 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Functionalized hybrid magnetic catalytic systems on micro- and nanoscale utilized in organic synthesis and degradation of dyes
Fatemeh
Ganjali
,
Amir
Kashtiaray
,
Simindokht
Zarei-Shokat
,
Reza
Taheri-Ledari
 * and
Ali
Maleki
* and
Ali
Maleki
 *
*
Catalysts and Organic Synthesis Research Laboratory, Department of Chemistry, Iran University of Science and Technology, Tehran 16846-13114, Iran. E-mail: r_taheri94@chem.iust.ac.i; maleki@iust.ac.ir; Fax: +98-21-73021584; Tel: +98-21-73228313
First published on 9th February 2022
Abstract
Herein, a concise review of the latest developments in catalytic processes involving organic reactions is presented, focusing on magnetic catalytic systems (MCSs). In recent years, various micro- and nanoscale magnetic catalysts have been prepared through different methods based on optimized reaction conditions and utilized in complex organic synthesis or degradation reactions of pharmaceutical compounds. These biodegradable, biocompatible and eco-benign MCSs have achieved the principles of green chemistry, and thus their usage is highly advocated. In addition, MCSs can shorten the reaction time, effectively accelerate reactions, and significantly upgrade both pharmaceutical synthesis and degradation mechanisms by preventing unwanted side reactions. Moreover, the other significant benefits of MCSs include their convenient magnetic separation, high stability and reusability, inexpensive raw materials, facile preparation routes, and surface functionalization. In this review, our aim is to present at the recent improvements in the structure of versatile MCSs and their characteristics, i.e., magnetization, recyclability, structural stability, turnover number (TON), and turnover frequency (TOF). Concisely, different hybrid and multifunctional MCSs are discussed. Additionally, the applications of MCSs for the synthesis of different pharmaceutical ingredients and degradation of organic wastewater contaminants such as toxic dyes and drugs are demonstrated.
1. Introduction
The synthesis of pharmaceutical compounds as the foundation of the pharmaceutical industry conventionally requires complex synthetic routes. However, this problem can be alleviated by using appropriate catalysts in the synthetic process.1–4 Also, the discharge of dangerous and toxic drugs in water, which has far-reaching effects on the aquatic ecosystem and the health of living creatures, has become a major problem. One solution to this issue is to catalytically degrade drugs into low-risk components. However, separating homogeneous catalysts from the reaction medium is difficult, which is why recent research has focused on the use of heterogeneous catalytic systems.5 Heterogeneous catalytic systems render the catalyst and products in separable phases to speed up the effortless separation and lessen the reaction time with a higher yield.6Generally, among the extensive and diverse collection of heterogeneous catalytic systems, magnetic catalytic systems (MCSs) have attracted increasing attention from scientists due to their substantial benefits.7–9 Also, nanomaterial-based catalysts are highly regarded owing to their high aspect ratio.10–12 Initially, magnetic catalysts facilitate the purification and workup process.13 As reported, for the removal of a magnetic catalyst, a simple process is to hold an external magnet under the reaction flask and decant the medium, where a magnetic field of 20 emu† g−1 or higher is required.14 Furthermore, in the case of utilizing nanoscale magnetic catalysts, high reaction yields are obtained using a small amount of nanocatalyst due to their high aspect ratio. These heterogeneous catalysts are affordable and satisfactory species for industrial applications.15
Another reason to employ heterogeneous MCSs is their facile separation. Heterogeneous MCSs retain their stability even after alternating recycling runs, as long as the structural stability of the catalyst is maintained. Indeed, metallic-based heterogeneous MCSs such as iron demonstrate no significant decline in catalytic performance over several sequential recycling procedures, where thermogravimetric analysis can confirm these claims.16 In addition, many studies have been devoted to functionalizing MCSs or combining them with other substances to enhance the functions and applications of catalytic systems.17–21 Reportedly, a heterogeneous MCS, i.e., amine-functionalized Fe3O4@SiO2 nanoparticles grafted with gallic acid (GA), (Fe3O4@SiO2–NH2–GA), presented not only a high yield of α-aminonitriles via one-pot synthesis but also exhibited enhanced reusability and structural stability.22
Considering that most MCSs are composed of inorganic or natural components,23 they are environmentally benign and considered in the green chemistry spectrum.24 The most renowned and forerunner species of MCSs is highly magnetic iron oxide nanoparticles (Fe3O4 NPs) synthesized in an alkaline environment, i.e., pH > 11, via a facile co-precipitation route from Fe2+ and Fe3+ ions. The resulting Fe3O4 NPs can be modified with an SiO2 layer to inhibit the further oxidation of Fe3O4 to the less magnetic Fe2O3 species. The silica-coated Fe3O4 NPs have several surface hydroxyl groups as active sites, which can bind to other species through covalent bonding.25,26
Ferrites are another class that can form MCSs. Various ferrites such as zinc ferrite (ZnFe2O4), nickel ferrite (NiFe2O4), and cobalt ferrite (CoFe2O4), with different dopants, can be employed in the preparation of MCS. These materials exhibit different properties, that is, Ni ions in NiFe2O4 are easily exposed to reduction compared with the Fe ions.27 ZnFe2O4 has a narrow bandgap, moderate photocatalytic activity, and chemical and thermal stability.28 CoFe2O4 has average saturation magnetization, strong anisotropy, and excellent mechanical and chemical stabilities.29 Together with these inorganic systems, well-suited, lightweight, and innately magnetic species such as pumice‡ are utilized in MCSs. Another advantage of pumice is its highly porous structure, which is formed when gaseous species are emitted from it.30,31
These moderate MCSs have caused pharmaceutical synthesis to proceed in aqueous media, which was previously done in organic solvents. Safari et al. designed Fe3O4 located in the space between the lamellae and exterior surface of the montmorillonite (MMT) support, namely, MMT@Fe3O4, as an eco-friendly catalyst for the synthesis of indeno[1,2-b]indolone in aqueous media with gentle conditions, high yield, and convenient recovery.32 Further, in another report, Pd NPs were immobilized on melamine-functionalized magnetic chitosan beads (Fe3O4/CS–Me@Pd) to catalytically reduce p-nitrophenol and to conduct Suzuki reaction in aqueous medium.33
In this study, melamine played a particularly important role, which not only acts as a platform for the excellent surface distribution of Pd(II) but also strongly interacts with Pd(II) to minimize the leaching of the metal NPs. Another notable advantage of this work was its high catalytic activity, as authenticated by the high TON and TOF under mild conditions. Similarly, MCSs are vital for the degradation of pharmaceuticals with high yield in aqueous medium. As a remarkable example, Kargae et al. synthesized a ZnFe2O4@CMC nanobiocomposite to remove ciprofloxacin (CIP), an antibiotic of the fluoroquinolones group, with good removal efficiency (87%) in synthetic samples without introducing any extra oxidizing agents. This nanophotocatalyst followed the “green chemistry” protocols and showed good chemical stability after five times reuse.34,35 Also, Taghavi et al. introduced the MCS FeNi3/SiO2/CuS for the photocatalytic degradation of tetracycline under simulated solar light.36 Facile recyclability and advanced photocatalytic decomposition, which degrades complex cyclic compounds into simple linear ingredients, and ultimately turn them into CO2 and H2O, are two remarkable points in their report.
Magnetic biochar (MBC) composites are members of MCSs, which exhibit advanced magnetic properties when loaded with magnetic NPs, together with the advantages of biochar such as eco-friendliness and biodegradable raw materials including biomass waste, sugarcane bagasse, rice straw, peanut shells, and herb remains.37,38 For instance, Reddy et al. introduced two MBC systems with biodegradable raw materials, namely iron-loaded rice husk biochar (Fe-RHB) and coir pith biochar (Fe-CPB) for the decomposition of Acid Red 1 (AR1) organic dye, which resulted in nearly complete decolorization.39
Herein, we aim to focus on MCSs and their applications in the synthesis of different pharmaceuticals and degradation of organic pollutants (dyes and versatile drugs) with an overview of the latest related literature. Certainly, our purpose is to concisely review the current literature associated with catalytic systems consisting of magnetic components to pave the way for the synthesis of pharmaceutical compounds and their degradation. Also, we briefly summarize hybrid and multifunctional MCSs. Besides, we discuss the various properties of MCSs such as magnetization, reusability, structural stability, turnover number (TON), and turnover frequency (TOF).
2. Classification of the magnetic micro- and nanostructures
Here, a classification of MCSs is presented to give a qualified overview on catalytic systems implemented for the synthesis of pharmaceutical compounds to detail the unique advantages in each case.2.1. Magnetic metal oxides
Extensive studies on metallic particles have demonstrated their excellent properties in the catalytic, optical, electronic, magnetic, and antibacterial fields.40 Moreover, metallic nanoparticles and metal oxides have attracted attention due to their unique structure, electrical conductivity and electrocatalytic activity.41 Gold NPs enhance the therapeutic effects against tumors with low toxicity.42 Besides, gold and silver particles are exploited in biomedicine and drug delivery.43 Silver NPs are applied in tissue engineering, coating medical implants, and boosting injury curing according to their antimicrobial and anti-inflammatory potential.44 The wide application diversity such as catalysis, preparation of nanocomposites, and production of electronic pieces of noble metallic NPs such as Ag, Au, Pd, Cu, and platinum group in pure or alloy form has persuaded scientists to focus on investigating these species.40,45–48One of the most famous and widely used species with ferrimagnetic properties is iron oxide magnetic nanoparticles (Fe3O4 MNPs). Pure Fe3O4 NPs are not usually utilized in electrochemical techniques because pure metallic NPs do not have sufficient chemical stability in air and are immediately oxidized. Also, pure Fe3O4 MNPs tend to form large aggregates due to their intense dipole–dipole attraction. Thus, approaches for the protection of MNPs during their synthesis and applications are of great importance. These methods vary from grafting with organic parts to coating them with polymeric, organic, and inorganic layers such as silica or carbon. Furthermore, these coating layers promote the properties of MNP. Specifically, the chemical stability of NPs prevents their aggregation and agglomeration, their toxicity is reduced, and the effective interactions or coactions between NPs with other particles or various ligands are enhances. Magnetic nanoparticles can be placed next to other nanoparticles in electrocatalytic applications and sensors.41 MNPs are used in composite materials, which involves two main steps, i.e., preparation of MNPs, and then surface modification. Fe3O4 MNPs have abundant surface hydroxyl groups, which are active to bind to other organic structures through covalent bonds.
As one of the brightest examples, Karimi Zarchi et al. reported a magnetic catalyst, palladium on surface-ameliorated Schiff base complex, Fe3O4@Pd–Schiff-base MNPs, as presented in Fig. 1.49 Due to the attached Pd complex, the magnetic saturation dropped from 62.10 emu g−1 for the Fe3O4 MNPs to 54.04 emu g−1 for the catalyst. This magnetic catalyst was utilized in the synthesis of 5-substituted 1H tetrazole (Fig. 2) and benzamide (Fig. 3). As a crucial fact, this reaction started from the insertion of the nontoxic K2[Ni(CN)4] inorganic azide source in aryl halide via a one-pot procedure. It should be highlighted that the metal leaching study for this system was negligible, as proven by hot filtration and ICP-AES tests, which confirmed the strong binding of palladium to the active sites on the surface of the catalyst. Consequently, due the low leaching and high stability and reusability of the catalyst, it could be recycled five times without any reduction in its catalytic performance. In some cases, 99% yield was obtained for 5-substituted 1H tetrazole and benzamide under the optimum condition of 5.7 h, which is comparable with long-time reactions in organic solvents.
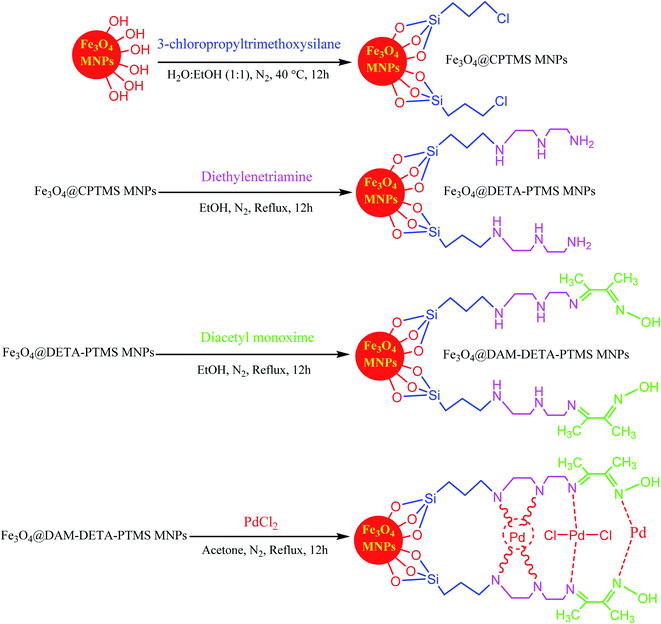 | ||
| Fig. 1 Synthesis and structure of Fe3O4@Pd–Schiff-base MNPs. This figure was adapted with permission from Journal of Organometallic Chemistry, 2019, 880, 196–212.49 | ||
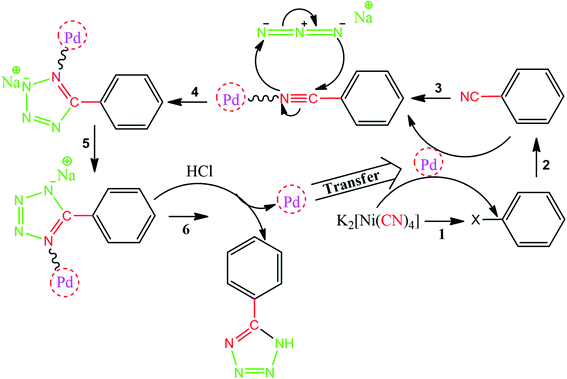 | ||
| Fig. 2 Suggested mechanism for the synthesis of 5-substituted 1H tetrazoles. This figure was adapted with permission from Journal of Organometallic Chemistry, 2019, 880, 196–212.49 | ||
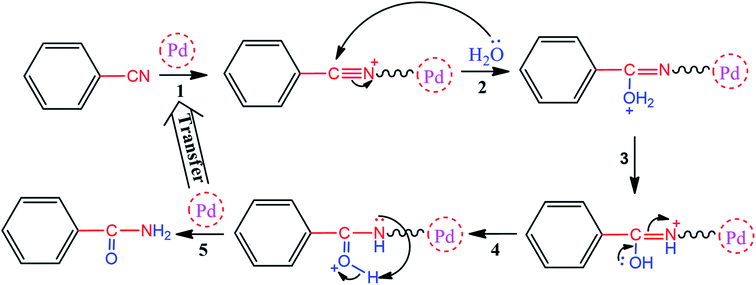 | ||
| Fig. 3 Proposed mechanism for the synthesis of benzamide. This figure was adapted by permission from Journal of Organometallic Chemistry, 2019, 880, 196–212.49 | ||
The combination of pure metallic catalysts with magnetic agents through surface modification routes is considered in another approach. Herein, the active catalytic sites are together with the magnetic feature. As a related example, Elazab et al. proposed a highly active reduced graphene oxide-supported Pd/Fe3O4 NP catalyst with a uniform dispersion of palladium NPs on its surface. This catalyst exhibited very high catalytic activity under both batch and continuous reaction conditions with 100% conversion in the 4-bromobenzaldehyde Suzuki cross-coupling reaction with phenylboronic acid, as shown in Fig. 4.50
 | ||
| Fig. 4 Suzuki cross-coupling reaction with Pd–Fe3O4/RGO heterogeneous catalyst. This figure was adapted with permission from H. A. Elazab, 2019 (DOI: 10.9767/bcrec.14.3.3518.478-489).50 | ||
The structural defects in graphene sheets enhanced the catalytic activity and the interactions with anchored NPs. The remarkable benefits of adopting the flow chemistry method include a shorter reaction time and higher conversion rate and selectivity than the ordinary batch approach. As an important point, the reduction reaction conducted by microwave irradiation resulted in rapid heating of the reaction mixture. Palladium inhibited the agglomeration of the products during the graphene oxide reduction process.
In another work, the focus was the core–shell structure of the Fe3O4 magnetic core and SiO2 protecting shell that, which was functionalized with ortho-phenylenediamine (PDA) and Pd ions to form (Fe3O4/o-PDA–Pd), as depicted in Fig. 5.17 A general fact that should be considered in the case of core–shell structures owning a magnetic core is that a stronger external magnetic field is required given that there is an inverse relationship between the core covering layers and its magnetic property.
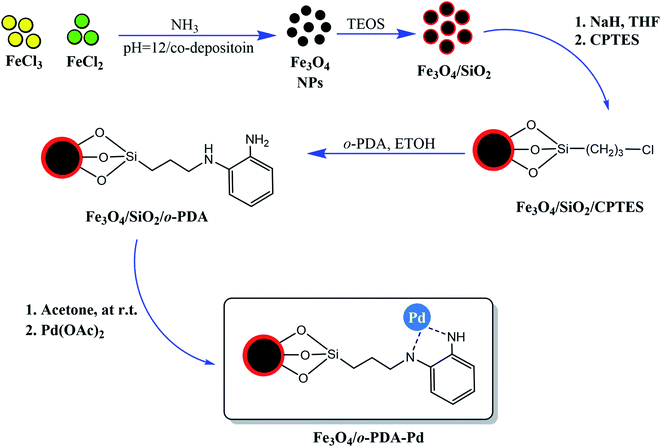 | ||
| Fig. 5 Preparation of Fe3O4/o-PDA–Pd heterogeneous magnetic nanocatalyst. This figure was adapted with permission from Journal of Physics and Chemistry of Solids, 2020, 136, 109200.17 | ||
MNPs are prepared via physical and chemical preparation routes. Physical methods such as ball milling can be divided into ordinary ball milling method51 and high-energy ball milling method.52 The chemical procedures for the preparation of MNPs include co-precipitation,53 hydrothermal,54 pyrolysis,55 sol–gel,56 microemulsion,57 sonochemical,58 electrodeposition,59 and polyol methods.60
Ferrites as a member of ferrimagnetic ceramics with the general formula of MFe2O4 (M signifies bivalent metal ions including Mn, Fe, Co, Ni, Cu, and Zn) are well known because of their physical and magnetic properties, electrical resistance, high chemical stability, and low cost.61–63 According to the initial crystal lattice of ferrites, their structures are garnet, hexagonal, and spinel.64–66 Among these structures, normal and inverse spinel ferrites are very attractive. Cobalt and iron oxides have higher magnetic properties compared to other ferrites. The properties of cobalt ferrites can be altered by applying different methods such as chemical substitution, heat treatment, and sudden cooling.67Fig. 6 shows the characteristics, synthesis routes, and applications of CoFe2O4.68
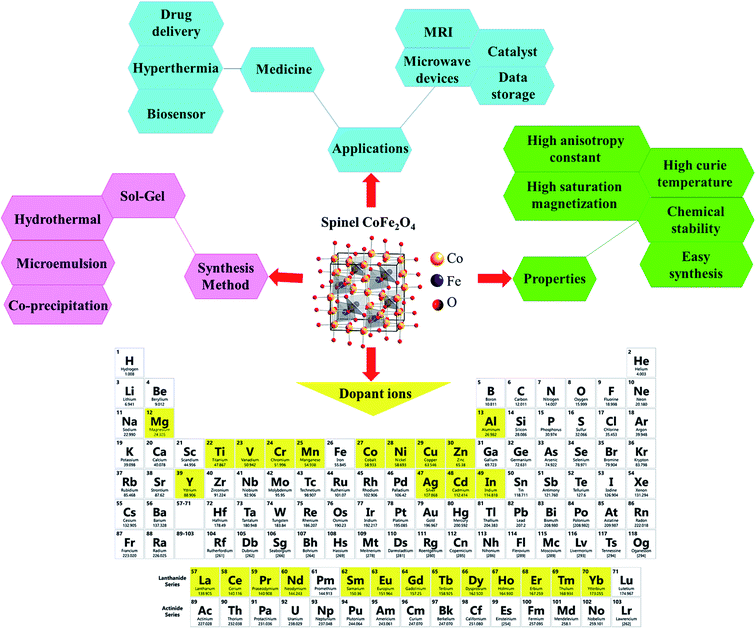 | ||
| Fig. 6 Characteristics, synthesis methods, and applications of CoFe2O4. This figure was adapted with permission from Ceramics International, 2020, 46, 18391–18412.68 | ||
In the ferrites category, copper spinel ferrites (CuFe2O4) are appealing candidates due to their strong anisotropy, high coercivity, acceptable magnetic saturation, and excellent mechanical and chemical stabilities at high temperatures. The other relevant superiorities of CuFe2O4 MNPs include their simple modification, surface functionalization, and good reusability and catalytic activity.69 In the study by Maleki et al., nanoscale ZnS/CuFe2O4 was used as a magnetic hybrid heterogeneous catalyst via the co-precipitation approach, which was retrieved by an external magnet from the reaction mixture. This magnetic hybrid composite catalyzed 2,4,5-triaryl-1H-imidazoles. The other advantages of this synthesized catalyst were its cost-effectiveness and reusability for five times in alternating cycles without any discernible reduction in its catalytic activity.70 As one of the precious instances of the catalytic function of NiFe2O4 for the synthesis of pharmaceutical components, Hamidinasab et al. prepared a magnetically recoverable organoacid-decorated NiFe2O4 nanocatalyst for the multicomponent synthesis of some phthalazine-trione and benzo[4,5] imidazo[1,2-a]pyrimidine derivatives, which complied with green chemistry protocols (Fig. 7).71 As previously stated, the abundant OH groups on TiO2-coated nickel ferrite NPs act as active sites to bond with (3-chloropropyl) trimethoxysilane. Subsequently, nucleophilic attack occurs between the chlorine leaving group on NiFe2O4@TiO2–chloropropylsilane and OH on diethanolamine (DEA) as a nucleophile. Lastly, another nucleophilic substitution takes place between the surface OH groups on nano-NiFe2O4@TiO2–SiO2–Pr–DEA and chlorine leaving group on chlorosulfonic acid to constitute the nano-NiFe2O4@TiO2–SiO2–Pr–DEA–OSO3H hybrid nanocatalyst. According to the formation of titania and other layers around the NiFe2O4 core, the magnetic saturation decreased from 35.1 to 11.1 emu g−1 at high magnetic fields up to 8000 Oe. Both nano-NiFe2O4@TiO2–SiO2–Pr–DEA–OSO3H and neat NiFe2O4 exhibited superparamagnetism. In this regard, it is reasonable to use neat NiFe2O4 as an inorganic magnetic catalyst with higher magnetic saturation and more convenient workup due to the work reported by Kaviyarasu et al.72 In their study, neat NiFe2O4 was prepared via the microwave combustion method (MCM) and conventional combustion method (CCM). The magnetic saturation value at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe magnetic field increased from 40.76 emu g−1 for CCM to 42.49 emu g−1 for MCM because of the narrow and limited distribution of NPs in MCM. Plenty of parameters affect saturation magnetization. Indeed, the magnetic saturation increases with the crystallinity of the NPs.73 On the contrary, as stated by Dai et al., an increase in calcination temperature is inversely proportional to the saturation magnetization given that defects and strain are abundant in the product during the calcination process, although some are relieved throughout the procedure.74
000 Oe magnetic field increased from 40.76 emu g−1 for CCM to 42.49 emu g−1 for MCM because of the narrow and limited distribution of NPs in MCM. Plenty of parameters affect saturation magnetization. Indeed, the magnetic saturation increases with the crystallinity of the NPs.73 On the contrary, as stated by Dai et al., an increase in calcination temperature is inversely proportional to the saturation magnetization given that defects and strain are abundant in the product during the calcination process, although some are relieved throughout the procedure.74
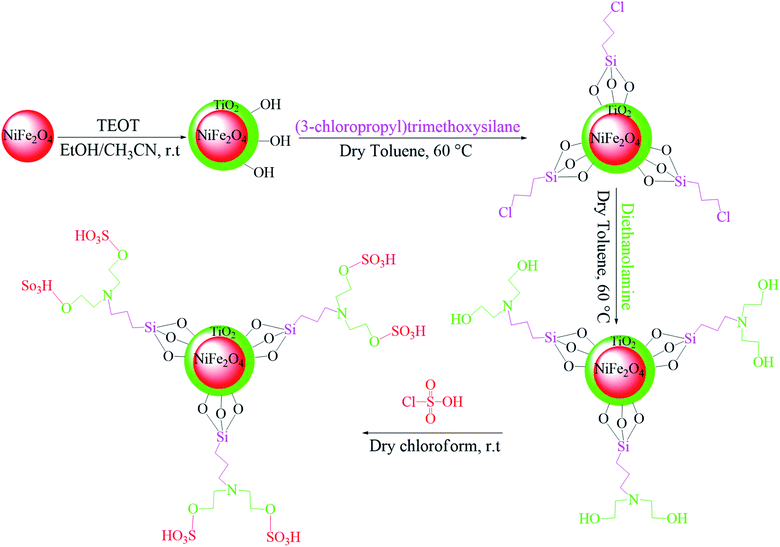 | ||
| Fig. 7 Approach for the preparation of the NiFe2O4@TiO2–SiO2–Pr–DEA–OSO3H nanocatalyst (r.t: room temperature). This figure was adapted with permission from Chem. Sel., 2019, 4, 17–23.71 | ||
Elsewhere, as another type of MFe2O4, ZnFe2O4 MNPs and alginic acid (as a biopolymer) nanocomposite were recently synthesized, as shown in Fig. 8, and applied in an efficient catalyst for the practical synthesis of 2-amino-3-cyano-4H-pyran derivatives.75
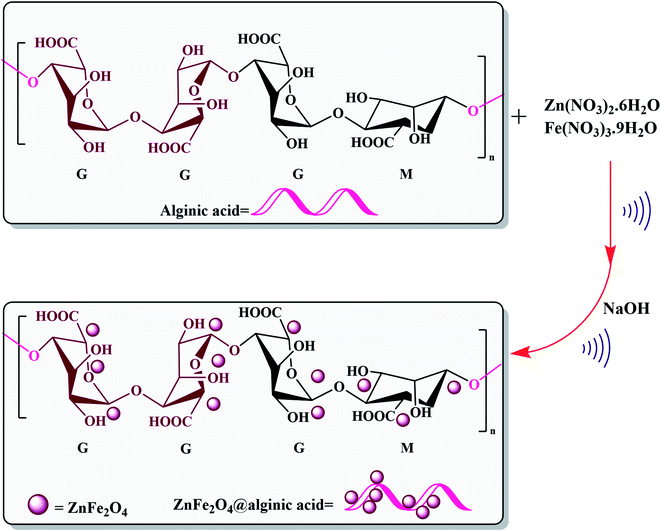 | ||
| Fig. 8 Synthesis of ZnFe2O4@alginic acid. This figure was adapted with permission from Polyhedron, 2019, 171, 193–202.75 | ||
The distinctive benefits of this process are mild conditions, short reaction time, facile workup, high yield, and green synthesis procedure. As a disadvantage, the magnetic saturation of the prepared catalyst (17.06 emu g−1) was lower than the neat ZnFe2O4 MNPs, which could be ascribed to the magnetically neutral alginic acid structure present in the catalyst. However, despite the decreased magnetic saturation of the catalyst, it could be simply separated from the reaction environment.
2.2. Magnetic frameworks
Metal–organic frameworks (MOFs) are novel porous materials composed of ions or clusters of metals linked to organic ligands with coordination bonds. MOFs are not only magnificent adsorbents but also catalysts due to their consistent distribution of single-site or extremely small metal species, high porous structure, large surface area, and abundant active sites.76 Magnetic MOF nanocomposites are an example of materials with facile separation and improved catalytic activity. However, one of the main challenges that still need to be solved is how to assemble MOFs on the surface of MNPs without modifying their surface.77 The combination of Fe3O4 nanoparticles with Fe-MOFs endows the composite catalyst some advantages, such as easy magnetic separation, reusability, and other enhancements such as photo-Fenton performance. Fe3O4 nanoparticles can be in situ lodged into the MOF structure, acting as a magnetic core in magnetic MOF core–shell structures.78 Bian et al. synthesized a novel hierarchical core–shell Fe3O4@PDA–Pd@[Cu3(BTC)2] nanocomposite via a layer by layer assembly method, as shown in Fig. 9.77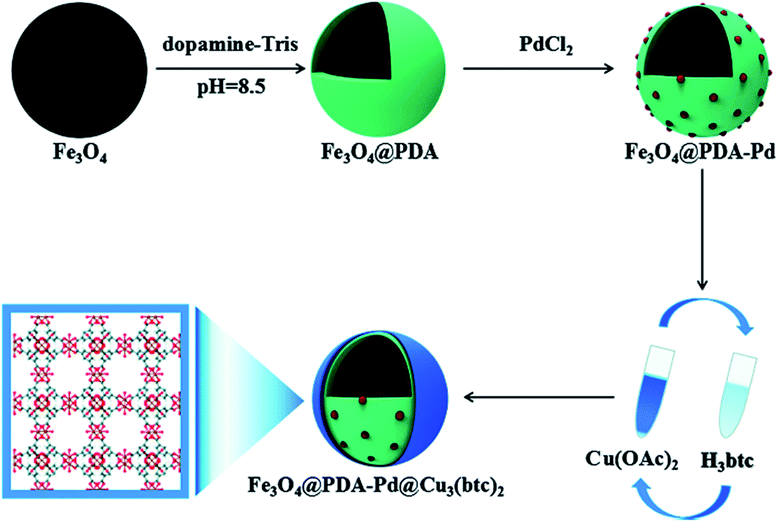 | ||
| Fig. 9 Schematic of the procedure for the synthesis of the Fe3O4@PDA–Pd@[Cu3(BTC)2] nanocomposite. This figure was adapted with permission from ChemCatChem, 2018, 10, 1446–1454.77 | ||
There are also magnetic frameworks with inserted second-row transition metals. This inclusion extends the performance of these materials to various application ranges due to their advanced covalency, redox capability, and last-row spin–orbit coupling compared to their first-row analogs. Mononuclear niobium and molybdenum sites in MOFs display a wide variety of second-and third-row transition metals for stronger magnetic coupling in frameworks.79
The preferred method for the synthesis of magnetic MOFs is the solvothermal method, sometimes coupled with microwave and ultrasonication treatment.78,80 Recently, Espallargas et al. synthesized 2D Fe-based magnetic MOF nanosheets named MUV-1-X via a liquid exfoliation method. The resulting crystalline layers had favourable lateral size and thickness, which retained the structural magnetic properties.81
Besides, MOFs are well-defined solid structures to carry immobilized enzymes in a robust biocatalytic system. Enzymes are natural biocatalysts, demonstrating high selectivity and catalytic performances for the synthesis of chemicals and pharmaceuticals. Although the porosity of MOFs plays a vital role in their structure and performance, hierarchically porous MOFs introduce an adaptable pore distribution from the micro to meso-scale to provide better accessibility to immobilize large molecules such as enzymes. In the work by Zheng et al., they have prepared a magnetic hierarchically porous core–shell Fe3O4@MOF structure through the formation of modulator-induced defects. Then, amidase was immobilized on this magnetic carrier. The properties of this magnetic MOF biocomposite were optimized, resulting in a high enzyme loading, high catalytic yield, thermal and storage stability, and reusability in comparison with the free enzyme and similar structures without hierarchical porosity.82 In another investigation, Zhang et al. focused their efforts on the preparation of a magnetic metal–organic framework, NiFe2O4@MOF-5.
NiFe2O4 is a spinel ferrite structure with high saturation magnetization and strong chemical stability. The combination of ferrite and MOF resulted in excellent magnetic susceptibility, which allows easy separation and reusability.83 Song et al. reported the synthesis of the (Fe3O4@Au@MIL-100(Fe)) structure via a reaction including three successive steps. These steps included a solvothermal reaction, Au seed-induced growth and low-temperature cycling self-assembly, as shown in Fig. 10. This magnetic nanocatalyst acted as a peroxide mimic for the catalytic oxidation of the 3,3,5,5-tetramethylbenzidine (TMB) substrate. A prevalent non-devastating analytical technique used in chemical and biological analyses is surface-enhanced Raman scattering.84 The vibrational modes of a molecule show a unique and fingerprint-like spectrum in the SERS technique to show valuable inherent structural information. In situ SERS spectroscopy can be employed to monitor the whole reaction. However, MOFs themselves do not have excellent SERS enhancement ability. Thus, the use of MOF composites with noble metals can modify their signal enhancement ability. According to the distinctive structure and catalytic characteristics of an Fe-based magnetic MOF, under photo-irradiation with the assistance of ascorbic acid (AA), enhance photo-induced catalytic oxidation was achieved.85
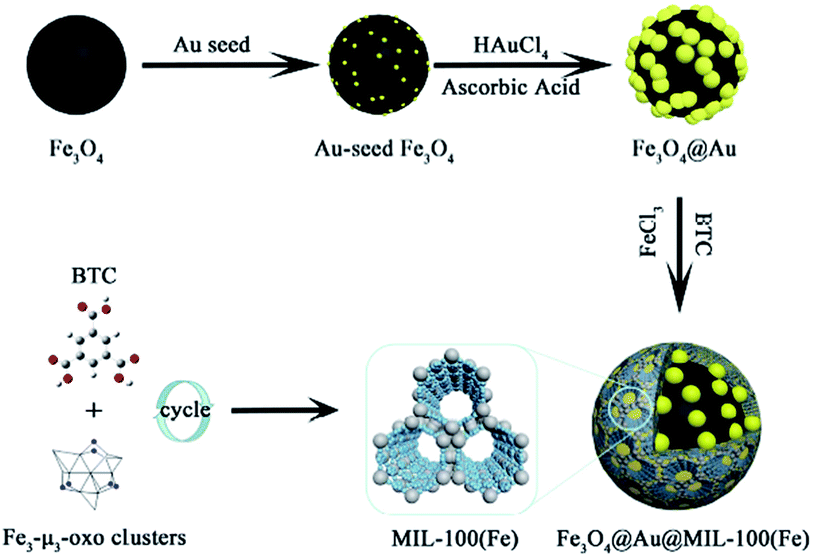 | ||
| Fig. 10 Illustration of the synthetic route to obtain magnetic MOF-based nanocatalysts. This figure was adapted with permission from ACS Applied Materials & Interfaces, 2018, 10, 25726–25736.85 | ||
Covalent organic frameworks (COFs) are organic porous structures, which have strong couplings among their building blocks. According to their characteristics including high chemical and thermal stability, structural resilience, and simple surface modification, COFs can be applied in various applications such as sensors, gas storage systems, catalytic approaches and many others. The density of COFs is lower than that of MOFs, resulting in enhanced stability in different acidic and basic pH and redox conditions. COFs have high sustainability to endure rough conditions without losing their crystallinity and orderly structure. COFs are one of the best candidates for heterogeneous catalysis. They can act as a host for MNPs in host–guest supramolecular structures. COFs are well isolated to prevent the agglomeration of MNPs and their large spatial pore distribution and channel permeability allow access to catalytically active substances as a nanoreactor. MNPs have desirable properties, and thus have received significant attention from scientists, ranging from effortless magnetic recovery, low toxicity, and the ability to have various morphologies to low cost. Thus, due to the excellent properties of MNPs and COFs, their combination as magnetic COFs uncovers a vast, fascinating world of materials.86
Additionally, MNPs can enhance the efficiency of the procedure and reusability of the catalyst. Cai et al. synthesized a unique Gypsophila bouquet-shaped magnetic COF through facile mechanochemical grinding followed by crystallization. In this work, amino-functionalized Fe3O4 nanoparticles were grafted to the Tp monomer (COF monomer). Then, Tp and Pa-1 (another monomer) were combined, resulting the formation of the magnetic TpPa-1. This magnetic COF exhibited superparamagnetic property and a large surface area due to its porous structure. It was used to extract trace analytes such as polycyclic aromatic hydrocarbons (PAHs) from ecological samples. The preparation and function of the bouquet-like magnetic TpPa-1 sorbent are illustrated in Fig. 11.87
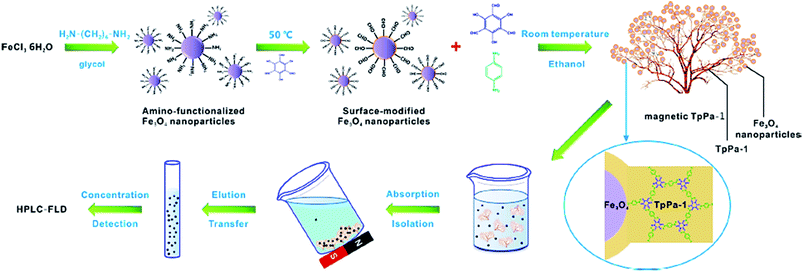 | ||
| Fig. 11 Schematic showing the synthesis and function of the bouquet-like magnetic TpPa-1 sorbent. This figure adapted with permission from ACS Applied Materials & Interfaces, 2017, 9, 2959–2965.87 | ||
2.3. Natural-based magnetic systems
Considering that green synthesis and biocompatibility are vital aspects in catalytic systems, the presence of natural-based environmentally friendly magnetic compounds appear to be suitable for scaling up procedures, simplicity in final purification processes and use in industrial applications. Versatile natural species such as clays and polymers have become the focus of scientists after biocompatibility.30 Intrinsically magnetic natural species such as pumice, magnetite, hematite, ilmenite, and pyrrhotite (monoclinic Fe7S8) are utilized in competent catalytic systems. Magnetite, iron(II and III) oxide, a with brownish-black color, has the highest magnetic properties among the natural-based minerals. The chemical composition of pure hematite (Fe2O3) consists of about 70% iron and 30% oxygen by weight. Magnetite is used in magnetic-derived catalysts in various processes. Magnetite NPs are employed in biomedical, environmental, and ferrofluid applications.88–90 However, unlike magnetite, hematite has weak attraction to a magnetic field. Hematite is applied as a pigment mineral, healing stone, and catalyst with magnetic separation ability.91 Additionally, the paramagnetic ilmenite (FeTiO3) and the ferrimagnetic pyrrhotite (monoclinic Fe7S8) have emerged as great magnetically separable catalysts in different reactions.92,93 Volcanic pumice magnetic particles (VPMPs) are light-colored, highly porous volcanic rocks with an intrinsic magnetic property of 30 emu g−1. VPMPs are biocompatible and have high surface functionalization capacity.94 Furthermore, the physical properties of VPMPs, such as lightweight porous structures, are well suited for filtration and concrete construction applications. The chemical structure of pumice consists of a silica network, aluminum oxide, and aluminum silicate, and similar to other minerals, it has small amounts of the other metal oxides. Also, pumice can be functionalized with other species due to its chemical structure and abundant OH groups.952.4. Hybrid micro and nanoscale magnetic composites
Recently, organic–inorganic hybrid materials have been implicated as heterogeneous catalysts given that they exhibit the advantages of both homogeneous and heterogeneous catalysts in organic synthesis.96 The chemically bonded hybrid materials have been described as materials with covalent or partially covalent bonds. Hybrid catalysts consist of two distinguished phases with completely different properties and functionalities, which have synergistic combinations to develop overall catalytic properties.97In one of the most recent reports, Sedaghat et al. introduced an efficient hybrid catalyst with antibacterial property for the synthesis of 5-substituted-1H-tetrazoles.98 In this regard, a Cu2+–Schiff base complex was anchored on magnetic mesoporous silica NPs to form Fe3O4@MCM-41–SB–Cu (Fig. 12). The possibility to load high amounts of copper on the catalyst is due to the presence of many coordination sites in the bis-Schiff base ligand. Besides, a coordination bond is formed between the oxygen and nitrogen atoms of the supported Schiff base and Cu2+ ions. Significantly, the long catalyst linkers in this study resulted in easy access by the reactants to the active sites of the catalyst compared to the studies with short linkers.
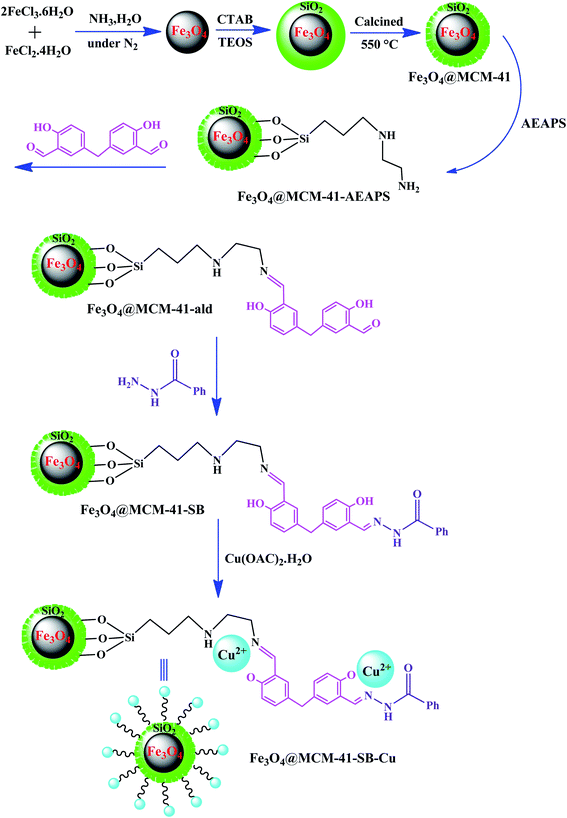 | ||
| Fig. 12 Synthetic route of functionalized magnetic Fe3O4@MCM-41–SB–Cu hybrid catalyst. This figure was adapted with permission from Applied Organometallic Chemistry, 2020, 34, e5572.98 | ||
From a mechanistic point of view, during the synthesis of 5-substituted-1H-tetrazoles, the interaction between Cu2+ ions and oxygen atoms of the aldehyde and nitrogen atoms of the nitrile increases the electrophilicity of the aldehyde and nitrile, respectively, which promotes further interactions. The high catalytic performance and non-toxicity of the Cu catalysts led to excellent catalytic yields (69–95%) in this reaction.
Ferrites can also act as the magnetic core of hybrid catalysts. As an example, a hybrid nanocomposite of organoacid-decorated NiFe2O4 was prepared. The advantages of the used MNPs included not only their superparamagnetic behavior and effortless separation from the reaction environment, but also considerable surface area, low toxicity, good stability, and easy surface functionalization.71 Another example of ferrites is CuFe2O4@Si–Imid–PMo, which consists of an acidic ionic liquid based on the imidazolium cation and phosphomolybdic acid anion, both immobilized on the CuFe2O4@SiO2 core–shell magnetic structure (Fig. 13).99 This catalyst was applied in the synthesis of 2,4,5-trisubstituted imidazole derivatives. Matching with previous studies involving several functionalization steps on a magnetic core, the magnetic saturation of this catalyst decreased from 25.85 to 24.1 emu g−1 under an applied magnetic field of 104 Oe compared to the neat CuFe2O4, which is ascribed to its non-magnetic silica shell and immobilized ionic liquid. However, the magnetization of this catalyst was sufficient for easy magnetic separation. The CuFe2O4@Si–Imid–PMo catalyst improved the production of the intermediate during the reaction, while it increased the electrophilicity of the electrophiles according to its role as a Brønsted acid center.
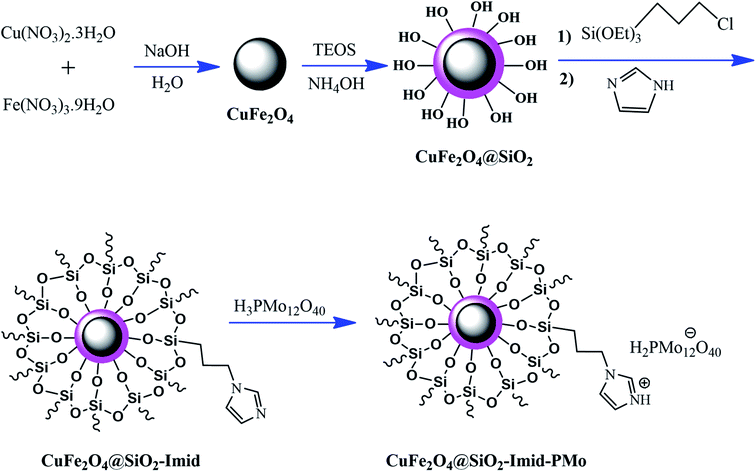 | ||
| Fig. 13 Synthesis of CuFe2O4@Si-Imid-PMo catalyst. This figure was adapted with permission from: Quarterly Journal of Iranian Chemical Communication, 2019, 7, 271–282.99 | ||
In a recent study reported by Bodaghifard et al., a magnetic core–shell structure was functionalized by polymer for the green synthesis of 2-amino-3-cyanopyridine derivatives.100 This hybrid heterogeneous catalyst contained poly N,N-dimethylaniline-formaldehyde supported on Fe3O4@SiO2 (PDMAF-MNPs), as depicted in Fig. 14. It should be noted that the reduction in magnetic saturation compared to its pure magnetic core is much greater in the case of polymer linkers compared to other short organic likers, as stated in a previous study. According to the silica shell and polymeric linker around Fe3O4, the magnetic saturation declined from 53.5 to 31.1 emu g−1 in high magnetic fields up to 8000.0 Oe. The unpaired electron pairs of nitrogen in this eco-friendly catalyst expedited the Knoevenagel condensation of benzaldehyde and malononitrile and also Michael addition of cyclohexanone by taking the hydrogen of its components to form intermediates and adducts in the synthesis of 2-amino-3-cyanopyridine derivatives. This catalyst resulted in efficient isolated yields in the range of 74–93%. This retrievable catalyst was recycled six times with no distinctive structural alteration and change in its catalytic behavior.
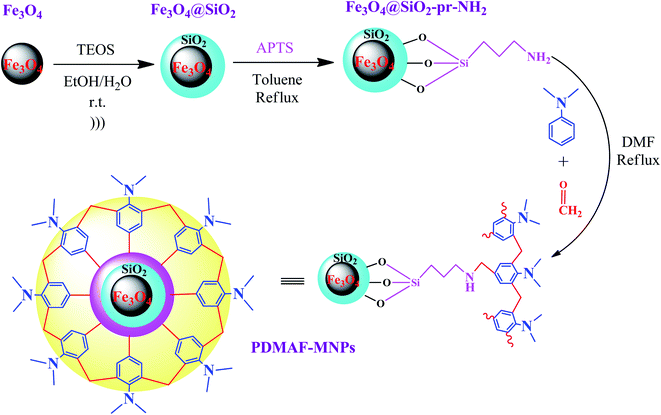 | ||
| Fig. 14 Synthesis of poly N,N-dimethylaniline-formaldehyde supported on silica-coated Fe3O4 magnetic nanoparticles (PDMAF-MNPs). This figure was adapted with permission from Research on Chemical Intermediates, 2020, 46, 1629–1643.100 | ||
As another strategy, conventional biocompatible and naturally occurring catalysts are combined with magnetic particles through surface modification approaches. Consequently, the active sites of the catalyst also exhibit magnetic property. For example, Maleki et al. designed an efficient magnetic hybrid catalyst, as shown in Fig. 15, for the synthesis of biologically active polyhydroquinoline derivatives.101 In the design of natural polymer-based hybrid catalysts, dextrin, which originates from natural polysaccharide resources, is beneficial due to its many effective advantages such as abundant active functional groups, non-toxicity and biocompatibility, availability, environmentally friendly nature, ability to prevent unwanted side reactions, and stereoselectivity in some organic reactions. Thus, the question arises, how can magnetic dextrin effectively act as a hybrid biocatalyst in pharmaceutical synthesis reactions?
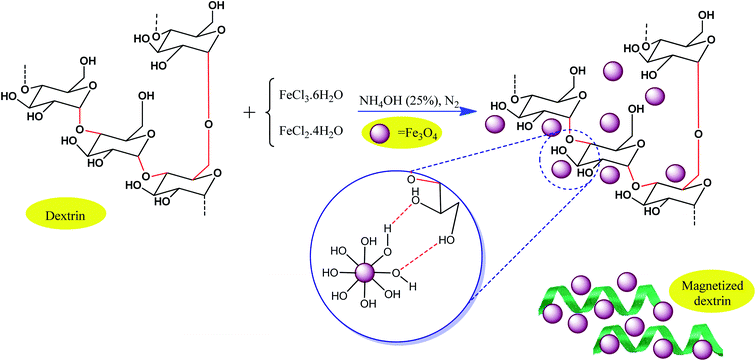 | ||
| Fig. 15 Process for the preparation of magnetic dextrin. This figure was adapted with permission from Materials Science and Engineering: C, 2020, 109, 110502.101 | ||
On the one hand, the convenient separation of the catalyst is ascribed to iron oxide MNPs with acceptable particle distribution, high surface area to volume ratio, and superparamagnetic feature. On the other hand, its abundant reactive functional groups give the catalyst the ability to interact with other organic components via hydrogen bonds derived from the its hydroxyl groups and the electronegative atoms of the functional groups in the other material. Accordingly, the electrophilicity of the designed materials would be enhanced, finally facilitating the reaction. The synthesis of polyhydroquinoline derivatives via the asymmetric Hantzsch reaction took advantage of this catalyst to overcome the limitations in previous studies such as tedious workup process, harsh reaction conditions, and unsafe catalysts by presenting an appropriate yield (70–95%), short reaction time (15–45 min), and five times recycling. Also, polyhydroquinoline derivatives have numerous pharmaceutical applications, for example, vasodilators, anti-atherosclerotic, hepatoprotective, antitumor, bronchodilator, antidiabetic, and calcium channel blockers.102
In another study, Ru3+ and carboxymethylcellulose (CMC) in the magnetic RuIII@CMC/Fe3O4 hybrid catalyst (Fig. 16) exhibited a noticeable synergistic effect during each step in the synthesis of polyhydroquinoline derivatives.103
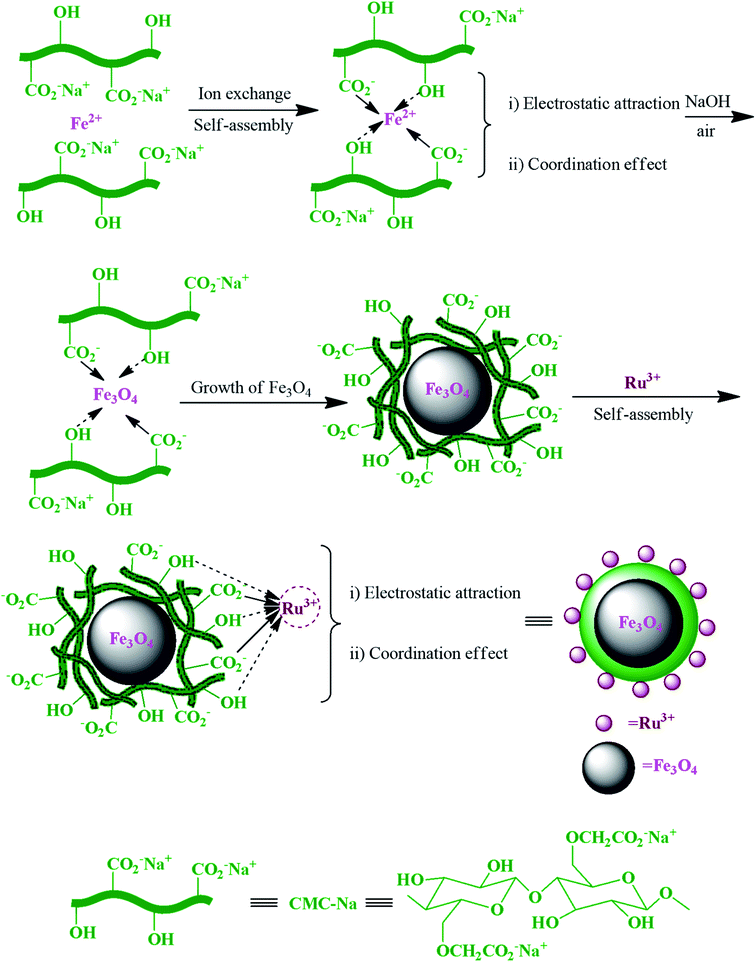 | ||
| Fig. 16 Schematic of the preparation of magnetic RuIII@CMC/Fe3O4 organic/inorganic hybrid catalyst via self-assembly. This figure was adapted with permission from: Molecular Diversity, 2019, 23, 421–442.103 | ||
This advantage of the above-mentioned hybrid catalyst is attributed to the Ru3+ Lewis acid, which can be chelated with the carboxyl and free hydroxyl groups of CMCs. Ru3+ activated β-keto ester (4) to facilitate further nucleophilic attack in the condensation of hydrazine (3). Also, it acts as a center through which an electron transfers to give the enol (B) form from keto (A). Besides, Ru3+ and hydrogen bonding advance the Knoevenagel condensation of a carbonyl compound (1) with malononitrile (2). It should be noted that the Ru3+ and hydroxyl groups of CMCs are active catalytic sites to promote the Michael addition of the reaction intermediates (B and C), as displayed in Fig. 17.
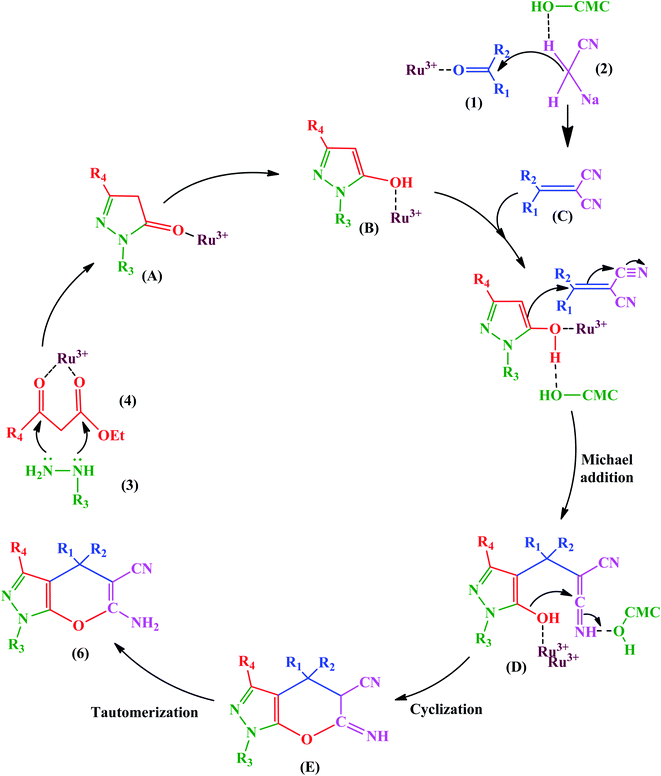 | ||
| Fig. 17 Proposed mechanism for synthesis of pyrano[2,3-c]pyrazoles. This figure was adapted with permission from Molecular Diversity, 2019, 23, 421–442.103 | ||
In the recent report on the use of hybrid catalysts for the synthesis of pharmaceuticals by Maleki et al., they demonstrated the use of an eco-friendly solid-state hybrid catalyst.94 Specifically, in this study, they successfully demonstrated the synergistic impacts of ultrasonic waves and a natural-based magnetic cellulose/pumice hybrid catalyst for the synthesis of 2,4,5-triarylimidazoles. The mechanism for the synthesis of imidazole with respect to the role of the catalyst is shown in Fig. 18. The hydroxyl groups on cellulose activate the carbonyl group of benzaldehyde (1) and benzil (3) to further form intermediate I and II, respectively. According to the physical aspects, the highly porous structure of pumice created good electronic interactions between the components due to its very high surface area. Finally, because of the magnetic property of pumice, the catalyst was recycled for ten successive runs without any significant loss in its catalytic functionality.
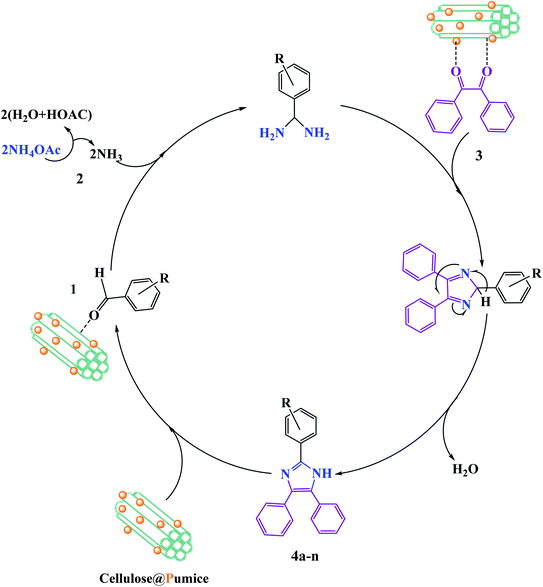 | ||
| Fig. 18 Suggested reaction mechanism for the synthesis of 2,4,5-triarylimidazoles (4a–n) utilizing a magnetic cellulose/pumice hybrid catalyst. This figure was adapted with permission from Journal of Physics and Chemistry of Solids, 2020, 142, 109443.94 | ||
Reportedly, a heterogeneous hybrid nanocomposite was synthesized via the co-precipitation method. Although the catalytic activity of each component of the composite was not significant, the hybrid structure showed much higher activities due to the composition of the materials. Alternatively, the reported hybrid structure offers characteristic properties and applications that are not achievable in each component alone. The synergistic effect of the metals and metal oxides in a hybrid catalyst scaffold expedites the organic reaction more efficiently.70,104Table 1 summarizes the information of some efficient magnetic catalytic systems, highlighting their target organic reactions and reaction yields.
| Entry | Catalyst | Function | Yielda (%) | Ref. |
|---|---|---|---|---|
| a Isolated yield. b Tetraphenyl porphyrin copper hybrid nanoflowers. c Tetraphenyl porphyrin cobalt hybrid nanoflowers. d Peroxymonosulfate. e Alginate. f 3-Chloropropyltrimethoxysilane. g L-Arginine. h [Fe3O4@–SiO2@R-NHMe2][H2PO4]. i Shilajit. j β-Cyclodextrin. k Graphene oxide. l Polyacrylamide. m Dicyandiamide. n Chitosan. o Cyclodextrin. p Magnetic graphene quantum dots. q Hydroxyapatite. r 1,3,5-Tris(2-hydroxyethyl) isocyanurate-1,3-propylene covalently functionalized MCM-41. s Diethylenetriamine penta. t Palladium-containing dicationic bipyridinium-supported periodic mesoporous organosilica (PMO). | ||||
| 1 | TPP@CuhNfsb and TPP@CohNfsc | Hydrogenation of nitrobenzenes | 52–98% | 105 |
| 38–98% | ||||
| 2 | Fe3O4/PVA-10%Ag | Reduction of nitrobenzene derivatives | 89–99 | 106 |
| 3 | MNPs@C/UV/PMSd | Catalytic oxidative degradation of acetaminophen | 97.4% | 107 |
| 4 | CoFe@rGO | Epoxide ring opening reaction with various aromatic amines of cyclohexene oxide | 86–94% | 108 |
| Cyclopentane oxide | 85–94% | |||
| Styrene oxide | 82–92% | |||
| 5 | Fe3O4@Alge@CPTMSf@Argg | Synthesis of pyrazole derivatives | 90–97% | 109 |
| 6 | [FSRN][H2PO4]h | Synthesis of pyrimido[4,5-b]quinolines | 79–96% | 110 |
| 7 | γFe2O3@Shi@Cu2O | Synthesis of 1,4-disubstituted-1,2,3-triazoles | 50–98% | 111 |
| 8 | Fe3O4@Cu–β-CDj | Synthesis of dihydropyrano[2,3-c]pyrazoles | 89–98% | 112 |
| 9 | Pd@GOk/Fe3O4/PAAl/DCAm | Sonogashira reaction of various halides with terminal alkynes | 80–97% | 113 |
| 10 | ZnS–ZnFe2O4 | Synthesis of 2,4,5-triaryl-1H-imidazoles | 51–95% | 114 |
| 11 | Pd@CSn–CDo–MGQDsp | Hydrogenation of nitro compounds | 80–97% | 115 |
| 12 | α-Fe2O3@Hapq@Cu | Synthesis of 1,4-disubstitued triazoles | 40–97% | 116 |
| 13 | Fe3O4@SiO2–guanidine–poly acrylic acid | Synthesis of 4H-benzo[b]pyrans and dihydropyrano[c]chromenes | 95–98% | 117 |
| 14 | Ferroferric oxide nanocatalyst@mesoporous activated carbon | Degradation of acetaminophen | 98.6% | 118 |
| 15 | MCM41–Pr–THEICr | Synthesis of 9-(aryl)-3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione derivatives | 68–92% | 119 |
| 16 | Fe–DPMPs | Synthesis of tri-substituted imidazole derivatives | 65–92% | 120 |
| 17 | Fe3O4@xanthan gum | 2-Amino-3-cyano-4H-pyran derivatives | 84–96% | 121 |
| 18 | Pd NPs@Fe3O4/chitosan/pumice hybrid beads | Cyanation of aryl halides | 80–98% | 122 |
| 19 | ZnO/g-C3N4 | Synthesis of biologically interesting small molecules of thiazolidinones | 57–97% | 123 |
| 20 | Pd@Bipy–PMOt | Suzuki cross-coupling reactions of various aryl halides and bromic acid substrates | 6–98% | 124 |
3. Application of micro and nanoscale magnetic catalytic systems in the degradation of pharmaceutical compounds
In recent decades, the discharge of toxic substances, including inorganic heavy metals and organic contaminants such as pesticides, herbicides, dyes and pharmaceuticals in water and sewage has become major human and ecology issues.125–128 Thus, a wide variety of water decontamination methods utilizing micro-and nano-adsorbents and/or photocatalysts have been developed.129–131 In addition, microwave- and ultrasonic-assisted pollutants catalysis approaches have gained wide interest in this field due to their synergistic effect of converging the dispersed microwave and ultrasound fields onto the reactive sites of the catalyst.60,132,133 These synergetic methods produce thermal or discharge effects around the catalyst, which lead to the in situ degradation of contaminants.134 Generally, microwave technology reduces the activation energy and organic reaction time due to the following reasons: firstly, the catalysts absorb microwaves to produce a thermal effect, which can create abundant hot spots to speed up organic reactions. Secondly, the coupling effects of microwaves and catalysts that have high wave absorption capacity result in a non-thermal effect, which can generate hydroxyl radicals with strong oxidation potential, leading to an enhancement in the oxidation rate of organic materials. As the most recent example, Riaz et al. prepared MnO2 nanorods and ZnMn2O4 nanostructures with hexagonal shapes for the microwave-assisted catalytic decomposition of 4-nitrophenol (p-NP) solution.135 ZnMn2O4 exhibited a high degradation efficiency of up to 85% in just 30 min. As displayed in Fig. 19a, similar to the photocatalytic degradation conducted under visible light, hot spots with high temperatures are produced on the ZnMn2O4 absorbent upon exposure to microwave irradiation. The water and dissolved O2 molecules produce ˙OH and H˙, and O2˙− radicals by the heat absorbance from the hot spots, respectively.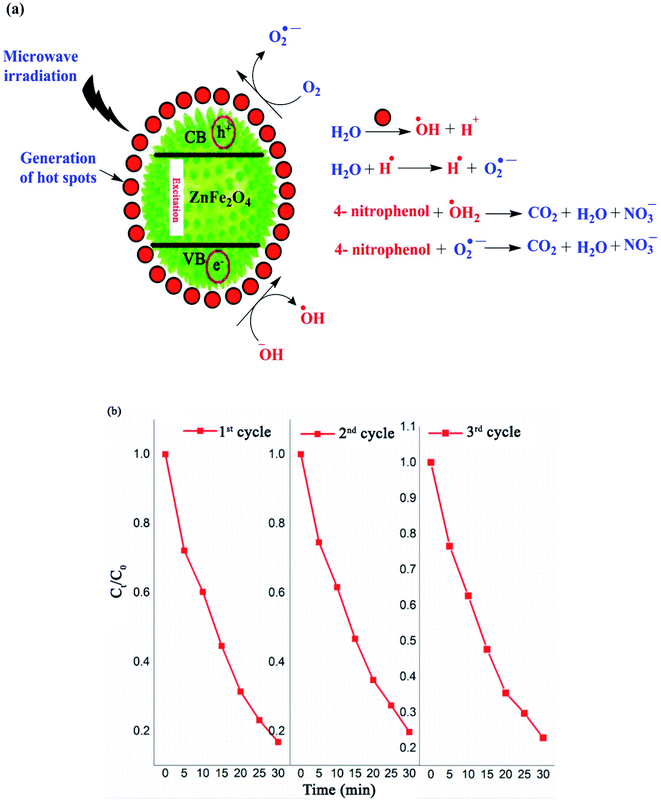 | ||
| Fig. 19 (a) Mechanism for the degradation of 4-nitrophenol using ZnMn2O4 catalyst. (b) Recyclability of spinel ZnMn2O4 absorbent during three degradation cycles under microwave irradiation. This figure was adapted with permission from Journal of Materials Research and Technology, 2020, 9, 9709–9719.135 | ||
Similarly, the ZnMn2O4 absorbent contributes to producing more ˙OH and O2˙− radicals. The electrons in the valence band (VB) of the ZnMn2O4 absorbent are excited and transported to the conduction band (CB), which causes holes (h+) in the VB and electrons (e−) in the CB. The holes oxidize H2O to produce ˙OH and the electrons react with dissolved O2 to produce O2˙−. Eventually, this mechanism process facilitates the degradation of p-NP to CO2, H2O, and nitrate. According to the scavenging experiment results, it was found that the produced ˙OH radicals acted as the major active species in the decomposition compared to O2˙−. Besides, the ZnMn2O4 absorbent had a slight reduction in activity (from 83% to 79%) after three successive p-NP microwave-assisted degradation cycles, as shown in Fig. 19b.
Another study was devoted to investigating the synergistic effect of ultrasound irradiation power, catalyst dosage, solution pH, etc. on tetracycline (TC) ultrasound-assisted degradation using Fe/N–C-x hybrid/H2O2 Fenton-like catalysts (x stands for iron salt molar ratio (Fe(NO3)3·9H2O)).136 Unlike previous reports, the removal of TC decreased from 93.8 to only 86.3 in 80 min by altering the pH from 3 to 11, indicating that this catalyst has high removal efficiencies in a wide pH range (Fig. 20a). The TC degradation increased concurrently with an increase in Fe/N–C-2 concentration, which was attributed to the generation of more ˙OH radicals, resulting from the decomposition of H2O2 (Fig. 20b). Terephthalic acid, as a fluorescence probe, was employed to authenticate the amount of ˙OH radicals produced. A slight increase in the fluorescence response was assigned to the hydroxyl radicals produced by Fe/N–C-2 catalysis (Fig. 20c). Given that one of the most effective parameters to achieve superior degradation efficiencies is ultrasound irradiation, different ultrasonic powers (0, 40, 60, 80, 100, and 120 W) were applied to monitor their effects on the system (Fig. 20d). The increase in ultrasonic power was observed to be directly proportional to the TC degradation efficiency, confirming the synergistic catalytic effect of the Fe/N–C-2 catalyst and ultrasound waves.
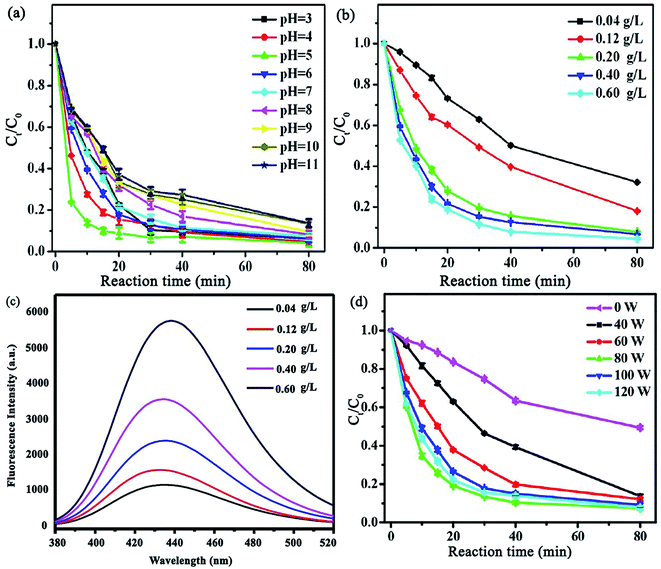 | ||
| Fig. 20 Effect of various parameters on TC removal: (a) solution pH, (b) Fe/N–C-2 concentration, and (c) influence of Fe/N–C-2 concentration (g L−1) on ˙OH production utilizing a terephthalic acid fluorescence probe. (Reaction conditions: 60 mM terephthalic acid concentration; 60 mM H2O2; reaction temperature: 25 °C; 80 W US power; and 80 min reaction time). (d) Ultrasonic power. This figure was adapted with permission from: ACS Omega, 2018, 3, 15870–15878.136 | ||
Moreover, consecutive ultrasound exposure removed the byproducts; thus, the surface of the catalyst was free for further reactions. Furthermore, a higher ultrasonic power generates more cavitational bubbles with extremely high pressure and temperature, which collapse and produce solution turbulence, leading to elevated intensity of mixing between the catalyst and contaminant.137 Nevertheless, it should be noted that an additional increase in ultrasonic power resulted in lower TC degradation because the maximum growth and collapse of the cavitation bubbles occur together with the compression cycle in the cavitation process, resulting in attenuated cavitation action at high ultrasound irradiation intensity. Accordingly, 80 W was chosen as the optimized ultrasonic power.
Precisely, various magnetic catalytic systems and the pioneering and most powerful photocatalysts are discussed briefly. As the first and foremost example of photocatalysts, TiO2 is well-known due to its chemical stability, non-toxicity, and comparatively low cost. As stated above, photocatalytic degradation is triggered when a photon is sufficiently excited to over the bandgap energy value of a semiconductor. Semiconductor materials with a wide bandgap, such as TiO2 with a bandgap of 3.2 eV in the anatase state, are less active under visible light irradiation.138 UV irradiation is the most potent energy source for the degradation of pollutants; however, it poses a risk to human health and has detrimental effects on the eyes.139 Thus, various strategies have been developed to produce visible light-active TiO2 photocatalysts such as metal doping,140 non-metal doping,141 codoping with different semiconductors,142 and dye sensitization.143 The presence of metal dopants in the structure of TiO2 delays the recombination of charge carriers by electron trapping, changing its bandgap energy and physical characteristics.
For instance, Ag co-doped TiO2 nanostructures grafted on Fe3O4 NPs were synthesized through a facile and affordable co-precipitation approach. They were applied for the degradation of dibutyl phthalate (DBP), which is a toxic ecological pollutant (Fig. 21a).144 The degradation intermediates such as butyl phthalate, diethyl phthalate, dipropyl phthalate, methyl benzoate, and benzoic acid were detected by gas chromatography/mass spectrometry (GC-MS) analysis, and the two possible decomposition routes are illustrated in Fig. 21b. During the reaction, the generated excitons by light stimulation of TiO2 are transferred to the surface of the catalyst to react with OH−, O2, H2O, and other species. The ˙OH radicals break DBP in two possible ways, as follows: the first way is to break the C–O bond of DBP to form monobutyl phthalate accompanied by the breakage of another C–O bond to produce phthalic acid.
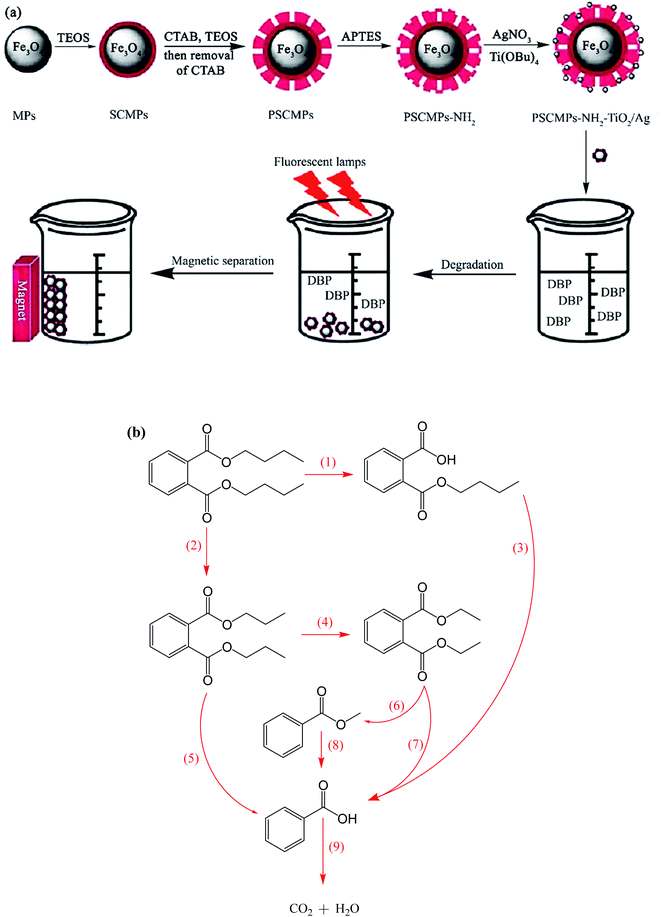 | ||
| Fig. 21 (a) Synthesis of magnetic photocatalysts and their application for the degradation of dibutyl phthalate under visible irradiation. (b) Suggested photocatalytic degradation routes for DBP. This figure was adapted with permission from Water Science and Technology, 2020, 81, 790–800.144 | ||
The further decomposition of phthalic acid generates benzoic acid, which breaks into CO2 and H2O. The second path is the degradation of the DBP molecules from various sides of its carbon chain to form dipropyl phthalate and diethyl phthalate as the main products. In the subsequent reaction stage, methyl benzoate and benzoic acid are generated by the actions of ˙OH radical. Similarly, benzoic acid degrades to CO2 and H2O. The DBP degradation efficiency reached 74%, which remained almost the same after five cycles, proving the high stability and reusability of the catalyst.
Another strategy to simplify the degradation of pharmaceuticals using a catalytic system is to narrow the absorption range of TiO2 to the visible light region. Besides the abovementioned method, another way includes doping non-metal species in the oxygen sites of the TiO2 structure, creating oxygen defects, which reduce the bandgap energy of non-metal-doped TiO2 and lowers the energy level of its VB. As an example, a nitrogen-doped TiO2/SiO2/Fe3O4 magnetic nanocomposite (NTSF) exhibited 96.32% efficiency for the degradation of naproxen (NPX) under the optimum conditions.145 As predicted, nitrogen doping improves the photocatalytic activity of TiO2 because it reduces the bandgap energy of the composite to 2.9 eV. Thus, it can be photocatalytically active under purple light-emitting diode (LED) illumination. Also, SiO2 acts as an efficient agent in the nanocomposite to enhance its specific surface area, lower its bandgap and obstruct the state change of TiO2 from anatase to rutile. The reduction in magnetic saturation from 45.40 emu g−1 for Fe3O4 to 30.45 emu g−1 for NTFS is attributed to the non-magnetic SiO2 layer and heating during the calcination process. However, NTSF was reused in four consecutive cycles without any significant reduction in its efficiency. In general, as previously stated, reactive oxidative species (ROS) such as ˙OH, O2˙−, h+, and e− promote photocatalytic reactions (Fig. 22a). Various scavenger agents were applied to evaluate the level of ROS participation in the photocatalytic reactions. The photocatalytic activity of NTSF in the degradation of NPX under the optimized conditions was investigated in the presence and absence of different scavengers (Fig. 22b). The greater the rate of degradation, the more effective role the radical plays in the degradation process. Ammonium oxalate (AO), benzoic acid (BA), p-benzoquinone (BQ), and K2Cr2O7 are the appropriate scavengers for h+, ˙OH, O2˙−, and e−, respectively. The degradation rate was considerably reduced with the addition AO, BQ, K2Cr2O7, and BA. Thus, it was concluded that the destruction of NPX was mainly conducted by ˙OH and h+ played a minor role in the degradation process.
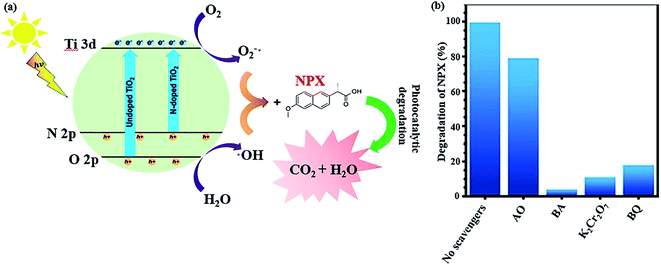 | ||
| Fig. 22 (a) Mechanism for the photocatalytic decomposition of NPX by co-doped TiO2. (b) NPX degradation efficiency with the photocatalyst in the presence and absence of various scavengers. This figure was adapted with permission from Journal of Environmental Science and Health, Part A, 2019, 54, 1254–1267.145 | ||
In a recent study by Sayadi et al., the degradation of naproxen (NPX) was conducted using ZnFe2O4@TiO2/Cu under solar light irradiation.146 The participation of ZnFe2O4 with a low bandgap of 2.11 eV in the composite photocatalyst caused it to possess a narrow bandgap of about 2.62 eV, which resulted in photocatalytic activity under visible light. Initially, to balance the total energy of the system, the electrons in the copper NPs transfer to TiO2 given that they have higher energy levels (Fig. 23). Simultaneously, when NPX contacts the ZnFe2O4@TiO2/Cu photocatalyst, the electrons in the VB of TiO2 and ZnFe2O4 transfer to their CB by excitation from sunlight irradiation. The fate of these stimulated electrons can be predicted in different ways. The produced electrons in the CB of TiO2 by solar light irradiation are transferred to Cu. In the next step, they may first transfer from the CB of ZnFe2O4 to the CB of TiO2 and then move to Cu.
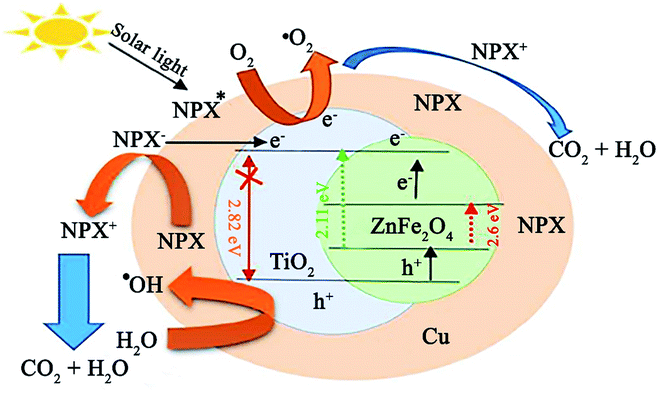 | ||
| Fig. 23 Plausible mechanism for the photocatalytic degradation of NPX under a solar light source. This figure was adapted with permission from Journal of Cleaner Production, 2020, 268, 122023.146 | ||
Further, direct electron injection from the CB of ZnFe2O4 to Cu NPs is plausible. Accordingly, the ZnFe2O4@TiO2/Cu photocatalyst can diminish the recombination rate of excitons. The holes in the VB of ZnFe2O4 and TiO2 are involved in the degradation of NPX and ˙OH generation, while the photogenerated electrons react with O2 for the production of more O2˙−. Lastly, the resultant drugs turn into H2O and CO2 by ˙OH, O2˙−, and h+ radicals. This catalyst exhibited not only high removal efficiency for NPX (80.73%) but also exhibited superior reusability, where after five runs of degradation, 72.31% degradation was attained. In addition, facile recovery of this core–shell ZnFe2O4@TiO2/Cu structure was achieved due to its magnetic saturation of 26.45 emu g−1, which is comparable with that of other magnetic TiO2 photocatalysts.
Also, a magnetic TiO2–graphene oxide–Fe3O4 composite was prepared by He et al. for the photo-Fenton decomposition of amoxicillin.147 Although each structural moiety of this photocatalyst has its unique beneficial properties, an excellent synergic effect occurred after they were combined. For instance, graphene oxide (GO) with properties such as 2D carbonaceous monolayer structure, high surface area, and electrical conductivity was employed as a template for binding nanoparticles. Besides, Fe3O4 has high chemical and thermal stability compared to other oxides and it aids the simple recovery of the catalyst. According, all these parts in the TiO2–graphene oxide–Fe3O4 composite aimed to enhance the catalytic role, durability and separation property deficiencies of TiO2 by its combination with the high-conductive (GO) and high-magnetic recovery ability (Fe3O4) of its components.
ZnO is another useful photocatalyst that contributes in catalytic systems to the degradation of organic contaminants. In a recent report, Maleki et al. prepared a ZnO/Fe3O4@pumice photocatalyst for the degradation of methylene blue (MB) under green light irradiation with a maximum photocatalytic efficiency of 85.5% (Fig. 24a).148 As another strategy, the improvement in magnetic property obtained through composing magnetic pumice micro-plates and Fe3O4 NPs was proved by the ∼20 emu g−1 increase in the magnetic saturation of Fe3O4@pumice at ±15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe magnetic field. The simple retrievability of the photocatalyst for eight cycles was attributed to its high magnetic property. The same as-proposed mechanisms for TiO2 and ZnO nanorods (NRs) was stimulated when exposed to green LED light.
000 Oe magnetic field. The simple retrievability of the photocatalyst for eight cycles was attributed to its high magnetic property. The same as-proposed mechanisms for TiO2 and ZnO nanorods (NRs) was stimulated when exposed to green LED light.
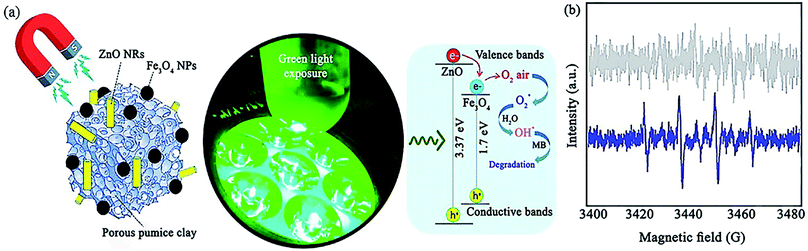 | ||
| Fig. 24 (a) Illustration of the photocatalytic mechanism for the degradation of MB with synergistic effect between green light exposure and ZnO/Fe3O4@pumice photocatalyst. (b) ESR spectra for DMPO scavenger of ZnO/Fe3O4@pumice photocatalyst at ambient temperature. This figure was adapted with permission from Materials Research Bulletin, 2020, 130, 110946.148 | ||
Regarding the resemblance of the energy level of the CB of ZnO and Fe3O4, the same number of holes produced in their CB results from the electron transfer from the VB of ZnO to the VB of Fe3O4. This procedure somehow reduces the electron–hole pair recombination. Therefore, electron accumulation in the VB of ZnO enhances the production of O2˙ from the O2 molecules in the air. Moreover, ˙OH radicals are generated from water during the oxidization process of the holes (h+). In this report, erythrocyte sedimentation rate (ESR) analysis was performed to screen the formation of the ˙OH radical (Fig. 24b). Accordingly, 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as a conventional spin-trap reagent was applied. It was concluded that ˙OH radicals play a vital role in the degradation of MB compared to O2˙ radicals because there was no distinctive signal in the ESR spectrum when the reaction with ZnO/Fe3O4@pumice was carried out in the dark. However, the DMPO–OH adduct exhibited four quartet peaks under green-light exposure. Considering that the individual bandgap of ZnO is 3.32 to 3.37 eV, it absorbs UV radiation, which is not very favorable. Thus, the composition process with Fe3O4 owing to its bandgap of 1.7 eV leads to more effective photocatalytic degradation and efficient energy harvesting ability in comparison with bare ZnO NRs.
It should also be noted that the contribution of magnetic nano spinel ferrites to 2D graphene family nanomaterials result in preferential properties compared to their individual catalytic systems. Graphene-based nano spinel ferrites (GNSFs) are potentially cost-effective and environmentally friendly materials and possess improved physical and chemical characteristics such as restrained particle aggregation, boosted active surface area, and simple magnetic removal for the recycling process.149,150 These magnetic catalysts participate in both adsorptive and degradation reactions.151 GNSFs show incomparably better adsorption efficiency than individual graphene-based nanomaterials, i.e., graphene, GO, and rGO. As a prominent example, rGO/Bi2Fe4O9 displayed a maximum adsorption capacity (qm) of 3.95 mg g−1 for bisphenol-A, which is significantly higher than that of Bi2Fe4O9 (0.74 mg g−1) and GO/Bi2Fe4O9 (1.72 mg g−1).152 This remarkable result is related to the enhanced surface area of rGO (∼2600 m2 g−1) and π–π stacking interactions between the benzene ring of bisphenol-A and surface functional groups of rGO. GNSFs demonstrate a high degree of light-harvesting features and a broader visible light absorption spectrum because of the participation of graphene in magnetic catalytic systems. In the optimized state, it was found that as the graphene content increases, the absorption of visible light increases.
Regarding the study done by Liu et al., graphene in GNSFs acts as an acceptor and mediator agent for photogenerated electrons, which impedes the recombination of e−–h+ pairs during the transition process. The role of rGO relies on its substantial π–π network, which retains the electrons, and its lower Fermi energy compared to the CB of ZnFe2O4.153 Reportedly, manganese ferrite/graphene oxide (MFO–GO) was applied for the adsorption of MB from an aqueous solution with a maximum adsorption capacity (qm) of about 177.3 mg g−1 (Fig. 25).154 The magnetic saturation of the MFO NPs was 20 emu g−1; however, the magnetic saturation for MFO–GO was reduced with an increase in the GO content from 12.2 to 3.2 emu g−1, altering the GO content from 10% to 50%, respectively. Also, MFO–GO showed excellent reusability after five cycles. From a mechanistic aspect, there are four possibilities for the removal of MB dye in neutral solution. The first is associated with electrostatic/ionic interactions between the MB molecules with a positive charge and surface OH groups of GO and MFO. The second reason is assigned to the abundant active binding sites of the basal planes and GO edges, including carboxyl (–COOH), epoxy (C–O), and hydroxyl (–OH) oxygen-containing functional groups, which act as major adsorption sites for MB. The third factor is related to the π–π interactions between the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds of MB and π electrons of the benzene ring with the π electrons on the surface of GO. The forth item to be considered is associated with the synergistic effect of adsorption and photocatalysis, which result in the degradation of MB. As other effective items, the Mn/Fe synergistic mechanism in MFO catalysts for the decomposition of MB should be considered. As stated above, by considering the critical role of GO, more oxygen-containing functional groups can be obtained by increasing the content of GO, which agrees with the results of enhanced adsorption activity. Thus, the adjustment of the GO content and MFO NPs in composite systems can ultimately optimize the MB adsorption mechanism.
C double bonds of MB and π electrons of the benzene ring with the π electrons on the surface of GO. The forth item to be considered is associated with the synergistic effect of adsorption and photocatalysis, which result in the degradation of MB. As other effective items, the Mn/Fe synergistic mechanism in MFO catalysts for the decomposition of MB should be considered. As stated above, by considering the critical role of GO, more oxygen-containing functional groups can be obtained by increasing the content of GO, which agrees with the results of enhanced adsorption activity. Thus, the adjustment of the GO content and MFO NPs in composite systems can ultimately optimize the MB adsorption mechanism.
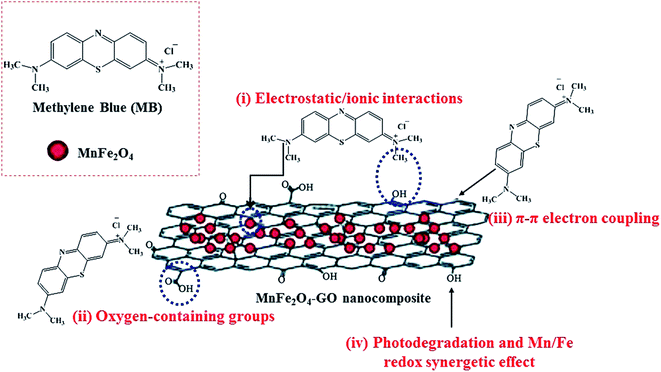 | ||
| Fig. 25 Illustration of methylene blue (MB) adsorption mechanism on GO–MnFe2O4 nanocomposites. This figure was adapted with permission from RSC Advances, 2018, 8, 12376–12389.154 | ||
In addition to the mentioned magnetic photocatalytic systems, magnetic biochar (MBC) from the biochar composite family not only exhibits the advantageous features of biochar (BC) but can also be magnetically separated from the reaction flask.155 Moreover, due to the good contribution and dispersal of photocatalysts on the MBC support, their recovery and performance are enhanced, and their aggregation will be effectively inhibited.156 MBC-catalyzed decomposition systems competently decrease the organic content of pollutants, which can be traced by total organic carbon (TOC) analysis.157,158 Additionally, the degraded contaminants may be more noxious and toxic than the individual pollutants given that the mineralization of organic pollutants does not completely occur.159 Fortunately, researchers have proven the detoxification effect of MBC-catalyzed degradation systems, which is an excellent feature compared to other degradation systems.160 In a recent study conducted by Xie et al., a Bi2WO6/Fe3O4/BC photocatalyst was synthesized via a hydrothermal method to degrade ofloxacin (OFL) and ciprofloxacin (CIP) upon exposure to a visible LED.161 The intermediate products of OFL and CIP exhibited less toxicity against Escherichia coli bacteria than the original pharmaceutical contaminants. As depicted in Fig. 26, by irradiating Bi2WO6 with a visible LED light, e− transfers from its VB (1.89 eV) to CB (−0.85 eV). Given that the reduction potential of O2/O2˙− (−0.33 eV vs. NHE) is lower than the CB potential of Bi2WO6, oxygen molecules can be reduced to form O2˙− by the electrons in the CB. However, the VB potential of Bi2WO6 is not positive enough to generate ˙OH (+2.40 eV vs. NHE). In this regard, the ˙OH production proceeds via the oxygen-containing functional groups of BC. The cascade electron transition first transfers e− to O2 to form O2˙−. Subsequently, H2O2 is produced under the actions of the electron. Finally, the produced H2O2 is degraded to form ˙OH. As mentioned, the main and vital factors to produce ˙OH in these catalytic degradation systems are the oxygen-containing functional groups on BC. Specifically, the h+, O2˙−, and ˙OH radicals are involved in both the degradation and mineralization of OFL and CIP. Table 2 presents some examples of magnetic catalytic systems applied for degradation of the pharmaceutical ingredients with high removal efficiency.
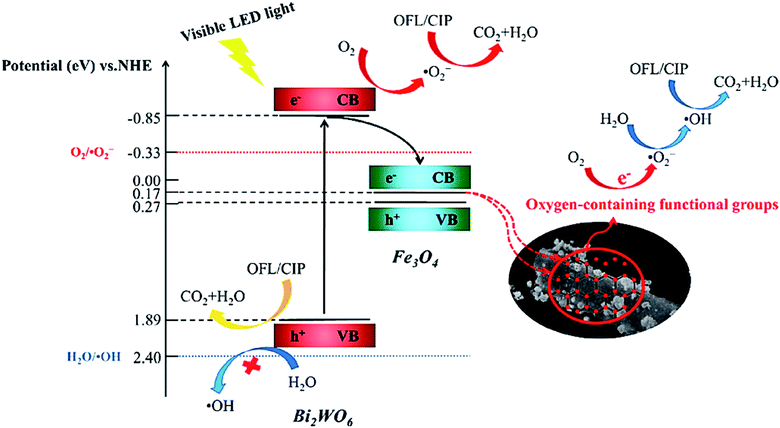 | ||
| Fig. 26 Plausible mechanism for photocatalytic degradation of OFL and CIP by Bi2WO6/Fe3O4/BC under visible LED light irradiation. This figure was adapted with permission from Science of The Total Environment, 2021, 764, 142879.161 | ||
| Entry | Catalyst | Function | Pollutant | Pollutant removal (%) | Ref. |
|---|---|---|---|---|---|
| a Biochar. b Reduced graphene oxide. c Carbon-bridge-modified malonamide (MLD)/g-C3N4 (CN)/Fe3O4. d Carboxymethyl cassava starch (CMCS)-functionalized Fe3O4. e Polypyrrole. f Sn0.15Mn0.85Fe2O4. | |||||
| 1 | Fe3O4@BCa | Fenton-like degradation | Metronidazole (MNZ) | 100 | 162 |
| 2 | γ-Fe2O3@BC | Degradation | Tetracycline (TC) | 82.24 | 163 |
| 3 | rGOb/gadolinium doped ZnFe2O4 (GZFG) | Adsorption | Levofloxacin (LVX) | 86 | 164 |
| 4 | Carbon-bridge-modified MLD/CN/Fe3O4c | Fenton-like degradation | Tetracycline (TC) | 95.8 | 165 |
| 5 | CMCS@Fe3O4d | Adsorption | Doxorubicin hydrochloride (DOX) | 85.46 | 166 |
| 6 | MnFe2O4/Bi2MoO6/PPye | Adsorption | Ketoprofen (KET) and indomethacin (IDM) | 87.03, 86.24 | 167 |
| 7 | SMFf | Photocatalytic degradation | Diclofenac (2-(2-(2,6-dichlorophenylamino)phenyl) acetic acid) (DCF) | 99 | 168 |
| 8 | CuFe2O4/Bi2O3 | Degradation | Lomefloxacin (LOM) | 77.19 | 169 |
| 9 | MoS2/CuFe2O4 | Degradation | Fluoxetine | 97.7 | 170 |
| 10 | Fe3O4/g-C3N4 | Photocatalytic degradation | Tetracycline (TC) | 99.8 | 171 |
| 11 | Mn2O3–Fe3O4@BC | Degradation | Naphthalene | 77.1 | 172 |
| 12 | Ag–CuFe2O4@WO3 | Photocatalytic degradation | Gemfibrozil (GEM) and Tamoxifen (TAM) | 81, 83 | 173 |
| 13 | Magnetite supported on multi-walled carbon nanotubes | Catalytic wet peroxide oxidation | Diclofenac (DCF) and naproxen (NAP) | 54, 19 | 174 |
| 14 | Ag3PO4/rGO/CoFe2O4 | Adsorption | Levofloxacin (LVF) | 90.7 | 175 |
| 15 | rGO/NiFe2O4 | Photocatalytic degradation | MB, MO, RhB | 99.1, 47.1, 82.2 | 176 |
| 16 | Bi2O2CO3–CoFe2O4@BC | Photocatalytic degradation | Paraquat (PQT) | 99.3 | 177 |
| 17 | CoFe2O4 NPs | Photocatalytic degradation | Atenolol (ATL) | 90 | 178 |
| 18 | Iron oxide/cellulose | Adsorption | Ciprofloxacin (CIP) | 92.01 | 179 |
| 19 | ZnO/Fe2O3 | Photocatalytic degradation | Sulfamethoxazole (SMX) | 95.2 | 180 |
| 20 | Fe0@BC | Fenton-like degradation | Trichloroethylene (TCE) | 98.9 | 181 |
4. Application of micro and nanoscale magnetic catalytic systems in the synthesis of pharmaceutical compounds
In recent decades, the use of catalytic systems and their functionalities have attracted great attention due to their benefits in pharmaceutical synthesis for scaling up production. However, there are numerous issues in the field of catalysis. These issues include environmental impact, process safety, mass and heat transfer restrictions, and optimizing reaction conditions, i.e., temperature, pressure, time, solvents, scaled-up procedures, reusability and stability, catalyst deactivation, and recovery. Among the catalytic systems, heterogeneous and magnetic catalysts meet the needs for convenient catalyst recovery from the reaction flask with only an external magnet. As a brilliant example, the Fe3O4/o-PDA–Pd nanocatalyst developed by Maleki et al. was applied in the Suzuki–Miyaura coupling reaction, in which biphenyls were prepared from aryl halides and phenylboronic acids, as shown in Fig. 27.17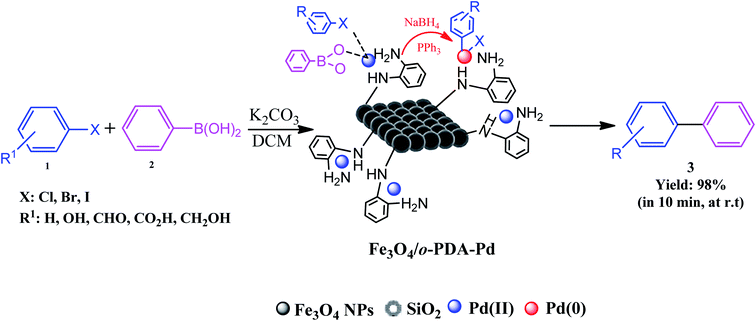 | ||
| Fig. 27 Schematic of Suzuki–Miyaura cross-coupling reaction conducted using magnetic Fe3O4/o-PDA–Pd nanocatalyst at room temperature (r.t). This figure was adapted with permission from Journal of Physics and Chemistry of Solids, 2020, 136, 109200.17 | ||
The active pharmaceutical biphenyl compounds resulting from this reaction, including losartan, flurbiprofen, and tarenflurbil, are used as resources to treat hypertension and diabetic nephropathy,182 signs of osteoarthritis and rheumatoid arthritis,183 and Alzheimer's disease and prostate cancer,184 respectively. The surface functionalization through covalent bonding occurs between the amine functional group of PDA and chloropropyl carbons of silane, which are bonded to chlorine. Eventually, the unpaired electron pairs in the amines interact with the empty orbitals in Pd. Due to the reaction mechanism, the electron interactions among the three main components involving the oxygen atoms of aryl halides and phenylboronic acid and divalent palladium ions lead to the final covalent binding. Subsequently, under basic conditions, Pd(II) was subjected to reduction to Pd(0) by sodium borohydride reducing agent. The reproducibility tests indicated ten times recycling for this catalyst without any reduction in its catalytic activity. It should be highlighted that by applying this catalyst in the Suzuki–Miyaura coupling reaction, approximately 98% yield was achieved in 10 min. In another study, Beitollahi et al. developed an environmentally friendly and facile method for the synthesis of an Fe3O4@cellulose nanocrystal/Cu nanocomposite (Fe3O4@CNC/Cu). Henceforth, a graphite screen-printed electrode (GSPE) modified with Fe3O4@CNC/Cu was applied as a sensor for the electrochemical oxidation of venlafaxine. Fig. 28 displays the possible electrooxidation mechanisms.41
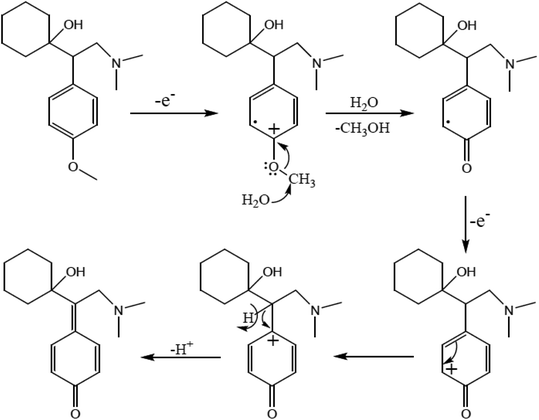 | ||
| Fig. 28 Proposed mechanism for the electrooxidation of venlafaxine at the surface of Fe3O4@nano-cellulose/Cu nanocomposite/GSPE. This figure was adapted with permission from Industrial & Engineering Chemistry Research, 2020, 59, 4219–4228.41 | ||
Mo et al. developed a magnetic recyclable metal–organic framework catalyst. They synthesized cyclohexenone derivatives under solvent-free conditions. Cyclic enone derivatives are acknowledged to be precious intermediates in pharmaceuticals and natural products. Cyclohexanone units containing C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups in their structure exist in many synthetic and medicinally natural products such as the fungus A. flavus YIM DT 10012, and Trachyspermum roxburghianum. Additionally, they can be applied in drugs, pesticides, and polymers.185–187 As depicted in Fig. 29 and 30, Zhang et al. researched the catalytic solvent-free synthesis of cyclohexanone derivatives through aldehyde and acetoacetanilide condensation reactions utilizing the heterogeneous γ-Fe2O3@SiO2/IRMOF-3 MOF catalyst. The catalyst was easily separated with an external magnet in this environmentally benign approach and retrieved several times without any changes in its catalytic activity.188
C groups in their structure exist in many synthetic and medicinally natural products such as the fungus A. flavus YIM DT 10012, and Trachyspermum roxburghianum. Additionally, they can be applied in drugs, pesticides, and polymers.185–187 As depicted in Fig. 29 and 30, Zhang et al. researched the catalytic solvent-free synthesis of cyclohexanone derivatives through aldehyde and acetoacetanilide condensation reactions utilizing the heterogeneous γ-Fe2O3@SiO2/IRMOF-3 MOF catalyst. The catalyst was easily separated with an external magnet in this environmentally benign approach and retrieved several times without any changes in its catalytic activity.188
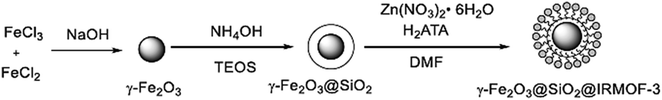 | ||
| Fig. 29 Schematic showing the preparation of the γ-Fe2O3@SiO2/IRMOF-3 catalyst. This figure was adapted with a permission from Journal of Catalysis, 2020, 387, 39–46.188 | ||
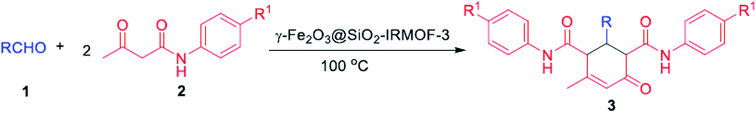 | ||
| Fig. 30 Synthesis of cyclohexenone derivatives using γ-Fe2O3@SiO2/IRMOF-3 catalyst. This figure was adapted with permission from Journal of Catalysis, 2020, 387, 39–46.188 | ||
Maleki et al. introduced magnetic pumice in a novel and well-designed magnetic composite, which led to the effortless recycle of the catalyst from the reaction mixture. In this work, palladium nanoparticles as the main catalytic active sites were well-distributed on the VPMP@CLS structure. For the Suzuki–Miyaura cross-coupling catalytic reactions, as a heterogeneous catalytic system, the Pd2+ nanoparticles were reduced to Pd- and biphenyl pharmaceutical derivatives were produced. This product has high importance in pharmaceutical compounds. It can be applied in cancer therapy and treatment of arteriosclerosis, osteolytic disorders, and ophthalmic disorders, and used as integrin antagonists.30 In another study by Maleki et al., a pumice magnetic volcanic rock and cellulose matrix nanocomposite was synthesized. This heterogeneous biodegradable nanocomposite was employed in the synthesis of 1,4-dihydropyridine derivatives, and the reaction outline is presented in Fig. 31. Given that 1,4-dihydropyridine derivatives have a wide variety of applications in antihypertensive and anticancer drugs, there synthesis procedures require significant consideration.189
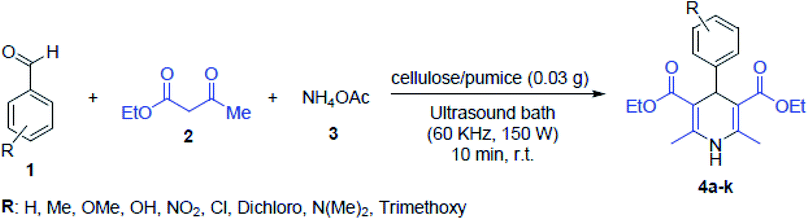 | ||
| Fig. 31 Synthetic pathway for 1,4-dihydropyridine derivatives using cellulose/pumice nanocomposite catalyst via sonication at room temperature (r.t). This figure was adapted with permission from Solid State Sciences, 2020, 101, 106141.189 | ||
Based on the advantages of heterogeneous hybrid magnetic nanocatalysts, organo-sulfonic acid tags attached to magnetic titania-coated NiFe2O4 nanoparticles were employed to form nano-NiFe2O4@TiO2–SiO2–Pr–DEA–OSO3H nanocatalysts to advance the green synthesis process.71 In this regard, the prepared hybrid nanocatalyst was utilized in the synthesis of pharmaceutical components such as 2H-indazolo[2,1-b]phthalazine-triones (Fig. 32a) and benzo[4,5]imidazo[1,2-a]pyrimidine derivatives through multicomponent reactions (MCRs) under moderate and green reaction conditions (Fig. 32b). In addition to good catalytic reusability (8 times), for the synthesis of 2H-indazolo[2,1-b]phthalazine-triones, a high reaction yield (97%) in just 5 min at 90 °C was obtained using only 20 g of the catalyst in a one-pot, three-component, solvent-free reaction, which is an accomplishment compared to the time taken when the reaction was performed with neat NiFe2O4. Moreover, for the synthesis of benzo[4,5]imidazo[1,2-a]pyrimidine derivatives at 110 °C under solvent-free conditions, 95% yield was obtained. Significantly, phthalazine-trione derivatives are well-known heterocycles used in the pharmaceutical and biological fields including vaso-relaxants,190 antifungal,191 antimicrobial,192 anti-cancer,193 and anti-inflammatory agents.194 Besides, benzo[4,5]imidazo[1,2-a]pyrimidines are biologically related fused pyrimidine derivatives, which demonstrate highlighted therapeutic and biological characteristics such as antimicrobial,195 anti-inflammatory,196 protein kinase inhibitor,197 and anticancer.198
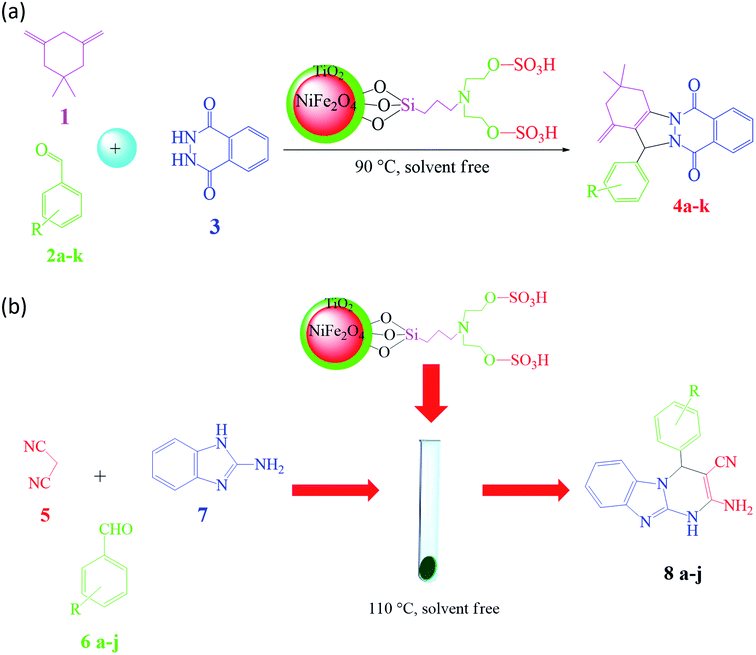 | ||
| Fig. 32 (a) Schematic of the synthetic route for 2H-indazolo[2,1-b]phthalazine-triones. This figure was adapted with permission from Chem. Sel., 2019, 4, 17–23.71 (b) Schematic of the synthetic approach for benzo[4,5]imidazo[1,2-a]pyrimidines. This figure was adapted with permission from Chem. Sel., 2019, 4, 17–23.71 | ||
In this report, a hybrid magnetic nanocomposite, ZnS/CuFe2O4, was applied as a heterogeneous catalyst to synthesize 2,4,5-triaryl-1H-imidazole derivatives through the one-pot condensation of various aromatic aldehydes, benzyl and ammonium acetate, as depicted in Fig. 33. The imidazole nucleus acts as the leading scaffold to form significant biological active molecules with antibacterial, antifungal, anti-inflammation, anticancer, antiviral, anti-diabetic, anti-allergic, analgesic and herbicidal functionalities.199,200 Some aromatic aldehydes were used in the synthesis of 2,4,5-triaryl-1H-imidazole derivatives. The aromatic aldehydes produced the desirable corresponding products in 82–92% yield due to their electron withdrawing and donating substituents.
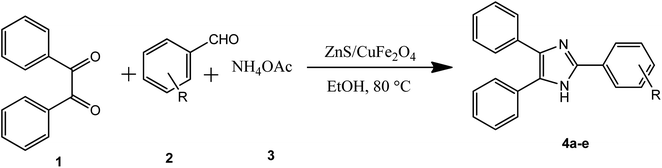 | ||
| Fig. 33 Schematic showing the synthesis of 2,4,5-triaryl-1H-imidazole derivatives. This figure was adapted with permission from Multidisciplinary Digital Publishing Institute Proceedings, 2019, pp. 44 (DOI: 10.3390/ecsoc-23-06654).70 | ||
According to Maleki's report, 2-amino-3-cyano-4H-pyran derivatives were synthesized using the ZnFe2O4@alginic acid heterogeneous nanocatalyst through the condensation reaction of dimedone (1), aromatic aldehydes (2) and malononitrile (3) in ethanol at room temperature (Fig. 34).
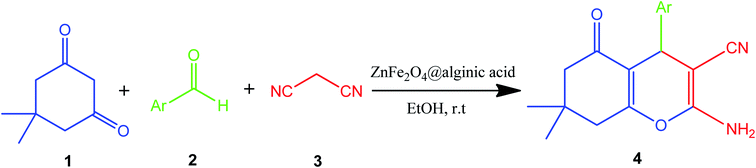 | ||
| Fig. 34 Synthesis of 2-amino-3-cyano-4H-pyran derivatives at room temperature (r.t). This figure was adapted with permission from: Polyhedron, 2019, 171, 193–202.75 | ||
Precisely, due to the synergistic effect of the active sites of alginic acid (several hydroxyl and carboxylic acid groups in its structure) and Lewis acid centers of ZnFe2O4, the hybrid catalyst demonstrated a remarkable yield of 93% in 10 min under the optimized conditions compared to its individual components. In the first stage, the heterogeneous nanocatalyst activates the carbonyl groups of dimedone and aldehyde in two paths from a mechanistic view. The formation of a hydrogen bond results from the hydroxyl or carboxylic acid active groups in alginic acid as the organic constituent of the catalyst or Lewis acid centers of ZnFe2O4 as the inorganic part. Then, the activated aldehyde and dimedone are exposed to catalytic Knoevenagel condensation to form molecule (5). Afterwards, the malononitrile as a C–H acid attacks the intermediate through Michael addition. In the next step, an intramolecular cyclization happens for molecule (5) to form intermediate (7). Eventually, the product formation is implemented by tautomerization of intermediate (7). The proposed mechanism is shown in Fig. 35.
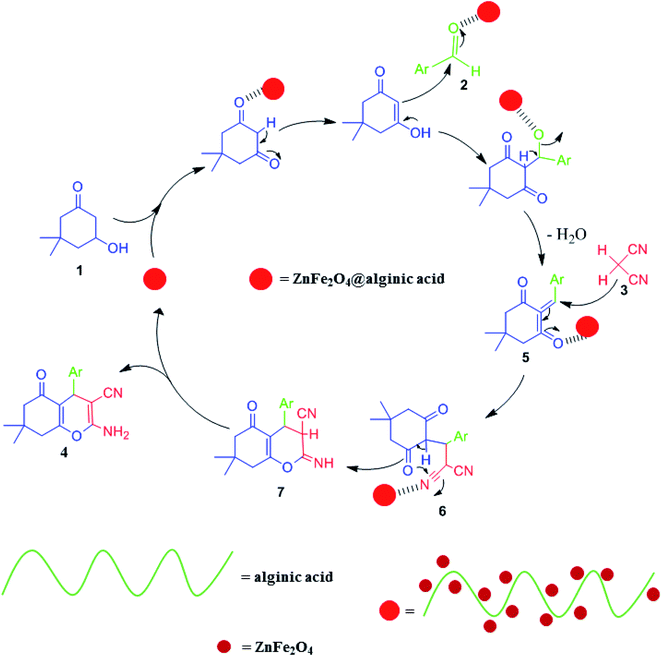 | ||
| Fig. 35 Suggested mechanism for the synthesis of 2-amino-3-cyano-4H-pyran derivatives. This figure was adapted with permission from Polyhedron, 2019, 171, 193–202.75 | ||
The high reaction yield in the range of 83–95% for ten 2-amino-3-cyano-4H-pyran derivatives should be highlighted to indicate the other strengths of this study. Also, a convenient purification procedure with high stability and reusability for five sequential catalytic cycles was carried out. Notably, the 4H-pyran family and their derivatives are the main parts in the production of natural and chemical molecules. They exhibit extensive favorable pharmaceutical applications such as anticancer, diuretic, spasmolytic, antibacterial, anti-HIV, antimalarial, anti-inflammatory, antihyperglycemic and dyslipidemia activities.201,202 Besides, they have demonstrated therapeutic effects in some neurodegenerative disorders such as Parkinson's disease and Alzheimer's disease.203 Also, a magnetic MOF catalyst, NiFe2O4@MOF-5, was applied as a heterogeneous catalyst to synthesize 2-substituted alkyl and aryl(indolyl) kojic acid derivatives through the solvent-free, one-pot, three-component reaction of aldehyde, indole, and kojic acid.83Table 3 lists some of the magnetic catalytic systems that have been suitably utilized for the synthesis of pharmaceutical compounds under mild reaction conditions.
| Entry | Catalyst | Function | Yielda (%) | Ref. |
|---|---|---|---|---|
| a Isolated yield. b Fibrous nano-silica. c Chitosan. d Magnetic phosphonium ionic liquid. e 2-(7-Amino-4-methyl-2-oxo-2H-chromen-3-yl) acetic acid. f Highly-ordered periodic mesoporous organosilica. g Magnetic zeolite nanocomposite. h Graphene oxide. i Nano-fibrillated cellulose. j Imidazole. k Salophen. l 2-Aminothiophenol. m Triethylenglycol monomethyl ether. n Copper/Schiff base complex immobilized on amine-functionalized silica mesoporous magnetic nanoparticles. o Fe3O4-magnetized N-pyridin-4-amine-functionalized graphene oxide. | ||||
| 1 | Nano-Fe3O4@(HSO4)2 | Synthesis of 2-amino-3-cyanopyridines and hexahydroquinoline derivatives | 53–89.4, 67.97–98.4 | 204 |
| 2 | WO3ZnO/Fe3O4 | Synthesis of 2-substituted benzimidazole derivatives | 88–98 | 205 |
| 3 | Fe3O4@KCC-1b–npr–NH2 | Synthesis of sulfonamide derivatives | 85–97 | 206 |
| 4 | CoFe2O4 MNPs | Synthesis of 2,4,5-trisubstituted imidazoles | 83–93 | 207 |
| 5 | Fe3O4@CSc–Co | Synthesis of aryl nitriles and biaryls | 60–85, 62–91 | 208 |
| 6 | MPILd | Synthesis of 2′-aminobenzothiazolomethylnaphthols and amidoalkyl naphthols | 87–96, 75–94 | 209 |
| 7 | Pd@Fe3O4/AMOCAAe | Suzuki and Sonogashira cross-coupling reactions | 60–100, 79–96 | 210 |
| 8 | γ-Fe2O3@cellulose-OSO3H | Synthesis of 2,4-dihydropyrano[2,3-c]pyrazole and spiro[indoline-3,4′-pyrano[2,3-c]pyrazole derivatives | 84–99, 89–95 | 211 |
| 9 | Cu NPs@Fe3O4-chitosan | Synthesis of amino- and N-sulfonyl tetrazoles | 81–90 | 212 |
| 10 | Pd NPs @Fe3O4/lignin/chitosan | Cyanation of aryl halides and coupling reactions | 60–97, 70–97 | 213 |
| 11 | Iron oxide@PMOf-PrSO3H | Synthesis of imidazopyrimidine derivatives | 88–96 | 214 |
| 12 | Fe3O4@SiO2@(CH2)3–urea–benzimidazole sulfonic acid | Synthesis of 2-amino-3-cyano pyridine derivatives | 70–92 | 215 |
| 13 | γ-Fe2O3/Cu@cellulose | Synthesis of 1,4-dihydropyridine derivative and polyhydroquinolines | 80–93, 80–98 | 216 |
| 14 | Cu NPs/MZNg | Synthesis of 1,2,3-triazoles | 90–98 | 217 |
| 15 | Fe3O4@SiO2@Si(CH2)3Cl with morpholine tags | Synthesis of hexahydroquinolines and 2-amino-4,6-diphenylnicotinonitriles | 78–87, 81–95 | 218 |
| 16 | SrFeGOh | Synthesis of β-enamino ketones | 80–98 | 219 |
| 17 | Fe3O4@NFCi-ImjSalophkCu | Synthesis of 1,4-disubstituted 1,2,3-triazoles | 75–97 | 220 |
| 18 | Pd–γ-Fe2O3-2-ATPl–TEG–MMEm | C–C cross-coupling reactions including cyanation reaction of iodobenzene with K4[Fe(CN)6]·3H2O, fluoride-free Hiyama reaction of halobenzenes with triethoxyphenylsilane, and Suzuki reaction of various aryl halides with phenylboronic acid | 52–99, 51–96, 80–98 | 221 |
| 19 | MMNPsn | Synthesis of thiazolidinones | 93–98 | 222 |
| 20 | [Fe3O4@GON–(pyridin-4-amine)]o | Synthesis of 4H-chromenes and dihydropyrano[2,3-c]pyrazole derivatives | 10–98, 30–98 | 223 |
5. Magnetization in micro and nanoscale materials
Magnetic nanoparticles (MNPs) were studied over 50 years ago just based on the specific difference in their high surface-to-volume ratio to bulk materials.224 Since then, impressive signs of the synthesis progress of MNPs have been seen regarding their sizes, shapes, compositions, and core–shell structures.225 The sensitivity and efficiency of MNPs are directly related to their higher saturation magnetization (maximum magnetization possible). The effects of geometry and shape such as composition variation are pivotal factors in enhancing their magnetic properties.226 Core–shell MNPs are dependent on the ligand and the surface interactions of nanoparticles, the effect of the relative thickness of their shell, and size of the coated nanoparticles.227 Briefly, the size, shape, composition and core–shell design in an appropriate synthesis are fundamentals of nano magnetization, which play vital roles in tuning the magnetic properties. Also, the intrinsic and extrinsic magnetic properties of materials should be considered. Atomic and molecular structure define the magnetic dipole and net magnetization to determine the diamagnetic, paramagnetic, ferromagnetic, ferrimagnetic, and antiferromagnetic properties.228,229 As can be seen in Fig. 36, the behavior of a material is entirely different due to the adjustment and response of its magnetic dipoles in the presence and absence of an external magnetic field.230 Superparamagnetic property has special importance because of drug delivery and MRI. Magnetic particles are referred to as superparamagnetic due to their magnetic properties upon the removal of an external field.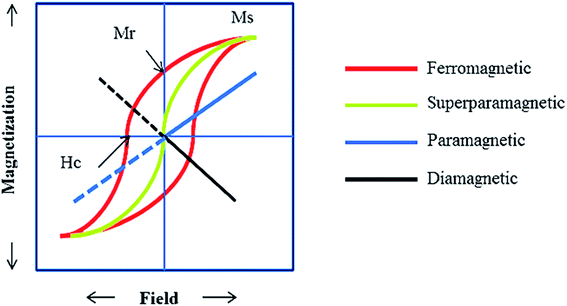 | ||
| Fig. 36 Magnetic behavior under the influence of an applied field. This figure was adapted with permission from Int. J. Mol. Sci., 2013, 14, 15977–16009.230 | ||
The effect of temperature changes the behavior of ferromagnetic and ferrimagnetic nanoparticles if they are considered in the superparamagnetic category (Fig. 37). This is the case when the measuring time is longer than the relaxation time; however, when the measurement time is shorter than the relaxation time, the nanoparticles are in a “blocked” (ferromagnetic) regime.230,231
| TB = KV/25kB = K(4τr03/3)/25kB | (1) |
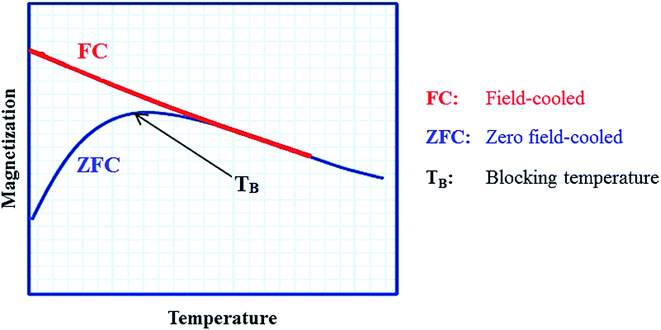 | ||
| Fig. 37 Effect of temperature. This figure was adapted with permission from Int. J. Mol. Sci., 2013, 14, 15977–16009.230 | ||
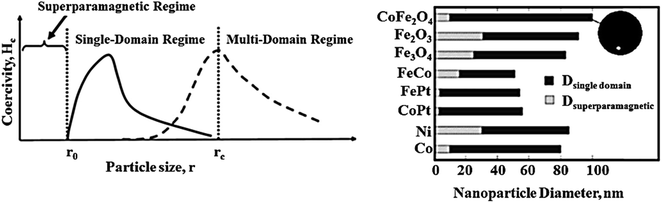 | ||
| Fig. 38 Particle size and its effect on magnetization. This figure was adapted with permission from Int. J. Mol. Sci., 2013, 14, 15977–16009.230 | ||
We discussed the size of nanoparticles in the previous subsection regarding the relationship between nanoparticle size and magnetic properties, although there is another pivotal factor. The shape of nanoparticles plays a vital role in the differentiation of their magnetic properties. Nanorods, nanodiscs, nanowires, nanoflowers and tetrapods are some different shapes that exhibit varying properties. CoFe2O4 (cube and sphere) differs in coercivity, γ Fe2O3 (cube and sphere) differs in coercivity and higher TB, FePt (cube, octapod and cuboctahedron) differs in coercivity and TB, and Fe3O4 (cube and sphere) differs in TB.234 Researchers relate these differences to the less surface pinning according to fewer missing coordinating oxygen atoms.235 In addition to the unique morphology-generated gradient for the magnetic field, a higher surface-area-to-volume ratio is related to this variation, where more protons are present close to the magnetic field. However, no conclusion favours a particular shape, although MNPs with flat surfaces demonstrate promising biomedical applications to eliminate large aggregates in the body rather than gathering in the tumor.236
Finally, we discuss the composition of MNPs for the specific magnetic characteristic determination that defines their main behavior. Precursor concentration, synthesis route, and the dopant character specifically impact the magnetic properties of composites.237 Varying the precursor ratio affect the coercivity and Ms (emu g−1) and changes the magnetic properties. The cation distribution in the octahedral and tetrahedral sites, in addition to the nature of the cation itself, defines their proper replacement by dopants (Fig. 39). Cationic exchange is an applicable method to produce various structures for specific applications.230,238
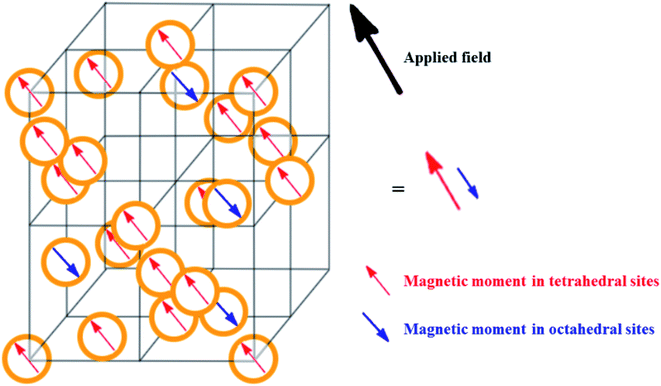 | ||
| Fig. 39 Spinel structure of ferrites, tetrahedral and octahedral. This figure was adapted with permission from Int. J. Mol. Sci., 2013, 14, 15977–16009.230 | ||
6. Reusability of micro and nanoscale magnetic catalytic systems
One of the most significant parameters of catalytic systems is their reusability, which affects their commercial applicability, suitability, and sustainability. Also, the reusability of catalysts plays a vital role in the catalytic oxidation process and impacts the cost of operation and catalyst separation in various applications.239 Catalysts are introduced to optimize successive reaction cycles after their recovery, rinsing, and drying to investigate their reusability.159,240–242 Magnetic catalysts have higher reusability due to their facile workup using only a magnet. In this case, due to the lower metal leaching, the catalytic efficiency increases.243 Maleki et al. introduced a volcanic-based mesoporous magnetic catalytic system, pumice, with excellent reusability (six consecutive times), which authenticates the beneficial industrial applications of this natural-based catalyst, as shown in Fig. 40.244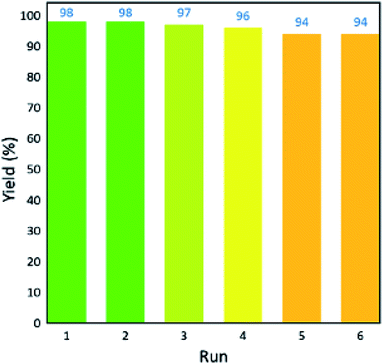 | ||
| Fig. 40 Reusability diagram of the natural igneous pumice as a catalyst. This figure was adapted with permission from Research on Chemical Intermediates, 2020, 46, 4113–4128.244 | ||
It was proved that the experimental procedure increased the reusability of the catalyst. For example, the regeneration of magnetic nanoscale zero-valent iron (nZVI)@Ti3C2-based MXene nanosheets by treatment with dilute HCl demonstrated an optimal method to enhance their reusability. The surface inactivation of the nZVI particles (nZVIPs) was caused by the dense iron-based oxide layer covering the active sites, resulting in poor reusability.245 A slight loss in activity is mainly attributed initially to the leaching of metal ions from the catalyst during the reaction, recovery, and washing steps, resulting in a loss of active sites. Secondly, some residual by-products on the catalysts may cause the active sites to be obstructed, reducing the degradation yield of organic pollutants. Moreover, in highly porous catalysts, pore blockage during the reaction is highly probable, leading to a decrease in catalytic activity.172,246Table 4 displays some magnetic catalytic systems with improved reusability.
| Entry | Catalyst | Improved properties | Ref. |
|---|---|---|---|
| 1 | Magnetic graphene oxide | Excellent catalytic efficiency, operational durability, and recyclability | 258 |
| 2 | Hydrophobic virus-like organosilica nanoparticles | Improved pH and thermal resistance, high tolerance to organic solvents and long-term storage stability | 259 |
| 3 | Chitosan-cross-linked magnetic nanoparticles | Superior separation and biocatalytic properties | 260 |
| Excellent storage stability and reusability | |||
| 4 | Barium ferrite magnetic microparticles | Enhanced thermostability and recyclability for three successive batches | 261 |
| 5 | Glutathione-coated gold magnetic nanoparticles | Enhanced storage and reusability stability. Immobilized biocatalyst showed good stability after ten repeated cycles | 262 |
| 6 | Ionic liquid-modified magnetic chitosan composites | Enhanced thermal stability and reusability after ten cycles of reuse | 263 |
Generally, in the scope of catalyst reusability, the chemical, physical, and mechanical stability of a catalysts should be monitored to accommodate industrial requirements and environmental considerations. From a mechanistic view, as long as the shape and structure of a magnetic catalyst are maintained, it means that it will display high structural strength in successive recovery cycles and is not degraded.247
For instance, Boruah et al. introduced an ammonia-modified graphene sheet (AG) with Fe3O4 MNPs decorated on its surface. This magnetic catalyst with the synergistic effect caused by amide-functionalized graphene and Fe3O4 MNPs hamper the rate of electron–hole pair recombination, leading to recyclability for ten cycles, representing the high structural stability of the AG/Fe3O4 photocatalyst. Besides its enhanced stability and convenient separation, the high photocatalytic yield of this magnetic catalyst shows its potential for scaled-up applications.248
Fe3O4 anchored on reduced graphene oxide (rGO) through urushiol facile cross-linking (Fe3O4–U-rGO) exhibited a recycle stability of up to seven continuous cycles. One of the impressive factors affecting the structural stability is the urushiol molecule, which creates robust coordination to metal oxides and connects other materials via its structural phenolic hydroxyl groups. Due to the enhanced Fenton reactions of the Fe3O4–U-rGO magnetic composite, the generation of iron sludge and undesirable decomposition of H2O2 to H2O and O2 were inhibited.249
Also, for acceptable catalytic performance, metal leaching as a factor affecting the stability of catalysts under harsh conditions such as acidic medium should be considered. The coherence, correlation, and overall physical properties of the structure should be maintained.250 To investigate the catalyst activation after recycling cycles, comparing the adsorptions bands in the FT-IR spectrum of the recovered catalyst and the original one seems necessary.251 In this case, another work applied a heterogeneous magnetic catalyst, i.e., an immobilized polymeric sulfonated ionic liquid on core–shell-structured Fe3O4/SiO2 composite (Fe3O4/SiO2-PIL), where the conversion of oil did not decrease significantly after five cycles of reutilizing, displaying the favorable recyclability of the catalyst. The durable structural stability of the catalyst indicated a strong attachment between the active acidic species and Fe3O4/SiO2 support. The reusability results verified a desirable heterogeneous catalyst from economic and environmental aspects compared to homogeneous catalysts, highlighting its applicability in the industrial production of biodiesel.252
It can be claimed that a magnetic catalyst is chemically stable when the deconjugation and chemical structure alteration of its active sites do not happen. In the case of metal oxide-based catalysts, sonication is proposed as an efficient way to recover magnetic catalysts given that agglomeration may occur during the catalytic procedure. The combination of magnetite NPs, i.e., Fe3O4, and metal oxide NPs such as ZnO with a high agglomeration propensity can enhance the efficiency of the catalytic system through the adsorption of the target contaminants. The enhancement caused by magnetization correlates with the reduction in metal oxide agglomeration and catalytic performance improvement by the Fe2+ active octahedral sites of Fe3O4 reacting with H2O2 molecules, resulting in the formation of ˙OH and HO2˙ radicals, which eventually enhances the reusability of sonocatalysts and metal oxides.250 Besides, in ultrasound-assisted catalysis systems denoted as sonocatalysis, the activation of the sonocatalyst occurs under ultrasound irradiation, which accelerates the degradation rate via the production of extra reactive species. In this respect, many sonocatalysts, namely ZnO, TiO2–NiO, and TiO2, have been activated by ultrasound irradiation.253–255
7. Structural stability of micro and nanoscale magnetic catalytic systems
Stability is one of the critical parameters affecting the performance of catalysts. Different factors can affect their stability, such as pH,256 temperature,239 and reaction conditions. As the temperature increases, the dye removal rate increases. Peroxymonosulfate catalyzed by reusable graphite felt/ferriferous oxide dissociated into sulfate and hydroxyl radicals via thermolytic division of its O–O bond.Additionally, a higher temperature produces more free radicals.239 Metal ion leaching is one reason that causes catalyst deactivation. In this case, the catalyst is immersed in aqueous solutions for a certain number of days. Subsequently, the leached metal concentration demonstrates the stability of the catalyst.257 Besides, the agglomeration of NPs diminishes the catalytic activity and stability.243 Magnetic nanoscale zero-valent iron (nZVI)@Ti3C2-based MXene nanosheets exhibited enhanced catalytic reactivity and stability due to its synergistic effect. Specifically, the Ti3C2-based MXene prevents the agglomeration of the nZVI particles (nZVIPs) and enhances the electron transition between the magnetic particles with a diameter of 10–40 nm.245
Different analyses are used to explore the structural stability of catalysts. Fig. 41a–c show the FT-IR, EDS, and SEM analyses of recovered magnetic pumice catalysts after six successive recycling cycles. The peaks at 580, 950, 1000, and 3390 cm−1 correspond to the characteristic peaks of the catalyst. Two new peaks emerged at 3600 cm−1, which are related to the ALO–H bonds in the internal alumina network and prove the presence of voids in pumice after complete rinsing. The EDS analysis did not display any changes in the structural elements of the recovered catalyst. Eventually, according to the SEM images, the structure, morphology, dispersion, and uniformity of the heterogeneous catalyst remained unchanged even after six recycling cycles.244
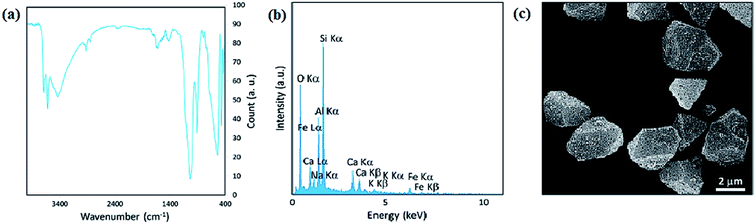 | ||
| Fig. 41 (a) FT-IR spectrum, (b) EDX spectrum, and (c) SEM image of the recovered pumice microparticles after six recycling cycles. This figure was adapted with permission from Research on Chemical Intermediates, 2020, 46, 4113–4128.244 | ||
8. Turnover number (TON) and turnover frequency (TOF)
The turnover frequency (TOF) first widened into the scope of heterogeneous catalysis according to Boudart's definition, as follows: “Certainly, the catalytic activity, must be assigned to the exposed number of surface atoms of a determined kind. Thus, to put catalytic activity in words, it is a turnover number equal to the number of reactant molecules converted per minute per catalytic site for specified reaction conditions”.264 Also, the authentic IUPAC Gold Book defines the turnover frequency term as: “Commonly called the turnover number, N, and determined, as in enzyme catalysis, as molecules that react per active site in unit time”.265 TOF, turnover frequency (TOF = (yield/time)/amount of catalyst (mol)). TON, turnover number (TON = yield/amount of catalyst (mol)).266 Alternatively, to evaluate intrinsic properties such as catalytic efficiency and recycling factors, TOF and TON are two significant parameters. The TON is determined as the number of molecules that experience transformation by ratio of active sites to products in the presence of 1 g catalyst. Also, the TOF is calculated as TON/degradation time.267 Numerous methods that mainly utilize homogeneous metal complexes and heterogeneous systems for the hydration of nitriles have been reported. Alumina, potassium fluoride-doped Al2O3 and phosphates, silica-supported manganese oxides, modified hydroxyapatite, and ruthenium hydroxide coated on alumina and ferrites are examples of heterogeneous systems.Nonetheless, due to their deficiency, laboriousness product and catalyst separation, requirement of inert atmosphere to conduct reactions for air-sensitive metal catalysts, poor catalyst reusability, very low TONs were obtained.268 In hydrogenation reactions of alkynes or alkenes, catalysts such as noble metals NPs represent high turnover numbers (TONs).269 Hamishehkar et al. synthesized glutathione-decorated gold-magnetic NPs (GSH-AuMNPs) as a competent recyclable catalyst. To express intrinsic activity, the TOF was concluded to be 12.5 h−1. This catalyst with high catalytic activity is a good candidate for biomedical and pharmaceutical applications.270 Koukabi et al. designed an air- and moisture-stable poly(2-acrylamido-2-methyl-1-propane sulfonic acid)-stabilized magnetic palladium catalyst with a core–shell structure. This catalyst was applied in Suzuki–Miyaura and Mizoroki–Heck reactions to produce coupling products with TON and TOF values of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 143 and 4900 and 28
143 and 4900 and 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 296 and 7424, respectively.271
296 and 7424, respectively.271
9. Multifunctional micro and nanoscale magnetic catalytic systems
A wide variety of multifunctional systems based on magnetic nanoparticles (MNPs) and derivative nanocomposites has amazed scientists because of their manifold applications using one material. These materials are used in various fields, such as sensing, adsorption, medicine, energy, and electronics. Recently, Yang et al. synthesized a 3D coral-like layered porous magnetic eggshell membrane (ESM)/CuFe2O4 nanocomposite through a green and affordable route from useless materials, exhibiting multifunctional applications including adsorption, catalysis, and antibacterial properties in water treatment against Gram-positive bacteria Staphylococcus aureus (S. aureus) and Gram-negative bacteria Escherichia coli (E. coli). The maximum adsorption capacity of Congo red (CR) was evaluated to be 199.23 mg g−1. Further, the catalytic activity of 4-nitrophenol reduction using this magnetic nanocomposite was high, and the attained degradation rate was 98.9% in 5.5 min.272Besides, multifunctional magnetic NiFe2O4@TiO2/Pt nanocomposites were prepared through the sol–gel procedure. In this work, the high performance of rapid photo-degradation of two azo dyes as water pollutants under UV-vis light, together with antibacterial activity against Escherichia coli (E. coli) bacteria were demonstrated.273 Kayani et al. worked on the sol–gel synthesis of magnetic ZnO NPs with different Fe dopant concentrations, ranging from 1–17%. Their studies confirmed the ferromagnetic behaviour of all the Fe-doped NPs. The highest antibacterial performance against Escherichia coli (E. coli) and Pseudomonas aeruginosa (P. aeruginosa) was acquired at 14–17% Fe doping content. The 1% Fe-doped ZnO photocatalyst revealed superior methylene blue (MB) degradation under sunlight.274 Ensafi et al. synthesized a magnetic spinel Fe2CuO4/rGO nanocomposite as a catalyst and adsorbent. The pyrolytic products resulting from the catalytic pyrolysis of discarded tires were liquid (pyrolytic fuel), gas (combustion gas), and char (activated carbon via gasification procedure). These products confirm the generation of valuable materials from waste. This nanocomposite acted as an adsorbent for mercury(II) elimination from sewage, which corresponded to the Langmuir isotherm with a maximum adsorption capacity of 1250 mg g−1.275
Another study focused on an approach for the one-pot synthesis of a CoNi alloy-reduced graphene oxide ((CoNiD)60RGO40) multifunctional nanocomposite, which acted as a remarkable catalyst for the 4-nitrophenol reduction reaction with its kapp value determined to be 20.55 × 10−3 s−1 and 80–93% yield in the Knoevenagel condensation reaction. The easy separation of this magnetic nanocomposite and perfect performance of the recycled catalyst were noticeable. Also, this snowflake-like dendritic nanocomposite operated as an active electrode in supercapacitors. The high performance of this supercapacitor was exhibited by its specific capacitance of 501 F g−1 (at 6 A g−1) and energy density of 21.08 W h kg−1 at a power density of 1650 W kg−1. The synthesis of the snowflake-like dendritic (CoNiD)60RGO40 nanocomposite and its catalytic and supercapacitor applications were presented in this study.276
Multifunctional and stimuli-responsive MNP- and derivative nanocomposite-based systems have emerged from nanobiotechnology with significant impacts on drug delivery, cancer diagnosis and treatment. These systems overcome the limitations of conventional therapeutic methods for cancer in recent decades. Among them, Fe3O4 MNPs have attracted attention from scientists due to their innate magnetic resonance imaging (MRI), drug delivery, biocatalytic activity, magnetic hyperthermia treatment (MHT), and stimuli-responsive therapy for multimodal approaches. The size and morphology are factors that determine the properties of these MNPs. There are three main routes presented for the synthesis of Fe3O4 MNPs, including physical, biosynthetic, and chemical approaches. Physical synthesis is a method that cannot entirely direct the size to the nano-scale. The biosynthetic process faces shortcomings of long synthesis time, low yield reaction, and broad size distribution. Thus, to overcome these disadvantages, chemical synthesis tends to be employed with superiorities such as facile procedure, low cost, high yield, and monodispersed MNPs. The various applications of Fe3O4 MNPs and their chemical synthetic strategies are presented in Fig. 42.277
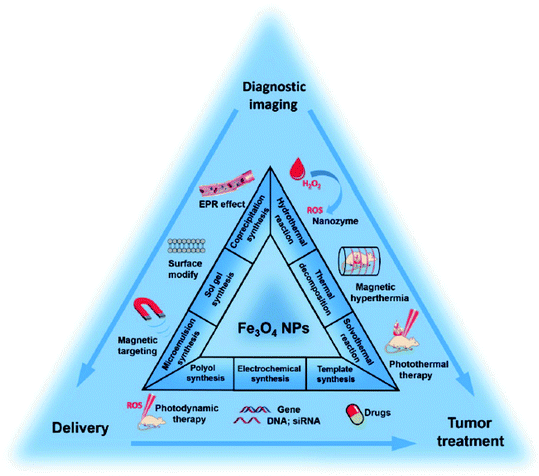 | ||
| Fig. 42 Scheme showing the synthesis of magnetic Fe3O4 NPs and their cancer diagnosis and healing applications. This figure was adapted with permission from Theranostics, 2020, 10, 6278.277 | ||
To better interact with biological media, surface-modified Fe3O4 MNPs with good biocompatibility, high stability, and stimuli responsiveness are applied in magnetic nanoscale systems. Ligand substitution and encapsulation are strategic processes. An interchange between the intrinsic surface hydrophobic ligands of MNPs and hydrophilic ligands take place to aid in their dispersity in the biological environment. However, although this process retains the initial hydrodynamic size of the MNPs, it causes metal atom leakage and surface flaws. Thus, the encapsulation/self-assembly process is employed to protect MNPs and to maintain their physical and chemical characteristics. The different functionalizing agents and surface ligands are depicted in Fig. 43.278 Feng et al. introduced poly(lactic-co-glycolic acid) (PLGA) drug-loaded magnetic Janus particles (DMJPs) as a multifunctional nanoscale system. In these three-sectioned Janus particles, Fe3O4 MNPs effectively released paclitaxel anticancer drugs with the assistance of an external magnetic field.279
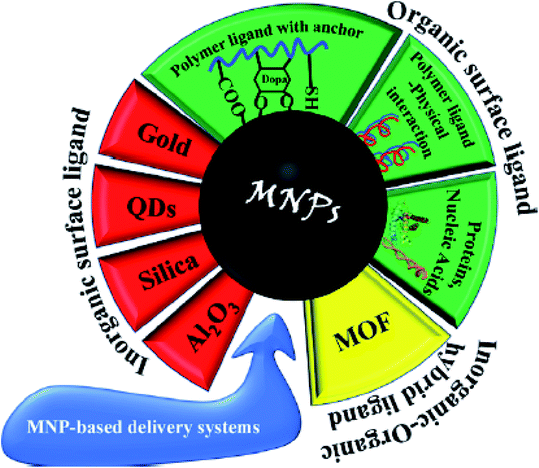 | ||
| Fig. 43 Illustration of MNP-based delivery systems and their versatile surface ligands (including inorganic surface ligands, organic surface ligands, and inorganic–organic hybrid ligands). This figure was adapted with permission from Journal of Physics and Chemistry of Solids, 2021, 148, 109661.278 | ||
Another theranostic nanosystem is multifunctional gold MNPs. According to their nanoscale and inimitable physicochemical properties, they display thriving multimodal imaging and cancer treatment applications. Also, from the biosafety perspective of Au MNPs, several types of studies proved their low toxicity in biomedical applications.280
Multifunctional catalytic systems are supplementary systems that improve the catalytic activity through the interaction of more than one catalyst functionality.281 A chitosan/ionic liquid multifunctional catalyst was prepared with high catalytic activity and selectivity for the synthesis of dimethyl carbonates (DMC) due to its nucleophilic sites such as hydroxyl and amine groups and also absorption sites for CO2.282 Additionally, magnetic multifunctional catalytic systems are beneficial due to their ease of separation using a permanent external magnet to recycle the catalysts. According to the desirable conversion and selectivity of multifunctional catalysts applied in the green oxidation method of benzyl alcohol, Ye et al. introduced Pd/Fe3O4@mCeO2 yolk–shell microspheres, as presented in Fig. 44. The catalyst exhibited a good performance of 80.5% conversion of benzyl alcohol and benzaldehyde selectivity of 94.8%.283
 | ||
| Fig. 44 Mechanism of the green oxidation method for benzyl alcohol with Pd/Fe3O4@mCeO2 yolk–shell microsphere catalyst. This figure was adapted with permission from Catalysis Letters, 2016, 146, 1321–1330.283 | ||
In another study, NiFe–NiFe2O4 was synthesized via the solution blow spinning (SBS) method, which exhibited good electrocatalytic activity in the oxygen evolution reaction (OER) with a noteworthy turnover frequency (TOF) of 4.03 s−1.284 Another electrocatalyst for the OER and also water splitting, resulting in the production of pure H2, is cobalt ferrite (CoFe2O4) powder obtained using agar–agar from Rhodophyta. The TOF of this electrocatalyst was 8.8 × 10−2 s−1.285 Also, Nagashri et al. prepared a cost-effective copper(II) complex with a 1,10-phenanthroline derivative catalyst for H2 evolution as a fuel with a TON and TOF of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 and 8100, respectively.286 Moreover, multifunctional catalytic systems present a satisfactory performance in the Suzuki–Miyaura and Mizoroki–Heck cross-coupling reactions. In this regard, the oxygen insensitive and phosphine-free MNP/HPG–CA/Pd catalyst with a turnover frequency of 372 h−1 was effortlessly recovered and reused many times.287 Similarly, a one-pot hydrothermal-synthesized Pd–Fe3O4/rGO nanocomposite with a TOF of up to 1449 h−1 was applied in C–C coupling reactions.288
600 and 8100, respectively.286 Moreover, multifunctional catalytic systems present a satisfactory performance in the Suzuki–Miyaura and Mizoroki–Heck cross-coupling reactions. In this regard, the oxygen insensitive and phosphine-free MNP/HPG–CA/Pd catalyst with a turnover frequency of 372 h−1 was effortlessly recovered and reused many times.287 Similarly, a one-pot hydrothermal-synthesized Pd–Fe3O4/rGO nanocomposite with a TOF of up to 1449 h−1 was applied in C–C coupling reactions.288
Magnetic core–shell-structured multifunctional heterogeneous catalysts exhibit synergistic effects from each constituent, such as preferable magnetic properties, catalytic activity, and structural stability. A layer-by-layer assembled Fe3O4@PDA-Pd@[Cu3(BTC)2] nanocomposite was employed for the reduction of 4-nitrophenol and Suzuki–Miyaura cross-coupling reactions.77Table 5 summarizes the information of some magnetic catalytic systems that exhibit versatility in chemical reactions.
| Entry | Catalyst | Active site | Main function | TON and TOF | Ref. |
|---|---|---|---|---|---|
| 1 | FeS2/CoS2 nanosheets | “S” vacancies | Electrocatalyst | TOF = 0.446 (s−1) | 289 |
| 2 | Co@MOF | Co | Catalyst | TOF = 0.8 (h−1) | 290 |
| 3 | Au/MOF-199 | Au, Cu content in MOF | Catalyst | TON = 363, TOF = 738 (h−1) | 291 |
| 4 | Ru(II)–PNN | Ru | Catalyst | TON = 4400, TOF = 2500 (h−1) | 292 |
| 5 | [κ4-Tptm]ZnOSiMe3 | Zn | Catalyst | TON = 105, TOF = 1.6 × 106 (h−1) | 293 |
| 6 | H3PW12O40 | Pt | Catalyst | TOF = 5.2 (h−1) | 294 |
| 7 | C18H26IrN3O7S·3.5H2O | OH | Catalyst | TON = 7280, TOF = 2600 (h−1) | 295 |
| 8 | Al/SiO2 | Al | Ethanol dehydration and m-xylene isomerization | TOF = 5.0 (×10−4 s−1 per site) | 296 |
| 9 | FeNi3N/NG | Ni, pyridinic N graphitic N, Fe | Electrocatalyst | TOF = 1.0 (s−1) | 297 |
| 10 | MoS2@NF | Mo | Catalyst | TOF = 2.54H2 s−1 | 298 |
10. Conclusion and future outlook
In this survey, a brief overview of the recent advancements in magnetic micro- and nanoscale catalytic systems was presented. Concurrently, their myriad of benefits and convenience, such as facile recovery and high chemical and structural stability, in the degradation and synthesis of assorted pharmaceutical compounds was discussed. Herein, the structure and properties of magnetic catalytic systems were classified, and all the recent accomplishments by researchers were briefly implied in this context. Furthermore, the synergistic effects between organic and inorganic catalysts as hybrid micro- and nanoscale magnetic composites were considered. In general, magnetic catalytic systems are developing rapidly. In this regard, researchers are trying to optimize previous processes both in terms of cost and time, as well as in terms of improving catalytic structures. Alternatively, it is imperative to focus on environmentally friendly structures and extracted materials from nature. These materials do not harm the environment, and are considered an opportunity in terms of mass production and industrial use.As a path to the future, attention to porous structures and frameworks is a priority. Due to their high porosity, these structures can act as reaction reactors and accelerate catalytic processes. These structures include metal–organic frameworks (MOFs) as well as a newer category, covalent organic frameworks (COFs). In addition to high porosity, COFs can also host magnetic nanoparticles, which aid the convenient separation of catalysts. Moreover, the prominent features of these frameworks are their lower density than MOFs and their high stability under acidic, alkaline, and redox conditions. Besides, attention should be given to magnetic heterostructures, which possess features beyond their single components, resulting in various applications in biomedicine and catalyst scopes. The insight into the reaction parameters, including ligands and counter anions, leads to the application of magnetic heterostructures in the desired fields. Regarding the biomedical application of magnetic heterostructures, the requirement of their long-term appraisal in terms of biotoxicity and metabolism of the magnetic heterostructures seems vital, while in terms of catalytic applications, stability enhancement has been highlighted as the major issue.299
Alternatively, the preparation approach of metal complexes containing immobilized MNPs enables phase tunability of the magnetic oxides applying a single path (Fe3O4, γ-Fe2O3, and α-Fe2O3). Even though the immobilization of MNPs on metal complexes results in higher activity and selectivity in the catalytic systems, the hydrophobicity may affect their catalytic performance. However, silica- and carbon-coated MNPs can prevent the aggregation of MNPs, providing functional groups to immobilize their active catalytic sites.300 Magnetic biochar is another magnetic catalytic system originating from a wide range of sources, which can be prepared via various routes and employed in the catalytic degradation of dyes, antibiotics, herbicides, and other organic contaminants. Although MBC catalysts exhibit the benefits of excellent stability, good reusability, and acceptable organic pollutant degradation yield, insight into many related facets to the mechanism of MBC is scarce. For instance, more environmental considerations in the case of metal leaching of some metal-loaded MBCs, profound studies on the routes for the regeneration of MBC catalysts, and concise research on the incomplete mineralization of degraded contaminants should be undertaken.38 Finally, the use of hybrid composites also requires more attention due to the catalytic benefits of both organic and inorganic compounds.
Table 6 lists the abbreviations used herein and their full definitions.
| Abbreviation | Definition |
|---|---|
| MNPs | Magnetic nanoparticles |
| MCRs | Multicomponent reactions |
| MOF | Metal–organic framework |
| COF | Covalent–organic framework |
| MCSs | Magnetic catalytic systems |
| PAHs | Polycyclic aromatic hydrocarbons |
| CIP | Ciprofloxacin |
| TOF | Turnover frequency |
| TON | Turnover number |
| DMC | Dimethyl carbonates |
| SBS | Solution blow spinning |
| OER | Oxygen evolution reaction |
| GA | Gallic acid |
| PLGA | Poly(lactic-co-glycolic acid) |
| MMT | Montmorillonite |
| GO | Graphene oxide |
| PDA | Ortho-phenylenediamine |
| nZVI | Nanoscale zero-valent iron |
| TMB | 3,3,5,5-Tetramethylbenzidine |
| AA | Ascorbic acid |
| CR | Congo red |
| MB | Methylene blue |
| GSPE | Graphitic screen-printed electrode |
| VPMP | Volcanic pumice magnetic particles |
| DEA | Diethanolamine |
| ESM | Eggshell membrane |
| MHT | Magnetic hyperthermia treatment |
| DMJP | Drug-loaded magnetic Janus particles |
| ICP-AES | Inductively coupled plasma atomic emission spectroscopy |
| MRI | Magnetic resonance imaging |
| MCM | Microwave combustion method |
| CCM | Conventional combustion method |
| CMC | Carboxymethylcellulose |
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors are grateful to the Iran University of Science and Technology (IUST) for their support.References
- R. Taheri-Ledari and A. Maleki, New J. Chem., 2021, 45, 4135–4146 RSC.
- M. S. Esmaeili, Z. Varzi, R. Taheri-Ledari and A. Maleki, Res. Chem. Intermed., 2021, 47, 973–996 CrossRef CAS.
- A. Maleki, R. Taheri-Ledari and M. Soroushnejad, ChemistrySelect, 2018, 3, 13057–13062 CrossRef CAS.
- W. Zhang, R. Taheri-Ledari, Z. Hajizadeh, E. Zolfaghari, M. R. Ahghari, A. Maleki, M. R. Hamblin and Y. Tian, Nanoscale, 2020, 12, 3855–3870 RSC.
- X. Chen, H. Zhang, M. Zhang, Y. Zou, S. Zhang and Y. Qu, Chem. Commun., 2020, 56, 1960–1963 RSC.
- Z. Varzi, M. S. Esmaeili, R. Taheri-Ledari and A. Maleki, Inorg. Chem. Commun., 2021, 125, 108465 CrossRef CAS.
- A. Maleki, M. Niksefat, J. Rahimi and R. Taheri-Ledari, Mater. Today Chem., 2019, 13, 110–120 CrossRef CAS.
- H. A. Elazab, Can. J. Chem. Eng., 2019, 97, 1545–1551 CrossRef CAS.
- R. Eivazzadeh-Keihan, R. Taheri-Ledari, N. Khosropour, S. Dalvand, A. Maleki, S. M. Mousavi-Khoshdel and H. Sohrabi, Colloids Surf., A, 2020, 587, 124335 CrossRef CAS.
- R. Eivazzadeh-Keihan, R. Taheri-Ledari, M. S. Mehrabad, S. Dalvand, H. Sohrabi, A. Maleki, S. M. Mousavi-Khoshdel and A. E. Shalan, Energy Fuels, 2021, 35(13), 10869–10877 CrossRef CAS.
- J. Rahimi, R. Taheri-Ledari and A. Maleki, Curr. Org. Synth., 2020, 17, 288–294 CrossRef CAS PubMed.
- R. Taheri-Ledari, W. Zhang, M. Radmanesh, N. Cathcart, A. Maleki and V. Kitaev, J. Nanobiotechnol., 2021, 19, 1–21 CrossRef PubMed.
- M. Miceli, P. Frontera, A. Macario and A. Malara, Catalysts, 2021, 11, 591 CrossRef CAS.
- A. Maleki, R. Taheri-Ledari, J. Rahimi, M. Soroushnejad and Z. Hajizadeh, ACS Omega, 2019, 4, 10629–10639 CrossRef CAS PubMed.
- G. Fan, M. Li, X. Chen, A. Palyanitsina and A. Timoshin, Energy Rep., 2021, 7, 2588–2593 CrossRef.
- R. Taheri-Ledari, M. S. Esmaeili, Z. Varzi, R. Eivazzadeh-Keihan, A. Maleki and A. E. Shalan, RSC Adv., 2020, 10, 40055–40067 RSC.
- A. Maleki, R. Taheri-Ledari, R. Ghalavand and R. Firouzi-Haji, J. Phys. Chem. Solids, 2020, 136, 109200 CrossRef CAS.
- R. Taheri-Ledari, S. M. Hashemi and A. Maleki, RSC Adv., 2019, 9, 40348–40356 RSC.
- R. Taheri-Ledari, A. Maleki, E. Zolfaghari, M. Radmanesh, H. Rabbani, A. Salimi and R. Fazel, Ultrason. Sonochem., 2020, 61, 104824 CrossRef CAS PubMed.
- Z. Hajizadeh, K. Valadi, R. Taheri-Ledari and A. Maleki, ChemistrySelect, 2020, 5, 2441–2448 CrossRef CAS.
- R. Taheri-Ledari, J. Rahimi and A. Maleki, Ultrason. Sonochem., 2019, 59, 104737 CrossRef PubMed.
- A. Maleki, S. Azadegan and J. Rahimi, Appl. Organomet. Chem., 2019, 33, e4810 CrossRef.
- M. S. Esmaeili, Z. Varzi, R. Eivazzadeh-Keihan, A. Maleki and H. Ghafuri, Mol. Catal., 2020, 492, 111037 CrossRef CAS.
- E. Rezaee Nezhad and R. Tahmasebi, Asian J. Green Chem., 2019, 3, 34–42 Search PubMed.
- S. Hossein Javadi, D. Zareyee, A. Monfared, K. Didehban and S. A. Mirshokraee, Phosphorus, Sulfur Silicon Relat. Elem., 2020, 195, 7–12 CrossRef CAS.
- R. Taheri-Ledari, W. Zhang, M. Radmanesh, S. S. Mirmohammadi, A. Maleki, N. Cathcart and V. Kitaev, Small, 2020, 16, 2002733 CrossRef CAS PubMed.
- F. Hezam, O. Nur and M. Mustafa, Colloids Surf. A Physicochem. Eng. Asp., 2020, 592, 124586 CrossRef CAS.
- P. A. Vinosha, A. Manikandan, R. Ragu, A. Dinesh, P. Paulraj, Y. Slimani, M. Almessiere, A. Baykal, J. Madhavan and B. Xavier, Environ. Pollut., 2021, 272, 115983 CrossRef CAS PubMed.
- L. Wu, Y. Yu, Q. Zhang, J. Hong, J. Wang and Y. She, Appl. Surf. Sci., 2019, 480, 717–726 CrossRef CAS.
- S. S. Soltani, R. Taheri-Ledari, S. M. F. Farnia, A. Maleki and A. Foroumadi, RSC Adv., 2020, 10, 23359–23371 RSC.
- R. Taheri-Ledari, M. Saeidirad, F. S. Qazi, A. Fazeli, A. Maleki and A. E. Shalan, RSC Adv., 2021, 11, 25284–25295 RSC.
- J. Safari and N. H. Nasab, Res. Chem. Intermed., 2019, 45, 1025–1038 CrossRef CAS.
- G. Wang, K. Lv, T. Chen, Z. Chen and J. Hu, Int. J. Biol. Macromol., 2021, 184, 358–368 CrossRef CAS PubMed.
- M. Malakootian, A. Nasiri, A. Asadipour and E. Kargar, Process Saf. Environ. Prot., 2019, 129, 138–151 CrossRef CAS.
- G. K. Kara, J. Rahimi, M. Niksefat, R. Taheri-Ledari, M. Rabbani and A. Maleki, Mater. Chem. Phys., 2020, 250, 122991 CrossRef CAS.
- N. Nasseh, B. Barikbin and L. Taghavi, Environ. Technol. Innov., 2020, 20, 101035 CrossRef CAS.
- X.-L. Chen, F. Li, H. Chen, H. Wang and G. Li, J. Environ. Chem. Eng., 2020, 8, 103905 CrossRef CAS.
- Z. Feng, R. Yuan, F. Wang, Z. Chen, B. Zhou and H. Chen, Sci. Total Environ., 2021, 765, 142673 CrossRef CAS PubMed.
- K. Rubeena, P. H. P. Reddy, A. Laiju and P. Nidheesh, J. Environ. Manage., 2018, 226, 320–328 CrossRef CAS PubMed.
- M. Sorbiun, E. Shayegan Mehr, A. Ramazani and A. Mashhadi Malekzadeh, Nanochem. Res., 2018, 3, 1–16 CAS.
- M. A. Khalilzadeh, S. Tajik, H. Beitollahi and R. A. Venditti, Ind. Eng. Chem. Res., 2020, 59, 4219–4228 CrossRef CAS.
- E. I. Hassanen, R. M. Korany and A. M. Bakeer, J. Biochem. Mol. Toxicol., 2021, 35, e22722 CrossRef CAS PubMed.
- O. Długosz, K. Szostak, A. Staroń, J. Pulit-Prociak and M. Banach, Materials, 2020, 13, 279 CrossRef PubMed.
- H. S. Sofi, T. Akram, N. Shabir, R. Vasita, A. H. Jadhav and F. A. Sheikh, Mater. Sci. Eng. C, 2021, 118, 111547 CrossRef CAS PubMed.
- P. G. Jamkhande, N. W. Ghule, A. H. Bamer and M. G. Kalaskar, J. Drug Deliv. Sci. Technol., 2019, 53, 101174 CrossRef CAS.
- M. Aadil, A. Rahman, S. Zulfiqar, I. A. Alsafari, M. Shahid, I. Shakir, P. O. Agboola, S. Haider and M. F. Warsi, Adv. Powder Technol., 2021, 32, 940–950 CrossRef CAS.
- M. Shahriari, M. A. Sedigh, Y. Mahdavian, S. Mahdigholizad, M. Pirhayati, B. Karmakar and H. Veisi, Int. J. Biol. Macromol., 2021, 172, 55–65 CrossRef CAS PubMed.
- R. Eyvazzadeh-Keihan, N. Bahrami, R. Taheri-Ledari and A. Maleki, Diam. Relat. Mater., 2020, 102, 107661 CrossRef CAS.
- M. A. K. Zarchi and S. S. A. D. M. Abadi, J. Organomet. Chem., 2019, 880, 196–212 CrossRef.
- H. A. Elazab, A. R. Siamaki, B. F. Gupton and M. S. El-Shall, Bull. Chem. React. Eng. Catal., 2019, 14(3), 478–489 CrossRef CAS.
- B. Aslibeiki and P. Kameli, Ceram. Int., 2020, 46, 20116–20121 CrossRef CAS.
- A. Scano, E. Magner, M. Pilloni, D. Peddis, F. Sini, S. Slimani and G. Ennas, J. Nanosci. Nanotechnol., 2021, 21, 2930–2934 CrossRef CAS PubMed.
- N. A. Neto, L. Nascimento, M. Correa, F. Bohn, M. Bomio and F. Motta, Mater. Chem. Phys., 2020, 242, 122489 CrossRef.
- S. V. Otari, S. K. Patel, V. C. Kalia and J.-K. Lee, Bioresour. Technol., 2020, 302, 122887 CrossRef CAS PubMed.
- S. Zhang, S. Zhu, H. Zhang, X. Liu and Y. Xiong, J. Anal. Appl. Pyrolysis, 2020, 147, 104806 CrossRef CAS.
- F. Moradnia, S. T. Fardood, A. Ramazani, B.-k. Min, S. W. Joo and R. S. Varma, J. Clean. Prod., 2021, 288, 125632 CrossRef CAS.
- I. Bibi, H. Maqbool, S. Iqbal, F. Majid, S. Kamal, N. Alwadai and M. Iqbal, Ceram. Int., 2021, 47, 5822–5831 CrossRef CAS.
- M. S. Amulya, H. Nagaswarupa, M. A. Kumar, C. Ravikumar and K. Kusuma, J. Phys. Chem. Solids, 2021, 148, 109661 CrossRef CAS.
- M. Jouyandeh, M. R. Ganjali, J. A. Ali, M. Aghazadeh, F. J. Stadler and M. R. Saeb, Prog. Org. Coat., 2019, 136, 105198 CrossRef CAS.
- A. Gallo-Cordova, S. Veintemillas-Verdaguer, P. Tartaj, E. Mazarío, M. d. P. Morales and J. G. Ovejero, Nanomaterials, 2021, 11, 1052 CrossRef CAS PubMed.
- M. B. Shyamaldas and C. Manoharan, J. Magn. Magn. Mater., 2020, 493, 165703 CrossRef CAS.
- S. Zare, D. Mehrabani, R. Jalli, M. Saeedi Moghadam, N. Manafi, G. Mehrabani, I. Jamhiri and S. Ahadian, J. Clin. Med., 2019, 8, 1418 CrossRef CAS PubMed.
- R. Bhowmik and M. Aswathi, Compos. B. Eng., 2019, 160, 457–470 CrossRef CAS.
- J. Milam-Guerrero, A. J. Neer and B. C. Melot, J. Solid State Chem., 2019, 274, 1–9 CrossRef CAS.
- H. Kaur, A. Marwaha, C. Singh, S. B. Narang, R. Jotania, S. Jacobo, A. Sombra, S. Trukhanov, A. Trukhanov and P. Dhruv, J. Alloys Compd., 2019, 806, 1220–1229 CrossRef CAS.
- H. Zheng, J. Luo, Q. Wu, P. Zheng, L. Zheng, M. Han and Y. Zhang, J. Magn. Magn. Mater., 2019, 479, 99–104 CrossRef CAS.
- M. Bansal, D. S. Ahlawat, A. Singh, V. Kumar and S. P. Rathee, Nanocomposites, 2020, 6, 158–164 CrossRef CAS.
- F. Sharifianjazi, M. Moradi, N. Parvin, A. Nemati, A. J. Rad, N. Sheysi, A. Abouchenari, A. Mohammadi, S. Karbasi and Z. Ahmadi, Ceram. Int., 2020, 46, 18391–18412 CrossRef CAS.
- M. Kazemi, M. Ghobadi and A. Mirzaie, Nanotechnol. Rev., 2018, 7, 43–68 CrossRef CAS.
- F. Hassanzadeh-Afruzi, S. Bahrami and A. Maleki, Proceedings, 2019, 41, 44 CrossRef.
- M. Hamidinasab and A. Mobinikhaledi, Chem. Sel., 2019, 4, 17–23 CAS.
- K. Kombaiah, J. J. Vijaya, L. J. Kennedy and K. Kaviyarasu, Mater. Chem. Phys., 2019, 221, 11–28 CrossRef CAS.
- M. A. Dheyab, A. A. Aziz, M. S. Jameel, O. A. Noqta, P. M. Khaniabadi and B. Mehrdel, Sci. Rep., 2020, 10, 1–8 CrossRef PubMed.
- G. Liu, B. Dai, Y. Ren, H. He and W. Zhang, Ceram. Int., 2021, 47, 11951–11957 CrossRef CAS.
- A. Maleki, Z. Varzi and F. Hassanzadeh-Afruzi, Polyhedron, 2019, 171, 193–202 CrossRef CAS.
- X. Zhang, Z. Chen, X. Liu, S. L. Hanna, X. Wang, R. Taheri-Ledari, A. Maleki, P. Li and O. K. Farha, Chem. Soc. Rev., 2020, 49, 7406–7427 RSC.
- R. Ma, P. Yang, Y. Ma and F. Bian, ChemCatChem, 2018, 10, 1446–1454 CrossRef CAS.
- X. Wang, X. Zhang, Y. Zhang, Y. Wang, S.-P. Sun, W. D. Wu and Z. Wu, J. Mater. Chem. A, 2020, 8, 15513–15546 RSC.
- M. E. Ziebel, J. C. Ondry and J. R. Long, Chem. Sci., 2020, 11, 6690–6700 RSC.
- S. Parvaz, R. Taheri-Ledari, M. S. Esmaeili, M. Rabbani and A. Maleki, Life Sci, 2020, 240, 117099 CrossRef CAS PubMed.
- L. León-Alcaide, J. López-Cabrelles, G. M. Espallargas and E. Coronado, Chem. Commun., 2020, 56, 7657–7660 RSC.
- C. Lin, K. Xu, R. Zheng and Y. Zheng, Chem. Commun., 2019, 55, 5697–5700 RSC.
- H.-Y. Zhang, X.-P. Hao, L.-P. Mo, S.-S. Liu, W.-B. Zhang and Z.-H. Zhang, New J. Chem., 2017, 41, 7108–7115 RSC.
- N. N. Yazgan, T. Bulat, A. Topcu, F. C. Dudak, I. H. Boyaci and U. Tamer, Food Chem., 2022, 372, 131235 CrossRef CAS PubMed.
- X. Ma, S. Wen, X. Xue, Y. Guo, J. Jin, W. Song and B. Zhao, ACS Appl. Mater. Interfaces, 2018, 10, 25726–25736 CrossRef CAS PubMed.
- R. K. Sharma, P. Yadav, M. Yadav, R. Gupta, P. Rana, A. Srivastava, R. Zbořil, R. S. Varma, M. Antonietti and M. B. Gawande, Mater. Horiz., 2020, 7, 411–454 RSC.
- S. He, T. Zeng, S. Wang, H. Niu and Y. Cai, ACS Appl. Mater. Interfaces, 2017, 9, 2959–2965 CrossRef CAS PubMed.
- M. Usman, J. Byrne, A. Chaudhary, S. Orsetti, K. Hanna, C. Ruby, A. Kappler and S. Haderlein, Chem. Rev., 2018, 118, 3251–3304 CrossRef CAS PubMed.
- Z. Shah, A. Dawar, I. Khan, S. Islam, D. L. C. Ching and A. Z. Khan, Case Stud. Therm. Eng., 2019, 13, 100352 CrossRef.
- P. Sangaiya and R. Jayaprakash, J. Supercond. Nov. Magn., 2018, 31, 3397–3413 CrossRef CAS.
- R. Kordnezhadian, M. Shekouhy, S. Karimian and A. Khalafi-Nezhad, J. Catal., 2019, 380, 91–107 CrossRef CAS.
- D. Xia, H. He, H. Liu, Y. Wang, Q. Zhang, Y. Li, A. Lu, C. He and P. K. Wong, Appl. Catal. B, 2018, 238, 70–81 CrossRef CAS.
- X. Li, X. Liu, C. Lin, H. Zhang, Z. Zhou, G. Fan, M. He and W. Ouyang, J. Hazard. Mater., 2019, 366, 402–412 CrossRef CAS PubMed.
- A. Maleki, S. Gharibi, K. Valadi and R. Taheri-Ledari, J. Phys. Chem. Solids, 2020, 142, 109443 CrossRef CAS.
- A. M. Zeyad, B. A. Tayeh and M. O. Yusuf, Constr. Build. Mater., 2019, 216, 314–324 CrossRef CAS.
- R. Taheri-Ledari, J. Rahimi and A. Maleki, Materials Research Express, 2020, 7, 015067 CrossRef CAS.
- R. Taheri-Ledari and A. Maleki, in Magnetic Nanoparticle-Based Hybrid Materials, Elsevier, 2021, pp. 619–636 Search PubMed.
- A. Ahmadi, T. Sedaghat, H. Motamedi and R. Azadi, Appl. Organomet. Chem., 2020, 34, e5572 CrossRef CAS.
- E. Teymoori, A. Davoodnia, A. Khojastehnezhad and N. Hosseininasab, Quarterly Journal of Iranian Chemical Communication, 2019, 7, 271–282 CAS.
- S. Asadbegi, M. A. Bodaghifard and A. Mobinikhaledi, Res. Chem. Intermed., 2020, 46, 1629–1643 CrossRef CAS.
- A. Maleki, F. Hassanzadeh-Afruzi, Z. Varzi and M. S. Esmaeili, Mater. Sci. Eng. C, 2020, 109, 110502 CrossRef CAS PubMed.
- V. N. Rathod, N. D. Bansode, P. B. Thombre and M. K. Lande, J. Chin. Chem. Soc., 2021, 68, 601–609 CrossRef CAS.
- Y. Chen, Z. Zhang, W. Jiang, M. Zhang and Y. Li, Mol. Divers., 2019, 23, 421–442 CrossRef CAS PubMed.
- A. Maleki, R. Taheri-Ledari and R. Ghalavand, Comb. Chem. High Throughput Screen., 2020, 23, 119–125 CrossRef CAS PubMed.
- S. Dayan, C. Altinkaynak, N. Kayaci, Ş. D. Doğan, N. Özdemir and N. K. Ozpozan, Appl. Organomet. Chem., 2020, 34, e5381 CrossRef CAS.
- J. Rahimi, R. Taheri-Ledari, M. Niksefat and A. Maleki, Catal. Commun., 2020, 134, 105850 CrossRef CAS.
- S. Jang, S. A. Hira, D. Annas, S. Song, M. Yusuf, J. C. Park, S. Park and K. H. Park, Processes, 2019, 7, 422 CrossRef CAS.
- A. Sheoran, J. Kaur, P. Kaur, V. Kumar, K. Tikoo, J. Agarwal, S. Bansal and S. Singhal, J. Mol. Struct., 2020, 1204, 127522 CrossRef CAS.
- S. Amirnejat, A. Nosrati and S. Javanshir, Appl. Organomet. Chem., 2020, 34, e5888 CAS.
- A. Zare, N. Lotfifar and M. Dianat, J. Mol. Struct., 2020, 1211, 128030 CrossRef CAS.
- F. Norouzi and S. Javanshir, BMC chem, 2020, 14, 1–16 CrossRef PubMed.
- F. Mirhashemi and M. A. Amrollahi, Res. Chem. Intermed., 2019, 45, 2549–2563 CrossRef CAS.
- M. Daraie, M. M. Heravi and S. S. Kazemi, J. Coord. Chem., 2019, 72, 2279–2293 CrossRef CAS.
- Z. Varzi and A. Maleki, Appl. Organomet. Chem., 2019, 33, e5008 CrossRef.
- M. Esmaeilzadeh, S. Sadjadi and Z. Salehi, Int. J. Biol. Macrmol., 2020, 150, 441–448 CrossRef CAS PubMed.
- B. Babaei, M. Mamaghani and M. Mokhtary, React. Kinet. Mech. Catal., 2019, 128, 379–394 CrossRef CAS.
- P. Mohammadi and H. Sheibani, Mater. Chem. Phys., 2019, 228, 140–146 CrossRef CAS.
- M. Noorisepehr, B. Kakavandi, A. A. Isari, F. Ghanbari, E. Dehghanifard, N. Ghomi and F. Kamrani, Sep. Purif. Technol., 2020, 250, 116950 CrossRef CAS.
- Z. Alirezvani, M. G. Dekamin and E. Valiey, ACS Omega, 2019, 4, 20618–20633 CrossRef CAS PubMed.
- F. Arpanahi and B. M. Goodajdar, J. Inorg. Organomet. Polym. Mater., 2020, 30, 2572–2581 CrossRef CAS.
- M. S. Esmaeili, M. R. Khodabakhshi, A. Maleki and Z. Varzi, Polycycl. Aromat. Compd., 2021, 41, 1953–1971 CrossRef CAS.
- T. Baran, Carbohydr. Polym., 2020, 237, 116105 CrossRef CAS PubMed.
- R. Mohammadi, H. Alamgholiloo, B. Gholipour, S. Rostamnia, S. Khaksar, M. Farajzadeh and M. Shokouhimehr, J. Photochem. Photobiol., 2020, 402, 112786 CrossRef CAS.
- A. Ahadi, S. Rostamnia, P. Panahi, L. D. Wilson, Q. Kong, Z. An and M. Shokouhimehr, Catalysts, 2019, 9, 140 CrossRef.
- L. Wöhler, G. Niebaum, M. Krol and A. Y. Hoekstra, Water Res. X, 2020, 7, 100044 CrossRef PubMed.
- R. R. Kalantary, G. Barzegar and S. Jorfi, Chemosphere, 2022, 286, 131667 CrossRef PubMed.
- H. Wang, K. Liu, L. Gao and W. Liu, Chin. J. Pestic. Sci., 2019, 21, 461–467 Search PubMed.
- Z. Fu and S. Xi, Toxicol. Mech. Methods, 2020, 30, 167–176 CrossRef CAS PubMed.
- T. Rasheed, N. Ahmad, J. Ali, A. A. Hassan, F. Sher, K. Rizwan, H. M. Iqbal and M. Bilal, Chemosphere, 2021, 129785 CrossRef CAS PubMed.
- S. Show, P. Chakraborty, B. Karmakar and G. Halder, Sci. Total Environ., 2021, 147327 CrossRef CAS PubMed.
- A. H. Asif, S. Wang and H. Sun, Curr. Opin. Green Sustain. Chem., 2021, 100447 CrossRef.
- R. Taheri-Ledari, J. Rahimi, A. Maleki and A. E. Shalan, New J. Chem., 2020, 44, 19827–19835 RSC.
- A. Sheikhmohammadi, E. Asgari, H. Nourmoradi, M. M. Fazli and M. Yeganeh, J. Environ. Chem. Eng., 2021, 9, 105844 CrossRef CAS.
- V. Soltaninejad, M. R. Ahghari, R. Taheri-Ledari and A. Maleki, Langmuir, 2021, 37, 4700–4713 CrossRef CAS PubMed.
- J. Zia, E. S. Aazam and U. Riaz, J. Mater. Res. Technol., 2020, 9, 9709–9719 CrossRef CAS.
- Y. Yang, X. Zhang, Q. Chen, S. Li, H. Chai and Y. Huang, ACS Omega, 2018, 3, 15870–15878 CrossRef CAS PubMed.
- S. Moradi, S. A. Sobhgol, F. Hayati, A. A. Isari, B. Kakavandi, P. Bashardoust and B. Anvaripour, Sep. Purif. Technol., 2020, 251, 117373 CrossRef CAS.
- J. Dong, Y. Kong, H. Cao, Z. Wang, Z. Zhang, L. Zhang, S. Sun, C. Gao, X. Zhu and J. Bao, J. Energy Chem., 2022, 64, 103–112 CrossRef.
- N. Bono, F. Ponti, C. Punta and G. Candiani, Mater, 2021, 14, 1075 CrossRef CAS PubMed.
- B. Rusinque, S. Escobedo and H. de Lasa, Catalysts, 2020, 10, 74 CrossRef CAS.
- C.-Y. Kuo, H.-K. Jheng and S.-E. Syu, Environ. Technol., 2021, 42, 1603–1611 CrossRef CAS PubMed.
- Y. Lin, Q. Wang, M. Ma, P. Li, V. Maheskumar, Z. Jiang and R. Zhang, Int. J. Hydrog. Energy, 2021, 46, 9417–9432 CrossRef CAS.
- G. Guo, H. Guo, F. Wang, L. J. France, W. Yang, Z. Mei and Y. Yu, Green Energy Environ, 2020, 5, 114–120 CrossRef.
- Z. Ding, Y. Liu, Y. Fu, F. Chen, Z. Chen and J. Hu, Water Sci. Technol., 2020, 81, 790–800 CrossRef CAS PubMed.
- Z. Amini, M. H. Givianrad, M. Saber-Tehrani, P. A. Azar and S. W. Husain, J. Environ. Sci. Health A, 2019, 54, 1254–1267 CrossRef CAS PubMed.
- N. Ahmadpour, M. H. Sayadi, S. Sobhani and M. Hajiani, J. Clean. Prod., 2020, 268, 122023 CrossRef CAS.
- Q. Li, H. Kong, P. Li, J. Shao and Y. He, J. Hazard. Mater., 2019, 373, 437–446 CrossRef CAS PubMed.
- R. Taheri-Ledari, K. Valadi, S. Gharibi and A. Maleki, Mater. Res. Bull., 2020, 130, 110946 CrossRef CAS.
- R. Taheri-Ledari, Classification of micro and nanoscale composites, Elsevier, 2022, pp. 1–21 Search PubMed.
- C. M. Park, Y. M. Kim, K.-H. Kim, D. Wang, C. Su and Y. Yoon, Chemosphere, 2019, 221, 392–402 CrossRef CAS PubMed.
- Y. Bao, Q. Yan, J. Ji, B. Qiu, J. Zhang and M. Xing, Trans. Tianjin Univ., 2021, 1–17 CAS.
- Z.-T. Hu, J. Liu, X. Yan, W.-D. Oh and T.-T. Lim, Chem. Eng. J., 2015, 262, 1022–1032 CrossRef CAS.
- S.-Q. Liu, X.-L. Zhu, Y. Zhou, Z.-D. Meng, Z.-G. Chen, C.-B. Liu, F. Chen, Z.-Y. Wu and J.-C. Qian, Catal. Sci. Technol., 2017, 7, 3210–3219 RSC.
- P. T. L. Huong, N. Tu, H. Lan, N. Van Quy, P. A. Tuan, N. X. Dinh, V. N. Phan and A.-T. Le, RSC Adv., 2018, 8, 12376–12389 RSC.
- Y. Yi, Z. Huang, B. Lu, J. Xian, E. P. Tsang, W. Cheng, J. Fang and Z. Fang, Bioresour. Technol., 2020, 298, 122468 CrossRef CAS PubMed.
- Y. Zhai, Y. Dai, J. Guo, L. Zhou, M. Chen, H. Yang and L. Peng, J. Colloid Interface Sci., 2020, 560, 111–121 CrossRef CAS PubMed.
- H. Zhou, X. Zhu and B. Chen, Sci. Total Environ., 2020, 724, 138278 CrossRef CAS PubMed.
- S. Li, Z. Wang, X. Zhao, X. Yang, G. Liang and X. Xie, Chem. Eng. J., 2019, 360, 600–611 CrossRef CAS.
- R. Taheri-Ledari, S. S. Mirmohammadi, K. Valadi, A. Maleki and A. E. Shalan, RSC Adv., 2020, 10, 43670–43681 RSC.
- C. Liu, L. Chen, D. Ding and T. Cai, Appl. Catal. B, 2019, 254, 312–320 CrossRef CAS.
- Z. Wang, X. Cai, X. Xie, S. Li, X. Zhang and Z. Wang, Sci. Total Environ., 2021, 764, 142879 CrossRef CAS PubMed.
- Y. Yi, G. Tu, P. E. Tsang and Z. Fang, Chem. Eng. J., 2020, 380, 122518 CrossRef CAS.
- J. Yu, L. Tang, Y. Pang, G. Zeng, J. Wang, Y. Deng, Y. Liu, H. Feng, S. Chen and X. Ren, Chem. Eng. J., 2019, 364, 146–159 CrossRef CAS.
- A. Naskar, H. Khan and S. Jana, J. Solgel Sci. Technol., 2018, 86, 599–609 CrossRef CAS.
- X. Zhang, B. Ren, X. Li, B. Liu, S. Wang, P. Yu, Y. Xu and G. Jiang, J. Hazard. Mater., 2021, 126333 CrossRef CAS PubMed.
- K. Fang, K. Li, T. Yang, J. Li and W. He, Int. J. Biol. Macromol., 2021, 184, 509–521 CrossRef CAS PubMed.
- Y. Wang, L. He, G. Dang, H. Li and X. Li, J. Colloid Interface Sci., 2021, 592, 51–65 CrossRef CAS PubMed.
- A. Abilarasu, P. S. Kumar, D.-V. N. Vo, D. Krithika, P. T. Ngueagni, G. J. Joshiba, C. F. Carolin and G. Prasannamedha, J. Environ. Chem. Eng., 2021, 9, 104875 CrossRef CAS.
- H. Zhang, Y. Song, L.-c. Nengzi, J. Gou, B. Li and X. Cheng, Chem. Eng. J., 2020, 379, 122362 CrossRef CAS.
- R. Bai, W. Yan, Y. Xiao, S. Wang, X. Tian, J. Li, X. Xiao, X. Lu and F. Zhao, Chem. Eng. J., 2020, 397, 125501 CrossRef CAS.
- K.-P. Cui, T.-T. Yang, Y.-H. Chen, R. Weerasooriya, G.-H. Li, K. Zhou and X. Chen, Environ. Technol., 2021, 1–14 CrossRef CAS PubMed.
- L. Li, C. Lai, F. Huang, M. Cheng, G. Zeng, D. Huang, B. Li, S. Liu, M. Zhang and L. Qin, Water Res., 2019, 160, 238–248 CrossRef CAS PubMed.
- M. H. Sayadi, N. Ahmadpour and S. Homaeigohar, Nanomaterials, 2021, 11, 298 CrossRef CAS PubMed.
- Y. Huaccallo-Aguilar, S. Álvarez-Torrellas, J. Martínez-Nieves, J. Delgado-Adámez, M. V. Gil, G. Ovejero and J. García, Catalysts, 2021, 11, 514 CrossRef CAS.
- Z. Hu, M. Ge and C. Guo, Chemosphere, 2021, 268, 128834 CrossRef CAS PubMed.
- J. Liang, Y. Wei, J. Zhang, Y. Yao, G. He, B. Tang and H. Chen, Ind. Eng. Chem. Res., 2018, 57, 4311–4319 CrossRef CAS.
- A. Kumar, A. Kumar, G. Sharma, H. Ala'a, M. Naushad, A. A. Ghfar, C. Guo and F. J. Stadler, Chem. Eng. J., 2018, 339, 393–410 CrossRef CAS.
- A. Mohseni-Bandpei, S. M. Ghasemi, A. Eslami, M. Rafiee, M. Sadani and F. Ghanbari, J. Photochem. Photobiol., 2021, 418, 113425 CrossRef CAS.
- A. Azizi, J. Iran. Chem. Soc., 2021, 18, 331–341 CrossRef CAS.
- P. Dhiman, A. Kumar, M. Shekh, G. Sharma, G. Rana, D.-V. N. Vo, N. AlMasoud, M. Naushad and Z. A. ALOthman, Environ. Res., 2021, 197, 111074 CrossRef CAS PubMed.
- J. Li, Y. Tao, S. Chen, H. Li, P. Chen, M.-z. Wei, H. Wang, K. Li, M. Mazzeo and Y. Duan, Sci. Rep., 2017, 7, 1–9 CrossRef PubMed.
- N. Suganthy, H. F. Aly, L.-G. Gunnarsson, G. Oboh, K. Muthusamy and M. Belkhelfa, Alzheimer's Disease & Treatment, Suganthy N, Department of Nanoscience and Technology, Science Campus, Alagappa University, Karaikudi, India, 2018, pp. 1–125 Search PubMed.
- C. Yang and T. Li, Eur. J. Pharm. Sci., 2021, 165, 105929 CrossRef CAS PubMed.
- E. Kaduševičius, Int. J. Mol. Sci., 2021, 22, 6637 CrossRef PubMed.
- W.-X. Chunyu, J.-Y. Zhao, Z.-G. Ding, X.-L. Han, Y.-X. Wang, J.-H. Ding, F. Wang, M.-G. Li and M.-L. Wen, Nat. Prod. Res., 2019, 33, 113–116 CrossRef CAS PubMed.
- A. Wisetsai, R. Lekphrom and F. T. Schevenels, Nat. Prod. Res., 2018, 32, 2499–2504 CrossRef CAS PubMed.
- Y. Yamamoto, N. Mori, H. Watanabe and H. Takikawa, Tetrahedron Lett., 2018, 59, 3503–3505 CrossRef CAS.
- G. Gao, J.-Q. Di, H.-Y. Zhang, L.-P. Mo and Z.-H. Zhang, J. Catal., 2020, 387, 39–46 CrossRef CAS.
- K. Valadi, S. Gharibi, R. Taheri-Ledari and A. Maleki, Solid State Sci., 2020, 101, 106141 CrossRef CAS.
- A. M. Inamuddin, A. Asiri and K Suvardhan, Green Sustainable Process for Chemical and Environmental Engineering and Science: Sonochemical Organic Synthesis, Elsevier, 2019 Search PubMed.
- R. Mozafari and F. Heidarizadeh, Polyhedron, 2019, 162, 263–276 CrossRef CAS.
- F. Sameri, A. Mobinikhaledi and M. A. Bodaghifard, Silicon, 2021, 1–12 Search PubMed.
- Y. F. Rego, C. M. da Silva, D. L. da Silva, J. G. da Silva, A. L. T. Ruiz, J. E. de Carvalho, S. A. Fernandes and A. de Fatima, Arab. J. Chem., 2019, 12, 4065–4073 CrossRef CAS.
- M. Hamidinasab, M. A. Bodaghifard and A. Mobinikhaledi, J. Mol. Struct., 2020, 1200, 127091 CrossRef.
- F. Bassyouni, M. Tarek, A. Salama, B. Ibrahim, S. Salah El Dine, N. Yassin, A. Hassanein, M. Moharam and M. Abdel-Rehim, Molecules, 2021, 26, 2370 CrossRef CAS PubMed.
- L. Z. Chen, H. Y. Shu, J. Wu, Y. L. Yu, D. Ma, X. Huang, M. M. Liu, X. H. Liu and J. B. Shi, Eur. J. Med. Chem., 2021, 213, 113174 CrossRef CAS PubMed.
- N. Abbas, G. S. Matada, P. S. Dhiwar, S. Patel and G. Devasahayam, Anticancer Agents Med. Chem., 2021, 21, 861–893 CrossRef CAS PubMed.
- N. Abbas, P. G. Swamy, P. Dhiwar, S. Patel and D. Giles, Pharm. Chem. J., 2021, 54, 1215–1226 CrossRef CAS.
- F. Al-Blewi, S. A. Shaikh, A. Naqvi, F. Aljohani, M. R. Aouad, S. Ihmaid and N. Rezki, Int. J. Mol. Sci., 2021, 22, 1162 CrossRef CAS PubMed.
- P. Nikitina, N. Bormotov, L. Shishkina, A. Y. Tikhonov and V. Perevalov, Russ. Chem. Bull., 2019, 68, 634–637 CrossRef CAS.
- F. Saghir, K. Hussain, M. N. Tahir, S. A. Raza, N. Shehzadi, S. Iftikhar, A. Shaukat, S. Naheed and S. Siddique, J. Medicinal plants and By-product, 2021, 10, 85–92 Search PubMed.
- A. M. El-Agrody, A. M. Fouda, M. A. Assiri, A. Mora, T. E. Ali, M. M. Alam and M. Y. Alfaifi, Med. Chem. Res., 2020, 29, 617–629 CrossRef CAS.
- M. Ghassemi and A. Maleki, SynOpen, 2021, 5(2), 100–103 CrossRef CAS.
- T. Akbarpoor, A. Khazaei, J. Y. Seyf, N. Sarmasti and M. M. Gilan, Res. Chem. Intermed., 2020, 46, 1539–1554 CrossRef CAS.
- B. Li, R. Tayebee, E. Esmaeili, M. S. Namaghi and B. Maleki, RSC Adv., 2020, 10, 40725–40738 RSC.
- S. Azizi, N. Shadjou and M. Hasanzadeh, Appl. Organomet. Chem., 2020, 34, e5321 CAS.
- S. Gupta and M. Lakshman, J. Med. Chem. Sci., 2019, 2, 51–54 CAS.
- H. H. Moghadam, S. Sobhani and J. M. Sansano, ACS Omega, 2020, 5, 18619–18627 CrossRef CAS PubMed.
- M. Torabi, M. Yarie, M. A. Zolfigol and S. Azizian, Res. Chem. Intermed., 2020, 46, 891–907 CrossRef CAS.
- T. Tamoradi, M. Daraie and M. M. Heravi, Appl. Organomet. Chem., 2020, 34, e5538 CAS.
- A. Maleki and V. Eskandarpour, J. Iran. Chem. Soc., 2019, 16, 1459–1472 CrossRef CAS.
- N. Motahharifar, M. Nasrollahzadeh, A. Taheri-Kafrani, R. S. Varma and M. Shokouhimehr, Carbohydr. Polym., 2020, 232, 115819 CrossRef CAS PubMed.
- T. Baran and I. Sargin, Int. J. Biol. Macromol., 2020, 155, 814–822 CrossRef CAS PubMed.
- A. Akbari, M. G. Dekamin, A. Yaghoubi and M. R. Naimi-Jamal, Sci. Rep., 2020, 10, 1–16 CrossRef PubMed.
- M. Torabi, M. Yarie and M. A. Zolfigol, Appl. Organomet. Chem., 2019, 33, e4933 CrossRef.
- A. Maleki, V. Eskandarpour, J. Rahimi and N. Hamidi, Carbohydr. Polym., 2019, 208, 251–260 CrossRef CAS PubMed.
- M. M. Khakzad Siuki, M. Bakavoli and H. Eshghi, Appl. Organomet. Chem., 2019, 33, e4774 CrossRef.
- S. Kalhor, M. Yarie, M. Rezaeivala and M. A. Zolfigol, Res. Chem. Intermed., 2019, 45, 3453–3480 CrossRef CAS.
- S. R. Mousavi, H. Sereshti, H. Rashidi Nodeh and A. Foroumadi, Appl. Organomet. Chem., 2019, 33, e4644 CrossRef.
- G. Bagherzade and H. Eshghi, RSC Adv., 2020, 10, 32927–32937 RSC.
- S. Sobhani, A. Habibollahi and Z. Zeraatkar, Org. Process Res. Dev., 2019, 23, 1321–1332 CrossRef CAS.
- L. Z. Fekri and S. Zeinali, Appl. Organomet. Chem., 2020, 34, e5629 CrossRef CAS.
- D. Azarifar and M. Khaleghi-Abbasabadi, Res. Chem. Intermed., 2019, 45, 199–222 CrossRef CAS.
- N. Malhotra, J.-S. Lee, R. A. D. Liman, J. M. S. Ruallo, O. B. Villaflores, T.-R. Ger and C.-D. Hsiao, Molecules, 2020, 25, 3159 CrossRef CAS PubMed.
- P. Bhatia, S. Verma and M. Sinha, J. Quant. Spectrosc. Radiat. Transf., 2020, 251, 107095 CrossRef CAS.
- J. G. Ovejero, A. Mayoral, M. Canete, M. Garcia, A. Hernando and P. Herrasti, J. Nanosci. Nanotechnol., 2019, 19, 2008–2015 CrossRef CAS PubMed.
- V. Zheltova, A. Vlasova, N. Bobrysheva, I. Abdullin, V. Semenov, M. Osmolowsky, M. Voznesenskiy and O. Osmolovskaya, Appl. Surf. Sci., 2020, 531, 147352 CrossRef CAS.
- D. S. Mathew and R. S. Juang, Chem. Eng. Sci., 2007, 129, 51–65 CrossRef CAS.
- O. Gorobets, S. Gorobets and M. Koralewski, Int. J. Nanomedicine, 2017, 12, 4371 CrossRef CAS PubMed.
- A. G. Kolhatkar, A. C. Jamison, D. Litvinov, R. C. Willson and T. R. Lee, Int. J. Mol. Sci., 2013, 14, 15977–16009 CrossRef PubMed.
- L. Yi, D. Yang, M. Liu, H. H. Fu, L. Ding, Y. Xu, B. Zhang, L. Pan and J. Q. Xiao, Adv. Funct. Mater., 2020, 30, 2004024 CrossRef CAS.
- D. Maity, G. Kandasamy and A. Sudame, in Nanotheranostics, Springer, 2019, pp. 245–276 Search PubMed.
- F.-Z. Bachar, C. Schröder and A. Ehrmann, J. Magn. Magn. Mater., 2021, 168205 CrossRef CAS.
- Y. Kumar, A. Sharma and P. M. Shirage, J. Alloys Compd., 2019, 778, 398–409 CrossRef CAS.
- T. Assefa, Y. Cao, J. Diao, R. Harder, W. Cha, K. Kisslinger, G. Gu, J. Tranquada, M. Dean and I. Robinson, Phys. Rev. B, 2020, 101, 054104 CrossRef CAS.
- Z. Zhou, L. Yang, J. Gao and X. Chen, Adv. Mater., 2019, 31, 1804567 CrossRef PubMed.
- A. Rasras, R. Hamdi, S. Mansour, A. Samara and Y. Haik, J. Magn. Magn. Mater., 2020, 513, 167009 CrossRef CAS.
- N. Jahan, J. Khandaker, S. Liba, S. Hoque and M. Khan, J. Alloys Compd., 2021, 869, 159226 CrossRef CAS.
- M. Ahmadi and F. Ghanbari, Mater. Res. Bull., 2019, 111, 43–52 CrossRef CAS.
- T. Baran, Catal. Letters, 2019, 149, 1721–1729 CrossRef CAS.
- Y. Li, S. Ma, S. Xu, H. Fu, Z. Li, K. Li, K. Sheng, J. Du, X. Lu and X. Li, Chem. Eng. J., 2020, 387, 124094 CrossRef CAS.
- R. M. Ali, M. R. Elkatory and H. A. Hamad, Fuel, 2020, 268, 117297 CrossRef CAS.
- X. Li, K. Cui, Z. Guo, T. Yang, Y. Cao, Y. Xiang, H. Chen and M. Xi, Chem. Eng. J., 2020, 379, 122324 CrossRef CAS.
- A. Maleki, K. Valadi, S. Gharibi and R. Taheri-Ledari, Res. Chem. Intermed., 2020, 46, 4113–4128 CrossRef CAS.
- Y. Ma, X. Lv, D. Xiong, X. Zhao and Z. Zhang, Appl. Catal. B, 2021, 284, 119720 CrossRef CAS.
- Z. Wang, C. Lai, L. Qin, Y. Fu, J. He, D. Huang, B. Li, M. Zhang, S. Liu and L. Li, Chem. Eng. J., 2020, 392, 124851 CrossRef CAS.
- N. Thomas, D. D. Dionysiou and S. C. Pillai, J. Hazard. Mater., 2021, 404, 124082 CrossRef CAS PubMed.
- P. K. Boruah, B. Sharma, I. Karbhal, M. V. Shelke and M. R. Das, J. Hazard. Mater., 2017, 325, 90–100 CrossRef CAS PubMed.
- X. Zheng, H. Cheng, J. Yang, D. Chen, R. Jian and L. Lin, ACS Appl. Nano Mater., 2018, 1, 2754–2762 CrossRef CAS.
- S. Dehghan, B. Kakavandi and R. R. Kalantary, J. Mol. Liq., 2018, 264, 98–109 CrossRef CAS.
- K. S. Al-Mawali, A. I. Osman, H. Ala’a, N. Mehta, F. Jamil, F. Mjalli, G. R. Vakili-Nezhaad and D. W. Rooney, Renewable Energy, 2021, 170, 832–846 CrossRef CAS.
- W. Xie and H. Wang, Renewable Energy, 2020, 145, 1709–1719 CrossRef CAS.
- R. Kumar, G. Kumar, M. Akhtar and A. Umar, J. Alloys Compd., 2015, 629, 167–172 CrossRef CAS.
- R. Vinoth, P. Karthik, K. Devan, B. Neppolian and M. Ashokkumar, Ultrason. Sonochem., 2017, 35, 655–663 CrossRef CAS PubMed.
- V. K. Gupta, A. Fakhri, S. Agarwal, A. K. Bharti, M. Naji and A. G. Tkachev, J. Mol. Liq., 2018, 249, 1033–1038 CrossRef CAS.
- X. Xu, S. Zong, W. Chen and D. Liu, Chem. Eng. J., 2019, 369, 470–479 CrossRef CAS.
- A. A. Oladipo, A. O. Ifebajo and M. Gazi, Appl. Catal. B, 2019, 243, 243–252 CrossRef CAS.
- F. Gao, Y. Guo, X. Fan, M. Hu, S. Li, Q. Zhai, Y. Jiang and X. Wang, Biochem. Eng. J., 2019, 143, 101–109 CrossRef CAS.
- Y. Jiang, H. Liu, L. Wang, L. Zhou, Z. Huang, L. Ma, Y. He, L. Shi and J. Gao, Biochem. Eng. J., 2019, 144, 125–134 CrossRef CAS.
- S. M. Hosseini, S. M. Kim, M. Sayed, H. Younesi, N. Bahramifar, J. H. Park and S.-H. Pyo, Biochem. Eng. J., 2019, 143, 141–150 CrossRef CAS.
- N. Mangkorn, P. Kanokratana, N. Roongsawang, A. Laobuthee, N. Laosiripojana and V. Champreda, J. Biosci. Bioeng., 2019, 127, 265–272 CrossRef CAS PubMed.
- M. Mohammadi, R. R. Mokarram, M. Ghorbani and H. Hamishehkar, Int. J. Biol. Macromol., 2019, 123, 846–855 CrossRef CAS PubMed.
- H. Suo, L. Xu, C. Xu, X. Qiu, H. Chen, H. Huang and Y. Hu, ACS Sustain. Chem. Eng., 2019, 7, 4486–4494 CrossRef CAS.
- M. Boudart, A. Aldag, J. Benson, N. Dougharty and C. G. Harkins, J. Catal., 1966, 6, 92–99 CrossRef CAS.
- R. L. Burwell Jr, in Advances in Catalysis, Elsevier, 1977, vol. 26, pp. 351–392 Search PubMed.
- S. Hemmati, M. Yousefi, M. H. Salehi, M. Amiri and M. Hekmati, Appl. Organomet. Chem., 2020, 34, e5653 CAS.
- S. Saha, A. Pal, S. Kundu, S. Basu and T. Pal, Langmuir, 2010, 26, 2885–2893 CrossRef CAS PubMed.
- V. Polshettiwar and R. S. Varma, Chem. -Eur. J., 2009, 15, 1582–1586 CrossRef CAS PubMed.
- R. N. Baig and R. S. Varma, Commun. Chem., 2013, 49, 752–770 RSC.
- M. Mohammadi, R. Rezaei Mokarram and H. Hamishehkar, J. Microencapsul., 2018, 35, 559–569 CrossRef CAS PubMed.
- M. Arghan, N. Koukabi and E. Kolvari, Appl. Organomet. Chem., 2018, 32, e4346 CrossRef.
- Y. Zhang, Y. Chen, Z.-W. Kang, X. Gao, X. Zeng, M. Liu and D.-P. Yang, Colloids Surf. A Physicochem. Eng. Asp., 2021, 612, 125874 CrossRef CAS.
- G. Nabiyouni and D. Ghanbari, J. Nanostruct., 2018, 8, 408–416 CAS.
- Z. N. Kayani, E. Abbas, Z. Saddiqe, S. Riaz and S. Naseem, Mater. Sci. Semicond. Process., 2018, 88, 109–119 CrossRef CAS.
- N. Zandi-Atashbar, A. A. Ensafi and A. H. Ahoor, J. Clean. Prod., 2018, 172, 68–80 CrossRef CAS.
- P. Makkar, M. Chandel, M. K. Patra and N. N. Ghosh, ACS Omega, 2019, 4, 20672–20689 CrossRef CAS PubMed.
- S. Zhao, X. Yu, Y. Qian, W. Chen and J. Shen, Theranostics, 2020, 10, 6278 CrossRef CAS PubMed.
- H. Y. Yang, Y. Li and D. S. Lee, Adv. Ther., 2018, 1, 1800011 CrossRef.
- Z.-Q. Feng, K. Yan, J. Li, X. Xu, T. Yuan, T. Wang and J. Zheng, Mater. Sci. Eng. C, 2019, 104, 110001 CrossRef CAS PubMed.
- P. Das, P. Fatehbasharzad, M. Colombo, L. Fiandra and D. Prosperi, Trends Biotechnol., 2019, 37, 995–1010 CrossRef CAS PubMed.
- J. Zhou, Multicatalyst System in Asymmetric Catalysis, John Wiley & Sons, 2014 Search PubMed.
- A. H. Tamboli, A. A. Chaugule and H. Kim, Fuel, 2016, 166, 495–501 CrossRef CAS.
- L. Kong, C. Wang, F. Gong, W. Zhu, Y. Zhong, X. Ye and F. Li, Catal. Letters, 2016, 146, 1321–1330 CrossRef CAS.
- R. A. Raimundo, V. D. Silva, E. S. Medeiros, D. A. Macedo, T. A. Simões, U. U. Gomes, M. A. Morales and R. M. Gomes, J. Phys. Chem. Solids, 2020, 139, 109325 CrossRef CAS.
- L. S. Ferreira, T. R. Silva, J. R. Santos, V. D. Silva, R. A. Raimundo, M. A. Morales and D. A. Macedo, Mater. Chem. Phys., 2019, 237, 121847 CrossRef CAS.
- E. E. Gladis, K. Nagashri and J. Joseph, Int. J. Hydrog. Energy., 2021, 46, 6573–6587 CrossRef CAS.
- M. Amini, M. Hajjari, A. Landarani-Isfahani, V. Mirkhani, M. Moghadam, S. Tangestaninejad, I. Mohammadpoor-Baltork and M. Ahmadi, Nanochem. Res., 2016, 1, 237–248 CAS.
- W. Fu, Z. Zhang, P. Zhuang, J. Shen and M. Ye, J. Colloid Interface Sci., 2017, 497, 83–92 CrossRef CAS PubMed.
- Y. Li, J. Yin, L. An, M. Lu, K. Sun, Y. Q. Zhao, D. Gao, F. Cheng and P. Xi, Small, 2018, 14, 1801070 CrossRef PubMed.
- S. Wang and X. Wang, Small, 2015, 11, 3097–3112 CrossRef CAS PubMed.
- Y. Jiang, X. Zhang, X. Dai, W. Zhang, Q. Sheng, H. Zhuo, Y. Xiao and H. Wang, Nano Res., 2017, 10, 876–889 CrossRef CAS.
- P. H. Dixneuf, Nat. Chem., 2011, 3, 578–579 CrossRef CAS PubMed.
- W. Sattler and G. Parkin, J. Am. Chem. Soc., 2012, 134, 17462–17465 CrossRef CAS PubMed.
- A. Alazman, D. Belic, E. F. Kozhevnikova and I. V. Kozhevnikov, J. Catal., 2018, 357, 80–89 CrossRef.
- L. Wang, N. Onishi, K. Murata, T. Hirose, J. T. Muckerman, E. Fujita and Y. Himeda, ChemSusChem, 2017, 10, 1071–1075 CrossRef CAS PubMed.
- M. Caillot, A. Chaumonnot, M. Digne and J. A. Van Bokhoven, ChemCatChem, 2014, 6, 832–841 CrossRef CAS.
- L. Liu, F. Yan, K. Li, C. Zhu, Y. Xie, X. Zhang and Y. Chen, J. Mater. Chem. A, 2019, 7, 1083–1091 RSC.
- M. A. R. Anjum, H. Y. Jeong, M. H. Lee, H. S. Shin and J. S. Lee, Adv. Mater., 2018, 30, 1707105 CrossRef PubMed.
- J. Xu, K. Zhu and Y. Hou, ACS Appl. Mater. Interfaces, 2020, 12, 36811–36822 CrossRef CAS PubMed.
- W. Yan, J. Wang, J. Ding, P. Sun, S. Zhang, J. Shen and X. Jin, Dalton Trans., 2019, 48, 16827–16843 RSC.
Footnotes |
| † Electromagnetic unit. |
| ‡ An igneous rock. |
| This journal is © The Royal Society of Chemistry 2022 |





