghee
Park
 *a and
Hong Seok
Kang
*a and
Hong Seok
Kang
 *d
*d
aDepartment of Advanced Materials Chemistry, Korea University, Sejong 339-700, Republic of Korea. E-mail: parkjh@korea.ac.kr
bInstitute for Application of Advanced Materials, Jeonju University, Chonbuk 55069, Republic of Korea
cDGIST Research Institute, DGIST, Daegu 42988, Republic of Korea
dDepartment of Nano and Advanced Materials, Jeonju University, Chonju, Chonbuk 55069, Republic of Korea. E-mail: hsk@jj.ac.kr
First published on 1st July 2022
Abstract
The polymorphism of nanostructures is of paramount importance for many promising applications in high-performance nanodevices. We report the chemical vapor deposition synthesis of Ga2S3 nanowires (NWs) that show the consecutive phase transitions of monoclinic (M) → hexagonal (H) → wurtzite (W) → zinc blende (C) when lowering the growth temperature from 850 to 600 °C. At the highest temperature, single-crystalline NWs were grown in the thermodynamically stable M phase. Two types of H phase exhibited 1.8 nm periodic superlattice structures owing to the distinctively ordered Ga sites. They consisted of three rotational variants of the M phase along the growth direction ([001]M = [0001]H/W) but with different sequences in the variants. The phases shared the same crystallographic axis within the NWs, producing novel core–shell structures to illustrate the phase evolution. The relative stabilities of these phases were predicted using density functional theory calculations, and the results support the successive phase evolution. Photodetector devices based on the p-type M and H phase Ga2S3 NWs showed excellent UV photoresponse performance.
1. Introduction
Nanowires (NWs) have emerged as well-defined one-dimensional building blocks of next-generation nanodevices.1,2 Unlike the thermodynamically stable phase of their bulk counterparts, NWs often adopt metastable phases. Therefore, the controlled synthesis of metastable structures and their characterization are very attractive topics. Gallium sesquisulfide (Ga2S3) is a semiconductor with a wide bandgap (Eg = ∼3 eV at room temperature).3 It has a defective crystal structure, in which one-third of the Ga sites are vacant and the S atoms are arranged almost perfectly in a closely packed hexagonal lattice.3–12 The different ordering in the Ga sublattice results in a polymorphism of four crystalline phases: the monoclinic α′ phase (space group Bb or Cc), hexagonal α phase (P61), wurtzite-type β phase (P63mc), and zinc blende (also known as sphalerite)-type γ phase (F43m). The α′ phase is exactly stoichiometric and thermodynamically stable. Conversion of the α′ phase into other phases has been observed under a small number of S vacancies (<0.03%) and high-temperature modifications.8,11,12 Both the α and α′ phases are categorized by the superstructures of the β phase. The α and β phases exist at temperatures higher than that of the γ phase.Previous studies of Ga2S3 bulk crystals or thin films (α′ phase) have focused on their optical properties, such as strong photoluminescence (PL) ranging from near-infrared to blue (400 nm) due to vacancy defects,13–17 excellent photoconductivity upon UV or blue irradiation,15,16,18,19 and infrared second-order nonlinear optical properties.20 The synthesis of α′-Ga2S3 NWs has been demonstrated using various methods, including chemical vapor deposition (CVD) and sulfurization of Ga2O3 NWs pre-synthesized by CVD or hydrothermal reaction.21–25 The Sutter group synthesized γ-Ga2S3 nanotubes by sulfurization of GaAs NWs.26 Since graphene-like two-dimensional (2D) layered materials have attracted much attention, the existence of 2D hexagonal crystal structures of Ga2S3 was theoretically predicted.27,28 Experimental studies demonstrated the synthesis of atomically thin layers of α′-, β-, and γ-Ga2S3 and their high-sensitivity UV photodetection.29–31 Nevertheless, there are few studies on the phase control of Ga2S3 nanostructures.
This work examines the phase-controlled growth of Ga2S3 NWs using CVD. Ga2S3 NWs with a monoclinic (α′) phase were successfully grown at 850 °C. As the growth temperature was gradually lowered to 600 °C, there was a sequential transition to the hexagonal (α) → wurtzite (β) → zinc blende (sphalerite) cubic (γ) phases. Atomically resolved transmission electron microscopy (TEM) revealed novel superlattice structures of the hexagonal phase in two different Ga arrangements, which were observed for the first time. The polymorphism produced distinctive core–shell structures in which the phases shared the same crystallographic axis. From this point on, the monoclinic, hexagonal, wurtzite, and zinc blende phases are respectively referred to by the English letters M, H, W, and C, instead of the Greek letters. First-principles calculations were performed on various polymorphic crystal structures involved in the phase evolution, and the results support the experimental results. Using the grown NWs, we fabricated photodetector devices and demonstrated their electrical and photoelectrical properties. Since polymorphism could be an important subject for optoelectronic devices, our work provides intriguing insights into their promising applications.
2. Experimental
2.1. Synthesis
Ga2S3 powders were placed in ceramic boats, which are loaded inside a quartz tube CVD reactor that is heated using an electrical furnace. A silicon (Si) substrate, on which a 5 nm-thick Au film was deposited, was positioned at a distance of 18 cm away from the powder source. The reactor was evacuated using a mechanical pump. Then argon gas is continuously supplied at a rate of 500 sccm during growth while the pressure maintains below 20 torr. The temperature of the powder sources is set to 900–950 °C. The substrate is maintained at 600–850 °C to synthesize the nanowire. Detailed experimental and methods are described in the ESI.†2.2. Calculation
First-principles calculations were performed using density functional theory (DFT) as implemented in the Vienna ab-initio simulation package (VASP).32,33 Electron–ion interactions were described using the projector-augmented wave (PAW) method with a plane-wave kinetic energy cutoff of 520 eV.34 For the exchange–correlation functional, the generalized gradient approximation (GGA) suggested by Perdew, Burke, and Ernzerhof (PBE) was employed.35 Structure optimization (both ion and lattice relaxation) was performed until the average force was <0.01 eV Å−1 and the final energy change was <10−8 eV. Uniform k-point meshes with a reciprocal-space resolution of 2π × 0.32 Å−1 were used. To calculate the total density of states (DOS) and band gap (Eg), modified Becke-Johnson (mBJ) exchange functional developed by Tran and Blaha, was adopted using the PBE correlation functional.363. Results and discussion
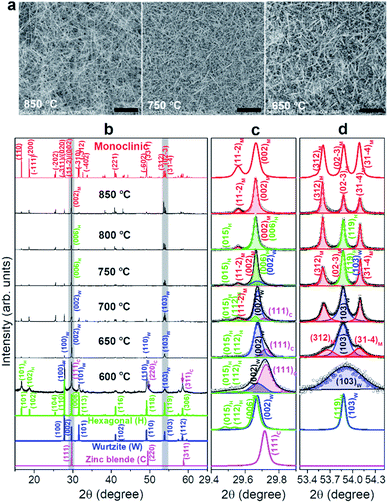
Fig. 1a shows the scanning electron microscopy (SEM) images of Ga2S3 NWs that were densely grown on a Si substrate at 850, 750, ad 650 °C. SEM images of all samples are shown in Fig. S1.† As the growth temperature decreased to 600 °C, the density of the NWs on the substrates decreased significantly. At 850 °C. the NWs had a straight and smooth surface. The high-resolution transmission electron microscopy (HRTEM) images are shown in Fig. S2.† The diameter of the NWs (average value: 150 nm) was uniform along a few tens of micrometers in length. As the temperature decreased to 650 °C, the NW morphology changed gradually to a tapered belt shape. The width (average value: 100 nm) was gradually reduced by more than half when approaching the tip. In Fig. S2,† we proposed a kinetically controlled growth model for the morphology change.The X-ray diffraction (XRD) patterns of the grown Ga2S3 NWs are shown in Fig. 1b. The XRD peaks of the samples grown at 850 °C are matched to the M phase. As the temperature decreased, some of the peaks were diminished or shifted in position because of the incorporation of other polymorphic phases. At 650 °C, the XRD pattern became closer to that of the W phase. The XRD peaks at 600 °C indicated the C phase. Fig. S3† displays the correlation that links the crystallographic axes of the M, H, W, and C phase unit cells. Neglecting the small distortion of the M phase, the following relationships hold:  , and
, and  . A correlation exists between the H and W phases,
. A correlation exists between the H and W phases,  and cH = 3cW. The M and H phases are superstructures of the W phase. Because the H phase can also be a superstructure of the M phase, its XRD includes the peaks of the M phase.
and cH = 3cW. The M and H phases are superstructures of the W phase. Because the H phase can also be a superstructure of the M phase, its XRD includes the peaks of the M phase.
The peaks at 2θ = 29.4°–29.9° are magnified in Fig. 1c. The samples grown at 850 °C exhibited (002)M and (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) )M peaks at 29.66° and 29.56°, respectively. As the growth temperature decreases to 800 °C, the (015)H peak appeared at 29.47°, suggesting an incorporation of the H phase. The peak position of (006)H appears to be coincident with that of (002)W. At 750 °C, the asymmetric band was resolved into two peaks corresponding to (002)M (at 29.66°) and (006)H/(002)W (at 29.67°). At both 700 and 650 °C, the main (29.67°) and shoulder (29.72°) peaks were assigned to the (002)W and (111)C peaks, respectively, indicating that the W phase became the major phase and the C phase started to emerge. At 600 °C, the main peak (29.72°) was assigned to (111)C and the minor peak (29.67°) to (002)W. The (111)C peak at the higher angle than the (001)W indicates that the lattice constant of C phase is smaller than that of W phase by 0.5%, based on the W–C phase relationship. It means that the Ga–Ga distance is shorter than that of H and W phases.
)M peaks at 29.66° and 29.56°, respectively. As the growth temperature decreases to 800 °C, the (015)H peak appeared at 29.47°, suggesting an incorporation of the H phase. The peak position of (006)H appears to be coincident with that of (002)W. At 750 °C, the asymmetric band was resolved into two peaks corresponding to (002)M (at 29.66°) and (006)H/(002)W (at 29.67°). At both 700 and 650 °C, the main (29.67°) and shoulder (29.72°) peaks were assigned to the (002)W and (111)C peaks, respectively, indicating that the W phase became the major phase and the C phase started to emerge. At 600 °C, the main peak (29.72°) was assigned to (111)C and the minor peak (29.67°) to (002)W. The (111)C peak at the higher angle than the (001)W indicates that the lattice constant of C phase is smaller than that of W phase by 0.5%, based on the W–C phase relationship. It means that the Ga–Ga distance is shorter than that of H and W phases.
Fig. 1d shows the (312)M, (02![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) )M, and (31
)M, and (31![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) )M peaks of samples grown at 850 °C. As the temperature decreased to 650 °C, the peaks became broader and the central (02
)M peaks of samples grown at 850 °C. As the temperature decreased to 650 °C, the peaks became broader and the central (02![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) )M peak became more intense due to the inclusion of the (119)H/(103)W peaks. At 600 °C, all peaks merged into the (103)W peak. The intensity also decreased significantly, implying that the C phase had become the major phase. This XRD peak analysis provides definite evidence for the M → H → W → C phase evolution, consistent with previous studies on bulk materials.8,11
)M peak became more intense due to the inclusion of the (119)H/(103)W peaks. At 600 °C, all peaks merged into the (103)W peak. The intensity also decreased significantly, implying that the C phase had become the major phase. This XRD peak analysis provides definite evidence for the M → H → W → C phase evolution, consistent with previous studies on bulk materials.8,11
The XPS and Raman spectra shown in Fig. S4 and S5,† respectively, confirming that the electronic structures are almost same for these samples. UV-visible absorption and PL spectra provide the similar Eg value (3.0 eV) for all samples, which is consistent with previous works (Fig. S6†).3,16,17,19
Fig. 2a shows the HRTEM images (measured using high-voltage TEM at 1.2 MV) and SAED patterns of M phase Ga2S3 NW (grown at 850 °C) at two zone axes ([010]M and [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 30]M). The NWs usually had a straight and smooth surface. The diameter (170 nm) was uniform along the growth direction of [001]M. As the TEM grid holder was tilted 30° to rotate the NW, the zone axis could be altered from [010]M to [
30]M). The NWs usually had a straight and smooth surface. The diameter (170 nm) was uniform along the growth direction of [001]M. As the TEM grid holder was tilted 30° to rotate the NW, the zone axis could be altered from [010]M to [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 30]M. The same SAED pattern was generated for the entire NW, confirming its single-crystalline nature. We note the following typical relation in crystallographic axes of the four phases: [103]M (normal to the shared (001)M plane) = [000
30]M. The same SAED pattern was generated for the entire NW, confirming its single-crystalline nature. We note the following typical relation in crystallographic axes of the four phases: [103]M (normal to the shared (001)M plane) = [000![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ]H = [0001]W = [111]C, [010]M = [
]H = [0001]W = [111]C, [010]M = [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 2
2![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0]H = [0
0]H = [0![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 10]W = [11
10]W = [11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) ]C, and [
]C, and [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 30]M = [01
30]M = [01![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0]H = [1
0]H = [1![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 10]W = [01
10]W = [01![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ]C.
]C.
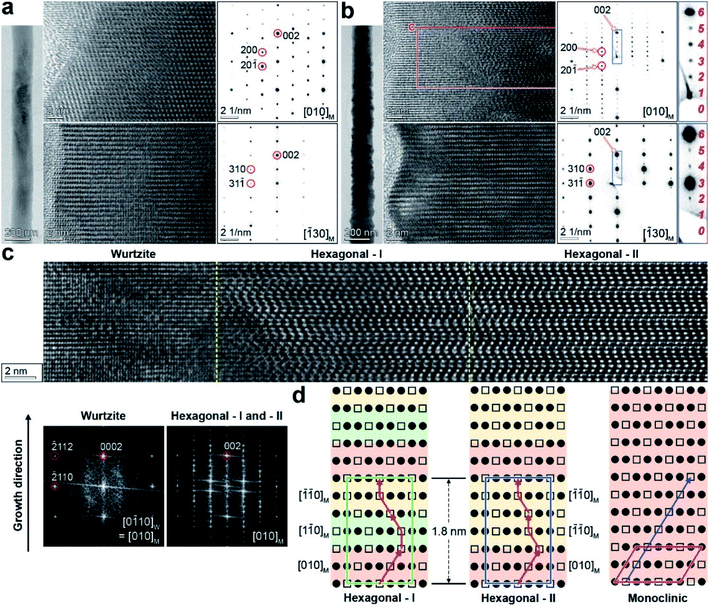
Fig. 2b shows the data for the Ga2S3 NWs grown at 750 °C. These NWs typically have a rough surface. The SAED pattern was indexed using the M phase, in order to show that they were measured at the same zone axes as those in Fig. 2a. The NWs had a superlattice structure with a uniform period of 1.8 nm, corresponding to the H phase (c = 18.05 Å).9 The growth direction was identified as [001]M = [0001]H/W. In both SAED patterns at the zone axes of [010]M and [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 30]M, the diffraction spots from two atomic layers along the [001]M growth direction are divided into six parts (the magnified images on the right).
30]M, the diffraction spots from two atomic layers along the [001]M growth direction are divided into six parts (the magnified images on the right).
Fig. 2c shows the lattice-resolved image of the marked area (8 nm × 50 nm with an extension to the core) in Fig. 2b (zone axis = [010]M). The middle and core parts (right) show lattice fringes that are distinct from the shell (left). The bright atomic sites corresponding to Ga vacancies are ordered in a zigzag pattern. Fast Fourier transform (FFT) images were generated for each part (bottom), and they revealed a single-crystalline W phase in the shell. The FFT image of the middle and core parts is the same as the corresponding SAED pattern.
The atomic arrangement in the middle and core parts was inferred from the TEM images at the zone axis of [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 2
2![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0]H (equivalent to [010]M), as shown in Fig. 2d. One-third of the Ga sites on the (001)M planes are vacant, as marked by the hollow squares, where the occupied Ga atoms are marked by the filled circles. The unit cell of the H phase is comprised of six atomic layers along the [0001]H (=[001]M) direction. The ordered Ga vacancy sites along the growth direction produce a zigzag pattern, as indicated by the arrow line. They are distinctive from the one-directional Ga vacancies of the M phase, as shown in the right panel. The atomic arrangement in the middle part is the same as that of the H phase reported for bulk materials.7,9,11 This H phase is referred to as the “H-I” phase. The superlattice structure can originate from the periodic stacking of the M phase variants separated by 120° rotations. Fig. S7† shows the structural models of the M phase for the [010]M, [1
0]H (equivalent to [010]M), as shown in Fig. 2d. One-third of the Ga sites on the (001)M planes are vacant, as marked by the hollow squares, where the occupied Ga atoms are marked by the filled circles. The unit cell of the H phase is comprised of six atomic layers along the [0001]H (=[001]M) direction. The ordered Ga vacancy sites along the growth direction produce a zigzag pattern, as indicated by the arrow line. They are distinctive from the one-directional Ga vacancies of the M phase, as shown in the right panel. The atomic arrangement in the middle part is the same as that of the H phase reported for bulk materials.7,9,11 This H phase is referred to as the “H-I” phase. The superlattice structure can originate from the periodic stacking of the M phase variants separated by 120° rotations. Fig. S7† shows the structural models of the M phase for the [010]M, [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0]M, and
0]M, and  projections by virtue of rotating 120° around the [001]M growth direction, which produces indistinguishable SAED and FFT images. The atomic arrangements of the three layers exactly match those of the [010]M, [1
projections by virtue of rotating 120° around the [001]M growth direction, which produces indistinguishable SAED and FFT images. The atomic arrangements of the three layers exactly match those of the [010]M, [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0]M, and
0]M, and  variants. The Ga vacancy sites are ordered by a sixfold screw rotation along the growth direction.
variants. The Ga vacancy sites are ordered by a sixfold screw rotation along the growth direction.
The superlattice structures at the core part have the same unit cell as that of the H phase, based on the SAED and FFT images. However, they have different atomic arrangements from those of the H phase shown in the middle part. This superlattice structure consisted of the three M phase variants ([010]M,  and
and  ) projected by 120° and 0° rotations around the [001]M growth direction. Fig. S7† shows the structural models of the M phase projected at the [010]M and
) projected by 120° and 0° rotations around the [001]M growth direction. Fig. S7† shows the structural models of the M phase projected at the [010]M and  zone axes. The Ga vacancy sites don't show a screw type ordering. This new superlattice structure has never been reported before, and we call it the “H-II” phase. The rough surface of the NWs probably resulted from the stacking of rotated M-phase variants. Structural disorders originating from the random stacking of rotational variants were previously reported for the M phase of Li- or Na-intercalated transition metal oxide materials.37,38 However, to the best of our knowledge, uniform stacking of the variants has never been observed for M-phase materials.
zone axes. The Ga vacancy sites don't show a screw type ordering. This new superlattice structure has never been reported before, and we call it the “H-II” phase. The rough surface of the NWs probably resulted from the stacking of rotated M-phase variants. Structural disorders originating from the random stacking of rotational variants were previously reported for the M phase of Li- or Na-intercalated transition metal oxide materials.37,38 However, to the best of our knowledge, uniform stacking of the variants has never been observed for M-phase materials.
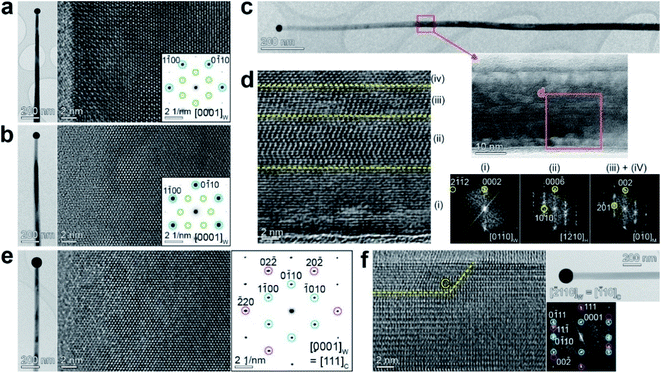
Fig. 3a and b show the HRTEM image and the corresponding SAED pattern of Ga2S3 NWs grown at 650 °C, measured at the zone axis of [0001]W. The “W” index was used here since it is the major phase, which also agrees with the XRD data (with minor H and C phases). The growth directions were identified as [1![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 10]W and [0
10]W and [0![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 10]W, respectively. The electron beam used in TEM was projected onto the basal plane of the belt-like NW with a tapered morphology. The SAED patterns show the stronger W and weaker H phase spots, indicating that the two NWs have different growth directions, but both contain the H phase.
10]W, respectively. The electron beam used in TEM was projected onto the basal plane of the belt-like NW with a tapered morphology. The SAED patterns show the stronger W and weaker H phase spots, indicating that the two NWs have different growth directions, but both contain the H phase.
Fig. 3c shows the HRTEM and the corresponding FFT images measured at the zone axis of [01![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0]W. The electron beam was projected onto the side facet of the belt-like NWs and perpendicular to the basal plane. The magnified image (marked area) shows the superlattice-structured H phase at the center. Fig. 3d clearly reveals the superlattice structure of the H phase in region (ii). FFT images were generated for the regions labeled (i)–(iv). The shell part (i) and the inner part (ii) show the W and H phases, respectively, confirming the coexistence of these two phases. The atomic arrangement of the H phase corresponds to a mixture of H-I and H-II structures. The core parts (iii) and (iv) were assigned to the twinned M phase at the [0
0]W. The electron beam was projected onto the side facet of the belt-like NWs and perpendicular to the basal plane. The magnified image (marked area) shows the superlattice-structured H phase at the center. Fig. 3d clearly reveals the superlattice structure of the H phase in region (ii). FFT images were generated for the regions labeled (i)–(iv). The shell part (i) and the inner part (ii) show the W and H phases, respectively, confirming the coexistence of these two phases. The atomic arrangement of the H phase corresponds to a mixture of H-I and H-II structures. The core parts (iii) and (iv) were assigned to the twinned M phase at the [0![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0]M and [110]M projections that overlapped after 120° rotations. The NW exhibits the W phase in the shell, the H phase in the middle, and the M phase in the core. The most stable M phase in the core and the metastable phase in the shell are supported by our growth model, in which the growth of shell parts is more driven under kinetically controlled conditions (see Fig. S3†).
0]M and [110]M projections that overlapped after 120° rotations. The NW exhibits the W phase in the shell, the H phase in the middle, and the M phase in the core. The most stable M phase in the core and the metastable phase in the shell are supported by our growth model, in which the growth of shell parts is more driven under kinetically controlled conditions (see Fig. S3†).
The HRTEM image and SAED pattern were measured for another NW at the zone axis of [0001]W (Fig. 3e). This NW has a growth direction of [0![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 10]W. The W and C phase SAED spots indicate that the C phase coexists with the W phase. Fig. 3f shows the HRTEM and corresponding FFT images measured by projecting the electron beam onto the side plane of the belt-like NW. The lattice fringes of the C phase were found at the surface (the region marked with the letter “C”). The growth direction was identified as [0
10]W. The W and C phase SAED spots indicate that the C phase coexists with the W phase. Fig. 3f shows the HRTEM and corresponding FFT images measured by projecting the electron beam onto the side plane of the belt-like NW. The lattice fringes of the C phase were found at the surface (the region marked with the letter “C”). The growth direction was identified as [0![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 10]W or [11
10]W or [11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) ]C. We monitored the growth direction for a few tens of NWs and found that 80% and 20% of them grew along [0
]C. We monitored the growth direction for a few tens of NWs and found that 80% and 20% of them grew along [0![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 10]W and [1
10]W and [1![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 10]W, respectively.
10]W, respectively.
Analysis results for the sample grown at 650 °C can be summarized as follows. (i) The tapered belt-like NWs show M → H → W → C phase evolution from the core to the shell, and the phases share the same crystallographic axis. The core–shell structures provide a panoramic view of the phase transition. (ii) The growth direction is [0![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 10]W or [1
10]W or [1![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 10]W, equivalent to [010]M and [
10]W, equivalent to [010]M and [![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) 31]M, respectively, unlike the [001]M growth direction of the M-phase NWs grown at 850 °C (shown in Fig. 2a). Rao et al. reported the [100]M growth direction for Ga2S3 NWs with a twinned structure synthesized using CVD at 650–800 °C.21 This growth direction is different from any of the directions in our sample, even if we convert the H (or W) phase axis into the M phase axis. Fig. S8† shows a schematic of the evolution of the growth direction, with a plausible model to explain the result.
31]M, respectively, unlike the [001]M growth direction of the M-phase NWs grown at 850 °C (shown in Fig. 2a). Rao et al. reported the [100]M growth direction for Ga2S3 NWs with a twinned structure synthesized using CVD at 650–800 °C.21 This growth direction is different from any of the directions in our sample, even if we convert the H (or W) phase axis into the M phase axis. Fig. S8† shows a schematic of the evolution of the growth direction, with a plausible model to explain the result.
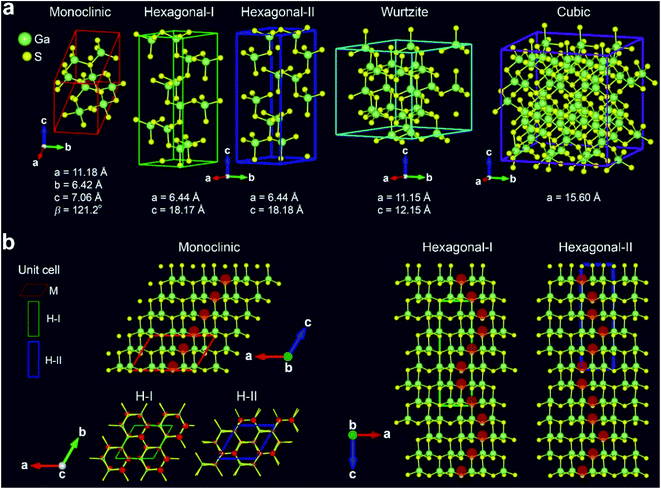
In order to support the phase evolution, we performed density functional theory (DFT) calculations using a supercell geometry with 20–180 atoms. Fig. 4a shows the optimized crystal structures for the M, H-I, H-II, W, and C phases of Ga2S3. For the M phase, the unit cell contains 8 Ga and 12 S atoms. The H phase was constructed using one H unit cell having Ga12S18 stoichiometry. For the W and C phases, the supercell was constructed from (3 × 3 × 2) and (3 × 3 × 3) unit cells with Ga24S36 and Ga72S108 stoichiometry, respectively. Since the Ga vacancy sites are unknown, we tried to find an optimized configuration using the supercell. The lattice constants of the supercell were within 0.7% difference of the experimental values.
Fig. 4b shows the atomic arrangement of the M, H-I, and H-II structures projected at the [010]M (=[![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 2
2![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0]H) zone axis. The ordered Ga vacancy sites are marked by the red-shaded discs. In the M structure, the Ga vacancies are formed uniformly along the c direction, which perfectly depicts the M phase. The H-I and H-II structures exhibit the ordered Ga vacancy sites at the different positions along the c direction, which matches the growth direction of Ga2S3 NWs. The distinctive zigzag pattern is consistent with the experimental data. Top view of H-I (zone axis = [0001]H) reveals the 6 Ga vacancy sites that were generated by a sixfold screw rotation with either right-handed (R) or left-handed (L) direction. In contrast, the Ga vacancies of H-II exhibit no such screw feature. The Ga vacancy sites of W and C phase are shown in Fig. S9.†
0]H) zone axis. The ordered Ga vacancy sites are marked by the red-shaded discs. In the M structure, the Ga vacancies are formed uniformly along the c direction, which perfectly depicts the M phase. The H-I and H-II structures exhibit the ordered Ga vacancy sites at the different positions along the c direction, which matches the growth direction of Ga2S3 NWs. The distinctive zigzag pattern is consistent with the experimental data. Top view of H-I (zone axis = [0001]H) reveals the 6 Ga vacancy sites that were generated by a sixfold screw rotation with either right-handed (R) or left-handed (L) direction. In contrast, the Ga vacancies of H-II exhibit no such screw feature. The Ga vacancy sites of W and C phase are shown in Fig. S9.†
The calculated total energy confirms that the M phase is the most stable one. The relative energy per Ga2S3 unit is 0.58 (H-I), 9.18 (H-II), 26.40 (W), and 389.82 meV (C), respectively, and therefore the stability order should be M > H > W > C. The M, H-I, H-II, and W phases are much more stable than the C phase. Furthermore, we calculated the formation energy (Ef) of each phase using the chemical potential of Ga and S atoms (at 0 K). The M, H-I, H-II, W, and C phases exhibit Ef = −5.103, −5.101, −5.095, −5.078, and −4.685 eV per Ga2S3 unit, respectively, following the same order of the relative energy. Overall, the total energy calculation can explain the successive phase evolution from the thermodynamically stable M phase to the metastable phases.
The mBJ band structures predicted bandgaps with an accuracy similar to the hybrid functionals or even to GW methods.39,40 Our calculation of mBJ band structure suggests that the direct bandgap at the Γ point is 1.92, 2.04, 2.07, and 2.11 eV, respectively, for M, H-I, H-II, and W phases (see Fig. S10†). The similar band gap of four phases (∼2 eV) is in reasonable agreement with our experimental values (3 eV). Since the unit cell of C phase consisted of 180 atoms, the bandgap was estimated using DOS, that is 1.41 eV. The PBE calculation predicted the respective values of 1.69, 1.68, 1.72, 1.81, and 0.30 eV, since it generally underestimates the band gap.
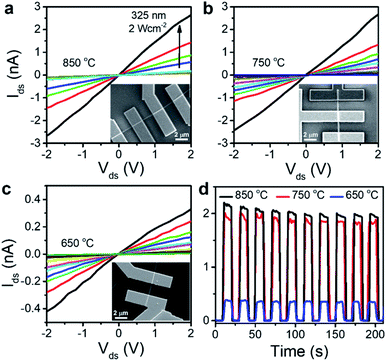
Now, we investigate the dependence of photoelectrical properties on the phases. Fig. 5a–c show the SEM images of photodetector devices fabricated using the NWs, as well as the linear I–V curves of source-drain (ds) measured under irradiation. The laser power density (P) is 0.02–2 W cm−2. The dark current (Idark) was 0.1 pA at Vds = 2 V. The NWs grown at 850 and 750 °C exhibited similar current changes upon light irradiation; however, the change was less for the device with NWs grown at 650 °C. Fig. 5d depicts the Ids–t curves for 10 light on–off cycles (10 s each), showing excellent stability and repeatability of the photocurrent. The rise/decay times were shorter than 1 s, and the photocurrent (Ip = Ilight − Idark) at 2 V was 2 nA for 850/750 °C and 0.4 nA for 650 °C.
Fig. S11† shows that the photocurrent increases almost linearly with the laser powder density, indicating that the Ga2S3 NW device possesses a highly efficient photoelectric conversion capability. The linear feature and the fast photocurrent response are probably related to the negligible trapping or scattering of hot carriers, which occurs at the surface defects and the interface between the NW surface and electrodes. The lower photocurrent level in NWs grown at lower temperatures is ascribed to the defect sites that act as trapping sites to decrease the concentration of photogenerated carriers. We suggest that the lattice mismatching C phase against the H/W phase produces the defect sites.
The photosensitivity (Ip/Idark) was 2 × 104 for the NWs grown at 850 °C. The spectral responsivity (R) is defined as the photocurrent generated (Ip) when light of unit intensity shines on the effective area of NW: R = Ip/PA, where P is the incident light intensity (2 W cm−2) and A is the effective area of the NW (200 nm in diameter and 2 μm in length). We obtained R = 1.8 × 104 A W−1. Another figure of merit of a photodetector is its specific detectivity (D*), which is defined as D* = R (A/2eIdark)1/2 when the noise from the dark current is small. We obtained D* = 3.5 × 1013 Jones (i.e., cm Hz1/2 W−1). Table S1† compares the parameters of our devices and previously reported photodetectors based on Ga2S3, demonstrating the excellent performance of our devices.
Conclusions
We synthesized high-crystalline Ga2S3 NWs on Au-deposited Si substrates by a CVD method utilizing the thermal evaporation of Ga2S3 powders. Decreasing the growth temperature from 850 °C to 600 °C induced sequential phase transitions from the M phase to the H, W, and C phases (M → H → W → C). The M-phase NWs grown at the highest temperature are single crystalline with the growth direction of [001]M. For the first time, we found two different H phases (H-I and H-II) with 1.8 nm periodic superlattice structures. The H-I and H-II phase exhibit the ordered Ga vacancy sites at different position along the growth direction. The H-I phase consists of three variants of the M phase, that is, the [010]M, [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0]M, and
0]M, and  . In H-II, the three variants are [010]M,
. In H-II, the three variants are [010]M,  , and
, and  projections. As the temperature decreases, conversion to the W and C phase is accompanied by evolution of the growth direction to [0
projections. As the temperature decreases, conversion to the W and C phase is accompanied by evolution of the growth direction to [0![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 10]W (=[
10]W (=[![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 10]C) and [1
10]C) and [1![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 10]W. The polymorphism of Ga2S3 produced distinctive core–shell structures that illustrate the phase evolution occurred by sharing the same crystallographic axis within the NWs. Using the DFT, extensive total energy calculations were carried out on four types of crystal structures, showing that the stability follows the order of M > H > W > C, which support the experimental phase transition. The H-I and H-II phases exhibit consistently the ordered Ga vacancy sites with a similar stability. The photodetector devices showed that the photoresponsivity can reach 1.8 × 104 A W−1. These results suggest a new strategy for enhancing the performance of NW-based UV photodetectors.
10]W. The polymorphism of Ga2S3 produced distinctive core–shell structures that illustrate the phase evolution occurred by sharing the same crystallographic axis within the NWs. Using the DFT, extensive total energy calculations were carried out on four types of crystal structures, showing that the stability follows the order of M > H > W > C, which support the experimental phase transition. The H-I and H-II phases exhibit consistently the ordered Ga vacancy sites with a similar stability. The photodetector devices showed that the photoresponsivity can reach 1.8 × 104 A W−1. These results suggest a new strategy for enhancing the performance of NW-based UV photodetectors.
Author contributions
K. P.: investigation (lead), synthesis and crystal analysis, D. K.: device fabrication, T. T. D.: calculation, M. B: calculation, G. M. Z.: calculation (supporting), J. S.: synthesis, I. S. K.: characterization, I. H. K.: characterization, M. K. J.: supervision (supporting), J. P.: project lead, writing, H. S. K.: calculation lead.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by 2014R1A6A1030732, 2017H1D3A1A01014082, 2018R1A2B2006474, 2020R1A6A3A01095689, 2020R1A2C2004392, and 2021H1D3A2A02096250 funded by the Ministry of Science and ICT. We also thank to the DGIST R&D Program of the Ministry of Science of Korea, ICT, Future Planning (21-IT-01) for financial support. This work was also supported by the national supercomputing resources including technical support (KSC-2021-CRE-0463). We would also like thank to Jeonju University for partial financial support. The HVEM measurements were supported by the KBSI under the R&D program (Project No. C140440).Notes and references
- N. P. Dasgupta, J. Sun, C. Liu, S. Brittman, S. C. Andrews, J. Lim, H. Gao, R. Yan and P. Yang, Adv. Mater., 2014, 26, 2137 CrossRef CAS PubMed.
- J.-L. Wang, M. Hassan, J.-W. Liu and S.-H. Yu, Adv. Mater., 2018, 30, 1803430 CrossRef PubMed.
- V. P. Mushjnskii, L. I. Palaki and V. V. Chebotaru, Phys. Status Solidi B, 1977, 83, K149 CrossRef.
- V. H. Hahn and G. Frank, Z. Anorg. Chem., 1955, 278, 333 CrossRef.
- J. Goodyear and G. A. Steigmann, Acta Crystallogr., 1963, 16, 946 CrossRef CAS.
- G. Collin, J. Flahaut, M. Gulttard and A.-M. Loireau-Lozach, Mater. Res. Bull., 1976, 11, 285 CrossRef CAS.
- N. H. Dung, P. Marie-Paule and D. S. Leila, Mater. Res. Bull., 1982, 17, 293 CrossRef.
- M. P. Pardo and M. Guittard, Mater. Res. Bull., 1987, 22, 1677 CrossRef CAS.
- A. Tomas, M. Guymon, M. P. Pardo, M. Guittard and J. Flahaut, Phys. Status Solidi A, 1988, 107, 775 CrossRef CAS.
- M. Guymon, A. Tomas, M. P. Pardo and M. Guittard, Phys. Status Solidi A, 1989, 113, K5 CrossRef.
- M. P. Pardo, M. Guittard, A. Chiloulet and E. A. Thomas, J. Solid State Chem., 1993, 102, 423 CrossRef CAS.
- C. Y. Jones and J. G. Edwards, J. Phys. Chem. B, 2001, 105, 2718 CrossRef CAS.
- T. Aono and K. Kase, Solid State Commun., 1992, 81, 303 CrossRef CAS.
- C. S. Yoon, F. D. Medina, L. Martinez, T. Y. Park, M. S. Jin and W.-T. Kim, Appl. Phys. Lett., 2003, 83, 1947 CrossRef CAS.
- H. F. Liu, K. K. A. Antwi, N. L. Yakovlev, H. R. Tan, L. T. Ong, S. J. Chua and D. Z. Chi, ACS Appl. Mater. Interfaces, 2014, 6, 3501 CrossRef CAS PubMed.
- C.-H. Ho and H.-H. Chen, Sci. Rep., 2014, 4, 6143 CrossRef CAS PubMed.
- Y. Zhang, C. Chen, C. Y. Liang, Z. W. Liu, Y. S. Li and R. Che, Nanoscale, 2015, 7, 17381 RSC.
- H. A. E. Shaikh, M. Abdal-Rahman, A. E. Belal and I. M. Ashraf, J. Phys. D: Appl. Phys., 1996, 29, 466 CrossRef CAS.
- C.-H. Ho, M.-H. Lin, Y.-P. Wang and Y.-S. Huang, Sens. Actuators, A, 2016, 245, 119 CrossRef CAS.
- M.-J. Zhang, X.-M. Jiang, L.-J. Zhou and G.-C. Guo, J. Mater. Chem. C, 2013, 1, 4754 RSC.
- R. Rao, H. Chandrasekaran, S. Gubbala, M. K. Sunkara, C. Daraio, S. Jin and A. M. Rao, J. Electron. Mater., 2006, 35, 941 CrossRef CAS.
- K. M. Othonos, M. Zervos, C. Christofides and A. Othonos, Nanoscale Res. Lett., 2015, 10, 304 CrossRef PubMed.
- M. Zervos, A. Othonos, V. Gianneta, A. Travlos and A. G. Nassiopoulou, J. Appl. Phys., 2015, 118, 194302 CrossRef.
- P. Wang, M. Liu, F. Mo, Z. Long, F. Fang, D. Sun, Y. Zhou and Y. Song, Nanoscale, 2019, 11, 3208 RSC.
- L. Wang and C. Tu, Nanotechnology, 2020, 31, 165603 CrossRef CAS PubMed.
- E. Sutter, J. S. French, A. Balgarkashi, N. Tappy, A. F. i Morral, J. C. Idrobo and P. Sutter, Nano Lett., 2019, 19, 8903 CrossRef CAS PubMed.
- W. Ding, J. Zhu, Z. Wang, Y. Gao, D. Xiao, Y. Gu, Z. Zhang and W. Zhu, Nat. Commun., 2017, 8, 14956 CrossRef CAS PubMed.
- C. F. Fu, J. Sun, Q. Luo, X. Li, W. Hu and J. Yang, Nano Lett., 2018, 13, 6312 CrossRef PubMed.
- M. M. Y. A. Alsaif, N. Pillai, S. Kuriakose, S. Walia, A. Jannat, K. Xu, T. Alkathiri, M. Mohiuddin, T. Daeneke, K. Kalantar-Zadeh, J. Z. Ou and A. Zavabeti, ACS Appl. Nano Mater., 2019, 2, 4665 CrossRef CAS.
- N. Zhou, L. Gan, R. Yang, F. Wang, L. Li, Y. Chen, D. Li and T. Zhai, ACS Nano, 2019, 13, 6297 CrossRef CAS PubMed.
- Y. Zheng, X. Tang, W. Wang, L. Jin and G. Li, Adv. Funct. Mater., 2020, 31, 2008307 CrossRef.
- G. Kresse and J. Furthmüller, Phys. Rev. B, 1996, 54, 11169 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15 CrossRef CAS.
- P. E. Blöchl, Phys. Rev. B, 1994, 50, 17953 CrossRef PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- F. Tran and P. Blaha, Phys. Rev. Lett., 2009, 102, 226401 CrossRef PubMed.
- A. K. Shukla, Q. M. Ramasse, C. Ophus, H. Duncan, F. Hage and G. Chen, Nat. Commun., 2015, 8, 8711 CrossRef PubMed.
- L. Xiao, Z. Ding, C. Chen, Z. Han, P. Wang, Q. Huang, P. Gao and W. Wei, iScience, 2020, 23, 100898 CrossRef CAS PubMed.
- J. Gusakova, X. Wang, L. L. Shiau, A. Krivosheeva, V. Shaposhnikov, V. Borisenko, V. Gusakov and B. K. Tay, Phys. Status Solidi A, 2017, 214, 1700218 CrossRef.
- A. Patra, S. Jana, P. Samal, F. Tran, L. Kalantari, J. Doumont and P. Blaha, J. Phys. Chem. C, 2021, 125, 11206 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2na00265e |
| ‡ K. P., D. K., and T. T. D. equally contributed as first author. |
| This journal is © The Royal Society of Chemistry 2022 |