Design and synthesis of chromophores with enhanced electro-optic activities in both bulk and plasmonic–organic hybrid devices†
Huajun
Xu
 a,
Delwin L.
Elder
a,
Delwin L.
Elder
 *ab,
Lewis E.
Johnson
*ab,
Lewis E.
Johnson
 *ab,
Wolfgang
Heni
*ab,
Wolfgang
Heni
 cd,
Yovan
de Coene
cd,
Yovan
de Coene
 e,
Eva
De Leo
e,
Eva
De Leo
 cd,
Marcel
Destraz
cd,
Norbert
Meier
c,
Wouter
Vander Ghinst
cd,
Marcel
Destraz
cd,
Norbert
Meier
c,
Wouter
Vander Ghinst
 e,
Scott R.
Hammond
e,
Scott R.
Hammond
 ab,
Koen
Clays
ab,
Koen
Clays
 e,
Juerg
Leuthold
e,
Juerg
Leuthold
 cd,
Larry R.
Dalton
cd,
Larry R.
Dalton
 a and
Bruce H.
Robinson
a and
Bruce H.
Robinson
 a
a
aDepartment of Chemistry, University of Washington, Seattle, WA 98195, USA. E-mail: lewisj@uw.edu; elderdl@uw.edu
bNonlinear Materials Corporation, Seattle, WA 98109, USA
cPolariton Technologies AG, 8803 Rüschlikon, Switzerland
dInstitute of Electromagnetic Fields, ETH Zurich, Gloriastrasse 35, Zurich 8092, Switzerland
eDepartment of Chemistry, University of Leuven, Celestijnenlaan 200D, 3001 Leuven, Belgium
First published on 23rd September 2021
Abstract
This study demonstrates enhancement of in-device electro-optic activity via a series of theory-inspired organic electro-optic (OEO) chromophores based on strong (diarylamino)phenyl electron donating moieties. These chromophores are tuned to minimize trade-offs between molecular hyperpolarizability and optical loss. Hyper-Rayleigh scattering (HRS) measurements demonstrate that these chromophores, herein described as BAH, show >2-fold improvement in β versus standard chromophores such as JRD1, and approach that of the recent BTP and BAY chromophore families. Electric field poled bulk devices of neat and binary BAH chromophores exhibited significantly enhanced EO coefficients (r33) and poling efficiencies (r33/Ep) compared with state-of-the-art chromophores such as JRD1. The neat BAH13 devices with charge blocking layers produced very large poling efficiencies of 11.6 ± 0.7 nm2 V−2 and maximum r33 value of 1100 ± 100 pm V−1 at 1310 nm on hafnium dioxide (HfO2). These results were comparable to that of our recently reported BAY1 but with much lower loss (extinction coefficient, k), and greatly exceeding that of other previously reported OEO compounds. 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BAH-FD
1 BAH-FD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BAH13 blends showed a poling efficiency of 6.7 ± 0.3 nm2 V−2 and an even greater reduction in k. 1
BAH13 blends showed a poling efficiency of 6.7 ± 0.3 nm2 V−2 and an even greater reduction in k. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BAH-BB
1 BAH-BB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BAH13 showed a higher poling efficiency of 8.4 ± 0.3 nm2 V−2, which is approximately a 2.5-fold enhancement in poling efficiency vs. JRD1. Neat BAH13 was evaluated in plasmonic–organic hybrid (POH) Mach–Zehnder modulators with a phase shifter length of 10 μm and slot widths of 80 and 105 nm. In-device BAH13 achieved a maximum r33 of 208 pm V−1 at 1550 nm, which is ∼1.7 times higher than JRD1 under equivalent conditions.
BAH13 showed a higher poling efficiency of 8.4 ± 0.3 nm2 V−2, which is approximately a 2.5-fold enhancement in poling efficiency vs. JRD1. Neat BAH13 was evaluated in plasmonic–organic hybrid (POH) Mach–Zehnder modulators with a phase shifter length of 10 μm and slot widths of 80 and 105 nm. In-device BAH13 achieved a maximum r33 of 208 pm V−1 at 1550 nm, which is ∼1.7 times higher than JRD1 under equivalent conditions.
New conceptsOver the past decade, trade-offs among molecular hyperpolarizability (β), optical loss, number density (ρN), and electric field poling-induced acentric order (〈cos3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ〉) of organic electro-optic (OEO) chromophores have frequently stymied improvement of in-device EO activity. New chromophores that are designed for an improved molecular hyperpolarizabity would suffer unacceptable optical loss or severe adverse dipolar aggregations. Most of the previous work of organic EO chromophore design is mainly focused on improving ordering by means of side-chain engineering or chromophore blending, as well as increasing chromophore number density. Here we adopt a theory-guided design process including quantum mechanical and statistical mechanical techniques to design a new generation of organic EO materials relevant to silicon–organic hybrid (SOH) and plasmonic–organic hybrid (POH) devices. Theory-guided design allows for screening of many structures by evaluating their calculated hyperpolarizabilities, band gaps, and dipole moments, and optimizing targets for multiple design criteria. As a result, the newly designed chromophores in this work present enhanced hyperpolarizabilities and EO activities in both bulk devices and POH modulators without unacceptable impacts on processibility or loss. These results demonstrate that the hyperpolarizability-loss-processibility trade-off is not insurmountable and can be effectively addressed by theory-guided design, which could lead to even higher performance OEO materials for applications in nanophotonic devices. θ〉) of organic electro-optic (OEO) chromophores have frequently stymied improvement of in-device EO activity. New chromophores that are designed for an improved molecular hyperpolarizabity would suffer unacceptable optical loss or severe adverse dipolar aggregations. Most of the previous work of organic EO chromophore design is mainly focused on improving ordering by means of side-chain engineering or chromophore blending, as well as increasing chromophore number density. Here we adopt a theory-guided design process including quantum mechanical and statistical mechanical techniques to design a new generation of organic EO materials relevant to silicon–organic hybrid (SOH) and plasmonic–organic hybrid (POH) devices. Theory-guided design allows for screening of many structures by evaluating their calculated hyperpolarizabilities, band gaps, and dipole moments, and optimizing targets for multiple design criteria. As a result, the newly designed chromophores in this work present enhanced hyperpolarizabilities and EO activities in both bulk devices and POH modulators without unacceptable impacts on processibility or loss. These results demonstrate that the hyperpolarizability-loss-processibility trade-off is not insurmountable and can be effectively addressed by theory-guided design, which could lead to even higher performance OEO materials for applications in nanophotonic devices.
|
1. Introduction
High-performance electro-optic (EO) modulators are key components for pushing the boundaries of optical communication, computing, sensor technology, and ultra-broadband signal processing at GHz–THz bandwidths.1–6 Over the past decade, considerable progress has been made with respect to plasmonic-organic hybrid (POH)2,3,5,7–9 and silicon-organic hybrid (SOH)10–19 technologies affording higher bandwidth (>500 GHz),20 smaller footprints (<20 μm2), better energy efficiency (<100 attojoule/bit),10,21 as well as dramatically lower VπL (the π-voltage–length product).5,22 State-of-the-art POH and SOH platforms combine the high intrinsic EO activity of organic chromophores with the tight confinement and improved overlap of electrical and optical fields achievable in nanophotonic devices, enabling ultra-compact, low-voltage devices permitting chip-scale integration with CMOS electronics.9,23–25 Achieving groundbreaking performance requires synergistic innovation from rational design of organic electro-optic (OEO) materials to device engineering and advancements in communication systems. As the active component for the Pockels effect, OEO materials offer coefficients up to 1000 pm V−1, with commercial materials producing over 300 pm V−1 (>10× lithium niobate), femtosecond (<30 fs) response times, and wide compatibilities to a variety of integration platforms and device structures.26–34 OEO materials can also be easily integrated into devices by low-cost, high-throughput methods such as spin-coating, micro-dispensing, or ink-jet printing. The EO activity of organic materials is derived from second-order nonlinear optical (NLO) properties of the chromophores and is proportional to the product of chromophore hyperpolarizability (β), electric field poling-induced acentric order of the chromophores (〈cos3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ〉), and chromophore number density (ρN).34–36 In past a decade, EO performance has been improved via strategies such as site-isolation, self-assembly, chromophore blending, and charge blocking layers (CBLs) to enhance the ρN〈cos3
θ〉), and chromophore number density (ρN).34–36 In past a decade, EO performance has been improved via strategies such as site-isolation, self-assembly, chromophore blending, and charge blocking layers (CBLs) to enhance the ρN〈cos3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ〉 value of bulk materials.26,28,31,33,37–49 Recent work50,51 has also shown substantial increases in hyperpolarizability due to theory-guided design, after years of little increase in β of reported chromophores.
θ〉 value of bulk materials.26,28,31,33,37–49 Recent work50,51 has also shown substantial increases in hyperpolarizability due to theory-guided design, after years of little increase in β of reported chromophores.
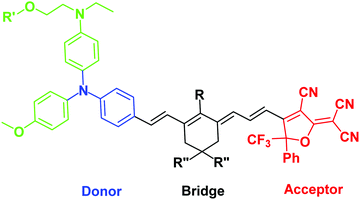 | ||
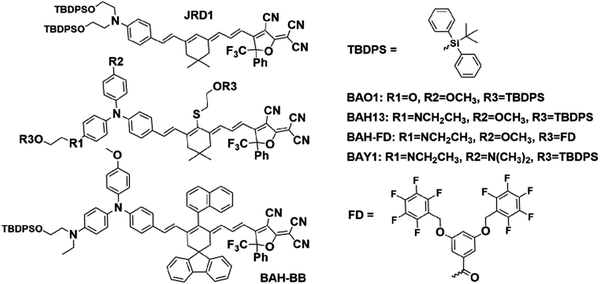
| Fig. 1 Generic structure of a “BAH” chromophore, showing the donor (primary donor in blue, secondary donors in green), bridge, and acceptor. | ||
Modern density functional theory (DFT) methods provide excellent prediction for linear/nonlinear optical properties of NLO chromophores and better understanding of structure–property relationships.25,52–54 Combining large-scale computation and theory-driven search, novel chromophore structures can be presented with enhanced hyperpolarizability while keeping dipole moment and band gap within acceptable constraints.53,55 In this work, a new series of NLO chromophores were demonstrated by using a bis(arylamino) hybrid (BAH) electron donor (Fig. 1). Hyperpolarizabilities were predicted via DFT calculations25,54,56 in chloroform with implicit solvent environments, and measured via hyper-Rayleigh scattering (HRS)57–59 measurements in chloroform. These experiments demonstrate large improvements in β and slight redshifts in their main charge-transfer (CT) band (29 nm to 45 nm) compared to the widely used JRD1.28,51 The chromophores have been engineered with bulky groups to prevent aggregation due to dipolar interactions. The presence of side-chain bulky groups was also helpful to improve chromophore solubility and film-forming property as well as reduce absorption.28,39,60 To realize a highly acentric alignment (〈cos3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ〉), strong electric fields were applied to the OEO thin films. To suppress the leakage current and get a more uniform electric field, titanium dioxide (TiO2) and hafnium dioxide (HfO2) thin films have been utilized as CBLs.28,61–63 The high dielectric constants of TiO2 and HfO2 allow the absolute majority of the poling and AC modulation field to be applied effectively across the micron-thick OEO layer. For electric field poling, the poled BAH13 films showed a significantly enhanced r33 value of 650 ± 110 pm V−1 on TiO2, higher than neat JRD1,28 which has a large r33 value (560 ± 90 pm V−1).28 The best r33 value (1100 ± 100 pm V−1) of BAH13 was achieved by using HfO2 as CBL, which is comparable to the performance of BAY1 but with greatly decreased absorption at communication wavelengths.50
θ〉), strong electric fields were applied to the OEO thin films. To suppress the leakage current and get a more uniform electric field, titanium dioxide (TiO2) and hafnium dioxide (HfO2) thin films have been utilized as CBLs.28,61–63 The high dielectric constants of TiO2 and HfO2 allow the absolute majority of the poling and AC modulation field to be applied effectively across the micron-thick OEO layer. For electric field poling, the poled BAH13 films showed a significantly enhanced r33 value of 650 ± 110 pm V−1 on TiO2, higher than neat JRD1,28 which has a large r33 value (560 ± 90 pm V−1).28 The best r33 value (1100 ± 100 pm V−1) of BAH13 was achieved by using HfO2 as CBL, which is comparable to the performance of BAY1 but with greatly decreased absorption at communication wavelengths.50
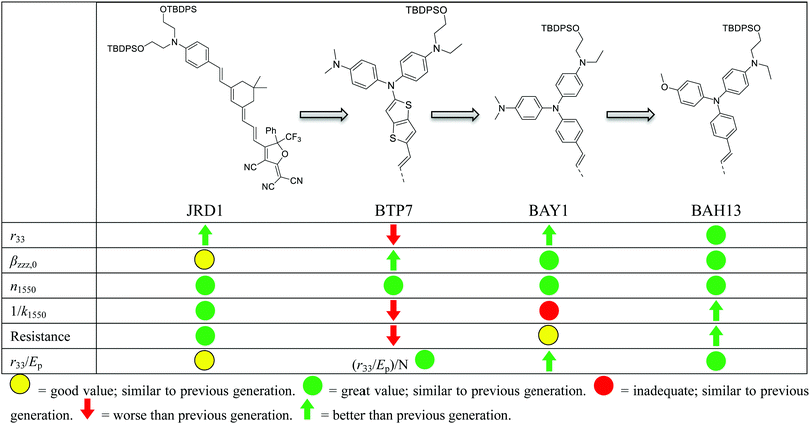
2. Results and discussion
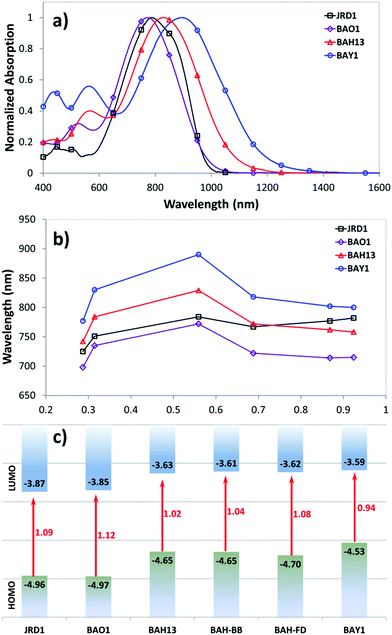
A key benefit of OEO materials versus most other NLO materials is that their structures have a broad parameter space for optimization of their optical and electronic properties. This has led to an evolution of structural design—guided by theory and computational modeling—to optimize EO performance. This evolution is illustrated in Table 1 with chromophore JRD1 as a starting point. JRD1 is based on the popular CLD-type chromophore structure using a dialkylamine donor, and ring-locked tetraene π-bridge, and a CF3-Ph-tricyanofuran acceptor. Virtually all the best OEO device results have used CLD-type chromophores. The key modification introduced with JRD1 was two sterically bulky tert-butyldiphenylsilyl (TBDPS) groups, which permitted the chromophore to be processed neat (without polymer host) and still pole efficiently, reaching record high r33 in bulk and in-device. The performance of JRD1 was later translated to a crosslinkable material, HLD, which could provide similar performance with long-term thermal stability.32 Despite the success of optimizing CLD-type chromophores, EO performance reached a limit that could only be improved by increasing β or through radically different design approaches such as sequential synthesis. After screening numerous design modifications, we discovered a class of molecules highlighted by BTP7 that had a powerful electron donor and huge enhancement in βzzz,0 (β tensor component parallel to the dipole moment of the chromophore).51 While this chromophore displayed excellent poling efficiency at low concentration, consistent with expectations for a higher βzzz,0 material, the transmittance (1/k) in the near IR was too low and the resistance was too low to permit poling as a neat material, and therefore r33 was not ultimately improved. The next modification kept the strong donor but increased aromatic character in the donor-bridge transition to better balance the donor. The result was chromophore BAY1, which exhibited improved resistance that permitted poling as a neat material and record high r33 > 1000 pm V−1, which is twice the EO coefficient of JRD1 and even higher than BaTiO3.64 However, the transmittance in the near IR was still lower than desired so another modification was needed. This paper introduces a new modification intended to increase the transmittance to an acceptable level for hybrid devices while maintaining ultra-high r33, high βzzz,0, high n, high resistance, and the good physical properties of the previous generation of chromophores. We synthesized three variants of the BAH structure shown in Fig. 2 that had different sterically bulky side-chain or bridge units. BAH13 is substituted with two pendant TBDPS groups, one on the donor end and one on the bridge. BAH-BB shares the same donor structures with BAH13 and includes rigid naphthyl and fluorenyl units on either side of the bridge designed to provide site isolation (reduce aggregation and anti-parallel dipolar coupling).30 BAH-FD was functionalized with fluoroaromatic dendrons (FD), which have been shown to blueshift the absorbance band and thereby reduce the absorption coefficient in the near IR. FD units are also known to promote favorable, cooperative intermolecular interactions in OEO materials resulting in increased poling performance.26,33,42,65,66 We also synthesized a BAH variant for exploring relative substitution effects on the secondary electron-donating groups (BAO1). Full synthetic details, characterization and official IUPAC names67 can be found in the ESI.†The UV-vis-NIR spectra of chromophores were measured in six solvents with varying dielectric constants and as thin films (Table S1 and Fig. S11, ESI†). Three BAH chromophores share the same donor–π bridge–acceptor (D–π–A) structure, which results in the similar absorption maxima (λmax) data and solvatochromic behavior. In chloroform, the (λmax) of BAH chromophores showed large redshifts (by 41–57 nm) relative to JRD1 in the primary charge transfer band. λmax is a good proxy for electron donor strength, and the redshift is evidence of the stronger electron-donating strength of (4-dialkylaminophenyl)(4-alkyloxyphenyl)phenyl-amino group (Table 3 and Fig. 3a). BAH chromophore absorbance in chloroform is blue shifted (by 61–77 nm) relative to BAY1 indicating that the BAH donor is intermediate between JRD1 and BAY1. BAO1 has the most blue shifted λmax (772 nm) in chloroform in the set, even more so than JRD1, confirming that the weaker donating ability of 4-alkoxyphenyl. The trends in donor strength are confirmed by highest-occupied molecular orbital (HOMO) estimations from cyclic voltammetry (Fig. 3c and Fig. S18 and Table S3, ESI†). The side chains connected to the conjugated bridge of BAH-FD and BAH-BB have a slight effect on the π-system and therefore allow us to fine tune the linear- and nonlinear optical properties. BAH-BB has a slight blueshift (8 nm) in chloroform relative to BAH13. BAH-FD has a 16 nm blueshift in chloroform relative to BAH13, which is partially ascribed to the quadrupolar interactions between perfluorinated aromatic rings and electron-rich segments of the chromophore π-system. Neat thin films had the same trends in λmax as in chloroform solution. These data serve as a guide for how to reduce optical loss in devices relative to BAY1.
The refractive index (n) and extinction coefficients (k) of unpoled films of neat chromophores and binary chromophore blends were measured using variable angle spectroscopic ellipsometry (VASE) (Table 2 and Fig. S12, ESI†). n1310 ranges from 1.96–1.81, and n1550 ranges from 1.87–1.75 for the BAH chromophores, BAO1, and their blends. BAH13 and BAH-BB have n values (n1310 = 1.95, 1.96, respectively) at the high end of the range. This observation is consistent with the general trend of refractive index increasing with number density and resonance effects. n values for BAH13 are very comparable to JRD1 and just a few percent lower than BAY1. The higher index of neat chromophore is significant in that the Pockels effect figure-of-merit (FOM) n3r33 has a cubic dependence on n, such that small index increases can lead to significant increases in performance. The measured refractive index values were used to calculate r33 in EO poling tests. The BAH chromophores exhibited low k values that were ∼10 to 50 times lower than BAY1, approximately equal to that of DLD164, the prior highest-performing chromophore in POH devices,55 and within the loss threshold for POH devices (k1550 ∼ 0.0055 = 0.19 dB μm−1).55 With the presence of FD groups, BAH-FD has greatly reduced k values at telecommunication wavelengths compared with the other BAH chromophores.
| Sample | n 1310 | n 1550 | k 1310 | k 1550 |
|---|---|---|---|---|
| 100 wt% JRD1 | 1.91 | 1.84 | 0.00010 | 0.000018 |
| 100% BAY1 | 2.02 | 1.90 | 0.133 | 0.0255 |
| 100 wt% BAO1 | 1.84 | 1.79 | 0.00099 | 0.000042 |
| 100 wt% BAH13 | 1.94 | 1.85 | 0.02126 | 0.001 |
| 100 wt% BAH-BB | 1.96 | 1.87 | 0.02906 | 0.00271 |
| 100 wt% BAH-FD | 1.81 | 1.75 | 0.00695 | 0.00041 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BAH-BB 1 BAH-BB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BAH13 BAH13 |
1.95 | 1.86 | 0.03395 | 0.00381 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BAH-FD 1 BAH-FD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BAH13 BAH13 |
1.84 | 1.78 | 0.00891 | 0.000504 |
Femtosecond HRS59 measurements were performed in chloroform solution using a custom-built setup at KU Leuven. Measurements were performed using a fundamental wavelength of 1300 nm and a repetition rate of 80 MHz. HRS intensity was corrected for absorption using a Beer–Lambert correction at the second harmonic wavelength. Resonance effects were approximated using the damped two-level model (TLM) and a linewidth (γ) of 0.1 eV (for details see ESI†). HRS results of BAH13, BAO1, and previously published comparison molecules are summarized in Table 3 (BAH-BB and BAH-FD were excluded since these two chromophores have the same D–π–A system and substitution points as BAH13 and likely have similar hyperpolarizabilities). DFT calculations were also employed to evaluate the theoretical values and compare with experiment. The hyperpolarizabilities of JRD1, BAY1, and BTP7 have been measured with the same methods and reported in our previous work.50,51 BAH13 exhibited a very large βzzz,0 that was a more than 2.5-fold improvement over JRD1 and only ∼10% less than BAY1. This BAH13 β is consistent with observations of λmax and the HOMO levels being intermediate between JRD1 and BAY1, but more similar to BAY1. BAO1 has an enhanced βzzz,0, 1.7 times that of JRD1, which is larger than expectations based on the computed βzzz,0, and the experimental λmax and HOMO level.
| Sample | λ max (nm) | TLMa factor | μ (D) | β zzz,1300 | β zzz,0 | β zzz,0/βJRD1 | Comp.dβ0/β0,JRD1 |
|---|---|---|---|---|---|---|---|
| a The damped two-level model factor used to correct for resonance effects on β (for details, see ESI). b Dipole moment, M062X/6-31+G(d) calculation in polarizable continuum model (PCM) implicit solvent. c HRS – femtosecond HRS at 1300 nm (βzzz,1300) in CHCl3, extrapolation to zero frequency (βzzz,0) using damped TLM (0.1 eV linewidth). Hyperpolarizabilities in 10−30 esu. Reference against solvent (CHCl3, using Campo et al. (2009) value of βzzz,0 ∼ 0.44 × 10−30 esu).59 d Computational predictions – M062X/6-31+G(d) in PCM CHCl3 at zero frequency, analytic differentiation, 2-carbon truncation on inactive side chains. | |||||||
| JRD1 | 784 | 3.13 | 31 | 3330 ± 50 | 1060 ± 20 | 1 ± 0.02 | 1 |
| BAY1 | 890 | 2.11 | 30.2 | 6420 ± 90 | 3040 ± 40 | 2.87 ± 0.04 | 1.47 |
| BAO1 | 772 | 3.76 | 26 | 6769 ± 630 | 1800 ± 170 | 1.70 ± 0.16 | 0.95 |
| BAH13 | 829 | 2.69 | 28.6 | 7207 ± 298 | 2680 ± 110 | 2.53 ± 0.10 | 1.16 |
| BAH-BB | 821 | 2.66 | 26.2 | — | — | — | 1.17 |
| BAH-FD | 813 | 2.77 | — | — | — | — | — |
Thin film devices of neat and binary chromophores sandwiched between indium tin oxide (ITO) and glass were prepared to evaluate their bulk EO activity. OEO films were spin cast from 8–10 wt% chromophores into 1,1,2-trichloroethane (TCE). To achieve higher and more uniform electric fields, thin films of TiO2 or HfO2 were used as CBLs between ITO and the OEO material. CBL layers were deposited by either sol–gel,61,62,68,69 electron beam physical vapor deposition (PVD), or atomic layer deposition (ALD) methods. Thin film devices were poled under DC electric fields (Ep) ranging from 20 to 90 V μm−1 with a heating rate of 10 °C per minute under nitrogen. During poling, the current and voltage across the device were collected along with an in situ r33 analysis at 1310 nm by the Teng–Man ellipsometric method.70,71 After cooling down below 35 °C, the poling field was removed, then r33 values were measured. The average poling efficiencies (r33/Ep) were determined by linear fitting of the electro-optic activity (in pm V−1) versus Ep. Higher poling efficiency represents better performance of translating microscopic hyperpolarizability into macroscopic EO response. The poling results are shown in Fig. 4, Fig. S10 (ESI†) and summarized in Table 4.
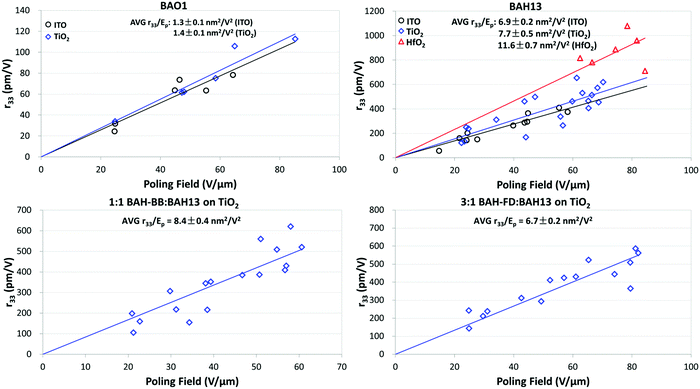 | ||
| Fig. 4 Poling curves for BAH chromophores (plots of r33vs. poling field). Ep is the average of poling fields at poling temperature and cooling down to 35 °C. | ||
| Sample | ρ N | CBL | Poling temp. (°C) | r 33/Epb (nm2 V−2) | Max. r33 (pm V−1) | Max. n3r33 (pm V−1) |
|---|---|---|---|---|---|---|
| a Number density (×1020 molecules per cm3, assumes mass density of 1 g cm−3). b Average from multiple poling experiments (Fig. 4 and Fig. S10, ESI). | ||||||
| 100% BAO1 | 4.43 | None | 82–85 | 1.3 ± 0.1 | 78 ± 8 | 490 ± 50 |
| TiO2 | 82–85 | 1.4 ± 0.1 | 113 ± 8 | 700 ± 50 | ||
| 100% BAH13 | 4.35 | None | 81–85 | 6.9 ± 0.2 | 410 ± 30 | 3000 ± 200 |
| TiO2 | 81–85 | 7.7 ± 0.5 | 650 ± 100 | 4700 ± 800 | ||
| HfO2 | 75–79 | 11.6 ± 0.7 | 1100 ± 100 | 7800 ± 1000 | ||
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BAH-FD 1 BAH-FD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BAH13 BAH13 |
3.46 | TiO2 | 65–68 | 6.7 ± 0.3 | 590 ± 50 | 3700 ± 300 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BAH-BB 1 BAH-BB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BAH13 BAH13 |
4.46 | TiO2 | 100–103 | 8.4 ± 0.3 | 620 ± 60 | 4600 ± 400 |
| 100% JRD1 | 5.33 | BCB | ∼93 | 3.4 ± 0.2 | 560 ± 70 | 3900 ± 500 |
| 100% BAY1 | 4.30 | TiO2 | 86–91 | 16.6 ± 0.5 | 1100 ± 100 | 9000 ± 1100 |
BAO1 and BAH13 displayed excellent film-forming properties on ITO and on all CBLs. A remarkable poling efficiency (6.9 ± 0.3 nm2 V−2) was achieved in the neat BAH13 devices, which is an approximately 2-fold improvement over neat JRD1 performance. The best performing neat BAH13 device without a CBL also afforded a very large r33 value of 410 ± 30 pm V−1, which is higher than some of the best OEO materials in the literatures. However, due to large leakage currents at high poling fields, the average poling fields on neat BAH13 devices with no barrier layer were limited to <60 V μm−1. CBLs were used to minimize leakage current and maximize achievable poling fields. TiO2 has been demonstrated as an effective CBL due to its large band gap, large dielectric constant, and excellent thin film processibility; we used layers of 20 nm (PVD or ALD) or 100 nm (sol–gel) in our devices. The neat BAO1 and BAH13 devices on TiO2 exhibited slight increases in poling efficiency relative to the devices without CBL. It is notable that the neat BAH13 on TiO2 achieved a poling efficiency increase of 0.8 nm2 V−2 and a very large r33 value of 650 ± 110 pm V−1, which is higher than the state-of-art JRD1 performance (560 ± 90 pm V−1). While the increase in slope was of marginal statistical significance (p = 0.116 for a two-tailed T-test, 0.058 for one-tailed), the TiO2 CBL enabled us of higher poling voltages and higher maximum EO activity. HfO2 has also been adopted as a CBL due to its large dielectric constant, larger band gap than TiO2, and high resistivity. Greatly reduced leakage currents were observed for the poling of neat BAH13 on HfO2, improving the actual poling fields to higher than 80 V μm−1. The neat BAH13 on HfO2 showed the highest performance, with poling efficiencies of 11.6 ± 0.7 nm2 V−2 and a record-high r33 value of 1100 ± 100 pm V−1, which is comparable with the best value of neat BAY1 and approximate to 2-fold enhancement compared to the previous highest reported OEO material JRD1.
BAH-BB and BAH-FD exhibited poorer film-forming characteristics as neat materials, with cracking upon drying leading to device failure from short or open circuit conditions during poling. Film forming could be greatly improved by blending with BAH13; 25 wt% and 50 wt% BAH13 were blended with BAH-FD and BAH-BB, respectively. Due to the impressive poling results of neat BAH13 on TiO2, the following evaluations of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BAH-BB
1 BAH-BB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BAH13 and 3
BAH13 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BAH-FD
1 BAH-FD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BAH13 blends were performed on TiO2, as well. The poling efficiency of 3
BAH13 blends were performed on TiO2, as well. The poling efficiency of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BAH-FD
1 BAH-FD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BAH13 was 6.7 ± 0.3 nm2 V−2, nearly a 2-fold improvement over neat JRD1 performance. This relatively lower poling efficiency is attributed to the low chromophore number density (3.46 × 1020 molecules per cm3) of 3
BAH13 was 6.7 ± 0.3 nm2 V−2, nearly a 2-fold improvement over neat JRD1 performance. This relatively lower poling efficiency is attributed to the low chromophore number density (3.46 × 1020 molecules per cm3) of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BAH-FD
1 BAH-FD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BAH13 compared with other BAH materials (4.35–4.46 × 1020 molecules per cm3). Also due to the lower chromophore number density, 3
BAH13 compared with other BAH materials (4.35–4.46 × 1020 molecules per cm3). Also due to the lower chromophore number density, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BAH-FD
1 BAH-FD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BAH13 device achieved higher maximum poling field (∼80 V μm−1) leading to a large r33 value of 590 ± 50 pm V−1, and a low absorbance at telecommunication wavelengths. Due to the very high Tg (127 °C) of BAH-BB, 1
BAH13 device achieved higher maximum poling field (∼80 V μm−1) leading to a large r33 value of 590 ± 50 pm V−1, and a low absorbance at telecommunication wavelengths. Due to the very high Tg (127 °C) of BAH-BB, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BAH-BB
1 BAH-BB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BAH13 was poled at 100–103 °C, and showed a higher poling efficiency of 8.4 ± 0.3 nm2 V−2 and yielded a maximum r33 of 620 ± 60 pm V−1.
BAH13 was poled at 100–103 °C, and showed a higher poling efficiency of 8.4 ± 0.3 nm2 V−2 and yielded a maximum r33 of 620 ± 60 pm V−1.
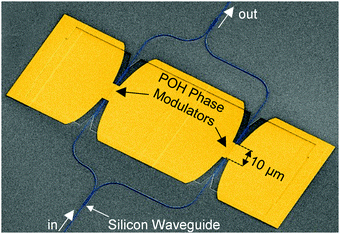
BAH13 was also evaluated in-device on a POH platform. POH devices feature large bandwidths, small footprint, low power consumption, and can be fabricated on top of CMOS electronics as a post-process.9 In POH phase modulators, light is coupled from a silicon strip waveguide into a metal–insulator–metal slot waveguide and converted to a surface plasmon polariton (SPP) mode at the gold surface. The SPPs propagate along the gold-dielectric interface. Plasmonics allows light confinement below the diffraction limit as well as enhanced light–matter interaction. The slot waveguide is filled with the OEO material, and the device is poled in a manner similar to bulk devices: the modulator is heated to the Tg, then an electric field is applied to the gold waveguide walls that serve as poling electrodes. The Pockels effect of the poled OEO material is used to encode an electrical signal on the phase of the propagating SPPs. At the end of the plasmonic waveguide, the phase modulated SPPs are converted back to photonic modes of the output silicon waveguide by the second taper structure. In this work, the phase plasmonic phase modulators are integrated in an imbalanced silicon–photonic push–pull Mach–Zehnder geometry,55Fig. 5, in which two phase modulators are oppositely driven. At an applied voltage Vπ, each phase modulator produces opposite quarter-wave phase shifts leading to complete destructive interference between the two arms, switching the modulator from the on- to the off-state. The EO performance at 1550 nm is determined in 10 μm long modulators with 80 and 105 nm wide slots operating in the 15–70 GHz range. To measure the on–off voltage Vπ, the wavelength-dependent intensity transfer function was recorded while applying DC voltages between ±1 V in steps of 0.5 V. By measuring the voltage-dependent spectral shift of transmission spectrum,55Vπ was measured to be 4.8 V and 6.5 V for 80 and 105 nm wide slots, respectively. Based on these drive voltages, the devices had voltage–length products VπL of 48 V μm and 65 V μm (Table 5). The electro-optic coefficient for BAH13 calculates to a maximum r33 of 197 and 208 pm V−1 in the modulators with 80 and 105 nm slot width, respectively. Further discussion on the differences between r33 values obtained in bulk devices at 1310 nm and narrow slot POH devices operating at 1550 nm is included in the ESI.† Based on these calculations and assuming negligible differences between 105 and 100 nm slots, this suggests a 1.7× improvement in r33 and a 1.8× improvement in n3r33 over JRD1 in POH devices.55 The best VπL product of BAH13 is 48 V μm, which is comparable to the best values previously reported.55 However, the present value was achieved in a much wider waveguide (80 nm vs. 40 nm), making fabrication more accessible. As the improvement was consistent with predictions using finite difference eigenmode (FDE) calculations (ESI†) on POH slot waveguides using our recent methodology,72 it suggests that VπL < 20 V μm could be obtained in narrower slots (Fig. 6), though potentially requiring CBLs and further optimization of poling protocols.
| BAH13 | JRD1 | |||
|---|---|---|---|---|
| w slot (nm) | 80 | 105 | 100 | 150 |
| r 33 (pm V−1) | 197 | 208 | 123 | 156 |
| n 3 r 33 (pm V−1) | 1251 | 1321 | 730 | 925 |
| V π L (V μm) | 48 | 65 | 112 | 146 |
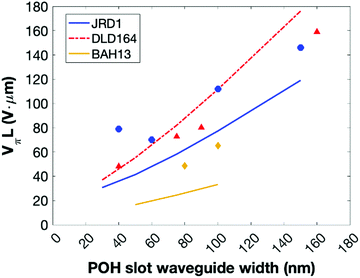 | ||
| Fig. 6 Comparison of measured (symbols) and computationally predicted (lines) VπL values for JRD1, DLD164,39,55 and BAH13 at 1550 nm in a POH MZI configuration. Measured values for JRD1 and DLD164 are from ref. 55; predictions use the methodology in ref. 72. No CBLs were included in the waveguide calculations. Calculations are discussed in ESI.† | ||
3. Conclusions
We synthesized and characterized four chromophores derived from theory-guided design and optimized to achieve high EO activity with acceptable loss and processibility; the highest-performing chromophore was also evaluated in POH devices. Due to the fine-tuned electron donating ability, the derived chromophores exhibited large molecular hyperpolarizabilities and controllable redshifts of absorption. To prevent dipolar interaction-induced aggregation, the chromophores were functionalized with side-chain bulky groups on donor and bridge moieties. In bulk devices BAH chromophores showed significantly improved EO performance including r33 values and poling efficiencies. Neat BAH13 devices with HfO2 CBL produced a very large maximum r33 value of 1100 ± 100 pm V−1 at 1310 nm. This r33 is equivalent to BAY1, with a much-improved absorption coefficient making it practical for use in EO modulators. 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BAH-FD
1 BAH-FD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BAH13 blends showed a lower poling efficiency of 6.7 ± 0.3 nm2 V−2 and a greatly reduced absorbance, which is important to the performance of modulators. 1
BAH13 blends showed a lower poling efficiency of 6.7 ± 0.3 nm2 V−2 and a greatly reduced absorbance, which is important to the performance of modulators. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BAH-BB
1 BAH-BB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BAH13 has the highest poling efficiency of 8.4 ± 0.4 nm2 V−2 and an improved Tg > 100 °C. The neat BAH13 was evaluated in POH modulators, achieving VπL < 50 V μm in substantially wider slots than prior record devices. In POH modulators, BAH13 exhibited high r33 values of 208 pm V−1 at 1550 nm, substantially exceeding the performance of prior chromophores evaluated in equivalent device architectures.
BAH13 has the highest poling efficiency of 8.4 ± 0.4 nm2 V−2 and an improved Tg > 100 °C. The neat BAH13 was evaluated in POH modulators, achieving VπL < 50 V μm in substantially wider slots than prior record devices. In POH modulators, BAH13 exhibited high r33 values of 208 pm V−1 at 1550 nm, substantially exceeding the performance of prior chromophores evaluated in equivalent device architectures.
Author contributions
LEJ, DLE, and BHR conceived the project. LEJ performed electronic structure calculations, electrodynamics calculations, and associated data analysis. LEJ, DLE, HX, and BHR contributed to design of novel compounds. HX synthesized and performed structural, linear optical, electro-optic, and thermal characterization on all compounds. DLE performed VASE characterization and assisted with other techniques and DLE and SRH contributed characterization protocols and assisted with interpretation of data. YdC and WVG performed all hyper-Rayleigh Scattering spectroscopy experiments and associated data analysis; KC and LEJ assisted with HRS data interpretation. SRH performed PVD and ALD depositions for CBLs. WH designed the POH devices and managed testing and in-device characterization; EDL and NM fabricated the POH devices, and MD performed poling experiments in POH devices. HX, LEJ, and DLE wrote the initial manuscript and led revision in response to reviewer comments. LEJ and DLE managed the project. BHR, LRD, KC and JL provided research-group level supervision, mentoring, and funding. All authors edited and approved the manuscript.Conflicts of interest
Competing Financial Interest Disclosure. Some work in this study is related to technology described in patent applications filed by the University of Washington, “Organic Electro-Optic Chromophores,” International Application No. PCT/US2020/054081, which have been licensed by Nonlinear Materials Corporation (NLM). L. E. J. is a co-founder and part-time employee of NLM and an equity holder in NLM. S. R. H and D. L. E. are part-time employees of NLM and equity holders in NLM. B. H. R. is an advisor to NLM and an equity holder in NLM. The terms of this arrangement have been reviewed and approved by the University of Washington in accordance with its policies governing outside work and financial conflicts of interest in research. W. H. is a co-founder, full-time employee, and equity holder in Polariton Technologies AG, which is commercializing POH technology. E. D. L., N. M., and M. D. are full-time employees of Polariton Technologies AG. J. L. is an advisor to and equity holder in Polariton Technologies AG. All other authors declare no competing financial interests.Acknowledgements
We gratefully acknowledge the financial support of the Air Force Office of Scientific Research (FA9550-19-1-0069), the National Science Foundation (IIP-2036514), the University of Washington College of Arts and Sciences, Flemish Fund for Scientific Research (FWO-V, G0A1817N), the University of Leuven (C16/16/003), EU-Project NEBULA (871658), and EU Project PlasmoniAC (871391). Part of this work was conducted at the Molecular Analysis Facility, a National Nanotechnology Coordinated Infrastructure (NNCI) site at the University of Washington, which is supported in part by funds from the National Science Foundation (awards NNCI-2025489, NNCI-1542101), the Molecular Engineering & Sciences Institute, and the Clean Energy Institute.Notes and references
- R. Bonjour, M. Burla, F. C. Abrecht, S. Welschen, C. Hoessbacher, W. Heni, S. A. Gebrewold, B. Baeuerle, A. Josten, Y. Salamin, C. Haffner, P. V. Johnston, D. L. Elder, P. Leuchtmann, D. Hillerkuss, Y. Fedoryshyn, L. R. Dalton, C. Hafner and J. Leuthold, Opt. Express, 2016, 24, 25608–25618 CrossRef CAS.
- M. Ayata, Y. Fedoryshyn, W. Heni, B. Baeuerle, A. Josten, M. Zahner, U. Koch, Y. Salamin, C. Hoessbacher, C. Haffner, D. L. Elder, L. R. Dalton and J. Leuthold, Science, 2017, 358, 630–632 CrossRef CAS PubMed.
- C. Haffner, D. Chelladurai, Y. Fedoryshyn, A. Josten, B. Baeuerle, W. Heni, T. Watanabe, T. Cui, B. Cheng, S. Saha, D. L. Elder, L. R. Dalton, A. Boltasseva, V. M. Shalaev, N. Kinsey and J. Leuthold, Nature, 2018, 556, 483–486 CrossRef CAS.
- Y. Salamin, I.-C. Benea-Chelmus, Y. Fedoryshyn, W. Heni, D. L. Elder, L. R. Dalton, J. Faist and J. Leuthold, Nat. Commun., 2019, 10, 5550 CrossRef CAS PubMed.
- S. Ummethala, T. Harter, K. Koehnle, Z. Li, S. Muehlbrandt, Y. Kutuvantavida, J. Kemal, P. Marin-Palomo, J. Schaefer, A. Tessmann, S. K. Garlapati, A. Bacher, L. Hahn, M. Walther, T. Zwick, S. Randel, W. Freude and C. Koos, Nat. Photonics, 2019, 13, 519–524 CrossRef CAS.
- I.-C. Benea-Chelmus, Y. Salamin, F. F. Settembrini, Y. Fedoryshyn, W. Heni, D. L. Elder, L. R. Dalton, J. Leuthold and J. Faist, Optica, 2020, 7, 498 CrossRef CAS.
- C. Hoessbacher, A. Josten, B. Baeuerle, Y. Fedoryshyn, H. Hettrich, Y. Salamin, W. Heni, C. Haffner, C. Kaiser, R. Schmid, D. L. Elder, D. Hillerkuss, M. Moller, L. R. Dalton and J. Leuthold, Opt. Express, 2017, 25, 1762–1768 CrossRef CAS PubMed.
- W. Heni, B. Baeuerle, H. Mardoyan, F. Jorge, J. M. Estaran, A. Konczykowska, M. Riet, B. Duval, V. Nodjiadjim, M. Goix, J.-Y. Dupuy, M. Destraz, C. Hoessbacher, Y. Fedoryshyn, H. Xu, D. L. Elder, L. R. Dalton, J. Renaudier and J. Leuthold, J. Lightwave Technol., 2020, 38, 2734–2739 CAS.
- U. Koch, C. Uhl, H. Hettrich, Y. Fedoryshyn, C. Hoessbacher, W. Heni, B. Baeuerle, B. I. Bitachon, A. Josten, M. Ayata, H. Xu, D. L. Elder, L. R. Dalton, E. Mentovich, P. Bakopoulos, S. Lischke, A. Krüger, L. Zimmermann, D. Tsiokos, N. Pleros, M. Möller and J. Leuthold, Nat. Electron., 2020, 3, 338–345 CrossRef CAS.
- S. Koeber, R. Palmer, M. Lauermann, W. Heni, D. L. Elder, D. Korn, M. Woessner, L. Alloatti, S. Koenig, P. C. Schindler, H. Yu, W. Bogaerts, L. R. Dalton, W. Freude, J. Leuthold and C. Koos, Light-Sci. Appl., 2015, 4, e255–e255 CrossRef CAS.
- C. Koos, J. Leuthold, W. Freude, M. Kohl, L. Dalton, W. Bogaerts, A. L. Giesecke, M. Lauermann, A. Melikyan, S. Koeber, S. Wolf, C. Weimann, S. Muehlbrandt, K. Koehnle, J. Pfeifle, W. Hartmann, Y. Kutuvantavida, S. Ummethala, R. Palmer, D. Korn, L. Alloatti, P. C. Schindler, D. L. Elder, T. Wahlbrink and J. Bolten, J. Lightwave Technol., 2016, 34, 256–268 CAS.
- C. Kieninger, Y. Kutuvantavida, D. L. Elder, S. Wolf, H. Zwickel, M. Blaicher, J. N. Kemal, M. Lauermann, S. Randel, W. Freude, L. R. Dalton and C. Koos, Optica, 2018, 5, 739 CrossRef CAS.
- C. Kieninger, C. Fullner, H. Zwickel, Y. Kutuvantavida, J. N. Kemal, C. Eschenbaum, D. L. Elder, L. R. Dalton, W. Freude, S. Randel and C. Koos, Opt. Express, 2020, 28, 24693–24707 CrossRef CAS.
- L. Alloatti, R. Palmer, S. Diebold, K. P. Pahl, B. Q. Chen, R. Dinu, M. Fournier, J. M. Fedeli, T. Zwick, W. Freude, C. Koos and J. Leuthold, Light-Sci. Appl., 2014, 3, e173–e173 CrossRef CAS.
- R. Palmer, S. Koeber, D. L. Elder, M. Woessner, W. Heni, D. Korn, M. Lauermann, W. Bogaerts, L. Dalton, W. Freude, J. Leuthold and C. Koos, J. Lightwave Technol., 2014, 32, 2726–2734 CAS.
- M. Lauermann, S. Wolf, P. C. Schindler, R. Palmer, S. Koeber, D. Korn, L. Alloatti, T. Wahlbrink, J. Bolten, M. Waldow, M. Koenigsmann, M. Kohler, D. Malsam, D. L. Elder, P. V. Johnston, N. Phillips-Sylvain, P. A. Sullivan, L. R. Dalton, J. Leuthold, W. Freude and C. Koos, J. Lightwave Technol., 2015, 33, 1210–1216 CAS.
- C. Kieninger, Y. Kutuvantavida, H. Miura, J. N. Kemal, H. Zwickel, F. Qiu, M. Lauermann, W. Freude, S. Randel, S. Yokoyama and C. Koos, Opt. Express, 2018, 26, 27955–27964 CrossRef CAS PubMed.
- S. Wolf, H. Zwickel, W. Hartmann, M. Lauermann, Y. Kutuvantavida, C. Kieninger, L. Altenhain, R. Schmid, J. Luo, A. K. Jen, S. Randel, W. Freude and C. Koos, Sci. Rep., 2018, 8, 2598 CrossRef PubMed.
- S. Ummethala, J. N. Kemal, A. S. Alam, M. Lauermann, A. Kuzmin, Y. Kutuvantavida, S. H. Nandam, L. Hahn, D. L. Elder, L. R. Dalton, T. Zwick, S. Randel, W. Freude and C. Koos, Optica, 2021, 8, 511 CrossRef.
- M. Burla, C. Hoessbacher, W. Heni, C. Haffner, Y. Fedoryshyn, D. Werner, T. Watanabe, H. Massler, D. L. Elder, L. R. Dalton and J. Leuthold, APL Photonics, 2019, 4, 056106 CrossRef.
- W. Heni, Y. Fedoryshyn, B. Baeuerle, A. Josten, C. B. Hoessbacher, A. Messner, C. Haffner, T. Watanabe, Y. Salamin, U. Koch, D. L. Elder, L. R. Dalton and J. Leuthold, Nat. Commun., 2019, 10, 1694 CrossRef PubMed.
- C. Haffner, W. Heni, Y. Fedoryshyn, J. Niegemann, A. Melikyan, D. L. Elder, B. Baeuerle, Y. Salamin, A. Josten, U. Koch, C. Hoessbacher, F. Ducry, L. Juchli, A. Emboras, D. Hillerkuss, M. Kohl, L. R. Dalton, C. Hafner and J. Leuthold, Nat. Photonics, 2015, 9, 525–528 CrossRef CAS.
- L. R. Dalton, B. H. Robinson, D. L. Elder, A. F. Tillack and L. E. Johnson, Presented in part at the SPIE Organic Photonics + Electronics, 2017, DOI:10.1117/12.2278795.
- W. Heni, Y. Kutuvantavida, C. Haffner, H. Zwickel, C. Kieninger, S. Wolf, M. Lauermann, Y. Fedoryshyn, A. F. Tillack, L. E. Johnson, D. L. Elder, B. H. Robinson, W. Freude, C. Koos, J. Leuthold and L. R. Dalton, ACS Photonics, 2017, 4, 1576–1590 CrossRef CAS.
- B. Robinson, L. Johnson, D. Elder, A. Kocherzhenko, C. Isborn, C. Haffner, W. Heni, C. Hoessbacher, Y. Fedoryshyn, Y. Salamin, B. Baeuerle, A. Josten, M. Ayata, U. Koch, J. Leuthold and L. R. Dalton, J. Lightwave Technol., 2018, 36, 5036–5047 CAS.
- T. D. Kim, J. W. Kang, J. Luo, S. H. Jang, J. W. Ka, N. Tucker, J. B. Benedict, L. R. Dalton, T. Gray, R. M. Overney, D. H. Park, W. N. Herman and A. K. Jen, J. Am. Chem. Soc., 2007, 129, 488–489 CrossRef CAS PubMed.
- T.-D. Kim, J. Luo, Y.-J. Cheng, Z. Shi, S. Hau, S.-H. Jang, X.-H. Zhou, Y. Tian, B. Polishak, S. Huang, H. Ma, L. R. Dalton and A. K. Y. Jen, J. Phys. Chem. C, 2008, 112, 8091–8098 CrossRef CAS.
- W. W. Jin, P. V. Johnston, D. L. Elder, A. F. Tillack, B. C. Olbricht, J. S. Song, P. J. Reid, R. M. Xu, B. H. Robinson and L. R. Dalton, Appl. Phys. Lett., 2014, 104, 243304 CrossRef.
- W. W. Jin, P. V. Johnston, D. L. Elder, K. T. Manner, K. E. Garrett, W. Kaminsky, R. M. Xu, B. H. Robinson and L. R. Dalton, J. Mater. Chem. C, 2016, 4, 3119–3124 RSC.
- D. L. Elder, C. Haffner, W. Heni, Y. Fedoryshyn, K. E. Garrett, L. E. Johnson, R. A. Campbell, J. D. Avila, B. H. Robinson, J. Leuthold and L. R. Dalton, Chem. Mater., 2017, 29, 6457–6471 CrossRef CAS.
- H. Xu, J. Liu, J. Liu, C. Yu, Z. Zhai, G. Qin and F. Liu, Mater. Chem. Front., 2020, 4, 168–175 RSC.
- H. J. Xu, F. G. Liu, D. L. Elder, L. E. Johnson, Y. de Coene, K. Clays, B. H. Robinson and L. R. Dalton, Chem. Mater., 2020, 32, 1408–1421 CrossRef CAS.
- X. H. Zhou, J. D. Luo, S. Huang, T. D. Kim, Z. W. Shi, Y. J. Cheng, S. H. Jang, D. B. Knorr, R. M. Overney and A. K.-Y. Jen, Adv. Mater., 2009, 21, 1976–1981 CrossRef CAS.
- L. R. Dalton, P. A. Sullivan and D. H. Bale, Chem. Rev., 2010, 110, 25–55 CrossRef CAS.
- P. A. Sullivan and L. R. Dalton, Acc. Chem. Res., 2009, 43, 10–18 CrossRef PubMed.
- J. Y. Wu, Z. A. Li, J. D. Luo and A. K. Y. Jen, J. Mater. Chem. C, 2020, 8, 15009–15026 RSC.
- P. A. Sullivan, H. Rommel, Y. Liao, B. C. Olbricht, A. J. Akelaitis, K. A. Firestone, J. W. Kang, J. Luo, J. A. Davies, D. H. Choi, B. E. Eichinger, P. J. Reid, A. Chen, A. K. Jen, B. H. Robinson and L. R. Dalton, J. Am. Chem. Soc., 2007, 129, 7523–7530 CrossRef CAS PubMed.
- S. J. Benight, D. B. Knorr Jr., L. E. Johnson, P. A. Sullivan, D. Lao, J. Sun, L. S. Kocherlakota, A. Elangovan, B. H. Robinson, R. M. Overney and L. R. Dalton, Adv. Mater., 2012, 24, 3263–3268 CrossRef CAS PubMed.
- D. L. Elder, S. J. Benight, J. S. Song, B. H. Robinson and L. R. Dalton, Chem. Mater., 2014, 26, 872–874 CrossRef CAS.
- M. Li, S. Huang, X.-H. Zhou, Y. Zang, J. Wu, Z. Cui, J. Luo and A. K. Y. Jen, J. Mater. Chem. C, 2015, 3, 6737–6744 RSC.
- J. Wu, J. Luo, N. Cernetic, K. Chen, K.-S. Chiang and A. K. Y. Jen, J. Mater. Chem. C, 2016, 4, 10286–10292 RSC.
- Z. A. Li, P. Chen, Y. Xie, Z. Li and J. Qin, Adv. Electron. Mater., 2017, 3, 1700138 CrossRef.
- J. Wu, B. Wu, W. Wang, K. S. Chiang, A. K.-Y. Jen and J. Luo, Mater. Chem. Front., 2018, 2, 901–909 RSC.
- F. Liu, S. Chen, S. Mo, G. Qin, C. Yu, W. Zhang, W.-J. Shi, P. Chen, H. Xu and M. Fu, J. Mater. Chem. C, 2019, 7, 8019–8028 RSC.
- F. Liu, M. Zhang, H. Xiao, Y. Yang, H. Wang, J. Liu, S. Bo, Z. Zhen, X. Liu and L. Qiu, J. Mater. Chem. C, 2015, 3, 9283–9291 RSC.
- H. Zhang, H. Xiao, F. Liu, F. Huo, Y. He, Z. Chen, X. Liu, S. Bo, L. Qiu and Z. Zhen, J. Mater. Chem. C, 2017, 5, 1675–1684 RSC.
- H. Zhang, Y. Yang, H. Xiao, F. Liu, F. Huo, L. Chen, Z. Chen, S. Bo, L. Qiu and Z. Zhen, J. Mater. Chem. C, 2017, 5, 6704–6712 RSC.
- Y. He, L. Chen, H. Zhang, Z. Chen, F. Huo, B. Li, Z. Zhen, X. Liu and S. Bo, J. Mater. Chem. C, 2018, 6, 1031–1037 RSC.
- Z. Chen, A. Zhang, H. Xiao, F. Huo, Z. Zhen, X. Liu and S. Bo, Dyes Pigm., 2020, 173, 107876 CrossRef.
- H. Xu, D. L. Elder, L. E. Johnson, Y. de Coene, S. R. Hammond, W. Vander Ghinst, K. Clays, L. R. Dalton and B. H. Robinson, Adv. Mater., 2021, 2104174 CrossRef CAS.
- H. Xu, L. E. Johnson, Y. de Coene, D. L. Elder, S. R. Hammond, K. Clays, L. R. Dalton and B. H. Robinson, J. Mater. Chem. C, 2021, 9, 2721–2728 RSC.
- L. E. Johnson, L. R. Dalton and B. H. Robinson, Acc. Chem. Res., 2014, 47, 3258–3265 CrossRef CAS PubMed.
- L. Johnson, D. Elder, A. Kocherzhenko, A. Tillack, C. Isborn, L. Dalton and B. Robinson, Presented in part at the SPIE Organic Photonics + Electronics, 2018.
- L. Johnson, H. Xu, Y. de Coene, D. Elder, K. Clays, L. Dalton and B. Robinson, Presented in part at the SPIE Nanoscience + Engineering, 2019.
- W. Heni, C. Haffner, D. L. Elder, A. F. Tillack, Y. Fedoryshyn, R. Cottier, Y. Salamin, C. Hoessbacher, U. Koch, B. Cheng, B. Robinson, L. R. Dalton and J. Leuthold, Opt. Express, 2017, 25, 2627–2653 CrossRef PubMed.
- L. Johnson, H. Xu, S. Hammond, D. Elder, S. Benight, Y. de Coene, J. Hesse-Withbroe, K. Clays, L. Dalton and B. Robinson, Presented in part at the SPIE Nanoscience + Engineering, 2020.
- K. Clays and A. Persoons, Phys. Rev. Lett., 1991, 66, 2980–2983 CrossRef CAS.
- K. Clays and A. Persoons, Rev. Sci. Instrum., 1992, 63, 3285–3289 CrossRef CAS.
- J. Campo, F. Desmet, W. Wenseleers and E. Goovaerts, Opt. Express, 2009, 17, 4587–4604 CrossRef CAS.
- A. F. Tillack and B. H. Robinson, J. Opt. Soc. Am. B, 2016, 33, E121–E129 CrossRef CAS.
- M. J. Alam and D. C. Cameron, J. Sol–Gel Sci. Technol., 2002, 25, 137–145 CrossRef CAS.
- S. Huang, T. D. Kim, J. D. Luo, S. K. Hau, Z. W. Shi, X. H. Zhou, H. L. Yip and A. K. Y. Jen, Appl. Phys. Lett., 2010, 96, 243311 CrossRef.
- Y. Enami, B. Yuan, M. Tanaka, J. Luo and A.-Y. Jen, Appl. Phys. Lett., 2012, 101, 123509 CrossRef.
- S. Abel, F. Eltes, J. E. Ortmann, A. Messner, P. Castera, T. Wagner, D. Urbonas, A. Rosa, A. M. Gutierrez, D. Tulli, P. Ma, B. Baeuerle, A. Josten, W. Heni, D. Caimi, L. Czornomaz, A. A. Demkov, J. Leuthold, P. Sanchis and J. Fompeyrine, Nat. Mater., 2019, 18, 42–47 CrossRef CAS.
- H. Xu, D. L. Elder, L. E. Johnson, B. H. Robinson and L. R. Dalton, ACS Appl. Mater. Interfaces, 2019, 11, 21058–21068 CrossRef CAS PubMed.
- X. Zang, G. Liu, Q. Li, Z. A. Li and Z. Li, Macromolecules, 2020, 53, 4012–4021 CrossRef CAS.
- ChemAxon, Marvin 21.9, 2021, https://chemaxon.com/.
- T. Nishide, S. Honda, M. Matsuura and M. Ide, Thin Solid Films, 2000, 371, 61–65 CrossRef CAS.
- H. Shimizu, T. Sato, S. Konagai, M. Ikeda, T. Takahashi and T. Nishide, Jpn. J. Appl. Phys., 2007, 46, 4209–4214 CrossRef CAS.
- C. C. Teng and H. T. Man, Appl. Phys. Lett., 1990, 56, 1734–1736 CrossRef CAS.
- Y. Shuto and M. Amano, J. Appl. Phys., 1995, 77, 4632–4638 CrossRef CAS.
- L. E. Johnson, D. L. Elder, S. J. Benight, A. F. Tillack, S. R. Hammond, L. R. Dalton and B. H. Robinson, Presented in part at the SPIE Organic Optics + Photonics, 2021.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures and analytical, UV/visible spectra in a variety of solvents, variable angle spectroscopic ellipsometry (VASE) data for films of chromophores and chromophore–polymer blends, HRS data analysis, computational methodology and additional DFT-calculated properties, poling data (plots of r33vs. poling field), glass transition temperatures (Tg) as determined by differential scanning calorimetry (DSC), decomposition temperatures (Td) as determined by thermogravimetric analysis (TGA), and cyclic voltammetry methods and data. See DOI: 10.1039/d1mh01206a |
| This journal is © The Royal Society of Chemistry 2022 |