Open Access Article
Open Access ArticleEnhanced formaldehyde gas-sensing response based on indium oxide nanowires doped with same-valence metal cations
J. Y.
Niu
,
B.
Hong
,
J. C.
Xu
 ,
Y. B.
Han
,
H. X.
Jin
,
D. F.
Jin
,
Y. X.
Zeng
,
X. L.
Peng
,
H. L.
Ge
and
X. Q.
Wang
,
Y. B.
Han
,
H. X.
Jin
,
D. F.
Jin
,
Y. X.
Zeng
,
X. L.
Peng
,
H. L.
Ge
and
X. Q.
Wang
 *
*
College of Materials Science and Chemistry, China Jiliang University, Hangzhou, 310018, China. E-mail: wxqnano@cjlu.edu.cn
First published on 26th July 2022
Abstract
Mesoporous indium oxides nanowires (In2O3 NWs) doped with Al, Sb and La were synthesized using a nanocasting method, and then the components, microstructures and morphology were characterized. All results indicate that the doped metals are well dispersed in the In2O3 NWs and hardly affect the NW microstructure and morphology. While the cations-doped concentration decreases greatly with the increasing radii of Al, Sb and La cations. The cation-doping not only causes the lattice distortion to improve the adsorbed oxygen on the surface, but also increases the ground-state resistance of the In2O3 NWs. In this way, the cation-doping greatly affects the gas-sensing behavior of formaldehyde gas. Considering the resistance in air and formaldehyde gas, the In1.984La0.016O3 NW sensor exhibits the best gas-sensing performance of 10 ppm formaldehyde gas with a response of 39.51 at 210 °C owing to the largest radius and lowest doping content of La3+.
1. Introduction
Formaldehyde (HCHO) is the simplest aldehyde, and is a colorless irritating gas.1 Long-term exposure to HCHO gas at a concentration higher than 0.1 ppm may cause serious eye irritation, a burning sensation in the throat, dyspnea, asthma-related symptoms and cancer.2 Considering the health hazards to humans and animals, it is very important to monitor the concentration of HCHO gas both indoors and outdoors.3 Therefore, test instruments and techniques should be well developed to detect and monitor HCHO gas in real-time.4 Nowadays, gas chromatography,5 high performance liquid chromatography,6 colorimetry-based derivatization,7 protein-based biosensing systems8 and gas sensors9,10 have been developed and used in formaldehyde monitoring.Gas sensors based on metal oxide semiconductors (MOS) have been well studied and developed to detect and monitor poisonous and harmful gases.11 The gas response of a MOS sensor is defined as the ratio of the resistance in target gas to that in air.12 For gas sensors it is important to control the microstructure of the material, to increase the specific surface area of MOS nanostructure. Nowadays, one-dimensional MOS nanostructures are widely studied as gas-sensing mediums due to their very large surface-to-volume ratio, high specific surface area and high porosity.13–15 In recent years, nanowires (NWs) have been used widely in gas sensors. For example, Wang et al. synthesized mesoporous Co3O4 NWs using a nanocasting method, and these NWs indicated an excellent gas-sensing performance for toluene gas.16 Hassen et al. fabricated rGO-W18O49 NWs, improving the gas-sensing response to toluene gas.17 However, microstructural regulation only improves the surface area to some extent.
As is known, heterogeneous doping can effectively adjust the carrier concentration of one-dimensional MOS nanostructures.18 Heterogeneous doping not only adjusts the resistance in the ground state but also causes lattice distortion of the MOS nanostructure. This lattice distortion results in more oxygen vacancies, which leads to more adsorbed oxygen on MOS nanostructures. Chen et al. synthesized high-valence-cation-doped NiO NWs, and the responses of the NiO NW sensor was greatly increased with doping of the high-valence donors.19 Mokoena et al. studied a Eu3+-doped NiO sensor for toluene gas detection, and the response was higher than for the NiO sensor.21
Due to its broad bandgap, low cost, low resistivity and high catalytic activity, In2O3 nanostructures as n-type MOS have been widely investigated all over the world to improve the gas-sensing performance.22 Jin et al. synthesized hierarchical porous In2O3 nanospheres, the sensor for which showed better selectivity for TEA and ethanol.23 Liu et al. prepared Sb-doped In2O3 microstructures for acetone detection, which showed a higher response compares with In2O3.24 Liang et al. synthesized Ca-doped In2O3 nanotubes, which showed excellent selectivity and a high response toward formaldehyde.25
In order to more accurately reveal the influence of lattice distortion from cation-doping, the interference of the carrier concentration, morphology, average particle size and specific surface area on the gas-sensing behavior of In2O3 nanostructures should be excluded.26 In this paper, a nanocasting method and the same process conditions were used to synthesize In2O3 NWs and cation-doped In2O3 NWs with a similar diameter, microstructure and specific surface area. The cations Al3+, Sb3+ and La3+ were doped into the In2O3 NWs using SBA-15 silica as a hard template. The influence of the doping content and cation radius on the microstructure and gas-sensing properties toward HCHO was investigated in detail. After optimizing the operating conditions of the In2O3 NW gas sensors, the physical mechanism was analyzed theoretically based on the doped semiconductor theory.
2. Experimental
2.1 Synthesis of In2O3 and cation-doped In2O3 NWs
All chemicals and reagents in this study were of analytical grade and used directly without further purification. The In2O3 NWs and Al-, Sb- and La-doped In2O3 NWs were synthesized using mesoporous SBA-15 silica as a hard template.19 Indium nitrates, aluminum chloride, antimony chloride, lanthanum nitrate and the SBA-15 powder were uniformly dissolved and dispersed in ethanol solution according to the atomic ratio of Si, i.e., (In + Al, Sb and La)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Al, Sb and La) = 2
(Al, Sb and La) = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05, and then n-hexane was added. After evaporating the above solution, the obtained powder samples were calcined at 260 °C using a muffle furnace. After washing to remove undecomposed soluble salts, the powder samples were sintered in air at 600 °C for 6 h and the SBA-15 silica was removed using a 2 M NaOH aqueous solution. Compared with the In2O3 molecular formula, the cation-doped In2O3 NWs were denoted as In2−xMxO3 (M = Al, Sb or La), in which x is the doping content calculated via EDS.
0.05, and then n-hexane was added. After evaporating the above solution, the obtained powder samples were calcined at 260 °C using a muffle furnace. After washing to remove undecomposed soluble salts, the powder samples were sintered in air at 600 °C for 6 h and the SBA-15 silica was removed using a 2 M NaOH aqueous solution. Compared with the In2O3 molecular formula, the cation-doped In2O3 NWs were denoted as In2−xMxO3 (M = Al, Sb or La), in which x is the doping content calculated via EDS.
2.2 Characterization of microstructures and gas-sensing properties
The phases, morphology and microstructures of undoped and cation-doped In2O3 NWs were characterized via XRD (SmartLabSE, 3 kW, Cu target, wavelength 0.154 nm, step 0.02°), TEM (JEOL, JEM-1400Flash, 120 kV), and EDS (HITACH, SU8010). Moreover, the bandgap and specific surface area were measured and deduced the from UV-visible adsorption spectra (UV3600 Spectrophotometer) and N2 adsorption–desorption isotherms (ASAP2020 Surface Area and Porosity Analyzer, −196 °C, relative pressure of 0.06 to 0.2), respectively.The gas-sensing tests were performed via static distribution using a CGS-4TPs gas-sensing measurement system with atmospheric air as the interference gas, where the relative humidity was held at about 30%. First, 2 mg In2O3 NWs or cation-doped In2O3 NWs and 1 ml deionized water were mixed together to form a homogeneous paste, which was brush-coated onto an Al2O3 ceramic substrate with interdigitated Ag–Pd electrodes. The as-prepared simulated sensors were dried in air at room temperature and then aged at 350 °C for 2 h in air to improve their stability. Considering the volume of the test chamber, the volume of HCHO liquid (37–40%), ammonia water (25–28%), water, ethanol, xylene, toluene and methanol were calculated, and then the above liquid was injected into the chamber and evaporated to a gas with a concentration from 1 ppm to 15 ppm. The response for n-type In2O3 NW sensors is defined as the ratio of the resistance in air (Rair) to that in HCHO gas (Rgas).13
3. Results and discussion
3.1 Microstructures and morphology of cation-doped In2O3 NWs
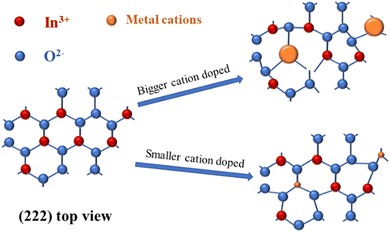
The phase analysis of In2O3 and cation-doped In2O3 NWs was performed using XRD, as shown in Fig. 1, in which all peaks are normalized according to the strongest (222) peak. The diffraction peaks of all the samples are consistent with the characteristic peaks of cubic In2O3 (PDF#71-2194). The diffraction peaks at 2θ values of 21.65, 30.76, 35.618, 51.188 and 60.85° correspond to the (211), (222), (400), (440) and (622) facets according to the standard card. The characteristic peaks belonging to Al-, Sb- and La-based compounds are not observed. The average crystallite sizes calculated using the Scherrer equation are listed in Table 1. In order to reveal the influence of doping with different cations on the lattice distortion of the In2O3 NWs, the (222) peaks of all the samples are enlarged and shown in Fig. 1b. Clearly, the (222) peak of the Al-doped In2O3 NWs is shifted to a higher angle and the La-doped sample shifted to a lower angle, while the Sb-doped In2O3 NWs are the same the In2O3 NWs due to the similar radius. According to Bragg's law:2dsin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = λ θ = λ | (1) |
 | ||
| Fig. 1 Full-angle range of XRD patterns (a) and shift of the (222) peak (b) for undoped In2O3 and cation-doped In2O3 NWs. | ||
| Sample | Crystallite size (nm) | Average pore size (nm) | S BET (m2 g−1) | E g (eV) | Response, S |
|---|---|---|---|---|---|
| In2O3 | 17.9 | 6.29520 | 57.37 | 3.34 | 26.16 |
| In1.838Al0.162O3 | 38.8 | 8.29376 | 53.55 | 3.39 | 28.73 |
| In1.816Sb0.184O3 | 45.8 | 7.82711 | 49.21 | 3.40 | 19.78 |
| In1.984La0.016O3 | 23.0 | 6.37254 | 57.29 | 3.36 | 39.51 |
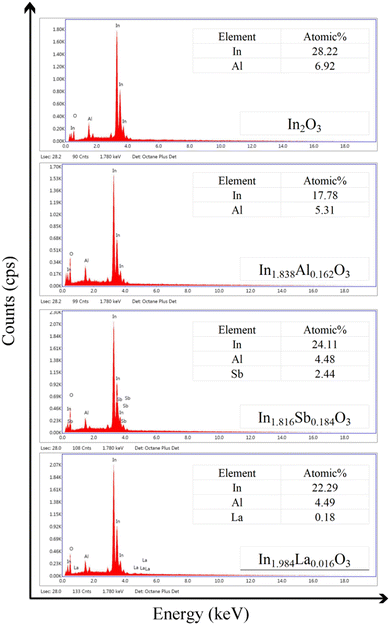
The components of In2O3 and cation-doped In2O3 NWs are characterized via EDS in Fig. 2. Considering the atomic percentage of all samples, cation-doped In2O3 NWs can be expressed as In1.838Al0.162O3, In1.816Sb0.184O3 and In1.984La0.016O3 NWs, respectively. Owing to the different cation radius values of In, Al, Sb and La in the initial solution, the probability of these cations entering the mesochannels of SBA-15 is different.19 The probability of entering the mesochannels decreases greatly with the increasing radius of the doped cation. As a result, the In1.838Al0.162O3 and In1.816Sb0.184O3 NWs present the highest doping concentration while the In1.984La0.016O3 NWs show the lowest doping concentration. Moreover, Fig. 3 shows the EDS spectra of In1.838Al0.162O3, In1.816Sb0.184O3 and In1.984La0.016O3 NWs, indicating that Al, Sb and La are well-dispersed in the In1.838Al0.162O3, In1.826Sb0.184O3 and In1.984La0.016O3 NWs. It can be seen easily from the number of bright spots that the La content is the lowest while the separate Al and Sb contents are much higher.
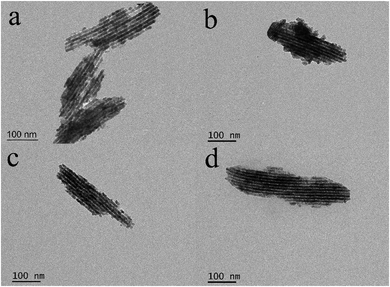
The morphology and microstructure of In2O3 and the cation-doped In2O3 NWs are observed using TEM in Fig. 4. It is shown that all samples exhibit mesoporous structures and that cation doping hardly affects the microstructure of the In2O3 NWs. The diameter of the nanowires is measured to be 8–9 nm and mesochannel size is about 2–3 nm.28 The N2 adsorption–desorption isotherms of In2O3 and the cation-doped In2O3 NWs are shown in Fig. 5. The curves of all samples exhibit type-IV adsorption isotherms, which are characteristic of mesoporous structures according to IUPAC. The BET surface area (SBET) and average pore size of all the samples calculated from the N2 adsorption–desorption isotherm data are given in Table 1. The specific surface area changes a little from 49.2 m2 g−1 to 57.4 m2 g−1. The pore size distribution diagrams of all the samples are shown in the insets of Fig. 5, in which the average pore size is mainly focused around 4–5 nm.
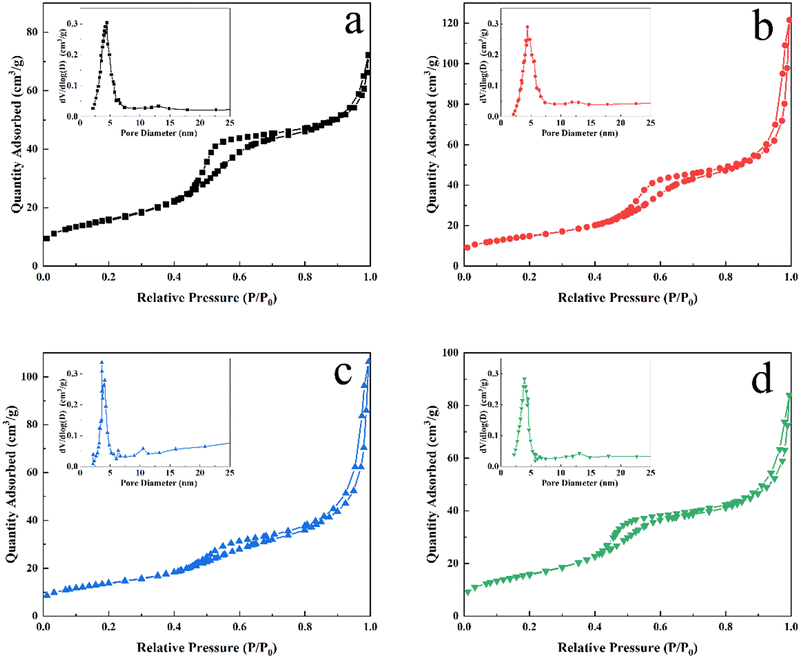 | ||
| Fig. 5 N2 adsorption–desorption isotherms and pore size distribution plots (inset) of (a) In2O3, and (b) Al-, (c) Sb- and (d) La-doped In2O3 NWs. | ||
The UV-visible absorption spectra of In2O3 and the cation-doped In2O3 NWs are used to calculate the bandgap (Eg) in Fig. 6. Eg is the basis of the optical absorption spectra according to eqn (2):29
| (αhν)1/n = A(hν − Eg) | (2) |
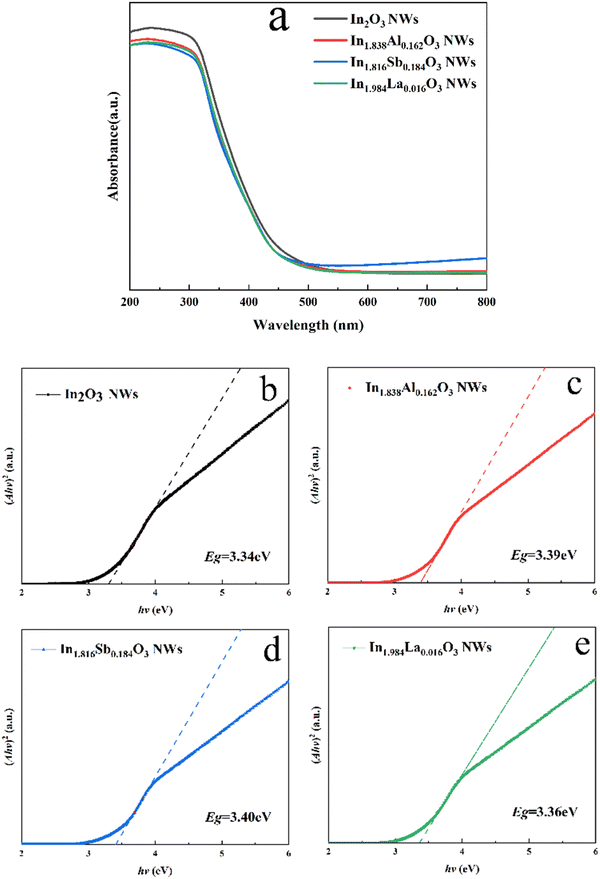 | ||
| Fig. 6 UV-vis spectrum of (a) In2O3 and the cation-doped In2O3 NWs; and (b–e) plots of (Ahν)2versus hν for (b) In2O3, and the (c) Al-, (d) Sb- and (e) La-doped In2O3 NWs. | ||
3.2 Gas-sensing performance of cation-doped In2O3 NWs sensors
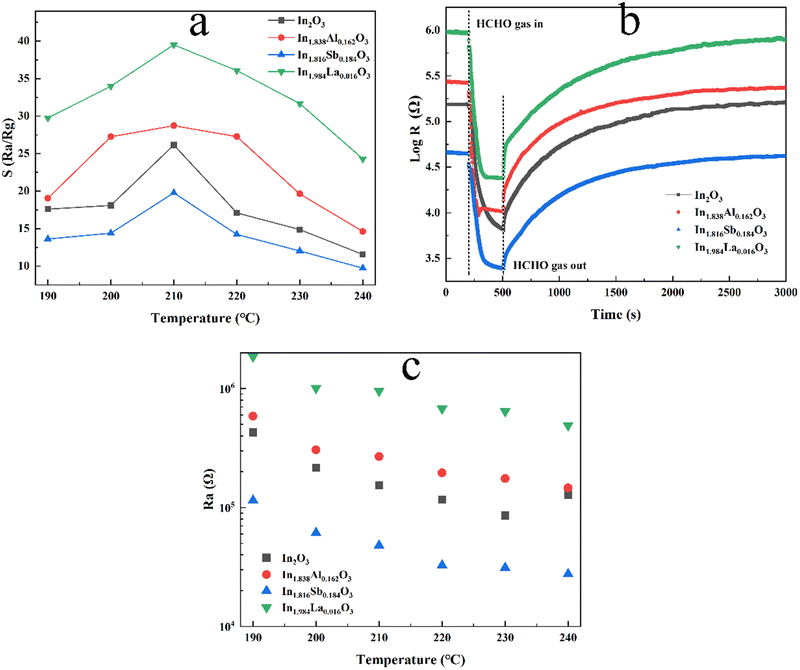
The gas-sensing behavior for 10 ppm HCHO gas of the In2O3 and cation-doped In2O3 NW sensors is characterized at operating temperatures from 190 to 240 °C in Fig. 7(a). For all the sensors, the response (S = Rair/Rgas) to HCHO gas increases initially up to a maximum value at 210 °C with increasing temperature, and then decreases to some extent. This phenomenon could be attributed to the balance of the surface chemical reaction and the HCHO gas diffusion. In this way, the operating temperature was confirmed to be 210 °C for all sensors in the subsequent experiments. Comparing all sensors, the response of the In1.984La0.016O3 and In1.838Al0.162O3 NWs sensors is higher than that of the In2O3 NW sensor, while the In1.186Sb0.184O3 NWs sensor shows the lowest response to HCHO gas at 210 °C. The change in resistance for In2O3 and the cation-doped In2O3 NW sensors to 10 ppm HCHO gas at 210 °C is given in Fig. 7(b), for which the resistance values in air and HCHO gas are also listed in Table 2. Owing to the electron-depletion layer at the shell of In2O3 NWs in air from adsorbed oxygen, Rair for all the sensors is much larger than Rgas. Furthermore, the differences in the resistance are relatively large due to the thickness differences of all the sensors. The Rair values of all the sensors at the different operating temperatures are given in Fig. 7(c), in which Rair decreases with the increasing temperature. The response and recovery time are defined as the time required for a change in the resistance to reach 90% of the equilibrium value after HCHO is injected and then removed. The response time for In2O3 and the cation-doped In2O3 NWs sensors is 80 s, 33 s, 77 s and 50 s, respectively. The response time of the cation-doped In2O3 NWs sensors is shorter than for the In2O3 NW sensor. This should be attributed to the destruction of the periodic potential field for cation doping, which facilitates the diffusion of molecular oxygen.40| Sample | R air (Ω) | R gas (Ω) |
|---|---|---|
| In2O3 NWs | 153![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 644 644 |
6650 |
| In1.838Al0.162O3 NWs | 267![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 635 635 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 389 389 |
| In1.816Sb0.184O3 NWs | 47943 | 2424 |
| In1.984La0.016O3 NWs | 954![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 557 557 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 202 202 |
The influence of the HCHO gas concentration from 1 ppm to 15 ppm on the response of In2O3 and the cation-doped In2O3 NW sensors at 210 °C is also discussed in Fig. 8(a). It was seen that the response of all the sensors increases with the HCHO gas concentration. HCHO gas first reacts with the adsorbed oxygen, and re-injects the extracted electrons back to the shell of In2O3 and the cation-doped In2O3 NWs. As the result, the resistance decreases greatly when in HCHO gas. The higher the HCHO gas concentration, the lower is the resistance value of all the sensors. Therefore, the response to HCHO gas increases greatly with the HCHO gas concentration for all sensors. Moreover, the relationship between the response (S) and the HCHO gas concentration (C) can be expressed in eqn (3) as:
| S = a(C)b + 1 | (3) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (S − 1) = blog (S − 1) = blog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (C) + log (C) + log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (a). (a). | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (S − 1) and log
(S − 1) and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (C) is plotted in Fig. 8(b) for In2O3 and the cation-doped In2O3 NW sensors, in which the response exhibits a good linear relationship with the HCHO gas concentration. Therefore, the HCHO gas concentration can be easily predicted according to the response value, indicating an excellent application in practice.
(C) is plotted in Fig. 8(b) for In2O3 and the cation-doped In2O3 NW sensors, in which the response exhibits a good linear relationship with the HCHO gas concentration. Therefore, the HCHO gas concentration can be easily predicted according to the response value, indicating an excellent application in practice.
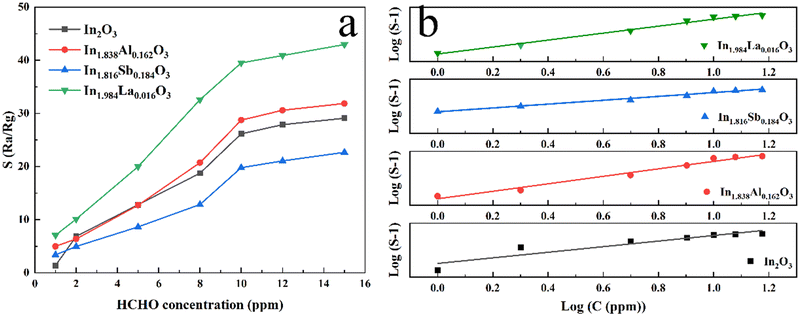 | ||
| Fig. 8 Concentration-dependent response curves (a) and the predicted concentration-response curves (b) for In2O3 and the cation-doped In2O3 NW sensors to HCHO gas at 210 °C. | ||
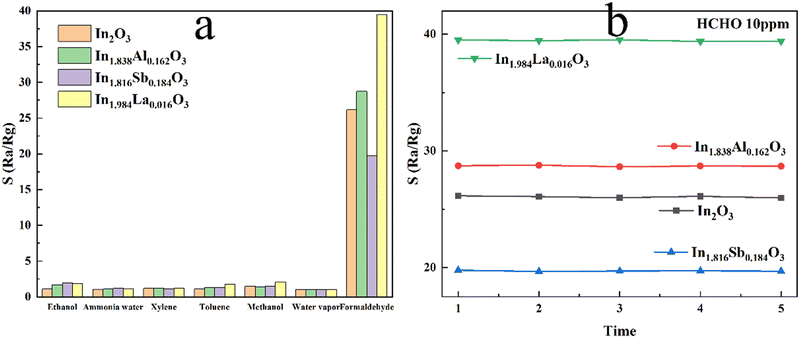
Furthermore, the selectivity of all the sensors towards 10 ppm ethanol, ammonia gas (ammonia water changed to ammonia gas after evaporation), water vapor, xylene, toluene, methanol and HCHO gases at 210 °C are given in Fig. 9(a). All sensors exhibit a much smaller response to water vapor than that of HCHO gas, indicating that the influence of humidity on the gas-sensing performance can be ignored. Clearly, the response to HCHO gas presents the highest value of all the gases, which indicates the excellent selectivity of the sensor to HCHO gas. In order to investigate the stability of In2O3 and the cation-doped In2O3 NW sensors, the response to 10 ppm HCHO gas was tested 5 times at 210 °C, as shown in Fig. 9(b). The response of all the sensors hardly changes, indicating the long-term stability of In2O3 and the cation-doped In2O3 NWs. Compared with the work of other researchers, the response to HCHO gas using the different gas-sensing media is listed in Table 3, in which the In1.984La0.016O3 NW sensor presents one of the highest responses to 10 ppm HCHO gas with an S value of about 40.
| Sensing material | T sens (°C) | HCHO concentration (ppm) | S | Ref. |
|---|---|---|---|---|
| α-Fe2O3 particles | 270 | 500 | 23 | 30 |
| ZnSnO3 cubic | 180 | 30 | 25 | 31 |
| rGO/ZnSnO3 microspheres | 110 | 10 | 12 | 32 |
| In2O3@rGO nanocubes | 225 | 25 | 88 | 33 |
| WOx/In2O3 nanosheets | 170 | 100 | 25 | 34 |
| SnO2 nanowires | 270 | 10 | 2.45 | 35 |
| Er-doped porous In2O3 nanotubes | 260 | 20 | 12 | 36 |
| 2 at% Al-doped ZnO | 320 | 50 | 6.8 | 37 |
| In1.984La0.016O3 NWs | 210 | 10 | 39.51 | This work |
3.3 Gas-sensing mechanism of cation-doped In2O3 NW sensors
For In2O3 and the cation-doped In2O3 NWs in air, the higher surface area adsorbed more oxygen molecules on the surface. The adsorbed oxygen molecules extract electrons from the surface shell and become O− at 210 °C to form an electron-depletion layer.38 As a result, the shell resistance in air (Rshell(air)) increases dramatically owing to the high surface area of the mesoporous structures. The more adsorptive O− that are present, the thicker electron-depletion layer, as shown in Fig. 10. When HCHO gas is injected, the HCHO gas molecules prefer to react with O− at the surface. The adsorptive O− decreases with the increasing HCHO gas concentration and more and more electrons are re-injected back to the shell of In2O3 and the cation-doped In2O3 NWs. Rgas hardly changes without the adsorbed O− at the extreme high concentration of HCHO gas. As a result, Rgas decreases and the response increases with the HCHO gas concentration.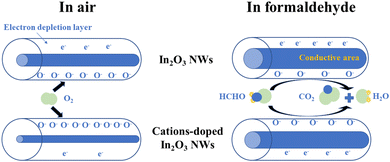 | ||
| Fig. 10 Theoretical schematic of the electron-depletion layer for In2O3 and the cation-doped In2O3 NWs in air and formaldehyde. | ||
The conduction pathway for n-type In2O3 NWs is equivalent to a series circuit of the core resistance (Rcore) and shell resistance (Rshell).29 In this way, the response can be rewritten as follows in eqn (5):
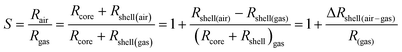 | (5) |
If a higher response is wanted, ΔRshell(air–gas) should be big enough and Rgas should be small as much as possible. The same-valent Al3+, Sb3+ and La3+ ions that are substituted for In3+ in the In2O3 NWs hardly change the carrier concentration of the In1.816Sb0.184O3, In1.838Al0.162O3 and In1.984La0.016O3 NWs compared with the undoped In2O3 NWs. Furthermore, the In2O3, In1.838Al0.162O3, In1.816Sb0.184O3 and In1.984La0.016O3 NWs are synthesized using SBA-15 silica as a hard template under the same chemical conditions and calcination temperature, so the microstructures, morphology and defects of all the samples should be similar. Only in this way can the influence of the lattice distortion caused by cation doping on the gas-sensing performance of the In1.816Sb0.184O3, In1.838Al0.162O3 and In1.984La0.016O3 NW sensors be truly revealed. As is known, cation-doping leads to lattice distortion of the In2O3 NWs for the different cation radii, resulting in a change in the oxygen vacancy concentration or chemical state of the adsorbed ions on the surface.39 At the same time, cation doping causes destruction of the periodic potential field, making the In2O3 lattice more open, which facilitates the diffusion of molecular oxygen through the In2O3 lattice.40
The radii of Al3+ (0.0535 nm) and La3+ (0.1032 nm) present a larger difference with that of In3+ (0.080 nm), whereas Sb3+ (0.076 nm) possesses a similar radius to In3+.41 Doping with the large La3+ radius causes the largest lattice distortion for the In1.984La0.016O3 NW lattice whereas doping with Al3+ and Sb3+, which have a smaller radius than La3+, bring about a smaller lattice distortion, as shown in schematic diagram in Fig. 11. Moreover, the cation-doped In2O3 NWs present the similar specific surface areas with a value of about 50 m2 g−1, which possesses the similar adsorbed oxygen owing to the mesoporous structures.42 As shown in Fig. 10, the larger lattice distortion results in more oxygen vacancies, which leads to the more adsorbed oxygen on the surface of the cation-doped In2O3 NWs.22 It was reported that rare-earth doping increases the surface alkalinity, which further improves the adsorbed oxygen of the In1.984La0.016O3 NWs.43 The more adsorbed oxygen leads to a larger change of ΔRshell(air–gas) according to eqn (5) for the In1.984La0.016O3 NWs sensor.
Moreover, cation-doping generates the lattice distortion of the In1.816Sb0.184O3, In1.838Al0.162O3 and In1.984La0.016O3 NWs, which scatters the electron transport and increases the Rgas value. The cation-doping content increases from In1.984La0.016O3 to In1.838Al0.162O3 and In1.816Sb0.184O3 NWs, leading to the highest Rgas for the In1.816Sb0.184O3 NWs and the lowest Rgas for the In1.984La0.016O3 NWs. According to eqn (5), the response of all the sensors to HCHO gas increases from 19.78 for In1.816Sb0.184O3 to 26.16 for In2O3, 28.73 for In1.838Al0.162O3 and 39.51 for In1.984La0.016O3. La-doping not only improves the adsorbed oxygen on the surface of the In2O3 NW sensor but also increases Rgas. Compared with the In2O3, In1.816Sb0.184O3 and In1.838Al0.162O3 NW sensors, the In1.984La0.016O3 NW sensor presents the highest response owing to the larger lattice distortion and the lowest doping content.
4. Conclusions
Mesoporous In2O3 and cation-doped In2O3 NWs have been synthesized using a nanocasting method. Different metals (Al, Sb and La) have been introduced to study the influence on the sensing performance. The characterization results show that both cation-doped In2O3 and undoped In2O3 NWs have a long-range-order mesoporous structure with a high specific surface area. All sensors present the best gas-sensing performance at the optimal working temperature of 210 °C, and the response of the La-doped In2O3 NWs reaches the maximum value of 39.51 for 10 ppm HCHO gas. The cation doping not only brings about lattice distortion to improve the adsorbed oxygen on the surface but also increases the ground-state resistance of the In2O3 NWs. Rare-earth doping increases the surface alkalinity, improving the sensor response towards HCHO. La-doping increases both the adsorbed oxygen and the lattice distortion of La-doped In2O3 NWs, and is responsible for the highly enhanced HCHO gas-sensing performance of the In1.984La0.016O3 NWs sensor.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This work was supported by the Natural Science Foundation of Zhejiang Province (LY16E030004 and LY20E020011) and the National Natural Science Foundation of China (U1809216).References
- N. Letellier, L. A. Gutierrez, C. Pilorget, F. Artaud, A. Descatha, A. Ozguler, M. Goldberg, M. Zins, A. Elbaz and C. Berr, Association Between Occupational Exposure to Formaldehyde and Cognitive Impairment, Neurology, 2022, 98, E633–E640 CrossRef CAS PubMed.
- T. Salthammer, S. Mentese and R. Marutzky, Formaldehyde in the indoor enviorment, Chem. Rev., 2010, 110, 2536–2572 CrossRef CAS PubMed.
- T. Y. Zhong, H. X. Li, T. M. Zhao, H. Y. Guan, L. L. Xing and X. Y. Xue, Self-powered/self-cleaned atmosphere monitoring system from combining hydrovoltaic, gas sensing and photocatalytic effects of TiO2 nanoparticles, J. Mater. Sci. Technol., 2021, 76, 33–40 CrossRef CAS.
- T. A. Vincent, Y. X. Xing, M. Cole and J. W. Gardner, Investigation of the response of high-bandwidth MOX sensors to gas plumes for application on a mobile robot in hazardous environments, Sens. Actuators, B, 2019, 279, 351–360 CrossRef CAS.
- Y. H. Kim, P. Kumar, E. E. Kwon and K. H. Kim, Metal-organic frameworks as superior media for thermal desorption-gas chromatography application: A critical assessment of MOF-5 for the quantitation of airborne formaldehyde, Microchem. J., 2017, 132, 219–226 CrossRef CAS.
- P. Wahed, M. A. Razzaq, S. Dharmapuri and M. Corrales, Determination of formaldehyde in food and feed by an in-house validated HPLC method, Food Chem., 2016, 202, 476–483 CrossRef CAS PubMed.
- X. C. Qin, R. Wang, F. Tsow, E. Forzani, X. J. Xian and N. J. Tao, A colorimetric chemical sensing platform for real-time monitoring of indoor formaldehyde, IEEE Sens. J., 2015, 15, 1545–1551 CAS.
- B. M. Woolston, T. Roth, I. Kohale, D. R. Liu and G. Stephanopoulos, Development of a formaldehyde biosensor with application to synthetic methylotrophy, Biotechnol. Bioeng., 2018, 115, 206–215 CrossRef CAS PubMed.
- J. N. Mao, B. Hong, H. D. Chen, M. H. Gao, J. C. Xu, Y. B. Han, Y. T. Yang, H. X. Jin, D. F. Jin, X. L. Peng, J. Li, H. L. Ge and X. Q. Wang, Highly improved ethanol gas response of n-type α-Fe2O3 bunched nanowires sensor with high-valence donor-doping, J. Alloys Compd., 2020, 827, 154248 CrossRef CAS.
- J. Q. Wei, X. Q. Li, Y. B. Han, J. C. Xu, H. X. Jin, D. F. Jin, X. L. Peng, B. Hong, J. Li, Y. T. Yang, H. L. Ge and X. Q. Wang, Highly improved ethanol gas-sensing performance of mesoporous nickel oxides nanowires with the stannum donor doping, Nanotechnology, 2018, 29, 245501 CrossRef PubMed.
- X. Q. Li, J. Q. Wei, J. C. Xu, H. X. Jin, D. F. Jin, X. L. Peng, B. Hong, J. Li, Y. T. Yang, H. L. Ge and X. Q. Wang, Highly improved sensibility and selectivity ethanol sensor of mesoporous Fe-doped NiO nanowires, J. Nanopart. Res., 2017, 19, 396 CrossRef.
- D. P. Li, B. B. Zhang, J. C. Xu, Y. B. Han, H. X. Jin, D. F. Jin, X. L. Peng, H. L. Ge and X. Q. Wang, Wide bandgap mesoporous hematite nanowire bundles as a sensitive rapid response ethanol sensor, Nanotechnology, 2016, 27, 185702 CrossRef PubMed.
- X. Q. Li, D. P. Li, J. C. Xu, Y. B. Han, H. X. Jin, B. Hong, H. L. Ge and X. Q. Wang, Calcination-temperature-dependent gas-sensing properties of mesoporous α-Fe2O3 nanowires as ethanol sensors, Solid State Sci., 2017, 69, 38–43 CrossRef CAS.
- D. Im, D. Kim, D. Jeong, W. I. Park, M. Chun, J. S. Park, H. Kim and H. Jung, Improved formaldehyde gas sensing properties of well-controlled Au nanoparticle-decorated In2O3 nanofibers integrated on low power MEMS platform, J. Mater. Sci. Technol., 2021, 38, 56–63 CrossRef.
- T. T. Zhou and T. Zhang, Recent progress of nanostructured sensing materials from 0D to 3D: overview of structure-property-application relationship for gas sensors, Small, Methods, 2021, 5, 2100515 CAS.
- L. Wang, S. Y. Song, B. Hong, J. C. Xu, Y. B. Han, H. X. Jin, D. F. Jin, J. Li, Y. T. Yang, X. L. Peng, H. L. Ge and X. Q. Wang, Highly improved toluene gas-sensing performance of mesoporous Co3O4 nanowires and physical mechanism, Mater. Res. Bull., 2021, 140, 111329 CrossRef CAS.
- M. Hassan, Z. H. Wang, W. R. Huang, M. Q. Li, J. W. Liu and J. F. Chen, Ultrathin tungsten oxide nanowires/reduced graphene oxide composites for toluene sensing, Sensors, 2017, 17, 2245 CrossRef PubMed.
- X. Q. Li, D. P. Li, J. C. Xu, H. X. Jin, D. F. Jin, X. L. Peng, B. Hong, J. Li, Y. T. Yang, H. L. Ge and X. Q. Wang, Mesoporous-structure enhanced gas-sensing properties of nickel oxides nanowires, Mater. Res. Bull., 2017, 89, 280–285 CrossRef CAS.
- H. D. Chen, K. L. Jin, J. C. Xu, Y. B. Han, H. X. Jin, D. F. Jin, X. L. Peng, B. Hong, J. Li, Y. T. Yang, J. Gong, H. L. Ge and X. Q. Wang, High-valence cation-doped mesoporous nickel oxides nanowires: nanocasting synthesis, microstructures and improved gas-sensing performance, Sens. Actuators, B, 2019, 296, 126622 CrossRef CAS.
- H. D. Chen, K. L. Jin, P. F. Wang, J. C. Xu, Y. B. Han, H. X. Jin, D. F. Jin, X. L. Peng, B. Hong, J. Li, Y. T. Yang, J. Gong, H. L. Ge and X. Q. Wang, Highly enhanced gas-sensing properties of indium-doped mesoporous hematite nanowires, J. Phys. Chem. Solids, 2018, 120, 271–278 CrossRef CAS.
- T. P. Mokoena, H. C. Swart, K. T. Hillie and D. E. Motaung, Engineering of rare-earth Eu3+ ions doping on n-type NiO for selective detection of toluene gas sensing and luminescence properties, Sens. Actuators, B, 2021, 347, 130530 CrossRef CAS.
- W. H. Zhang, S. J. Ding, Q. S. Zhang, H. Yi, Z. X. Liu, M. L. Shi, R. F. Guan and L. Yue, Rare earth element-doped porous In2O3 nanosheets for enhanced gas-sensing performance, Rare Met., 2021, 40, 1662–1668 CrossRef CAS.
- X. H. Jin, Y. W. Li, B. Zhang, X. T. Xu, G. Sun and Y. Wang, Temperature-dependent dual selectivity of hierarchical porous In2O3 nanospheres for sensing ethanol and TEA, Sens. Actuators, B, 2021, 330, 129271 CrossRef CAS.
- X. J. Liu, X. Y. Tian, X. M. Jiang, L. Jiang, P. Y. Hou, S. W. Zhang, X. Sun, H. C. Yang, R. Y. Cao and X. J. Xu, Facile preparation of hierarchical Sb-doped In2O3 microstructures for acetone detection, Sens. Actuators, B, 2018, 270, 304–311 CrossRef CAS.
- Q. H. Liang, X. X. Zou, H. Chen, M. H. Fan and G. D. Li, High-performance formaldehyde sensing realized by alkaline-earth metals doped In2O3 nanotubes with optimized surface properties, Sens. Actuators, B, 2020, 304, 127241 CrossRef CAS.
- Z. H. Wang, C. L. Hou, Q. M. De, F. B. Gu and D. M. Han, One-Step synthesis of Co-doped In2O3 nanorods for high response of formaldehyde sensor at low temperature, ACS Sens., 2018, 3, 468–475 CrossRef CAS PubMed.
- X. Y. Wang, J. J. Ma, Q. Ren, M. M. Wang, Z. Yang and J. H. Xin, Effects of Fe3+-doping and nano-TiO2/WO3 decoration on the ultraviolet absorption and gas-sensing properties of ZnSnO3 solid particles, Sens. Actuators, B, 2021, 344, 130223 CrossRef CAS.
- P. F. Cheng, Y. L. Wang, C. Wang, J. Ma, L. P. Xu, C. Lv and Y. F. Sun, Investigation of doping effects of different noble metals for ethanol gas sensors based on mesoporous In2O3, Nanotechnology, 2021, 32, 305503 CrossRef CAS PubMed.
- L. Wang, B. Hong, H. D. Chen, J. C. Xu, Y. B. Han, H. X. Jin, D. F. Jin, X. L. Peng, H. L. Ge and X. Q. Wang, The highly improved gas-sensing performance of α-Fe2O3-decorated NiO nanowires and the interfacial effect of p-n heterojunctions, J. Mater. Chem. C, 2020, 8, 3855–3864 RSC.
- W. X. Jin, S. Y. Ma, Z. Z. Tie, X. H. Jiang, W. Q. Li, J. Luo, X. L. Xu and T. T. Wang, Hydrothermal synthesis of monodisperse porous cube, cake and spheroid-like α-Fe2O3 particles and their high gas-sensing properties, Sens. Actuators, B, 2015, 220, 243–254 CrossRef CAS.
- W. W. Guo, B. Y. Zhao, L. L. Huang and Y. Z. He, One-step synthesis of ZnWO4/ZnSnO3 composite the enhanced gas sensing performance to formaldehyde, Mater. Lett., 2020, 277, 128327 CrossRef CAS.
- J. H. Sun, S. L. Bai, Y. Tian, Y. H. Zhao, N. Han, R. X. Luo, D. Q. Li and A. F. Chen, Hybridization of ZnSnO3 and rGO for improvement of formaldehyde sensing properties, Sens. Actuators, B, 2018, 257, 29–36 CrossRef CAS.
- R. K. Mishra, G. Murali, T. H. Kim, J. H. Kim, Y. J. Lim, B. S. Kim, P. P. Sahay and S. H. Lee, Nanocube In2O3@rGO heterostructure based gas sensor for acetone and formaldehyde detection, RSC Adv., 2017, 7, 38714–38724 RSC.
- Y. Y. Cao, Y. He, X. X. Zou and G. D. Li, Tungsten oxide clusters decorated ultrathin In2O3 nanosheets for selective detecting formaldehyde, Sens. Actuators, B, 2017, 252, 232–238 CrossRef CAS.
- I. Castro-Hurtado, J. Gonzalez-Chavarri, S. Morandi, J. Sama, A. Romano-Rodriguez, E. Castano and G. G. Mandayo, Formaldehyde sensing mechanism of SnO2 nanowires grown on-chip by sputtering techniques, RSC Adv., 2016, 6, 18558–18566 RSC.
- X. S. Wang, J. B. Zhang, L. Y. Wang, S. C. Li, L. Liu, C. Su and L. L. Liu, High response gas sensors for formaldehyde based on Er-doped In2O3 nanotubes, J. Mater. Sci. Technol., 2015, 31, 1175–1180 CrossRef CAS.
- K. Khojier, Preparation and investigation of Al-doped ZnO thin films as a formaldehyde sensor with extremely low detection limit and considering the effect of RH, Mater. Sci. Semicond. Process., 2020, 121, 105283 CrossRef.
- R. Prajesh, V. Goyal, M. Nahid, V. Saini, A. K. Singh, A. K. Sharma, J. Bhargava and A. Agarwal, Nickel oxide (NiO) thin film optimization by reactive sputtering for highly sensitive formaldehyde sensing, Sens. Actuators, B, 2020, 318, 128166 CrossRef CAS.
- S. K. Zhao, Y. B. Shen, A. Li, Y. S. Chen, S. L. Gao, W. G. Liu and D. Z. Wei, Effects of rare earth elements doping on gas sensing properties of ZnO nanowires, Ceram. Int., 2021, 47, 24218–24226 CrossRef CAS.
- Z. H. Wang, Z. W. Tian, D. M. Han and F. B. Gu, Highly sensitive and selective ethanol sensor fabricated with In-doped 3DOM ZnO, ACS Appl. Mater. Interfaces, 2016, 8, 5466–5474 CrossRef CAS PubMed.
- R. D. Shannon, Revised effective ionic radii and systematic studied of interatomic distances in halides and chalcogenides, Acta Crystallogr., 1976, 32, 751–767 CrossRef.
- J. Zhao, W. N. Wang, Y. P. Liu, J. M. Ma, X. W. Li, Y. Du and G. Y. Lu, Ordered mesoporous Pd/SnO2 synthesized by a nanocasting route for hydrogen sensing performance, Sens. Actuators, B, 2011, 160, 604–608 CrossRef CAS.
- A. Hastir, N. Kohli and R. C. Singh, Comparative study on gas sensing properties of rare earth (Tb, Dy and Er) doped ZnO sensor, J. Phys. Chem. Solids, 2017, 105, 23–34 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |