Open Access Article
Open Access ArticleGreen synthesis of silver nanoparticles using green tea leaf extract, characterization and evaluation of antimicrobial activity
Hiba Abbas
Widatalla
 *a,
Layla Fathi
Yassin
b,
Ayat Ahmed
Alrasheid
c,
Shimaa Abdel
Rahman Ahmed
b,
Marvit Osman
Widdatallah
a,
Sahar Hussein
Eltilib
a and
Alaa Abdulmoneim
Mohamed
d
*a,
Layla Fathi
Yassin
b,
Ayat Ahmed
Alrasheid
c,
Shimaa Abdel
Rahman Ahmed
b,
Marvit Osman
Widdatallah
a,
Sahar Hussein
Eltilib
a and
Alaa Abdulmoneim
Mohamed
d
aDepartment of Pharmacology, Faculty of Pharmacy, University of Medical Sciences and Technology, Khartoum, Sudan. E-mail: hiba127@gmail.com
bDepartment of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Medical Sciences and Technology, Khartoum, Sudan
cDepartment of Pharmacognosy, Faculty of Pharmacy, University of Medical Sciences and Technology, Khartoum, Sudan
dDepartment of Clinical Pharmacy, Faculty of Pharmacy, University of Medical Sciences and Technology, Khartoum, Sudan
First published on 18th January 2022
Abstract
The use of nanoparticles in medicine, nanomedicine, is very important to diagnose and treat diseases; among the various metallic nanoparticles, silver nanoparticles (AgNPs) are very popular due to their physical, chemical, and biological properties, encompassing a range of activities such as antiviral, antifungal, anti-inflammatory, and anticancer activities. In this study, the synthesis of AgNPs was conducted by the use of a nontoxic, ecofriendly method. Green tea (GT) leaf extract was used as a reducing agent to convert silver ions into free AgNPs. The UV-vis spectrum showed a peak at 410 nm, confirming the presence of AgNPs. A Fourier-transform infrared (FTIR) analysis of the GT extract and GT AgNPs display spectra that is identical to those of polyphenols, polysaccharides, and proteins. All the vibrational peaks in the GT extract spectrum were shifted in the AgNP spectrum, becoming narrower after the encapsulation of nanoparticles. The scanning electron microscopy (SEM) images confirm the presence of AgNPs with different sizes, ranging from 15 to 33 nm. Furthermore, the antibacterial activity of the synthesized AgNPs in three different concentrations (10, 20, and 50 mg ml−1) showed appreciable inhibition of bacterial growth against Staphylococcus aureus and Klebsiella sp. From the above findings, we can recommend the use of AgNPs from GT leaf extracts as an antimicrobial agent to treat chronic infections.
Introduction
Nanotechnology is a rapidly developing branch of science that incorporates many disciplines such as biology, chemistry, physics, food, medicine, electronics, aerospace, and medicine and it examines the design, manufacture, assembly, and characterization of materials that are smaller than 1–100 nm in size.1 Nanoparticles are being applied in many fields of science such as medical, cosmetic, biomedical, drug/gene delivery, environmental, energy science, photoelectrochemical, and optical applications.2 The wide range of uses of nanoparticles is due to their unique properties such as high surface-to-volume ratio, high surface energy, and unique mechanical, thermal, electrical, magnetic, and optical behaviors.3 The use of nanoparticles in medicine, also referred to as nanomedicine, is very important toward the diagnosis and treatment of diseases; in addition, it is important to determine the efficacy and safety of nanoparticles within biological systems.4 Among the most important types of nanoparticles, metallic nanoparticles have a wide range of applications.5The therapeutic efficacy of metallic nanoparticles is due to their optical property demonstrated by localized surface plasmon resonance.6 Among the various metal nanoparticles, silver nanoparticles (AgNPs) are very popular and have many uses in industry and biomedicine due to their physical and chemical properties in addition to their biological properties, which encompass a range of activities such as antiviral, antifungal, anti-inflammatory, and anticancer activities.7 Surface chemistry, size, size distribution, shape, particle morphology, particle composition, coating agglomeration, and dissolution rate are factors that affect the effectiveness of AgNPs inside biological systems.8
Silver is a nontoxic, safe inorganic antibacterial agent that is capable of killing about 650 types of disease-causing microorganisms.5,9 However, the emergence of bacterial strains resistant to metal ions has led to the search for new antibacterials.10 In 2015, the World Health Organization (WHO) published a report on a global antimicrobial resistance surveillance system concluding that it is very common for bacteria such as Escherichia coli, Staphylococcus aureus, Shigella sp., Klebsiella pneumoniae, and Salmonella sp. to show resistance toward one or several antibiotics as they are commonly present in communities and hospitals. The development of resistant machineries by bacteria has led to an increase in the rates of antibiotic resistance. All this motivates the search for a new alternative to conventional antimicrobials and antibiotics.10–12
Different methods have been developed for the synthesis of nanoparticles, and each method has its own advantages and disadvantages.13 There are physical and chemical methods for the synthesis of metallic nanoparticles. Although these methods produce particles of the desired characteristics, they usually involve high cost, require effort, and are potentially dangerous and toxic to the environment and living organisms. To overcome the shortcomings of these physical and chemical methods, the biological approach has been used to synthesize nanoparticles. Several biological resources such as plant extracts, microorganisms, milk, oilcake, and panchagavya have been used as alternatives to synthesize metal nanoparticles.14,15 Among the various green sources explored, microalgae provide worthwhile benefits with respect to their ease of growth and their ability to survive in extreme conditions (namely, pH and temperature).16 Numerous advantages of the biological synthesis of AgNPs have been acknowledged in recent years. Various plant materials and microorganisms have been identified as potential candidates for AgNPs synthesis. It has been suggested that the presence of specific proteins in plants and microorganisms can cause the reduction of Ag+ ions. The probable role of NADH-dependent nitrate reductase in the reduction of Ag+ ions has been suggested.17
Nevertheless, the synthesis of AgNPs using plant extracts is potentially advantageous over microorganisms because of its simple scale up.18 The use of microorganisms presents several challenges such as high cost, mass cultivation of microorganisms, maintenance of an aseptic environment, purification, and quantum of production.17 On the other hand, the ‘green’ method that involves the use of extracts obtained from plants is cost-effective, efficient and environment-friendly.19 Several studies have been performed in recent years to synthesize nanoparticles using various plant extracts such as Alternanthera dentata,20Carica papaya,21Coffea arabica,22Azadirachta indica,23 and Olea europaea.24 Furthermore, plant extracts derived from various species are regarded as a beneficial system for nanoparticle synthesis due to their tremendous capability to produce a variety of phytochemicals with a profound reducing potential.25 Plant metabolites like sugars, terpenoids, polyphenols, alkaloids, phenolic acids, and proteins play an important role in reducing metal ions into nanoparticles. These phytochemicals are also responsible for stabilizing the synthesized nanoparticles.26
In this study, the synthesis of AgNPs was conducted by the use of green tea (GT) leaf extracts. GT leaves are known for their high content of various phytochemical constituents that are involved in the reduction of metallic ions into nanoparticles.27 The synthesized AgNPs were characterized by using UV-vis spectrometry and FTIR. The morphology of the nanoparticles was imaged by scanning electron microscopy (SEM). The antibacterial activity of the synthesized GT AgNPs was evaluated.
Materials and methods
Materials
Methods
UV spectroscopy. The UV spectra were used to ascertain the formation of AgNPs. The AgNP solution was scanned over the range of 200–400 nm by using a Shimadzu UV spectrophotometer. The dilution of GT AgNP solution was required; a ratio of 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 was used. The same dilution ratio was used for the GT extract, which acted as the reference solution.
9 was used. The same dilution ratio was used for the GT extract, which acted as the reference solution.
Fourier-transform infrared (FTIR). The IR spectra of GT leaf aqueous extract and the centrifuged GT AgNPs sample were used to identify the possible chemical constituents involved in the synthesis and capping of GT AgNPs. The samples were analyzed by IRTracer-100 (Shimadzu). A pure KBr pellet was used for the background. The spectra were recorded from 400 to 4000 cm−1.
Scanning electron microscopy (SEM). SEM was used to characterize the morphology and particle size of AgNPs. A thin film of oven-dried GT AgNP sample was prepared and used over a carbon-coated copper grid via a TESCAN MIRA-3 instrument operated at an accelerated voltage of 20 kV.
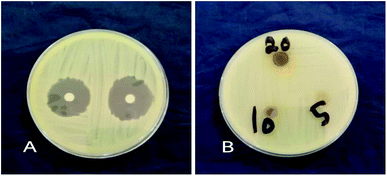
Antibacterial activity of GT AgNPs. The inhibition of bacterial growth of GTAgNPs was tested by using a disc-diffusion assay, as described by Sharma et al.28 with certain modifications. The antimicrobial effect of GT AgNPs was tested against Gram-positive bacteria (Staphylococcus aureus) and Gram-negative bacteria (Klebsiella spp). About 20 ml Mueller–Hinton agar were poured in sterile Petri dishes to prepare the base plates. About 0.1 ml standard bacterial stock suspensions (108–109 cfu ml−1) was streaked onto the base plates using a sterile cotton swab. A stock solution of 20 mg ml−1 GT AgNPs solution was prepared with DMSO; three dilutions of 5, 10, and 20 mg ml−1 were used. Further, 6 mm-diameter sterilized filter paper discs were immersed in the GT AgNP solutions and then placed on the surface of the test bacteria plates. After the plates were incubated for 24 h, the diameters of the inhibition zones were measured.
Results and discussion
UV spectroscopy
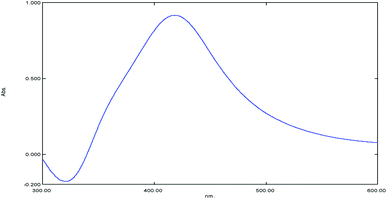
The UV-vis spectrometer is a spectral device that is widely used to confirm the formation of AgNPs in a colloidal solution via the surface plasmon resonance phenomena of metallic nanoparticles. This optical property is sensitive to size, shape, concentration, and agglomeration state of the nanoparticles produced.29Fig. 1 shows the observable change of yellowish GT into a brown color, which indicates the formation of GT AgNPs. The UV scan of the GT AgNP solution showed a distinct Gaussian-shaped peak at 410 nm. According to Grand et al.,30 the band corresponds to absorption by AgNPs is in the region of 400–450 nm, which is due to the excitation of surface plasmon vibration. Fig. 2 shows the UV spectrum of GT AgNPs.FTIR analysis
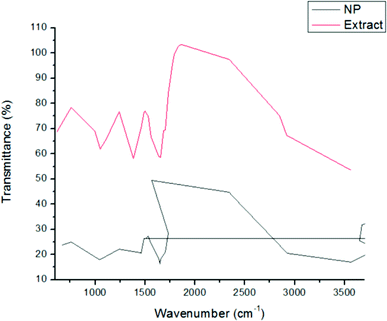
FTIR spectra of dried GT AgNPs and GT extracts (Fig. 3) were interpreted through the correlation of the absorption bands to the corresponding compounds for studying the phytochemical constituents involved in the reduction and capping of AgNPs. The GT extract showed distinguishing peaks at 3566.3, 2918, 2850.7, 1653.9, 1384, 1107, 1053, and 997.2 cm−1. The broad band at 3566.3 cm−1 is due to the O–H stretching of alcohol in polyphenols and N–H stretching in amines. The bands representing C–H stretching in alkanes and O–H stretching in carboxylic acids appear at 2918 and 2850.7 cm−1, respectively. The strong bands at 1653.9 and 1384 cm−1 correspond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic group and primary amide group of proteins (–CO–NH2), respectively; the 1107 and 1053 cm−1 peaks represent C–O–C stretching, and the strong band at 997.2 cm−1 represents C
C aromatic group and primary amide group of proteins (–CO–NH2), respectively; the 1107 and 1053 cm−1 peaks represent C–O–C stretching, and the strong band at 997.2 cm−1 represents C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending. A phytochemical analysis of GT aqueous extract indicates the presence of polyphenols such as gallic acid (GA), gallocatechin (GC), catechin (CE), epigallocatechin, protein, flavonoid, saponin, and glycosides.31 The most abundant constituent in GT is polyphenols, accounting for 24–36% in dry weight in the form of catechins.32 The obtained IR results are identical to those of polyphenols, polysaccharides, and proteins. The FTIR spectra of GT AgNPs reveal clear peaks at 3689.8, 2924.09, 1634, 1238.9, 1701, 1043, and 761.8 cm−1. The peaks at 3689.8, 2924.09, and 1634 cm−1 correspond to (O–H, N–H), C–H stretching, and N–H bending, respectively. The other peaks at 1238.9, 1043, and 1701 cm−1 can be attributed to aromatic amine stretching, C–O stretching, and C
C bending. A phytochemical analysis of GT aqueous extract indicates the presence of polyphenols such as gallic acid (GA), gallocatechin (GC), catechin (CE), epigallocatechin, protein, flavonoid, saponin, and glycosides.31 The most abundant constituent in GT is polyphenols, accounting for 24–36% in dry weight in the form of catechins.32 The obtained IR results are identical to those of polyphenols, polysaccharides, and proteins. The FTIR spectra of GT AgNPs reveal clear peaks at 3689.8, 2924.09, 1634, 1238.9, 1701, 1043, and 761.8 cm−1. The peaks at 3689.8, 2924.09, and 1634 cm−1 correspond to (O–H, N–H), C–H stretching, and N–H bending, respectively. The other peaks at 1238.9, 1043, and 1701 cm−1 can be attributed to aromatic amine stretching, C–O stretching, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ketone stretching, respectively. The final peak at 761.8 cm−1 corresponds to C–H bending. Changes in the chemical constituents of GT and GT AgNPs can be identified by comparing their FTIR spectra. All the vibrational peaks in the GT spectrum were shifted in the GT AgNP spectrum, becoming narrower after encapsulation of the nanoparticles. The structure–activity relationship of flavonoids plays a critical role in GT capacity to reduce several species; the abundance of the hydroxyl (HO) group makes epigallocatechin gallate a powerful antioxidant and a strong reducing agent for AgNP synthesis.33
O ketone stretching, respectively. The final peak at 761.8 cm−1 corresponds to C–H bending. Changes in the chemical constituents of GT and GT AgNPs can be identified by comparing their FTIR spectra. All the vibrational peaks in the GT spectrum were shifted in the GT AgNP spectrum, becoming narrower after encapsulation of the nanoparticles. The structure–activity relationship of flavonoids plays a critical role in GT capacity to reduce several species; the abundance of the hydroxyl (HO) group makes epigallocatechin gallate a powerful antioxidant and a strong reducing agent for AgNP synthesis.33
SEM analysis
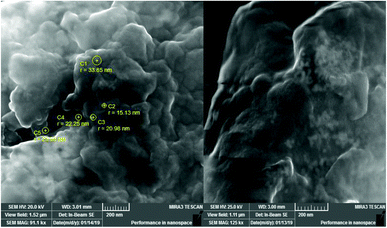
Fig. 4 shows the SEM images of GT AgNPs with different sizes below 50 nm (ranging from 15 to 33 nm), which are predominantly dispersed in the form of aggregates. The nanoparticles were not in direct contact with each other within the formed aggregates, which can be explained by the stabilizing action of capping agents present in the extract. These phytochemicals are known to play an active role in reducing and stabilizing metal nanoparticles.34Antibacterial activity
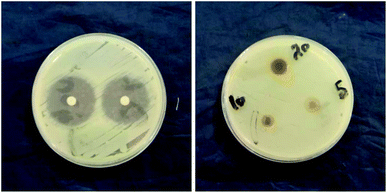
The antimicrobial activity of GT AgNPs has been reported in several studies.35–38 In this study, different concentrations (5, 10, and 20 mg ml−1) of GT AgNPs were tested against Staphylococcus aureus and Klebsiella sp. As shown in Fig. 5 and 6 GT AgNPs possessed moderate activity against the tested bacteria with inhibition zone ranging between 8–11 mm. A number of theories for antimicrobial actions of silver nanoparticles solution have been proposed. Some of the proposed mechanisms include changing the permeability of the cell membrane, which enables the interaction with DNA, proteins, and other phosphorus- and sulfur-containing cell constituents that are harmful to bacteria. Another mechanism is generating free radicals responsible for the damage of membrane and dissipation of the proton motive force, resulting in the disruption of membrane potential;39 however, the exact mechanism has not been fully understood. The three concentrations showed similar effectiveness in combating the tested species with the highest concentration having the highest activity. The inhibition zones obtained by GT AgNPs were insignificant when compared to standard drug. Results shown in Table 1.| Zone of Inhibition in mm | ||
|---|---|---|
| Klebsiella spp. | S. aureus | |
| GT AgNPs concentration (mg ml −1 ) | ||
| 20 | 10 | 11 |
| 10 | 8 | 8 |
| 5 | 9 | 10 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Antimicrobial agent (STD) | ||
| Ciprofloxacin (5 mcg) | 26 | 32 |
Conclusion
The synthesized silver nanoparticles were characterized using UV, FTIR and SEM. The morphology of GT AgNPs appear to be irregular and in the form of aggregates. GT AgNPs revealed antimicrobial activity against selected bacterial species. The difference in the FTIR spectra between the GT extract and GT AgNPs was used to indicate the functional groups involved in the reduction of Ag+ ions into nanoparticles.Author contributions
Hiba Abbas Widatallah was responsible for conceptualization, investigation, methodology, project administration and writing the original draft.Layla Fathi Yassin was responsible for conceptualization, investigation, methodology, project administration and writing the original draft.
Ayat Ahmed Alrasheid was responsible for conceptualization, investigation and supervision.
Shimaa Abdel Rahman Ahmed was responsible for conceptualization and investigation.
Marvit Osman Widdatallah was responsible for conceptualization and investigation.
Sahar Hussein Eltilib was responsible for conceptualization and investigation.
Alaa Abdulmoneim Mohamed was responsible for conceptualization and investigation.
Conflicts of interest
There are no conflicts to declare.References
- S. Fakhari, M. Jamzad and H. Kabiri Fard, Green Chem. Lett. Rev., 2019, 12(1), 19–24 CrossRef CAS.
- S. Ahmed, M. Ahmad, B. Swami and S. Ikram, J. Adv. Res., 2016, 7(1), 17–28 CrossRef CAS PubMed.
- G. Chen, I. Roy, C. Yang and P. Prasad, Chem. Rev., 2016, 116(5), 2826–2885 CrossRef CAS PubMed.
- B. Pelaz, C. Alexiou, R. Alvarez-Puebla, F. Alves, A. Andrews and S. Ashraf, et al. , ACS Nano, 2017, 11(3), 2313–2381 CrossRef CAS PubMed.
- V. Kumar and S. Anthony, Surf. Chem. Nanobiomater., 2016, 265–300 CAS.
- M. Rai, A. Ingle, S. Birla, A. Yadav and C. Santos, Crit. Rev. Microbiol., 2015, 42, 1–24 CrossRef PubMed.
- X. Zhang, F. Huang, G. Zhang, D. Bai, D. Massimo and Y. Huang, et al. , Int. J. Nanomed., 2017, 12, 7551–7575 CrossRef CAS PubMed.
- X. Zhang, Z. Liu, W. Shen and S. Gurunathan, Int. J. Mol. Sci., 2016, 17(9), 1534 CrossRef PubMed.
- S. Scandorieiro, L. de Camargo, C. Lancheros, S. Yamada-Ogatta, C. Nakamura and A. de Oliveira, et al. , Front. Microbiol., 2016, 7, 760 Search PubMed.
- J. Firdhouse and P. Lalitha, Prog. Biomater., 2015, 4(2–4), 113–121 Search PubMed.
- B. Ramalingam, T. Parandhaman and S. K. Das, ACS Appl. Mater. Interfaces, 2016, 8(7), 4963–4976 CrossRef CAS PubMed.
- D. M. Rudakiya and K. Pawar, 3 Biotech, 2017, 7(2), 1–2 CrossRef PubMed.
- M. Kim, S. Osone, T. Kim, H. Higashi and T. Seto, KONA Powder Part. J., 2017, 34, 80–90 CrossRef CAS.
- M. Govarthanan, M. Cho, J. H. Park, J. S. Jang, Y. J. Yi, S. Kamala-Kannan and B. T. Oh, J. Nanomater., 2016, 2016, 1–6 CrossRef.
- M. Govarthanan, Y. S. Seo, K. J. Lee, I. B. Jung, H. J. Ju, J. S. Kim, M. Cho, S. Kamala-Kannan and B. T. Oh, Artif. Cells, Nanomed., Biotechnol., 2016, 44(8), 1878–1882 CrossRef CAS PubMed.
- J. M. Jacob, R. Ravindran, M. Narayanan, S. M. Samuel, A. Pugazhendhi and G. Kumar, Curr. Opin. Environ. Sci., 2021, 20, 100163 Search PubMed.
- K. J. Lee, S. H. Park, M. Govarthanan, P. H. Hwang, Y. S. Seo, M. Cho, W. H. Lee, J. Y. Lee, S. Kamala-Kannan and B. T. Oh, Mater. Lett., 2013, 105, 128–131 CrossRef CAS.
- A. Sengottaiyan, R. Mythili, T. Selvankumar, A. Aravinthan, S. Kamala-Kannan, K. Manoharan, P. Thiyagarajan, M. Govarthanan and J. H. Kim, Res. Chem. Intermed., 2016, 42(4), 3095–3103 CrossRef CAS.
- V. V. Makarov, A. J. Love, O. V. Sinitsyna, S. S. Makarova, I. V. Yaminsky, M. E. Taliansky and N. O. Kalinina, “Green” nanotechnologies: synthesis of metal nanoparticles using plants, Acta Naturae, 2014, 6(1), 35–44 CrossRef CAS PubMed.
- D. A. Kumar, V. Palanichamy and S. M. Roopan, Spectrochim. Acta, Part A, 2014, 127, 168–171 CrossRef CAS PubMed.
- R. Sankar, P. Manikandan, V. Malarvizhi, T. Fathima, K. S. Shivashangari and V. Ravikumar, Spectrochim. Acta, Part A, 2014, 121, 746–750 CrossRef CAS PubMed.
- V. Dhand, L. Soumya, S. Bharadwaj, S. Chakra, D. Bhatt and B. Sreedhar, Mater. Sci. Eng., C, 2016, 58, 36–43 CrossRef CAS PubMed.
- A. S. Saifullah, M. Ahmad, B. L. Swami and S. Ikram, J. Radiat. Res. Appl. Sci., 2016, 9(1), 1–7 CrossRef.
- M. M. Khalil, E. H. Ismail, K. Z. El-Baghdady and D. Mohamed, Arabian J. Chem., 2014, 7(6), 1131–1139 CrossRef CAS.
- F. Ameen, S. AlYahya, M. Govarthanan, N. ALjahdali, N. Al-Enazi, K. Alsamhary, W. A. Alshehri, S. S. Alwakeel and S. A. Alharbi, J. Mol. Struct., 2020, 1202, 127233 CrossRef CAS.
- K. Parveen, V. Banse and L. Ledwani, AIP Conf. Proc., 2016, 1724, 020048 CrossRef.
- S. K. Nune, N. Chanda, R. Shukla, K. Katti, R. R. Kulkarni, S. Thilakavathy and K. V. Katti, J. Mater. Chem., 2009, 19(19), 2912–2920 RSC.
- V. Sharma, C. Chotia, V. Ganesan and G. S. Okram, Phys. Chem. Chem. Phys., 2017, 19, 14096–14106 RSC.
- E. Petryayeva and U. J. Krull, Anal. Chim. Acta, 2011, 706, 8–24 CrossRef CAS PubMed.
- J. Grand, B. Auguié and E. C. Le Ru, Anal. Chem., 2019, 91(22), 14639–14648 CrossRef CAS PubMed.
- D. A. Selvan, D. Mahendiran, R. S. Kumar and A. K. Rahiman, J. Photochem. Photobiol., B, 2018, 180, 243–252 CrossRef PubMed.
- L. Xing, H. Zhang, R. Qi, R. Tsao and Y. Mine, J. Agric. Food Chem., 2020, 67(4), 1029–1043 CrossRef PubMed.
- H. Al Rashid, A. Kundu, V. Mandal, P. Wangchuk, and S. C. Mandal, Herbal Medicine in India, 2020, DOI:10.1007/978-981-13-7248-3_39.
- P. Singh, S. Pandit, J. Garnæs, S. Tunjic, V. R. Mokkapati, A. Sultan, A. Thygesen, A. Mackevica, R. V. Mateiu, A. E. Daugaard and A. Baun, Int. J. Nanomed., 2018, 13, 3571 CrossRef CAS PubMed.
- S. Tang and J. Zheng, Adv. Healthcare Mater., 2018, 7(13), 1701503 CrossRef PubMed.
- B. Le Ouay and F. Stellacci, Nano Today, 2015, 10(3), 339–354 CrossRef CAS.
- A. K. Keshari, R. Srivastava, P. Singh, V. B. Yadav and G. Nath, J. Ayurveda Integr. Med., 2020, 11(1), 37–44 CrossRef PubMed.
- H. Gandhi and S. Khan, J. Nanomed. Nanotechnol., 2016, 7(2), 1000366 Search PubMed.
- H. M. Ibrahim, J. Radiat. Res. Appl. Sci., 2015, 8(3), 265–275 CrossRef.
| This journal is © The Royal Society of Chemistry 2022 |