 Open Access Article
Open Access ArticleMedicines for millions of patients†
David C.
Rees
 *
*
Astex Pharmaceuticals, 436 Cambridge Science Park, Cambridge, CB4 0QA, UK. E-mail: David.rees@astx.com
First published on 11th November 2021
Abstract
In this opinion piece I share personal anecdotes from three drug discovery projects, sugammadex an anaesthetic reversal agent from Organon Scotland, and ribociclib and erdafitinib, both oncology drugs arising from Astex UK collaborations with Novartis and Janssen respectively. These drugs have been used to treat millions of patients. The learnings from this research focus on innovation, teamwork, and collaborations. Drug discovery, even with its frustrations and disappointments can be a great career for scientists in industry, in academia, or in a not-for-profit institute, who want their research to alleviate human suffering.
Introduction
Suddenly, scientists are the heroines and heroes. After the roll out of Covid 19 vaccines in a record-breaking 12 months, the public spotlight is firmly focussed on science. And there are many more healthcare breakthroughs such as the revolution in cancer therapy based on immunology, and the promise of ‘one-and-done’ curative gene therapy treatments like Zolgensma for children with spinal muscular atrophy.Drug discovery research is changing in universities, in industry, and in collaboration. The pharmaceutical industry has moved a long way from the closed and secretive culture at the start of my career where everything was done in-house, and publications were usually only permitted for failed projects. Today, cross discipline activities like bioinformatics and chemical biology rely on team working across scientific departments and organisations. The models for collaborations are also changing, for example the Covid moonshot is an open science initiative using hundreds of crowdsourced medicinal chemists to design antivirals targeting MPro of SARS-CoV-2.1
Technologies are also changing at breakneck speed. Discoveries are made today that were previously near impossible. Designing selective kinase inhibitors used to be regarded as implausible given the promiscuity of ATP binding but today there are over 70 approved kinase drugs.2 More recently KRAS, known as the beating heart of cancer, was regarded as undruggable. But in 2021, building on ground-breaking research in academia and industry, sotorasib became the first KRAS drug approved for cancer.
Two of the stories described below also began with unconventional ideas: chemical chelation to reverse anaesthesia; and fragment based drug discovery (FBDD) using small, weak fragments as starting points for drug discovery. However, innovation, perseverance, and strong teamworking led to molecules that became launched drugs (Fig. 1).
 | ||
| Fig. 1 Three drugs, millions of patients. Sugammadex, erdafitinib, and ribociclib have been used to provide transformative treatments. | ||
Sugammadex
An anecdote from talking with anaesthetists in the mid-1990s was the dream to have an ‘off switch’ for general anaesthesia that would free up hospital beds in intensive care and facilitate faster patient recovery than was possible with the then-used reversal agents based on acetylcholinesterase inhibitors. Also, the anaesthetists wanted to address the rare but serious complications associated with residual neuromuscular blocking (NMB) activity in patients.Shortly after joining Organon I was thinking about this and came across an unrelated publication showing some chemical structures called cyclophanes that bound to steroids.3 This serendipity triggered a thought: could we use chemical chelation as a novel concept to reverse the anaesthetic effects of Organon's steroid-derived NMB drugs? Many people were sceptical, but inspired by a culture that supported innovation, a small team of highly-trained experimentalists decided to try to design a drug using host–guest chemistry for the first time.
The first chemical series was based on cyclophanes modified with COOH groups to increase reversible binding to Organon's cationic NMB drugs. Early results were encouraging because they showed that binding did indeed correlate with reversal of NMB-induced muscle relaxation in vitro (Ka ∼10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1, EC50 = 40 mM)4 (Fig. 2). We started to have corridor discussions about a ‘molecular-level vacuum cleaner’. But we were still a long way from a drug.
000 M−1, EC50 = 40 mM)4 (Fig. 2). We started to have corridor discussions about a ‘molecular-level vacuum cleaner’. But we were still a long way from a drug.
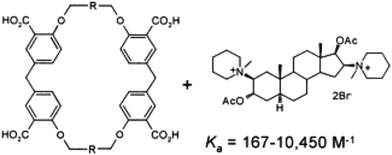 | ||
| Fig. 2 Anionic cyclophanes designed to chelate to NMBs and reverse their activity in vitro.4 | ||
A breakthrough came when biologists on the team tested a widely available cyclodextrin (CD) and discovered signs of reversal in vivo. This was a wow moment  . The chemists immediately wanted to design and synthesise more potent CD derivatives. The strategy was to increase binding affinity by attaching anionic groups (eg COOH) onto the CD via the hydroxyl sugar groups and to extend the lipophilic cavity.5
. The chemists immediately wanted to design and synthesise more potent CD derivatives. The strategy was to increase binding affinity by attaching anionic groups (eg COOH) onto the CD via the hydroxyl sugar groups and to extend the lipophilic cavity.5
Much effort led to Org 25969. X-ray crystallography of the co-complex with rocuronium confirmed the binary complex was consistent with the design hypothesis6 (Fig. 3) and the binding affinity is tight (ITC Ka = 1.05 × 10−7 M). Org 25969 was significantly faster at reversing rocuronium induced muscle relaxation in vivo than the clinically used reversal agents, eg the acetylcholinesterase inhibitor neostigmine.
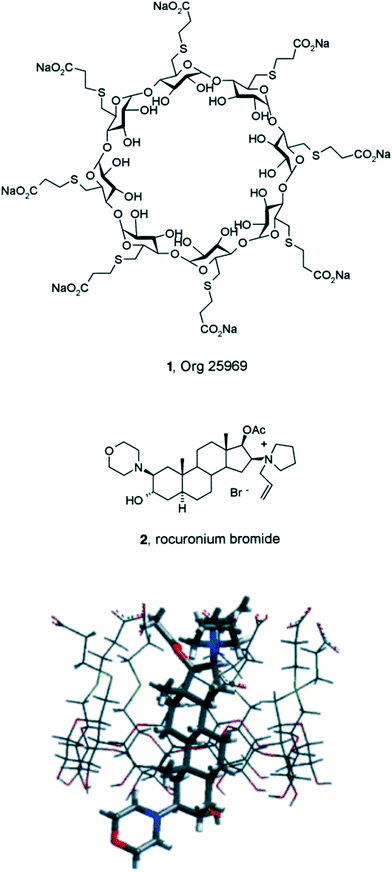 | ||
| Fig. 3 Structures of the synthetic cyclodextrin (CD) Org 25969 (sugammadex) (1) and the steroidal neuromuscular blocker drug, rocuronium (2). X-ray crystal structure of the Org 25969 rocuronium complex showing the four steroidal carbocyclic rings encapsulated within the lipophilic cyclodextrin cavity and the eight CD COOH groups near the quaternary nitrogen of rocuronium.6 | ||
Sugammadex (Bridion, Org 25969) was approved in 2008 as the first selective relaxant binding agent, after extensive preclinical and clinical development was performed by Organon, Schering Plough, and then Merck. Since then it has been used worldwide in hospitals in tens of millions of patients.7 Merck's annual sales of sugammadex in 2020 were US$1.2 billion,8 a remarkable success for a hospital-based drug. Anaesthetists have described sugammadex as the biggest breakthrough in anaesthesia in 25 years.9
Teamwork
You might expect that the research leading to Org 25969 involved camaraderie amongst experimental scientists across scientific departments at Organon, Scotland. That certainly was the case, but there was more. During the dark times when the chemists were struggling with selectively functionalising exactly 8 of the 24 CD hydroxy groups, motivation was maintained by great local team support and interactions with academic collaborators at Southampton and Glasgow Universities, Daresbury, and the Nijmegen University Medical Centre.6 As we shall see, this is a theme common to many successful drug discovery stories.Astex, a biotech success
Astex was founded in 1999 by Harren Jhoti, Sir Tom Blundell, and the late Chris Abell to tackle the problem that drug discovery is slow, expensive, and most compounds fail.Fragment-based drug discovery (FBDD) screens ultra-small compounds, which means a super high hit rate of low affinity but ligand efficient binders.10 However, industry was not ready for this approach. There was a widely held view that drug discovery was difficult enough starting with μM HTS hits – why make it even more difficult by going even weaker? Furthermore, how can such weak binding be differentiated from non-specific interactions? Astex's answer lay in pioneering an unusual use of protein X-ray crystallography as a screening method. Having identified fragments, there still remain huge challenges e.g., elaboration into much higher affinity, selective leads to name a few.
FBDD was an emerging technology which differentiated Astex at a time when the industry was searching for new lead discovery paradigms. This led big pharma companies to seek research collaborations with Astex. Two are described below.
Ribociclib
In 2005 Novartis, USA started a research alliance with Astex. This was a scientific marriage made in heaven with Astex's X-ray and FBDD technology complementing Novartis's internationally leading expertise in kinases and cancer drug discovery. The aim was to discover inhibitors of cyclin dependant kinases, such as CDK4/6, which control cell cycle progression and are dysfunctional in tumours, especially human breast cancers. Collectively several different hit finding approaches were mobilised but at that time there were no published crystal structures of CDK4 to guide the design of the very selective inhibitors that were required. Fortunately, the way the collaboration had been constructed was “all-in”, meaning that both parties shared all data regardless of where the experiments were done. This turned out to be critical because by working together in this open way the joint team established, and published, crystallography systems including CDK4 and CDK6.11 Now the team could leverage this together with Novartis's extensive oncology biology expertise, kinase assets, and HTS against CDK4 and CDK6 to get highly selective leads.12 One of these leads, which originated as a hit in global cross-screening across Novartis's kinase projects, was optimized to provide ribociclib.13A detailed account of CDK4/6 target validation, the design of ribociclib, its biology and use in combination studies, and the clinical trials has been published recently.13 The X-ray crystal structure of ribociclib bound to cyclin D1-CDK4 is shown in Fig. 4.
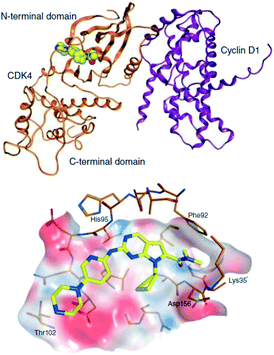 | ||
| Fig. 4 X-ray crystal structure of ribociclib bound to the hinge region of cyclin D1-CDK4 via hydrogen bonding; ribociclib is shown in yellow, CDK4 in gold, cyclin D1 purple.11,13 | ||
Following several years of extensive preclinical development and clinical trials by Novartis, ribociclib (Kisqali) was approved by the USA FDA under their breakthrough therapy designation program in March 2017 for the treatment of advanced breast cancer. The human tragedy of metastatic breast cancer, which takes a life in the USA approximately every 12 minutes, focuses the researchers and creates a strong team bond. Ribociclib was the second drug with this mechanism to be approved, after Pfizer's palbociclib, representing almost 50 years of breast cancer research in academia and industry. Ribociclib has achieved statistically significant overall survival for people with HR+/HER2− advanced breast cancer and is now being evaluated in adjuvant HR+/HER2− breast cancer.14 The sales for ribociclib in 2020 were US$ 687 million15 and are predicted to exceed US$1 bn.
Erdafitinib
Fibroblast growth factor receptors (FGFR1–4) belong to a family of receptor tyrosine kinases. Genetic alterations in FGFR family members are associated with tumour growth, metastasis, angiogenesis, and decreased survival.16Through a collaboration between the Northern Institute of Cancer Research at Newcastle University and Astex, early FGFR inhibitor fragments were identified.
A successful collaboration between Astex and Janssen led to erdafitinib, a pan-FGFR inhibitor with >50-fold selectivity over VEGFR2 in BaF3 cells.16 Janssen committed a major effort to the discovery and preclinical development and the subsequent clinical trials.
Fig. 5 shows the binding affinity of a quinoxaline containing fragment and of erdafitinib having increased binding interactions with the protein, and hence improved affinity and potency.16–18
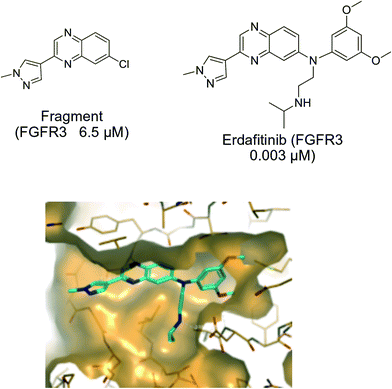 | ||
| Fig. 5 Binding affinity of a quinoxaline containing fragment and of JNJ-42756493 (erdafitinib). X-ray crystal structure of erdafitinib in FGFR1 is shown.16,17 | ||
In April 2019 erdafitinib (Balversa®) became the world's first launched FGFR kinase inhibitor. Data from metastatic urothelial carcinoma patients whose tumours had susceptible FGFR genetic alterations showed an overall response rate of 32%.19 Urothelial cancer, most frequently in the bladder, is in the top 10 most common causes of cancer death in the UK.
Perspectives and conclusions
There are, of course, many ingredients for successful drug discovery research, (people, serendipity, institutional knowledge and commitment, clinical tractability, financial support etc.,) but I am going to focus on just three learnings: teamwork, collaborations, and innovation.When I ask newly recruited Astex scientists what they notice about their move from academia into industry, their first comment usually refers enthusiastically to some aspect of teamworking. This culture is promoted in many drug discovery organisations because our research requires scientists with different skills and experiences, so teamworking within departments and across different disciplines or techniques is key for success. This gives a great opportunity for younger scientists to work alongside others with decades of experience and that is another way for the learning to flow. Furthermore, a strong teamworking spirit can keep a drug discovery project alive during the dark times that all of us encounter when progress is stalled, or the team loses the support of key stakeholders. This is when the team is really tested.
Whilst the overriding ethos is that team success is more important than individual recognition, high-performing teams of diverse scientists also nurture passionate individuals who encourage unorthodox views that might spark a breakthrough. All three of the stories described above had individual champions who kept the project alive at critical stages.
Research across scientific disciplines has always been embedded in drug discovery, but the stories here go further, they illustrate that a strong team spirit saved the sugammadex research at Organon. The experimental scientists who sweated the data and the champions who led to these drug candidates are the heroes and heroines of the story.
Secondly, collaborations between different organisations. This is different from the teamworking described above where I refer to individuals working together within the same organisation. Drug discovery is highly competitive and requires many different scientific disciplines, technologies, and expertise. Therefore, it is critical to choose the right collaborator(s), with the right experience, at the right time. And it is critical to ensure that all parties share 100% the same focus for a mutually agreed drug discovery project. Astex has been fortunate to have had collaborations with partners who were internationally leading in their own area. The teams had a spirit of strong mutual trust, information-sharing, and respect.
Industry-academia drug discovery collaborations are different from the networks that many of us are involved with on a weekly basis. Academic research is fundamentally important, it was Nobel prize-winning science that underpinned sugammadex (host guest chemistry, 1987), and ribociclib (protein X-ray crystallography), and selective C–H bond activation pioneered in academia is revolutionising the molecules chemists in industry can design, synthesise, and test today.20,21
This brings us to the third learning, innovation. In the stories here, certain individuals pursued an idea that was ‘off piste’ and they needed to convince others that was a good strategy. Many people would agree that there is an element of innovation, or maverick science in all successful drug discovery stories. To encourage this, we need to carve out ‘thinking time’. Short term deadlines, meetings, and emails do not help! The recent lab shut-downs caused by Covid have provided some unexpectedly good opportunities in this regard.
As we saw at the very beginning of the piece, drug discovery, even with its frustrations and disappointments can be a great career for scientists in industry, in academia, or in a not-for-profit institute, who want their research to alleviate human suffering. The science and culture in some industrial companies is similar to academia, albeit with a more overt commercial endpoint. The scientists are encouraged to publish, the research is strongly funded, and the culture supports teamworking within departments and across disciplines. And today there is growing research into diseases associated with less economically affluent countries, which remain an area of huge humanitarian need.
After 40 years I am still passionate about chemistry and drug discovery because when we are successful, what an impact! Getting an experimental drug candidate progressed into human trials and then hearing about the patients who benefit is why I come to work.
Conflicts of interest
David Rees is an employee of Astex, UK and previously was an employee of Organon, Scotland.Acknowledgements
I warmly acknowledge all scientists, too numerous to mention, who worked on the projects described, including those at Organon, and Astex, and our collaborators at Newcastle University, Novartis, and Janssen. I also thank a few individuals for their comments on this manuscript.Notes and references
- J. Chodera, A. A. Lee, N. London and F. von Delft, Crowdsourcing drug discovery for pandemics, Nat. Chem., 2020, 12(7), 581 CrossRef CAS PubMed.
- P. Cohen, D. Cross and P. A. Jänne, Kinase drug discovery 20 years after imatinib: progress and future directions, Nat. Rev. Drug Discovery, 2021, 20, 551–569 CrossRef CAS PubMed.
- F. Diederich and D. R. Carcanague, A Spacious Cyclophane Host for Inclusion Complexation of Steroids and [m.n] Paracyclophanes, Angew. Chem., Int. Ed. Engl., 1990, 29(7), 769–771 CrossRef.
- K. S. Cameron, L. Fielding, R. Mason, A. W. Muir, D. C. Rees, S. Thorn and M.-Q. Zhang, Anionic Cyclophanes as Potential Reversal Agents of Muscle Relaxants by Chemical Chelation, Bioorg. Med. Chem. Lett., 2002, 12(5), 753–755 CrossRef CAS.
- J. M. Adam, D. J. Bennett, A. Bom, J. K. Clark, H. Feilden, E. J. Hutchinson, R. Palin, A. Prosser, D. C. Rees, G. M. Rosair, D. Stevenson, G. J. Tarver and M.-Q. Zhang, Cyclodextrin-Derived Host Molecules as Reversal Agents for the Neuromuscular Blocker Rocuronium Bromide: Synthesis and Structure−Activity Relationships, J. Med. Chem., 2002, 45(9), 1806–1816 CrossRef CAS PubMed.
- A. Bom, M. Bradley, K. Cameron, J. K. Clark, J. Van Egmond, H. Feilden, E. J. MacLean, A. W. Muir, R. Palin, D. C. Rees and M. Q. Zhang, A novel concept of reversing neuromuscular block: chemical encapsulation of rocuronium bromide by a cyclodextrin-based synthetic host, Angew. Chem., Int. Ed., 2002, 41(2), 266–270 CrossRef.
- Merck Sharp & Dohme Corp, NDA 22225: Sugammadex Injection Anesthetic and Analgesic Drug Products Advisory Committee (AC) Meeting: Sugammadex Advisory Committee Briefing Document, Kenilworth, NJ, November 6, 2015, p. 10 Search PubMed.
- https://www.annualreports.com/HostedData/AnnualReports/PDF/NYSE_MRK_2020.pdf, Annual Report 2020, Merck & Co., Inc., 2020 Search PubMed.
- R. D. Miller, Sugammadex: An Opportunity to Change the Practice of Anesthesiology?, Anesth. Analg., 2007, 104(3), 477–478 CrossRef PubMed.
- C. W. Murray and D. C. Rees, The rise of fragment-based drug discovery, Nat. Chem., 2009, 1(3), 187–192 CrossRef CAS PubMed.
- P. J. Day, A. Cleasby, I. J. Tickle, M. O'Reilly, J. E. Coyle, F. P. Holding, R. L. McMenamin, J. Yon, R. Chopra, C. Lengauer and H. Jhoti, Crystal structure of human CDK4 in complex with a D-type cyclin, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 4166–4170 CrossRef CAS.
- Y. S. Cho, H. Angove, C. Brain, C. H.-T. Chen, H. Cheng, R. Cheng, R. Chopra, K. Chung, M. Congreve, C. Dagostin, D. J. Davis, R. Feltell, J. Giraldes, S. D. Hiscock, S. Kim, S. Kovats, B. Lagu, K. Lewry, A. Loo, Y. Lu, M. Luzzio, W. Maniara, R. McMenamin, P. N. Mortenson, R. Benning, M. O'Reilly, D. C. Rees, J. Shen, T. Smith, Y. Wang, G. Williams, A. J. A. Woolford, W. Wrona, M. Xu, F. Yang and S. Howard, Fragment-Based Discovery of 7-Azabenzimidazoles as Potent, Highly Selective, and Orally Active CDK4/6 Inhibitors, ACS Med. Chem. Lett., 2012, 3(6), 445–449 CrossRef CAS PubMed.
- C. T. Brain, R. Chopra, S. Kim, S. Howard and M. J. Sung, The Discovery of Kisqali® (Ribociclib): A CDK4/6 Inhibitor for the Treatment of HR+/HER2− Advanced Breast Cancer, in Successful Drug Discovery, 2021, pp. 273–289 Search PubMed.
- https://www.globenewswire.com/en/news-release/2021/04/21/2214060/0/en/TRIO-Completes-Enrollment-of-Phase-III-NATALEE-Breast-Cancer-Clinical-Trial.html .
- https://www.novartis.com/investors/financial-data/product-sales, Novartis Product Sales, 2020.
- T. P. S. Perera, E. Jovcheva, L. Mevellec, J. Vialard, D. De Lange, T. Verhulst, C. Paulussen, K. Van De Ven, P. King, E. Freyne, D. C. Rees, M. Squires, G. Saxty, M. Page, C. W. Murray, R. Gilissen, G. Ward, N. T. Thompson, D. R. Newell, N. Cheng, L. Xie, J. Yang, S. J. Platero, J. D. Karkera, C. Moy, P. Angibaud, S. Laquerre and M. V. Lorenzi, Discovery and Pharmacological Characterization of JNJ-42756493 (Erdafitinib), a Functionally Selective Small-Molecule FGFR Family Inhibitor, Mol. Cancer Ther., 2017, 16(6), 1010–1020 CrossRef CAS.
- C. W. Murray, D. R. Newell and P. Angibaud, A successful collaboration between academia, biotech and pharma led to discovery of erdafitinib, a selective FGFR inhibitor recently approved by the FDA, MedChemComm, 2019, 10(9), 1509–1511 RSC.
- Y. Loriot, A. Necchi, S. H. Park, J. Garcia-Donas, R. Huddart, E. Burgess, M. Fleming, A. Rezazadeh, B. Mellado, S. Varlamov, M. Joshi, I. Duran, S. T. Tagawa, Y. Zakharia, B. Zhong, K. Stuyckens, A. Santiago-Walker, P. De Porre, A. O'Hagan, A. Avadhani and A. O. Siefker-Radtke, Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma, N. Engl. J. Med., 2019, 381(4), 338–348 CrossRef CAS.
- https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-erdafitinib-metastatic-urothelial-carcinoma, FDA Press Release 12 April 2019.
- D. C. Blakemore, L. Castro, I. Churcher, D. C. Rees, A. W. Thomas, D. M. Wilson and A. Wood, Organic synthesis provides opportunities to transform drug discovery, Nat. Chem., 2018, 10(4), 383–394 CrossRef CAS.
- K. R. Campos, P. J. Coleman, J. C. Alvarez, S. D. Dreher, R. M. Garbaccio, N. K. Terrett, R. D. Tillyer, M. D. Truppo and E. R. Parmee, The importance of synthetic chemistry in the pharmaceutical industry, Science, 2019, 363(6424), eaat0805 CrossRef CAS PubMed.
Footnote |
| † Aspects of this opinion piece were included in a lecture at the September 2021 RSC SCI BMCS Medicinal Chemistry conference to mark the 2020 RSC BMCS Hall of Fame award. |
| This journal is © The Royal Society of Chemistry 2022 |

