Open Access Article
Open Access ArticleFabrication of a recyclable magnetic halloysite-based cobalt nanocatalyst for the efficient degradation of bisphenol A and malachite green†
Bhavya
Arora
 ,
Shivani
Sharma
,
Sriparna
Dutta
,
Sneha
Yadav
,
Pooja
Rana
,
Priyanka
and
R. K.
Sharma
,
Shivani
Sharma
,
Sriparna
Dutta
,
Sneha
Yadav
,
Pooja
Rana
,
Priyanka
and
R. K.
Sharma
 *
*
Green Chemistry Network Centre, Department of Chemistry, University of Delhi, New Delhi-110007, India. E-mail: rksharmagreenchem@hotmail.com; Fax: +91-011-27666250; Tel: +91-011-276666250
First published on 9th July 2022
Abstract
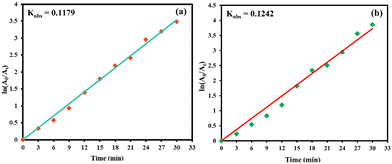
Worsening water quality has drawn considerable attention from the scientific fraternity owing to its serious impact on human health and environmental ecosystem. In this regard, magnetic halloysite-based organic–inorganic hybrid materials with a hollow nanotubular structure and surface tunable chemistry have emerged as an excellent platform for the efficient removal of recalcitrant water contaminants. The fabrication of nanocatalyst involves a simple yet versatile covalent immobilization strategy, wherein a chelating ligand, 2-benzoylpyridine (2-BPy) was grafted onto silane-functionalized magnetic halloysite nanotubes with subsequent anchoring of cobalt ions. Various physico-chemical techniques such as FT-IR spectroscopy, P-XRD, VSM, FE-SEM, TEM and XPS provided valuable insights into the crystallinity, magnetic attributes and morphology of the designed nanocomposites. The experimental results indicated that a Co(II)@2-BPy@APTES@MHNTs/H2O2 catalytic system not only exhibits immense catalytic potential in accelerating the degradation process but also shows impressive features such as shorter reaction time and ambient reaction conditions. With the assistance of coumarin fluorescent probe technique and radical scavenging studies, the mechanistic pathway has been proposed, and it has been found that in situ generated highly reactive hydroxyl radicals play a crucial role in achieving outstanding degradation efficiency. The kinetic characteristics of the degradation profile demonstrate that pseudo-first-order kinetics is followed with an apparent rate constant of 0.1179 min−1 for BPA and 0.1242 min−1 for MG. Fascinatingly, the splendid magnetic properties of the designed Co(II)@2-BPy@APTES@MHNTs furnished their facile recovery and reusability for nine subsequent runs. Remarkably, the new findings demonstrated here will deepen our understanding of the fabrication and utilization of heterogeneous catalysts for wastewater treatment via a greener approach.
Introduction
Clean water and sanitation have been globally recognized as one of the sustainable development goals (SDGs) outlined by various member countries of U.N. in the year 2015 when the entire world arrived at a common consensus of accomplishing the goals within the time frame of fifteen years, i.e. by 2030.1,2 The global population has not only been increasing every year but also resulting in increased urbanization and anthropogenic activities, which have, in turn, jeopardized the quality of water severely due to increased disposal of detrimental compounds in water bodies.3,4 Synthetic dyes, pesticides, pharmaceuticals, personal care products and organic pollutants are among the most common water pollutants that have exerted adverse effects on human survival and ecological environment even at low exposure levels. Bisphenol A (2,2-(4,4-dihydroxydiphenyl)propane, BPA) has been widely used as an important industrial precursor in the production of polyacrylates, polycarbonates, epoxy resins, polyester plastics, flame retardants and other chemical products such as baby bottles, food storage containers, toys, medicinal equipment, reusable drink containers, bar soaps, and shaving cream.5,6 Being highly soluble and stable in water, it gets easily leached from these materials and migrates into different aquatic streams, which ultimately results in the accumulation of BPA in the human body. Moreover, BPA has the tendency to mimic endogenous hormones, thereby causing sexual dysfunction, infertility, cancer (breast cancer, neuroblastoma), neurological disorders and cardiovascular diseases. Apart from this, synthetic water-soluble cationic dyes like malachite green (MG) that are used extensively in papermaking, food, leather, textile and cosmetic industries are carcinogenic, teratogenic and mutagenic, thus posing a serious threat.7,8 The toxicity associated with the contaminants has created an alarming situation, which needs to be tackled immediately at a realistic scale. Till date, various methods such as adsorption, biological treatment, coagulation processes, solvent extraction, precipitation, membrane separation, electrochemical oxidation and ion-exchange have been employed for the treatment of polluted water.9–11 However, these strategies are limited by a series of factors including incomplete degradation of effluents, sludge generation, harsh reaction conditions, high cost and tedious workup process. As a promising alternative in water remediation techniques, Fenton or Fenton-like reactions, one of the most efficient advanced oxidation processes (AOPs), have recently gained immense popularity due to excellent potency in degrading organic contaminants into harmless products.12 A plethora of methodologies are enlisted in the literature for the removal of BPA and MG, but these approaches encountered serious drawbacks such as lesser degradation efficiency, prolonged reaction time, harsh conditions and catalyst retrievability and reusability, which restrict their large-scale practical applicability.13–34 Therefore, a great array of endeavours need to be dedicated towards the fabrication and exploration of new catalytic materials, which not only overcome the abovementioned challenges but also meet the criteria of green chemistry.35–37 In this regard, engineered halloysite nanotube-based nanocomposites have recently emerged at the forefront as excellent heterogeneous catalytic systems. Naturally occurring one-dimensional halloysite nanotubes (HNTs) belong to a class of hydrated layered aluminosilicates with a chemical composition of Al2Si2O5(OH)4·2H2O in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 proportion of Al
1 proportion of Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si. The structure of HNTs consists of 15–20 rolled aluminosilicate layers, where the inner surface is composed of a gibbsite octahedral sheet of aluminol (Al–OH) groups, while the outer surface contains tetrahedral siloxane (Si–O–Si) groups.38,39 In recent years, HNTs have completely revolutionized the realm of materials science, due to the various fascinating properties such as hollow lumen structure, large surface area, high aspect ratio, great mechanical stability, high porosity, excellent thermal stability (up to 1200 °C), rich surface hydroxyl groups and tunable surface chemistry.40 By taking privilege of the aforementioned features, HNTs serve as promising heterogeneous solid supports in diverse areas such as drug delivery, catalysis, sensing, absorption, photonics, fibre spinning, rubber fillers, enzyme immobilization, and ion-exchange.41 Regardless of a copious number of benefits, HNTs still suffer from the problem of facile retrievability and reusability. In order to circumvent these challenges, incorporating the magnetic responses of Fe3O4 nanoparticles to the surface of HNTs is worth applauding, which furnished quick recoverability through the assistance of external magnets.42–44 Furthermore, organo-silane functionalization has provided an unprecedented platform for the introduction of desired active groups on the surface of materials.
Si. The structure of HNTs consists of 15–20 rolled aluminosilicate layers, where the inner surface is composed of a gibbsite octahedral sheet of aluminol (Al–OH) groups, while the outer surface contains tetrahedral siloxane (Si–O–Si) groups.38,39 In recent years, HNTs have completely revolutionized the realm of materials science, due to the various fascinating properties such as hollow lumen structure, large surface area, high aspect ratio, great mechanical stability, high porosity, excellent thermal stability (up to 1200 °C), rich surface hydroxyl groups and tunable surface chemistry.40 By taking privilege of the aforementioned features, HNTs serve as promising heterogeneous solid supports in diverse areas such as drug delivery, catalysis, sensing, absorption, photonics, fibre spinning, rubber fillers, enzyme immobilization, and ion-exchange.41 Regardless of a copious number of benefits, HNTs still suffer from the problem of facile retrievability and reusability. In order to circumvent these challenges, incorporating the magnetic responses of Fe3O4 nanoparticles to the surface of HNTs is worth applauding, which furnished quick recoverability through the assistance of external magnets.42–44 Furthermore, organo-silane functionalization has provided an unprecedented platform for the introduction of desired active groups on the surface of materials.
Enthused by the advantages offered by magnetic halloysite nanotubes (MHNTs) as potent heterogeneous catalytic supports and in conjugation with our recent research interest in the design and development of hybrid organic–inorganic nanomaterials for wastewater treatment and various organic transformations,45–49 we herein describe the fabrication of magnetically retrievable halloysite-based cobalt nanocatalysts (Co(II)@2-BPy@APTES@MHNTs). To our delight, we found that the newly designed cobalt-based catalyst reveals splendid catalytic efficiency in the oxidative degradation of noxious bisphenol A (BPA) and malachite green (MG) under optimal reaction conditions. With the combination of various marvellous features such as shorter reaction time, high degradation efficiency, quick magnetic separation, excellent stability and durability, the present protocol has proven its superiority to all previously reported ones. To the best of our understanding, this work presents the first example of a newly fabricated magnetic halloysite-based cobalt nanocatalyst that manifests great prospects for enhanced degradation as well as offers a practically operational and environmentally benign route, which aroused interest among the scientific fraternity for treatment of organic pollutants in water.
Results and discussions
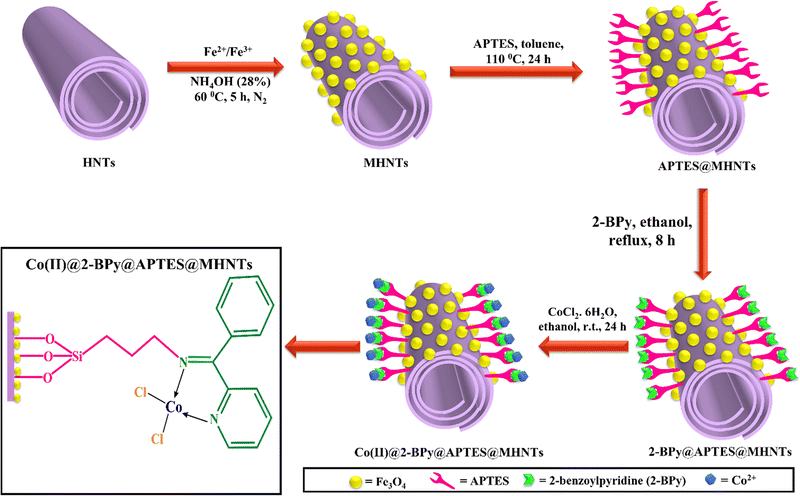
The catalyst preparation strategy fundamentally relies on the surface engineering of the magnetic halloysite nanotubes using a covalent grafting technique, which was conducted in a stepwise manner (Scheme 1). Initially, the halloysite nanotubes were decorated with ferrite nanoparticles by a co-precipitation technique.Anchoring of Fe3O4 NPs appeared to be a beneficial step because ferrite imparts imperishable magnetism to the catalyst, which facilitates its magnetic retrievability from the reaction media. Thereafter, the resulting MHNTs were functionalized with 3-aminopropyltriethoxysilane (APTES) to render the material amenable for further modification (ligand grafting and subsequently metalation). Apparently, APTES works as a good functionalizing agent for incorporating amino groups onto the surface of various matrices. Further, the immobilization of the ligand (2-BPy) onto the surface of APTES@MHNTs and metalation using cobalt chloride were achieved successively, which have been described in-depth in the experimental section. Moreover, the synthesized Co(II)@2-BPy@APTES@MHNTs nanocatalyst exhibits numerous unique characteristics such as enhanced degradation efficiency, incredible stability, ready recoverability, excellent recyclability and reusability. It is interesting to note that hydrogen peroxide (H2O2) acts as a fuel for generating active hydroxyl radical species, which further plays a crucial role towards expediting the degradation process. All the nanomaterials synthesized in steps were characterized thoroughly with the aid of advanced microscopic and spectroscopic techniques. Finally, the activity of the magnetic halloysite-supported cobalt catalyst was investigated in the degradation of BPA and MG.
Characterization of the designed catalyst
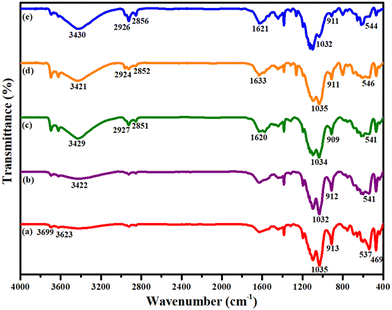
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibrations that validate the covalent immobilization of 2-BPy onto the surface of APTES@MHNTs via Schiff base condensation. Indeed, it is appreciable to mention that even after metalation of 2-BPy@APTES@MHNTs, all the inherent peaks appear in the final nanocatalyst, suggesting that the basic backbone structure remains completely intact. Nevertheless, a slight shift (i.e., 1621 cm−1) is observed in the value of absorption peak of –C
N stretching vibrations that validate the covalent immobilization of 2-BPy onto the surface of APTES@MHNTs via Schiff base condensation. Indeed, it is appreciable to mention that even after metalation of 2-BPy@APTES@MHNTs, all the inherent peaks appear in the final nanocatalyst, suggesting that the basic backbone structure remains completely intact. Nevertheless, a slight shift (i.e., 1621 cm−1) is observed in the value of absorption peak of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, which can be attributed to the coordinative linkage formation between the metal and the ligand.54,55
N, which can be attributed to the coordinative linkage formation between the metal and the ligand.54,55
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe to 10
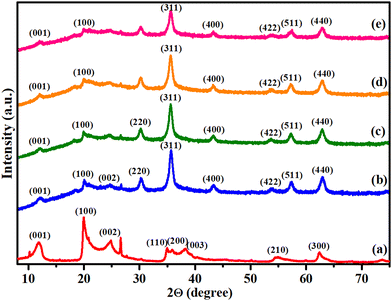
000 Oe to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe under atmospheric conditions. From Fig. 3, it can be clearly observed that the flat curve passes through the origin, indicating the absence of magnetic characteristics in pristine HNTs. However, for other synthesized nanocomposites, an S-shaped curve appeared, which implies that these are superparamagnetic in nature.
000 Oe under atmospheric conditions. From Fig. 3, it can be clearly observed that the flat curve passes through the origin, indicating the absence of magnetic characteristics in pristine HNTs. However, for other synthesized nanocomposites, an S-shaped curve appeared, which implies that these are superparamagnetic in nature.
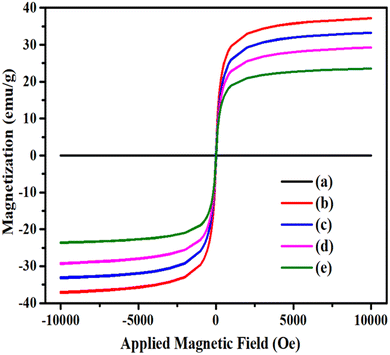 | ||
| Fig. 3 Magnetization curves obtained via VSM for (a) HNTs, (b) MHNTs, (c) APTES@MHNTs, (d) 2-BPy@APTES@MHNTs and (e) Co(II)@2-BPy@APTES@MHNTs. | ||
Further, it was observed that the saturation magnetization value of MHNTs (36.91 emu g−1) considerably decreased on moving to APTES@MHNTs (32.96 emu g−1) and 2-BPy@APTES@MHNTs (29.14 emu g−1). This gradual decrease in the Ms values could be ascribed to the grafting of surface-modifying entities onto bare MHNTs, which led to a reduction in surface moments of individual ferrite nanoparticles. Moreover, it is quite apparent from the VSM curves that the Ms value further dropped on moving from 2-BPy@APTES@MHNTs (29.14 emu g−1) to the final nanocatalyst (23.65 emu g−1), which was presumably due to the decoration of metal ions. Nevertheless, the net magnetism of the final nanocatalyst is found to be sufficiently good for its rapid and facile isolation from the reaction mixture with the aid of an external magnetic force.
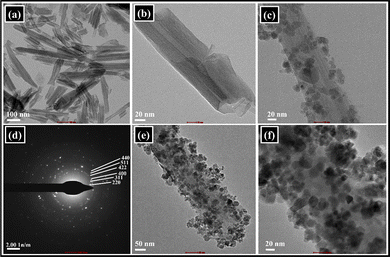 | ||
| Fig. 4 TEM images of (a and b) HNTs (100 nm, 20 nm), (c) MHNTs, (d) SAED pattern of Fe3O4 and (e and f) Co(II)@2-BPy@APTES@MHNTs (50 nm, 20 nm). | ||
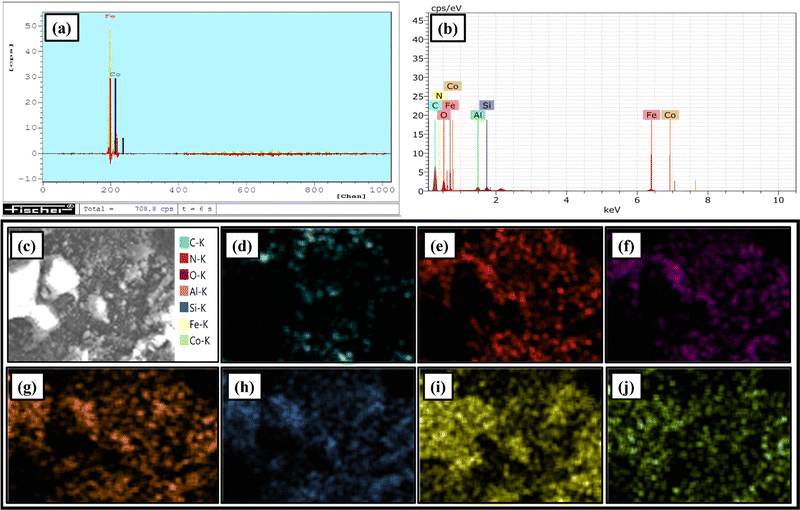
In addition, to shed light on the surface topographic attributes of HNTs, MHNTs and Co(II)@2-BPy@APTES@MHNTs, FE-SEM analysis was carried out. A thorough analysis of the FE-SEM micrograph of native HNTs (Fig. 5) revealed the tubular structure along with the smooth surface. Furthermore, from the FE-SEM image of MHNTs, it is quite evident that Fe3O4 particles with the spherical morphology have been appreciably decorated onto the surface of native HNTs.
Moreover, the FE-SEM images are in good agreement with the TEM images of Co(II)@2-BPy@APTES@MHNTs, authenticating that even after the grafting of discrete surface-modifying functionalities, the morphology remained intact.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–N
C and C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds. In addition, three characteristic peaks emerging at 529.57 eV, 531.41 eV and 532.08 eV in the core level spectra of O 1s are accordingly assigned to Fe–O, Al–O and Si–O.
C bonds. In addition, three characteristic peaks emerging at 529.57 eV, 531.41 eV and 532.08 eV in the core level spectra of O 1s are accordingly assigned to Fe–O, Al–O and Si–O.
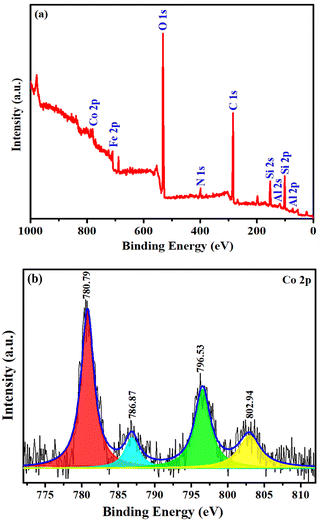 | ||
| Fig. 6 Full range survey scan XPS spectrum of (a) Co(II)@2-BPy@APTES@MHNTs and (b) core level region spectrum of Co 2p. | ||
Moreover, on moving to the high-resolution XPS spectrum of N 1s, the peak positioned at 399.63 eV corresponds well with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond, affirming the successful immobilization of ligands via Schiff base condensation.60 A detailed analysis of the high-resolution XPS spectrum of Fe 2p divulged two characteristic peaks at 709.63 eV (Fe 2p3/2) and 723.34 eV (Fe 2p1/2), which validated that iron is present in its +2 oxidation state. Moreover, the peaks at 711.35 (Fe 2p3/2) and 724.57 eV (Fe 2p1/2) are in good accordance with the trivalent state of iron, affirming the successful decoration of Fe3O4 NPs on the HNTs surface.61,62 Besides, the core level spectrum of Co 2p exhibits binding energy peaks at 780.79 eV and 786.87 eV, which match well with Co 2p3/2, while the peaks at 796.53 eV and 802.94 eV are correspondingly attributed to Co 2p1/2, which confirms the existence of +2 oxidation state of cobalt in Co(II)@2-BPy@APTES@MHNTs.63,64
N bond, affirming the successful immobilization of ligands via Schiff base condensation.60 A detailed analysis of the high-resolution XPS spectrum of Fe 2p divulged two characteristic peaks at 709.63 eV (Fe 2p3/2) and 723.34 eV (Fe 2p1/2), which validated that iron is present in its +2 oxidation state. Moreover, the peaks at 711.35 (Fe 2p3/2) and 724.57 eV (Fe 2p1/2) are in good accordance with the trivalent state of iron, affirming the successful decoration of Fe3O4 NPs on the HNTs surface.61,62 Besides, the core level spectrum of Co 2p exhibits binding energy peaks at 780.79 eV and 786.87 eV, which match well with Co 2p3/2, while the peaks at 796.53 eV and 802.94 eV are correspondingly attributed to Co 2p1/2, which confirms the existence of +2 oxidation state of cobalt in Co(II)@2-BPy@APTES@MHNTs.63,64
| Sr. No | Catalyst | Degradation efficiencya (%) | Degradation efficiencyb (%) |
|---|---|---|---|
| a Reaction conditions: 50 mL of BPA stock solution (68 mg L−1), 0.5 mL of H2O2, 8 mg of catalyst, pH = 7, 30 min, r.t., λmax = 276 nm. b Reaction conditions: 50 mL of MG stock solution (36 mg L−1), 0.8 mL of H2O2, 10 mg of catalyst, pH = 7, 30 min, r.t., λmax = 617 nm. | |||
| 1 | No catalyst | 20.30 | 25.88 |
| 2 | HNTs | 21.76 | 26.63 |
| 3 | MHNTs | 30.26 | 34.73 |
| 4 | APTES@MHNTs | 26.53 | 32.16 |
| 5 | 2-BPy@APTES@MHNTs | 23.19 | 28.14 |
| 6 | Co(II)@2-BPy@APTES@MHNTs | 96.93 | 97.89 |
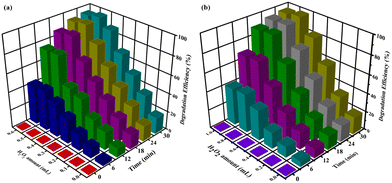
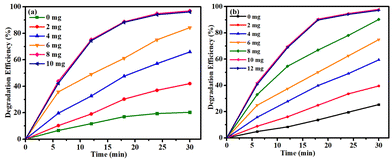
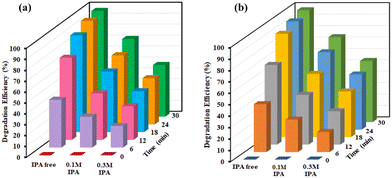
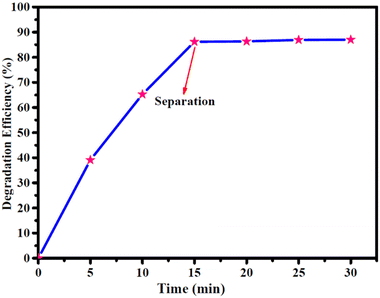
The obtained results indicated that under such conditions, the reaction failed to occur, suggesting the need of a potent catalyst in driving the degradation process. Furthermore, other nanocomposites were also screened under similar reaction profiles, but found to be totally ineffective for catalysing the reaction. A thorough analysis of the results concluded that by using Co(II)@2-BPy@APTES@MHNTs, outstanding catalytic degradation efficiency could be achieved. Next, we also evaluated the significance of oxidants in conducting the concerned degradation of BPA (Fig. S4, ESI†) and MG (Fig. S5, ESI†). Considering this, the reaction was performed in the absence of oxidant (i.e., only in the presence of catalyst). Almost negligible degradation efficiency of contaminants was observed, which led to the conclusion that presence of both the oxidant and catalyst is highly imperative in the reaction for attaining maximum degradation efficiency.
| H2O2 + ˙OH → H2O + ˙O2H |
| ˙O2H + ˙OH → H2O + O2 |
| Degradation efficiency (D%) = (A0 − At)/A0 × 100 |
| ln(A0/At) = kobst |
Conclusion
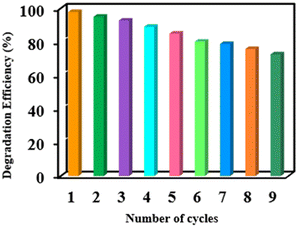
In conclusion, we have successfully demonstrated a facile and stepwise approach for fabricating magnetically separable Co(II)@2-BPy@APTES@MHNTs heterogenous nanocomposites, which involves covalent immobilization of 2-BPy onto the surface of amino-silane-functionalised magnetic halloysite nanotubes followed by metalation. Various physico-chemical techniques such as P-XRD, TEM, FE-SEM, XPS, EDX, ED-XRF and FT-IR spectroscopy provided a deep insight into the morphology, magnetization and crystallinity of the newly synthesized nanocomposites. Further, the catalytic activity of Co(II)@2-BPy@APTES@MHNTs was investigated for the first time in the oxidative degradation of BPA and MG, which were used as model water contaminants. Moreover, to achieve the maximum degradation efficiency of pollutants, different reaction parameters such as the amount of catalyst, oxidant dosage, pH and initial pollutant concentration were studied. During the process, the designed Co(II)@2-BPy@APTES@MHNTs exhibit a superior degradation efficiency of 97.89% (BPA) and 96.83% (MG) within 30 min. Besides, the presence of the magnetically responsive attribute of the heterogeneous Co(II)@2-BPy@APTES@MHNTs nanocatalyst offers effortless magnetic retrievability. Moreover, a shorter reaction time, exceptional degradation efficiency, high TON and TOF, absence of harsh chemicals, excellent recycling potential (up to nine consecutive runs), and ambient reaction conditions are some of the other interesting features that made the overall protocol highly sustainable and a better alternative to all other previously reported methodologies. Thus, we believe that the present work sheds light on the enormous potential of the designed nanocomposite, making it a promising candidate for eliminating recalcitrant water contaminants, as well as opens up new and promising possibilities for its usage in real-world applications.Experimental
Synthesis of MHNTs
The synthetic approach for the fabrication of a magnetically retrievable nanocatalytic support (MHNTs) was based on the well-known co-precipitation technique.71 Initially, the synthetic protocol involved the addition of 0.5 g of native HNTs to 400 mL of distilled water, which was further ultrasonicated for 15 min. After the uniform dispersion of HNTs, 1.165 g of FeCl3·6H2O and 0.5 g of FeCl2·4H2O were added and stirred vigorously at 60 °C in nitrogen atmosphere. With progress in time, the colour of the solution turned to yellowish orange. Subsequently, 28% of NH4OH solution was added dropwise to the above-mentioned reaction mixture till the pH of solution turned basic. The black precipitate of MHNTs was formed immediately as the base was added to the different salts of iron (Fe2+/Fe3+). The resulting solution was kept under stirring for further 5 h. Thereafter, the synthesized product was separated using an external magnet and successive washing was conducted with distilled water and ethanol until the pH of solution became neutral. Ultimately, the washed MHNTs were kept in an oven at 60 °C for drying.Synthesis of APTES@MHNTs
For introducing the silane-functionalizing agent (APTES) onto the surface of MHNTs, 1.5 g of MHNTs was added into a round-bottomed flask containing 50 mL of dried toluene and homogeneously dispersed via ultrasonic irradiation for 20 min.72 The flask was fitted with a rubber septum and a condenser, and the reaction mixture was vigorously stirred in a nitrogen atmosphere. Afterwards, 5 mL of APTES solution was injected in a dropwise manner into the above-mentioned solution, which was then placed in an oil bath at a temperature of 110 °C and refluxed for 24 h at the same temperature. Once the reaction was completed, it was cooled down to the ambient temperature and the solid product was separated via an external magnetic force. Finally, it was washed thoroughly with ethanol to remove the unreacted aminosilane residues and dried under vacuum at 80 °C.Synthesis of Co(II)@2-BPy@APTES@MHNTs
The synthesis of the Co(II)@2-BPy@APTES@MHNTs nanocatalyst was accomplished via a facile two-step procedure. At the outset, the fabrication of ligand-immobilized amine-functionalized MHNTs was carried out, which involved the uniform dispersion of 1.5 g of APTES@MHNTs into 25 mL of ethanol. Thereafter, to the above-mentioned reaction suspension, 2-BPy was added and kept under continuous refluxing for 8 h to afford the desired 2-BPy@APTES@MHNTs nanocomposite.73 After the reaction cooled down to room temperature, the resulting product was magnetically isolated, washed repeatedly and dried overnight in a vacuum drying chamber at 60 °C. In the subsequent step, an ethanolic solution of cobalt chloride was prepared to which 1.0 g of uniformly dispersed 2-BPy@APTES@MHNTs was added. Under vigorous stirring, the reaction was conducted at room temperature for 24 h. With the aid of an external magnet, the desired Co(II)@2-BPy@APTES@MHNTs were separated, extensively washed with ethanol and kept overnight in a vacuum chamber for drying.74General procedure for the reaction
To a 50 mL stock solution of BPA or MG taken in a round-bottomed flask, a certain amount of H2O2 was added. For the reaction to occur in a continuous manner, it was kept under vigorous stirring, and in the meantime, an appropriate amount of catalyst was added into the solution media. During this catalytic procedure, the reaction solution was withdrawn at predetermined time intervals using a syringe, and the catalyst was immediately retrieved using an external magnet. Further, using a UV-Vis spectrophotometer, changes in the absorbance intensity of the remaining supernatant solution were recorded during the reaction.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Bhavya Arora, Sneha Yadav, Pooja Rana and Priyanka greatly acknowledge Council of Scientific and Industrial Research (CSIR) and University Grants Commission (UGC), Delhi, India for awarding research fellowships. Also, the authors thank to USIC, University of Delhi, India for FT-IR, VSM, XRD assistance, Punjab University for FE-SEM studies, AIIMS for TEM analysis and IIT Roorkee for XPS analysis. We are also thankful to Faculty Research Programme Grant – IoE and Department of Science and Technology (DST) (Grant ID: DST/TMDEWO/WTI2K19/EWFH/2019/290(G)) for awarding project for carrying work related to water remediation.References
- X. Zhou, Z. Li, T. Zheng, Y. Yan, P. Li, E. A. Odey, H. P. Mang and S. M. N. Uddin, Environ. Int., 2018, 120, 246–261 CrossRef PubMed.
- V. Soltaninejad, M. R. Ahghari, R. Taheri-Ledari and A. Maleki, Langmuir, 2021, 37, 4700–4713 CrossRef CAS PubMed.
- J. Rahimi, R. Taheri-Ledari, M. Niksefat and A. Maleki, Catal. Commun., 2020, 134, 105850 CrossRef CAS.
- R. Taheri-Ledari, K. Valadi, S. Gharibi and A. Maleki, Mater. Res. Bull., 2020, 130, 110946 CrossRef CAS.
- D. Chen, F. Xiong, H. Zhang, C. Ma, L. Cao and J. Yang, ACS Omega, 2020, 5, 1198–1205 CrossRef CAS PubMed.
- X. Liang, G. Wang, X. Dong, G. Wang, H. Ma and X. Zhang, ACS Appl. Nano Mater., 2018, 2, 517–524 CrossRef.
- M. A. Salam, M. R. Abukhadra and A. Adlii, ACS Omega, 2020, 5, 2766 CrossRef PubMed.
- D. C. Ghosh, P. K. Sen and B. Pal, J. Phys. Chem. B, 2020, 124, 2048–2059 CrossRef CAS PubMed.
- V. Soltaninejad and A. Maleki, J. Photochem. Photobiol., A, 2021, 404, 112906 CrossRef CAS.
- A. Maleki, M. Mohammad, Z. Emdadi, N. Asim, M. Azizi and J. Safaei, Arab. J. Chem., 2020, 13, 3017–3025 CrossRef CAS.
- A. Maleki, Z. Hajizadeh, V. Sharifi and Z. Emdadi, J. Cleaner Prod., 2019, 215, 1233–1245 CrossRef CAS.
- W. Ma, K. Wang, S. Pan and H. Wang, Langmuir, 2019, 36, 6924–6929 CrossRef PubMed.
- C. M. Park, J. Heo and Y. Yoon, Chemosphere, 2017, 168, 617–622 CrossRef CAS PubMed.
- X. Yang, P. F. Tian, C. Zhang, Y. Q. Deng, J. Xu, J. Gong and Y. F. Han, Appl. Catal., B, 2013, 134, 145–152 CrossRef.
- V. Cleveland, J. P. Bingham and E. Kan, Sep. Purif. Technol., 2014, 133, 388–395 CrossRef CAS.
- S. A. Mirzaee, N. Jaafarzadeh, H. T. Gomes, S. Jorfi and M. Ahmadi, Chem. Eng. J., 2019, 370, 372–386 CrossRef CAS.
- T. Bao, M. M. Damtie, A. Hosseinzadeh, W. Wei, J. Jin, H. N. P. Vo, J. S. Ye, Y. Liu, X. F. Wang, Z. M. Yu, Z. J. Chen and B. J. Ni, J. Environ. Manage., 2020, 260, 110105 CrossRef CAS PubMed.
- L. Ma, H. He, R. Zhu, J. Zhu, I. D. Mackinnon and Y. Xi, Catal. Sci. Technol., 2016, 6, 6066–6075 RSC.
- B. Guo, T. Xu, L. Zhang and S. Li, J. Environ. Manage., 2020, 272, 111047 CrossRef CAS PubMed.
- M. P. Pachamuthu, S. Karthikeyan, R. Maheswari, A. F. Lee and A. Ramanathan, Appl. Surf. Sci., 2017, 393, 67–73 CrossRef CAS.
- K. Pan, C. Yang, J. Hu, W. Yang, B. Liu, J. Yang, S. Liang, K. Xiao and H. Hou, J. Hazard. Mater., 2020, 389, 122072 CrossRef CAS PubMed.
- X. Zhang, Y. Ding, H. Tang, X. Han, L. Zhu and N. Wang, Chem. Eng. J., 2014, 236, 251–262 CrossRef CAS.
- L. Zhang, D. Xu, C. Hu and Y. Shi, Appl. Catal., B, 2017, 207, 9–16 CrossRef CAS.
- M. Ahmadi, H. Rahmani, A. Takdastan, N. Jaafarzadeh and A. Mostoufi, Process Saf. Environ. Prot., 2016, 104, 413–421 CrossRef CAS.
- M. Ergüt, D. Uzunoğlu and A. Özer, J. Environ. Sci. Health A, 2019, 54, 786–800 CrossRef PubMed.
- Y. Hu, Y. Li, J. He, T. Liu, K. Zhang, X. Huang, L. Kong and J. Liu, J. Environ. Manage., 2018, 226, 256–263 CrossRef CAS PubMed.
- F. V. De Andrade, A. B. De Oliveira, G. O. Siqueira, M. M. Lage, M. R. de Freitas, G. M. de Lima and J. Nuncira, J. Environ. Chem. Eng., 2021, 9, 106232 CrossRef CAS.
- M. Hajnajafi, A. Khorshidi, A. G. Gilani and B. Heidari, Res. Chem. Intermed., 2018, 44, 3313–3323 CrossRef CAS.
- E. Yabalak and F. Elneccar, Biomass Convers. Biorefin., 2021, 1–13 Search PubMed.
- D. B. Jiang, Y. Yuan, D. Zhao, K. Tao, X. Xu and Y. X. Zhang, J. Nanoparticle Res., 2018, 20, 1–10 CrossRef CAS.
- K. Dutta, S. Bhattacharjee, B. Chaudhuri and S. Mukhopadhyay, J. Environ. Sci. Health A, 2003, 38, 1311–1326 CrossRef PubMed.
- S. Saha and A. Pal, Sep. Purif. Technol., 2014, 134, 26–36 CrossRef CAS.
- Y. Wu, S. Zeng, F. Wang, M. Megharaj, R. Naidu and Z. Chen, Sep. Purif. Technol., 2015, 154, 161–167 CrossRef CAS.
- Z. Li, Z. Chen, Q. Zhu, J. Song, S. Li and X. Liu, J. Hazard. Mater., 2020, 399, 123088 CrossRef CAS PubMed.
- A. Maleki, Z. Varzi and F. Hassanzadeh-Afruzi, Polyhedron, 2019, 171, 193–202 CrossRef CAS.
- Z. Varzi and A. Maleki, Appl. Organomet. Chem., 2019, 33, e5008 CrossRef.
- A. Maleki, Ultrason. Sonochem., 2018, 40, 460–464 CrossRef CAS PubMed.
- H. Hamza, A. M. Ferretti, C. Innocenti, K. Fidecka, E. Licandro, C. Sangregorio and D. Maggioni, Inorg. Chem., 2020, 59, 12086–12096 CrossRef CAS PubMed.
- M. Rouhi, M. Babamoradi, Z. Hajizadeh, A. Maleki and S. T. Maleki, Optik, 2020, 212, 164721 CrossRef CAS.
- C. E. Tas, E. B. Sevinis Ozbulut, O. F. Ceven, B. A. Tas, S. Unal and H. Unal, ACS Omega, 2020, 5, 17962–17972 CrossRef CAS PubMed.
- H. Zhang, T. Ren, Y. Ji, L. Han, Y. Wu, H. Song, L. Bai and X. Ba, ACS Appl. Mater. Interfaces, 2015, 7, 23805–23811 CrossRef CAS PubMed.
- A. Maleki, RSC Adv., 2014, 4, 64169–64173 RSC.
- A. Maleki, Tetrahedron Lett., 2013, 54, 2055–2059 CrossRef CAS.
- A. Maleki, Tetrahedron, 2012, 68, 7827–7833 CrossRef CAS.
- R. K. Sharma, B. Arora, S. Sharma, S. Dutta, A. Sharma, S. Yadav and K. Solanki, Mater. Chem. Front., 2020, 4, 605–620 RSC.
- B. Arora, S. Sharma, S. Dutta, A. Sharma, S. Yadav, P. Rana, P. Rana and R. K. Sharma, New J. Chem., 2022, 46, 5405–5418 RSC.
- S. Yadav, R. Dixit, S. Sharma, S. Dutta, B. Arora, P. Rana, B. Kaushik, P. Rana, A. Adholeya, M. B. Gawande and R. K. Sharma, Mater. Chem. Front., 2021, 5, 7343–7355 RSC.
- S. Yadav, R. Dixit, S. Sharma, S. Dutta, B. Arora, P. Rana, B. Kaushik, K. Solanki and R. K. Sharma, New J. Chem., 2022, 46, 10829–10843 RSC.
- S. Yadav, S. Sharma, S. Dutta, A. Sharma, A. Adholeya and R. K. Sharma, Inorg. Chem., 2020, 59, 8334–8344 CrossRef CAS PubMed.
- X. Wan, Y. Zhan, G. Zeng and Y. He, Appl. Surf. Sci., 2017, 393, 1–10 CrossRef CAS.
- Y. Zhang, X. He, J. Ouyang and H. Yang, Sci. Rep., 2013, 3, 1–6 Search PubMed.
- A. Maleki, Z. Hajizadeh and R. Firouzi-Haji, Microporous Mesoporous Mater., 2018, 259, 46–53 CrossRef CAS.
- Y. Xie, D. Qian, D. Wu and X. Ma, Chem. Eng. J., 2011, 168, 959–963 CrossRef CAS.
- G. R. Reddy and S. Balasubramanian, RSC Adv., 2015, 5, 53979–53987 RSC.
- H. Ebrahimiasl and D. Azarifar, Appl. Organomet. Chem., 2020, 34, e5359 CAS.
- A. B. Ganganboina, A. Dutta Chowdhury and R. A. Doong, ACS Sustainable Chem. Eng., 2017, 5, 4930–4940 CrossRef CAS.
- T. Zhu, C. Qian, W. Zheng, R. Bei, S. Liu, Z. Chi, X. Chen, Y. Zhang and J. Xu, RSC Adv., 2018, 8, 10522–10531 RSC.
- J. Pan, H. Yao, L. Xu, H. Ou, P. Huo, X. Li and Y. Yan, J. Phys. Chem. C, 2011, 115, 5440–5449 CrossRef CAS.
- S. C. Jee, M. Kim, S. K. Shinde, G. S. Ghodake, J. S. Sung and A. A. Kadam, Appl. Surf. Sci., 2020, 509, 145358 CrossRef CAS.
- A. Saroja and B. R. Bhat, Ind. Eng. Chem. Res., 2018, 58, 590–601 CrossRef.
- X. Song, L. Zhou, Y. Zhang, P. Chen and Z. Yang, J. Cleaner Prod., 2019, 224, 573–582 CrossRef CAS.
- R. Nagarajan, P. Gupta, P. Singh and P. Chakraborty, Dalton Trans., 2016, 45, 17508–17520 RSC.
- C. Ren, H. Li, R. Li, S. Xu, D. Wei, W. Kang, L. Wang, L. Jia, B. Yang and J. Liu, RSC Adv., 2016, 6, 33302–33307 RSC.
- J. Sun, G. Yu, L. Liu, Z. Li, Q. Kan, Q. Huo and J. Guan, Catal. Sci. Technol., 2014, 4, 1246–1252 RSC.
- F. Chen, X. Wu, R. Bu and F. Yang, RSC Adv., 2017, 7, 41945–41954 RSC.
- Y. Shen, Z. H. Zhang and K. J. Xiao, RSC Adv., 2015, 5, 91846–91854 RSC.
- A. M. I. Jayaseeli, A. Ramdass and S. Rajagopal, Polyhedron, 2015, 100, 59–66 CrossRef.
- E. Spier, U. Neuenschwander and I. Hermans, Angew. Chem., Int. Ed., 2013, 52, 1581–1585 CrossRef CAS PubMed.
- D. D. Dionysiou, M. T. Suidan, I. Baudin and J. M. Laîné, Appl. Catal., B, 2004, 50, 259–269 CrossRef CAS.
- X. Guo, K. Wang and Y. Xu, Mater. Sci. Eng., B, 2019, 245, 75–84 CrossRef CAS.
- A. A. Kadam, J. Jang and D. S. Lee, ACS Appl. Mater. Interfaces, 2017, 9, 15492–15501 CrossRef CAS PubMed.
- A. B. Ganganboina, A. D. Chowdhury and R. A. Doong, Electrochim. Acta, 2017, 245, 912–923 CrossRef CAS.
- T. Baran, İ. Sargın, M. Kaya, P. Mulerčikas, S. Kazlauskaitė and A. Menteş, Chem. Eng. J., 2018, 331, 102–113 CrossRef CAS.
- P. Chakraborty, S. Purkait, S. Mondal, A. Bauzá, A. Frontera, C. Massera and D. Das, CrystEngComm, 2015, 17, 4680–4690 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ma00500j |
| This journal is © The Royal Society of Chemistry 2022 |