Open Access Article
Open Access ArticlePhotoactivated carbon dots inducing bacterial functional and molecular alterations†
Xiuli
Dong
a,
Ping
Wang
b,
Cristian E.
Rodriguez
a,
Yongan
Tang
c,
Sophia
Kathariou
*d,
Ya-Ping
Sun
*b and
Liju
Yang
 *a
*a
aDepartment of Pharmaceutical Sciences, Biomanufacturing Research Institute and Technology Enterprise, North Carolina Central University, Durham, North Carolina, USA. E-mail: lyang@nccu.edu
bDepartment of Chemistry, Clemson University, Clemson, South Carolina 29634, USA. E-mail: syaping@clemson.edu
cDepartment of Mathematics and Physics, North Carolina Central University, Durham, NC 27707, USA
dDepartment of Food, Bioprocessing & Nutrition Sciences, North Carolina State University, Raleigh, NC 27695, USA. E-mail: skathar@ncsu.edu
First published on 29th June 2022
Abstract
Carbon dots (CDots) of small carbon nanoparticles with oligomeric polyethylenimine for surface functionalization, coupled with visible light exposure, were found highly effective in the inactivation of bacterial pathogens. In this study, using a representative strain of a major foodborne pathogen – Listeria monocytogenes, as a target, the effects of the CDots treatment at sublethal concentrations on bacterial functions/behaviors related to the biofilm formation ability/potential, including cell attachment and swimming motility, were assessed. On the consequence at molecular level, the expression levels of the genes that are related to cell attachment/adhesion, motility, flagellar synthesis, quorum sensing, and environmental stress response and virulence were found all being up-regulated.
Introduction
Listeria monocytogenes is a foodborne opportunistic Gram-positive pathogen and is widespread in the environment. L. monocytogenes causes listeriosis, which is the third leading cause of death among major pathogens commonly transmitted by food.1 Particularly troubling is the fact that it can persist for an impressively long period of time, a decade or longer in some cases, on floors, drains and equipment within food industry premises.2,3 In addition, the biofilm formation by L. monocytogenes in food processing environments and food-contact surfaces results in even more persistence and stress resistance, which have long been recognized as especially problematic for food safety, presenting major challenges for the food industry.4 Many chemical and physical antimicrobial agents/strategies have been studied for the control of foodborne pathogens and their biofilms5–12 with some success, but the tough challenges still call for novel strategies for more effective yet low-cost solutions. In recent years, major achievements in the development of nanotechnology and its successful integration with biotechnology offer excellent opportunities to address the challenges in the control of persistent foodborne pathogens. In this regard, the strategy/methodology based on the potent antimicrobial properties of visible/natural light-activated carbon “quantum” dots or carbon dots (CDots)13–15 has been found highly promising for the effective and efficient inactivation of various bacterial pathogens,16–23 including multidrug-resistant pathogens and foodborne persistent pathogens.18,22,23CDots are generally small carbon nanoparticles with various surface passivation schemes,13–15 each with a carbon nanoparticle core (mostly <10 nm in diameter) and a thin shell of soft materials (organic or biological molecules) serving the surface passivation function. In our original exploration of the antimicrobial function of CDots, we found and demonstrated that CDots with visible/natural light activation are highly potent antibacterial agents against model Gram-positive and Gram-negative bacteria such as Bacillus subtilis and Escherichia coli.20 Since then, we have explored and validated the use of CDots in various structural configurations for the inactivation of model bacteria and selected bacterial pathogens,16,18,19,21,22 as well as their inhibitory effect on biofilm formation.17 For example, we recently reported that CDots are highly effective for the inactivation of several drug-resistant Listeria strains isolated from different foodborne outbreaks,23 and also nonpathogenic L. innocua UAM003-1A which is streptomycin and tetracycline resistant and an important reservoir for resistance determinants transferable to L. monocytogenes.24 The results from all these studies have shown consistently that light-activated CDots represent a new class of highly effective antimicrobial agents. Notably, unlike photosensitizers based on conventional semiconductor nanoparticles such as colloidal TiO2 that require hazardous UV irradiation, CDots are readily activated by benign visible/natural light, such as the light from a commonly used household LED lamp, which afford them particularly suitable for applications in food processing facilities and other human friendly environments under manmade visible light or natural ambient light conditions.
In the pursuit of more comprehensive mechanistic understanding of the photoinduced antimicrobial function of CDots, in this study, we selected L. monocytogenes as a target to examine the biological consequences occurred in the cells at the functional and molecular levels upon the treatment by CDots with visible light activation. We determined the minimal inhibitory concentration (MIC) of CDots for the inhibition of cell growth, the minimal bactericidal concentration (MBC), and the minimal concentration for inhibition of biofilm formation, and evaluated the effects of the CDots treatment on cell attachment and swimming motility, which are functions related to the biofilm formation ability/potential. At the molecular level, we examined the alterations in the expression levels of genes that are related to the functions of cell attachment, swimming motility, flagellar synthesis, biofilm formation, environmental stress response and virulence.
Materials and methods
CDots
CDots with oligomeric polyethylenimine (PEI, average molecular weight ∼600, from Polysciences) for dot surface functionalization, thus PEI-CDots, were prepared by using the method reported previously.22,25 In the method, small carbon nanoparticles with an average diameter around 5 nm were harvested from the commercially acquired carbon nano-powders (US Research Nanomaterials, Inc.), and the nanoparticles were surface functionalized by PEI in the microwave-assisted thermal reaction.26,27 The as-prepared sample was purified via dialysis against fresh water to remove free PEI and other impurities, followed by vigorous centrifuging to retain the supernatant as an aqueous solution of PEI-CDots. The results from the characterization by using microscopy and optical spectroscopy techniques were in agreement with those of similarly prepared samples reported previously.28 For the relevance to their photoinduced activities in this study, the PEI-CDots were verified for strong optical absorptions in the visible spectrum, with the observed absorption spectrum essentially unchanged from that of the small carbon nanoparticles, the same as what was found and reported previously.22,25 The PEI-CDots concentration expressed in this study was the concentration of carbon in the core carbon nanoparticles of the dots, which was measured in solution by absorption and calculated with the use of the separately determined absorptivity values of the carbon. The stock aqueous solution of PEI-CDots was stored in the dark at room temperature and used in antibacterial experiments. Characterizations of PEI-CDots’ properties are included in ESI.†Bacteria culture preparation
L. monocytogenes 10403S cells were grown in 10 mL tryptic soy broth (TSB) by inoculating with a single colony streaked on TSB plate at 37 °C overnight with constant agitation at 225 rpm in an Excella E24 incubator shaker (New Brunswick Scientific). The cells were then centrifugated at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g (Beckman Coulter Life Sciences, Indianapolis, IN, USA) for 5 min, and washed twice with phosphate buffer saline (PBS) buffer. The cell pellet was re-suspended and diluted in PBS or TSB to the desired cell concentration for further experiments.
000 × g (Beckman Coulter Life Sciences, Indianapolis, IN, USA) for 5 min, and washed twice with phosphate buffer saline (PBS) buffer. The cell pellet was re-suspended and diluted in PBS or TSB to the desired cell concentration for further experiments.
Minimum inhibitory concentration (MIC) assay, minimum bactericidal concentration (MBC) assay, and inhibition of biofilm formation
MIC and MBC assays are commonly used to evaluate the potency of an antimicrobial material in terms of the concentration at which it inhibits the growth of (MIC) or completely kills (MBC) 1 × 106 challenge microorganisms during 18–20 h incubation period at 35 ± 2 °C. In this study, the MIC assay was performed using the standard micro dilution method.29 Briefly, L. monocytogenes prepared as above was diluted to the concentration of ∼2 × 106 CFU mL−1 in TSB. The aqueous solution of PEI-CDots was 2-fold serial diluted with deionized water (DI–H2O), followed by mixing the resulting PEI-CDots solutions with bacterial cells at vol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) vol = 1
vol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The mixtures, containing ∼1 × 106 CFU mL−1 cells and different concentrations of CDots, were vortexed vigorously and then aliquoted into a 96-well plate at the volume of 150 μL per well. The negative control sample was the mixture of TBS and DI–H2O (vol
1. The mixtures, containing ∼1 × 106 CFU mL−1 cells and different concentrations of CDots, were vortexed vigorously and then aliquoted into a 96-well plate at the volume of 150 μL per well. The negative control sample was the mixture of TBS and DI–H2O (vol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) vol = 1
vol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) without cells and PEI-CDots. All samples were triplicated. The plate was placed on an Orbital shaker (BT Lab Systems, St. Louis, MO) with shaking at 300 rpm, and exposed to visible light from a commercially acquired household daylight LED bulb by CREE (omnidirectional 815 lumens) placed at ∼10 cm above the surface of the plate for 1 h. The plate was then incubated at 37 °C in the incubator for 20 h. The optical density at 595 nm (OD595) values of the samples were measured before the treatment and after the 20 h incubation using the Max M5 spectrophotometer (Molecular Devices, LLC, Sunnyvale, CA). The changes in OD595 values were used to indicate the growths of the cells. The MIC of CDots was the minimum concentration that showed no bacterial growth, e.g. no change in OD595 after 20 h incubation compared to the OD measured before the treatment.
1) without cells and PEI-CDots. All samples were triplicated. The plate was placed on an Orbital shaker (BT Lab Systems, St. Louis, MO) with shaking at 300 rpm, and exposed to visible light from a commercially acquired household daylight LED bulb by CREE (omnidirectional 815 lumens) placed at ∼10 cm above the surface of the plate for 1 h. The plate was then incubated at 37 °C in the incubator for 20 h. The optical density at 595 nm (OD595) values of the samples were measured before the treatment and after the 20 h incubation using the Max M5 spectrophotometer (Molecular Devices, LLC, Sunnyvale, CA). The changes in OD595 values were used to indicate the growths of the cells. The MIC of CDots was the minimum concentration that showed no bacterial growth, e.g. no change in OD595 after 20 h incubation compared to the OD measured before the treatment.
The MBC assay was performed using the same protocol for the CDots treatment of the cells, but examining the colony formation on solid brain heart infusion (BHI) agar after 48 h incubation instead of monitoring the growth of cells in liquid medium. Briefly, aliquots of 5 μL samples treated with different concentrations of CDots or the controls were placed on BHI agar plates and then incubated at 37 °C for 48 h. The MBC value was the lowest CDots concentration at which no bacterial colonies were observed on the BHI agar plates after 48 h incubation.
For biofilm formation assays, the same CDots treatment conditions were used as those for the MIC test above, except that the plates were incubated at 37 °C for 24 h. After the incubation, the plate was processed in 3 repeats of the following steps: immersing-in-water and dumping out, to thoroughly remove the planktonic cells in each well. The plates were then dried and stained using the crystal violet staining method to measure the formation of biofilm on the surface of the well. Briefly, crystal violet solution (0.07%, 180 μL) was added to each well, and the plate was incubated at room temperature for 10 min, and then the staining solution was discarded. The plate was immersed in tap water with shaking motions for 10 s and then water was dumped out, and the same procedure was repeated 3 times. After pat dried on paper towels, 200 μL of 30% acetic acid was added to each well, and the plate was incubated at room temperature for 15 min with gentle shaking. The optical density at 550 nm (OD550) was determined using the Max M5 spectrophotometer (Molecular Devices, LLC, Sunnyvale, CA), and the OD550 value was used as the measure of biofilm formation by each sample.
Swimming motility assay
Swimming motility of L. monocytogenes cells was assessed by growing the cells on semi-solid BHI agar (0.3% agar) plates at 28 °C for 24 h, with the change in the radius of the growth ring indicating the swimming motility alteration.30 In the experiment, freshly grown L. monocytogenes cells (1 mL) were washed twice with PBS and re-suspended in 5 mL PBS. The cells were treated with CDots at the concentration of 0, 5, and 10 μg mL−1 in a 96-well plate on the shaker under the visible light at room temperature for 1 h. After the treatment, aliquots of 1.5 μL CDots-treated cells and the controls (without CDots) were placed on the center of the semi-solid BHI agar (0.3% agar) in 6-well plates, and the radius of the swimming ring were measured after 24 h incubation at 28 °C.Bacterial cell attachment assay
For bacterial cell attachment assay, overnight grown L. monocytogenes cells (10 mL) were washed and diluted to 1/4 in PBS. The cells were then treated with CDots at the concentration of 0, 5, and 10 μg mL−1 in a 96-well plate under visible light at room temperature for 1 or 2 h without shaking. The blanks were those sharing the same reaction systems with PBS replacing the cell suspension. After the treatments, the planktonic cells in each well were removed, and the plate was immersed in tap water with gentle shaking for 10 s. After dumping out water, the plate was dried on a hot plate at 60 °C for 40 min. The attached cells were measured by the crystal violet staining method, using the same procedure as described in the biofilm formation assay. The OD at 550 nm (OD550) was used as the measure of attached cells in each sample.Determination of expression levels of target genes by reverse transcription real time PCR (qRT-PCR)
L. monocytogenes cells were treated with 0 or 10 μg mL−1 CDots under visible light for 1 h. The samples were collected and washed twice with PBS to remove CDots. Total RNA was extracted using the RiboPure RNA Purification Kit for Bacteria (Life Technologies, Carlsbad, CA). The RNA quality and concentration were examined using NanoDrop Spectrophotometer (Thermo Fisher Scientific, Wilmington, Delaware). Reverse transcription was performed for cDNA synthesis by using the SuperScript™ IV VILO™ Master Mix with ezDNase™ Enzyme (Invitrogen, Thermo Fisher Scientific, Carlsbad, CA) according to the manufacturer's instruction.The target genes selected in this study were associated with biofilm formation potential including quorum sensing genes, motility associated genes, and flagellar synthesis genes, environmental stress and virulence, and the genes related to starvation response were tested for the effects of environmental stress caused by CDots.
qRT-PCR reactions were prepared in triplicate, and each reaction (20 μL) contained 10 μL of 2 × Power SYBR™ Green PCR Master Mix (Applied Biosystems by Life Technologies, Carlsbad, CA), 0.8 μL of 7 μM reverse primer, 0.8 μL of 7 μM forward primer, 2 μL cDNA, and 6.4 μL nuclease-free water. The primers for the evaluated genes are according to previously published sequences,31–34 and are listed in Table 1. They were custom synthesized by Integrated DNA Technologies, Inc. (Coralville, Iowa). The housekeeping gene 16S rRNA was used as an endogenous control to normalize the data. The reactions were performed in QuantStudio™ 6 Flex Real-Time PCR System (Applied Biosystems by Life Technologies, Carlsbad, CA) with the program as follows: an initial hold step at 95 °C for 10 min, and then 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The 2−ΔΔCt method was used to analyze the relative gene expression by the use of QuantStudio™ 6 and 7 Flex System Software (Applied Biosystems by Life Technologies, Carlsbad, CA).
| Target gene | Function/protein product | Primer sequence (5′ to 3′) |
|---|---|---|
| 16S rRNA | Used as internal control in this study | ACCGTCAAGGGACAAGCA |
| GGGAGGCAGCAGTAGGGA | ||
| agrA | Quorum sensing response regulator | ATGAAGCAAGCGGAAGAAC |
| TACGACCTGTGACAACGATAAA | ||
| agrC | A sensor for the autocrine signal | GGGGTCAATCGCAGGTTTTG |
| CTTTAAGTTCGTTGGTTGCCGTA | ||
| agrD | A putative quorum-sensing peptide | AAATCAGTTGGTAAATTCCTTTCTAG |
| AATGGACTTTTTGGTTCGTATACA | ||
| degU | A putative response regulator | CAGTCCATTCACAGTTGGCATA |
| GCGAAGGTATCAAGCGAATTTTAG | ||
| flaA | Flagellin | CTGGTATGAGTCGCCTTAG |
| CATTTGCGGTGTTTGGTTTG | ||
| flgE | Flagellar hook protein | AATGCCAACACGACAGGATA |
| TTTGTTCCAGCGTAAAGTCC | ||
| fliG | Flagellar switch protein | CCGCCCTTATTATTTGGAGC |
| CGAGTTTAGCAATTCCTCCTG | ||
| motB | Flagellar motor rotation | TTCTGTTTGCCTCCAGTTC |
| CTCTTGTTCGTTTGCTTCTTTC | ||
| relA | Regulate the starvation responses | TGCGATGCCGAAGTCGAATA |
| GCAACCCCGTATTCAGCGAT | ||
| sigB | The global regulator of the stress response | TGGATTGCCGCTTACCAAGAA |
| TCGGGCGATGGACTCTACTA | ||
| inlA | A virulence factor | ACTTGGCAGTGGAGTATGGA |
| CTGAAGCGTCGTAACTTGGTC | ||
| hly | Listeriolysin O | AACCAGATGTTCTCCCTGTA |
| CACTGTAAGCCATTTCGTCA | ||
| gap | Glyceraldehyde-3P-dehydrogenase | GAACTGGAACACGTTGAGCA |
| TCCAAAAGGTGACTTCCGTC | ||
| plcA | Phosphatidylinositol-specific phospholipase C | AGCCTAGCAGCCATTTCTATC |
| CCGTATTCCTGCTTCTAGTTGT | ||
| murA | Cell wall hydrolase | AGCGCAGACGAAACAGCGCC |
| AGGAGTGGCCGTTGCTGATGC |
Results and discussion
MIC and MBC of CDots on planktonic L. monocytogenes and CDots’ effect on inhibition of biofilm formation
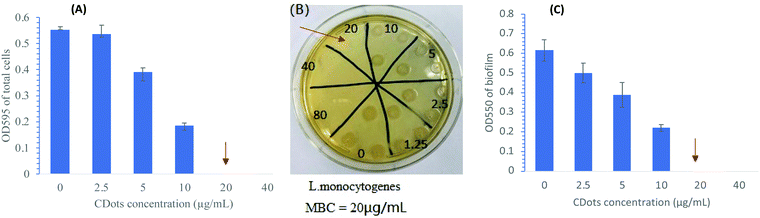
The MIC of CDots with visible light treating L. monocytogenes cells (∼1 × 106 CFU mL−1) were determined by monitoring the growth of cells post-treatment with various concentrations of 2-fold diluted CDots ranging from 2.5 to 80 μg mL−1. Fig. 1(A) shows the net OD changes of the CDots-treated L. monocytogenes cells after 20 h incubation at 37 °C compared to that before the treatment. Clearly, cell samples treated with CDots at concentrations of 5 and 10 μg mL−1 showed partial growth inhibition, with the degree of inhibition increasing with the CDots concentration used in the treatment, whereas samples treated with CDots at concentrations ≥20 μg mL−1 showed complete growth inhibition. The results indicate that the MIC of CDots with visible light activation was around 20 μg mL−1 for L. monocytogenes cells under the testing condition.The same CDots-treated samples were examined using MBC assay on their ability of colony formation on BHI agar plate after 48 h incubation. The prolonged incubation time gave sufficient time for sublethally damaged cells to recover and grow into colony. Thus, the MBC assay determines the minimal concentration of CDots required to completely kill all the cells in the treatment. Fig. 1(B) shows the picture of a representative plate with colony formation by L. monocytogenes samples that were treated with different concentrations of CDots with visible light activation. All samples treated with CDots at concentrations ≤10 μg mL−1 were able to form colonies, whereas samples treated with CDots at concentrations ≥20 μg mL−1 were not, indicating that the MBC of CDots is around 20 μg mL−1 for L. monocytogenes at the stated treatment condition, rather similar to the MIC value determined above.
Further, L. monocytogenes samples after the same treatment with visible light activated CDots were examined for effects on their ability to form biofilms by using the crystal violet staining method described above. The treated cells in the 96-well plate were incubated at 37 °C for 24 h. After removal of the planktonic cells, the plates were washed, dried and stained with the crystal violet, and the OD values at 550 nm were used for the evaluation of biofilm formation of the samples. Fig. 1(C) shows the OD550 of the stained biofilms formed by L. monocytogenes cells that were treated with CDots at concentrations ranging from 2.5 to 80 μg mL−1 under visible light for 1 h. According to the results, the biofilm formation by the CDots-treated cells was reduced in a dose-dependent manner with increasing CDots concentrations. Treatments with <20 μg mL−1 CDots partially prevented the formation of biofilm, while treatments with ≥20 μg mL−1 CDots completely prevented the formation of biofilm by the treated cells. As discussed above, the MIC and MBC for the CDots treatment were found to be around 20 μg mL−1, the same dot concentration apparently required and sufficient for the prevention of any biofilm formation. Thus, the observed no growth, no colony formation, and no biofilm formation by the cells post-treatment with 20 μg mL−1 CDots and visible light must all be due to the complete cell death in the treated samples. In further investigation, L. monocytogenes cells were treated with sublethal concentrations of CDots (5 and 10 μg mL−1) for an evaluation on effects of the treatment on the cellular functions/behaviors in terms of functional and molecular alterations of the cells.
Effects of CDots treatment on L. monocytogenes cell attachment and swimming motility
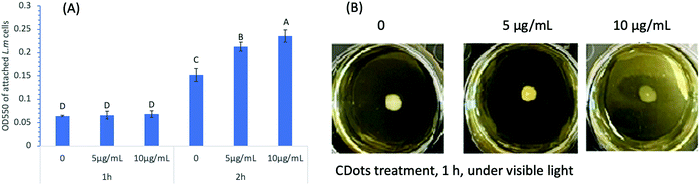
The cell adhesion/attachment potential has been a focus of extensive investigations for the understanding of L. monocytogenes’ persistence and biofilm formation ability, since cell adhesion/attachment is the first event when bacterial cells come in contact with a solid surface and the initial step of biofilm formation. To determine the effect of CDots treatment on cell attachment, L. monocytogenes cells (∼1 × 106 CFU mL−1) were treated with CDots at the concentrations of 5 and 10 μg mL−1 in a 96-well plate with exposure to the visible light for 1 h and 2 h. The cell attachment was measured by using the same protocol described above. Fig. 2(A) shows the results of cell attachment of CDots-treated L. monocytogenes samples, which was apparently dependent on the treatment time. For 1 h, the treatment did not have significant effects on the attachment of L. monocytogenes cells (p ≤0.05) to the surface of the wells (polystyrene), but for 2 h, there was an increase of about 2-fold in the cell attachment vs the 1 h treatment (p ≤ 0.05) at each treatment condition. The cell attachment was also more substantial when higher CDots concentrations were used in the treatment, with increases for the same 2 h treatment by 39.9% and 54.7% at CDots concentrations of 5 and 10 μg mL−1, respectively, from those of the untreated control samples. The results show that the treatment of CDots with visible light exposure could increase the cell attachment on the surface of 96-wells, likely a result of changes in bacterial biophysical status and behavior of the treated cells due to their response to the environmental stress induced by light-activated CDots.It is generally understood that the bacterial attachment process is complex, influenced by many factors such as material surface properties, hydrodynamic flow, bacterial motility, environmental stress, etc.35,36 Besides, the cell surface is dynamic, with varying cell organelle, continuously sensing and changing in response to environmental stress and other changes.37 In the presence of antimicrobial reagents, cell attachment could be a defensive response,38 triggering distinctive adaptive behavior of bacteria biofilms.39 There have also been reports suggesting that different strains of Listeria cells with significantly different initial cell attachment/adhesion capacities could reach comparable levels of cell density after 72 h incubation.40 On what were observed in this study, the known highly reactive species generated by the light-activated CDots18 must be causing elevated environmental stress to trigger adaptive behaviors of the cells and stimulate cell attachment during short contact time. On the other hand, the reduced biofilm formation after 24 h incubation (Fig. 1(C)) was apparently a direct consequence of cell death caused by the CDots treatment.
Swimming motility is another function of cells related to the potential of biofilm formation. Therefore, we further examined the effect of CDots with visible light treatment on the swimming motility of L. monocytogenes cells. The tests were performed on semi-solid BHI agar plates, with the change in the radius of the swimming ring as an indication for the change in cells’ swimming motility. It is known that L. monocytogenes cells are motile via flagella at 30 °C and below, but not at 37 °C.41 In the experiments, we tested L. monocytogenes samples that were pre-treated with CDots at 5 and 10 μg mL−1 under visible light for 1 h, and examined their motility by subsequent growing the treated cells on semi-solid BHI agar plates at 28 °C for 24 h. As shown in Fig. 2(B), the radii of the swimming motility rings of the CDots-treated samples had no visible change compared to that of the control samples without the CDots treatment, suggesting no visible change in swimming motility of the L. monocytogenes cells pre-treated with CDots at sublethal concentrations (5 and 10 μg mL−1) for a relatively short period of time (1 h in the experiment).
Effect of CDots treatment on the expression of genes related to cell adhesion/attachment and quorum sensing
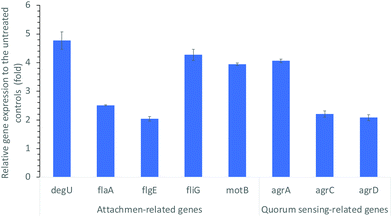
It is well known that environmental changes/stresses induce physicochemical alterations on bacterial behaviors. The initiation of biofilm formation is known as a result of a defensive reaction to the presence of antimicrobial reagents. For example, many of the antibacterial treatments trigger distinctive adaptive behavior of bacteria biofilms.39 Hoffman, et al.38 found that sub-inhibitory concentrations of aminoglycosides induced biofilm formation as a part of the defense response of E. coli and P. aeruginosa. Bacteria attachment as the initial step of biofilm formation is influenced by bacterial motility and other environmental conditions.35,36 The CDots treatment could definitely cause more environmental stress on bacteria and change bacterial biophysical status and behaviors, such as attachment/adhesion, swimming motility, and other functions related to biofilm formation. These behavioral/functional changes should be associated with and correlated to changes in the expression levels of related genes. In that regard, we examined the expression levels of genes related to biofilm formation including flagellar synthesis, the motility ability, and the quorum sensing system of L. monocytogenes.It is known that bacterial flagella play a role as adhesins in surface attachment42 and contribute to the adhesion and invasion of human epithelial cells,30 with some variation from strain to strain. The motility ability of flagella is also important in the early stage of biofilm formation,43 and integral to bacterial virulence.30 The genes associated with motility and flagellar synthesis and involved in the L. monocytogenes attachment include flaA, flgE, fliG, motB, and degU.44,45 In the experiment to probe alterations in the expression levels of these genes, L. monocytogenes cell samples treated with 10 μg mL−1 CDots with visible light and the untreated control cell samples were examined by reverse transcript real time PCR (qRT-PCR) using the primers and protocols described above in the Materials and Methods section. Fig. 3 shows the relative expression levels of the tested genes that are related to motility and flagellar synthesis after the CDots treatment. These genes were all up regulated substantially by 2.04- to 4.77-fold corresponding to the given CDots treatments, with degU showing the highest up-regulation (4.77-fold). DegU is involved in the regulation of flagellin expression on the post-transcriptional level, a putative response regulator involved in motility and virulence.46 The up-regulation of these genes agrees well with the results on the increased attachment of L. monocytogenes cells upon CDots treatments.
Quorum sensing (QS) system of L. monocytogens (agr system) is critical in its biofilm formation. QS is an intercellular communication system which bacteria use to coordinate their population density and control a variety of physiological processes.47 In L. monocytogenes, the QS is regulated by Agr system for intraspecies communication.48,49 It is the Agr system that regulates cell adhesion, biofilm formation,50,51 and infection of mammalian hosts.52Fig. 3 also shows the alterations in the expression levels of genes related to QS. As compared to the untreated controls, the CDots treatment substantially increased the expression of quorum-sensing gene agrA, agrC, and agrD, with 4.06-, 2.21-, and 2.08-fold up-regulation, respectively (Fig. 3). Apparently, the CDots treatment enhanced the QS in L. monocytogenes cells, triggering the cell self-defense response in the survived cells and the initiation of biofilm formation. However, the behavioral changes were associated with only the survived cells, whose numbers were limited, as the CDots treatment did kill the majority of L. monocytogenes cells. Consequently, in the CDots-treated samples there were less biofilm growth, and reduced biofilm formation, as observed in the experiments described above.
Effects of CDots treatment on the expression of genes related to environmental stress response and virulence
L. monocytogenes is a highly adaptable organism that can persist in a wide range of stress conditions such as low pH, low water activity, and low temperature. The stress tolerance of L. monocytogenes can be attributed in part to the general stress response of the organism by reconfiguring gene transcription to provide homeostatic and protective functions to cope with the stress.53 In our study, the L. monocytogenes cells post-treatment with 10 μg mL−1 CDots and visible light for 1 h were examined for the expression levels of several genes that are related to stress response and virulence. Fig. 4 shows the significant alterations in the expression levels of these genes.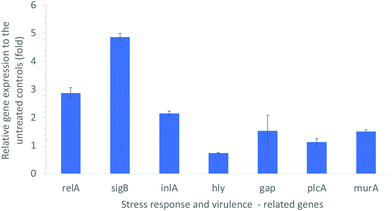 | ||
| Fig. 4 Alterations in the expression levels of environmental stress response- and virulence-related genes in L. monocytogenes, upon CDots treatment at 10 μg mL−1 for 1 h under visible light. | ||
The starvation response regulation gene relA is known as an essential gene for adhered cells on a model surface in response to nutrient deficiency.54,55 As shown in Fig. 4, the CDots treatment up-regulated the expression of relA gene by about 3-fold, suggesting that the treatment increased the stress responses of L. monocytogenes cells to resist the external harsh conditions. The CDots treatment also up-regulated the expression of sigB gene by about 5-fold. The sigB gene is a global regulatory gene of the stress response and closely related to virulence, and it also plays important roles in L. monocytogenes biofilm development.56 A deletion of sigB gene had been reported to affect the virulence phenotype of L. monocytogenes in murine and guinea pig models of infection.57 Other studies demonstrated that a L. monocytogenes sigB deletion mutant was more susceptible to environmental stresses such as acid stress,58 osmotic stress,59 and carbon starvation.60 The up-regulated expression of sigB gene indicated the increased stress response by L. monocytogenes cells to the environmental stress induced by the CDots treatment.
Other virulence related genes, including inlA (encoding a virulence factor), murA (autolysin), hly (encoding listeriolysin O), plcA (phosphatidylinositol-specific phospholipase C), and gap (encoding glyceraldehyde-3P-dehydrogenase), were also examined. The product of murA gene is similar to N-acetylmuramidase (autolysin), which is important for cell separation and autolysis of bacteria. The absence or malfunction of MurA results in virulence defects, e.g. diminished ability in adhesion to host cells.61 As shown in Fig. 4, the CDots treatment up-regulated the expression of murA by 1.51-fold, the expression of inlA by 2.15-fold, and gap by1.53-fold. However, the treatment slightly down-regulated hly gene expression by 26%, and kept the plcA expression almost the same. Gene hly encodes a cholesterol-binding, and pore-forming toxin (Listeriolysin O) that is essential for the bacterial escape from phagosomes.62 Listeriolysin O cooperates with two phospholipases C, plcA and plcB, for the effective escape from the vacuoles. The down-regulated expression of hly by the CDots treatment might slightly decrease the virulence of Listeriolysin O from L. monocytogenes bacteria, despite the almost the same plcA expression level.
Conclusions
CDots with visible light activation exhibited highly effective and efficient antimicrobial activities against foodborne L. monocytogenes. The MIC and MBC of PEI-CDots with 1 h light exposure for the inactivation of L. monocytogenes cells were found to be around 20 μg mL−1. The same dot concentration of 20 μg mL−1 and light exposure inhibited the treated cells from any biofilm. When sublethal concentrations of PEI-CDots (5 and 10 μg mL−1) were used, the treatment induces bacterial defensive responses, including the observed increases in cell attachment as the first step of the triggered adaptive behavior toward bacteria biofilms. Correspondingly at the molecular level, the expression levels of genes that are related to the cell attachment/adhesion, motility and flagellar synthesis, quorum sensing, and environmental stress response and virulence, were up-regulated. The results reported here, together with those from previous studies on the potent antibacterial activities of light-activated CDots,16–20 collectively contribute to the more comprehensive understanding of CDots as a new class of effective antimicrobial agents and their action mechanisms, and also to their practical applications in a variety of settings for combating bacterial pathogens and beyond.Author contributions
XD: conceptualization, data curation, investigation, methodology, writing – original draft; PW: conceptualization, data curation, investigation, methodology; CER: data curation, investigation; YT: conceptualization, funding acquisition, investigation, methodology, resources; SK: unding acquisition, methodology, resources; YPS: conceptualization, funding acquisition, project administration, resources, supervision, writing – review & editing; LY: conceptualization, funding acquisition, investigation, project administration, resources, uspervision, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from USDA grant 2019-67018-29689 and NSF grants 2102021 and 2102056 is gratefully acknowledged.References
- E. Scallan, R. M. Hoekstra, F. J. Angulo, R. V. Tauxe, M. A. Widdowson, S. L. Roy, J. L. Jones and P. M. Griffin, Foodborne illness acquired in the United States-major pathogens, Emerging Infect. Dis., 2011, 17, 7–15 CrossRef PubMed.
- B. Carpentier and O. Cerf, Review – persistence of listeria monocytogenes in food industry equipment and premises, Int. J. Food Microbiol., 2011, 145, 1–8 CrossRef PubMed.
- M. Gandhi and M. L. Chikindas, Listeria: A foodborne pathogen that knows how to survive, Int. J. Food Microbiol., 2007, 113, 1–15 CrossRef PubMed.
- T. Møretrø and S. Langsrud, Listeria monocytogenes: Biofilm formation and persistence in food-processing environments, Biofilms, 2004, 1, 107–121 CrossRef.
- M. G. Ammendolia, F. Iosi, B. De Berardis, G. Guccione, F. Superti, M. P. Conte and C. Longhi, Listeria monocytogenes behaviour in presence of non-UV-irradiated titanium dioxide nanoparticles, PLoS One, 2014, 9, e84986 CrossRef PubMed.
- W. M. Davison, B. Pitts and P. S. Stewart, Spatial and temporal patterns of biocide action against staphylococcus epidermidis biofilms, Antimicrob. Agents Chemother., 2010, 54, 2920–2927 CrossRef CAS PubMed.
- S. Galie, C. Garcia-Gutierrez, E. M. Miguelez, C. J. Villar and F. Lombo, Biofilms in the Food Industry: Health Aspects and Control Methods, Front. Microbiol., 2018, 9, 898 CrossRef PubMed.
- P. Gilbert, J. R. Das, M. V. Jones and D. G. Allison, Assessment of resistance towards biocides following the attachment of micro-organisms to, and growth on, surfaces, J. Appl. Microbiol., 2001, 91, 248–254 CrossRef CAS PubMed.
- J. A. Gray, P. S. Chandry, M. Kaur, C. Kocharunchitt, J. P. Bowman and E. M. Fox, Novel biocontrol methods for listeria monocytogenes biofilms in food production facilities, Front. Microbiol., 2018, 9, 605 CrossRef PubMed.
- M. W. Lechevallier, C. D. Cawthon and R. G. Lee, Inactivation of biofilm bacteria, Appl. Environ. Microbiol., 1988, 54, 2492–2499 CrossRef CAS PubMed.
- M. S. Nair, A. Upadhyay, S. Fancher, I. Upadhyaya, S. Dey, A. Kollanoor-Johny, J. Zhao and K. Venkitanarayanan, Inhibition and Inactivation of Escherichia coli O157:H7 Biofilms by Selenium, J. Food Prot., 2018, 81, 926–933 CrossRef CAS PubMed.
- B. Pitts, M. A. Hamilton, N. Zelver and P. S. Stewart, A microtiter-plate screening method for biofilm disinfection and removal, J. Microbiol. Methods, 2003, 54, 269–276 CrossRef CAS PubMed.
- Y.-P. Sun, Fluorescent carbon nanoparticles, US Pat. 7,829,772, 2010 Search PubMed.
- Y.-P. Sun, Carbon Dots – Exploring Carbon at Zero-Dimension, Springer International Publishing, 2020 Search PubMed.
- Y. P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. F. Wang, P. J. G. Luo, H. Yang, M. E. Kose, B. L. Chen, L. M. Veca and S. Y. Xie, Quantum-sized carbon dots for bright and colorful photoluminescence, J. Am. Chem. Soc., 2006, 128, 7756–7757 CrossRef CAS PubMed.
- M. M. Al Awak, P. Wang, S. Wang, Y. Tang, Y. P. Sun and L. Yang, Correlation of carbon dots’ light-activated antimicrobial activities and fluorescence quantum yield, RSC Adv., 2017, 7, 30177–30184 RSC.
- X. Dong, C. M. Overton, Y. Tang, J. P. Darby, Y.-P. Sun and L. Yang, Visible light-activated carbon dots for inhibiting biofilm formation and inactivating biofilm-associated bacterial cells, Front. Bioeng. Biotechnol., 2021, 9, 786077 CrossRef PubMed.
- X. L. Dong, L. Ge, D. I. Abu Rabe, O. O. Mohammed, P. Wang, Y. G. Tang, S. Kathariou, L. J. Yang and Y. P. Sun, Photoexcited state properties and antibacterial activities of carbon dots relevant to mechanistic features and implications, Carbon, 2020, 170, 137–145 CrossRef CAS.
- X. L. Dong, W. X. Liang, M. J. Meziani, Y. P. Sun and L. J. Yang, Carbon dots as potent antimicrobial agents, Theranostics, 2020, 10, 671–686 CrossRef CAS PubMed.
- M. J. Meziani, X. Dong, L. Zhu, L. P. Jones, G. E. LeCroy and F. Yang, Visible-Light-Activated Bactericidal function of carbon dots “Quantum” dots, ACS Appl. Mater. Interfaces, 2016, 8, 10761–10766 CrossRef CAS PubMed.
- D. I. Abu Rabe, M. M. Al Awak, F. Yang, P. A. Okonjo, X. Dong, L. R. Teisl, P. Wang, Y. Tang, N. Pan, Y. P. Sun and L. Yang, The dominant role of surface functionalization in carbon dots’ photo-activated antibacterial activity, Int. J. Nanomed., 2019, 14, 2655–2665 CrossRef CAS PubMed.
- D. I. Abu Rabe, O. O. Mohammed, X. L. Dong, A. K. Patel, C. M. Overton, Y. A. Tang, S. Kathariou, Y. P. Sun and L. J. Yang, Carbon dots for highly effective photodynamic inactivation of multidrug-resistant bacteria, Mater. Adv., 2020, 1, 321–325 RSC.
- X. Dong, P. Wang, J. P. Darby, Y. Tang, C. M. Overton, S. Kathariou, Y. P. Sun and L. Yang, Photoactivated carbon dots for inactivation of foodborne pathogens Listeria and salmonella, Appl. Environ. Microbiol., 2021, 87, e0104221 CrossRef PubMed.
- S. Katharios-Lanwermeyer, M. Rakic-Martinez, D. Elhanafi, S. Ratani, J. M. Tiedje and S. Kathariou, Coselection of cadmium and benzalkonium chloride resistance in conjugative transfers from nonpathogenic Listeria spp. to other Listeriae, Appl. Environ. Microbiol., 2012, 78, 7549–7556 CrossRef CAS PubMed.
- L. Ge, N. Y. Pan, J. R. Jin, P. Wang, G. E. LeCroy, W. X. Liang, L. J. Yang, L. R. Teisl, Y. A. Tang and Y. P. Sun, Systematic comparison of carbon dots from different preparations-consistent optical properties and photoinduced redox characteristics in visible spectrum and structural and mechanistic implications, J. Phys. Chem. C, 2018, 122, 21667–21676 CrossRef CAS.
- G. E. LeCroy, S. K. Sonkar, F. Yang, L. M. Veca, P. Wang, K. N. Tackett 2nd, J. J. Yu, E. Vasile, H. Qian, Y. Liu, P. G. Luo and Y. P. Sun, Toward structurally defined carbon dots as ultracompact fluorescent probes, ACS Nano, 2014, 8, 4522–4529 CrossRef CAS PubMed.
- F. Yang, G. E. LeCroy, P. Wang, W. Liang, J. Chen, K. A. S. Fernando, C. E. Bunker, H. Qian and Y.-P. Sun, Functionalization of carbon nanoparticles and defunctionalization—toward structural and mechanistic elucidation of carbon “Quantum” dots, J. Phys. Chem. C, 2016, 120, 25604–25611 CrossRef CAS.
- Y. Hu, M. M. Al Awak, F. Yang, S. J. Yan, Q. W. Xiong, P. Wang, Y. G. Tang, L. J. Yang, G. E. LeCroy, X. F. Hou, C. E. Bunker, L. X. Xu, N. Tomlinson and Y. P. Sun, Photoexcited state properties of carbon dots from thermally induced functionalization of carbon nanoparticles, J. Mater. Chem. C, 2016, 4, 10554–10561 RSC.
- CLSI, Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, approved standard-Eighth Edition. CLSI document M07-A8, Clinical and Laboratory Standards Institute, Wayne, (PA), 2009 Search PubMed.
- H. S. O'Neil and H. Marquis, Listeria monocytogenes flagella are used for motility, not as adhesins, to increase host cell invasion, Infect. Immun., 2006, 74, 6675–6681 CrossRef PubMed.
- A. Camejo, C. Buchrieser, E. Couve, F. Carvalho, O. Reis, P. Ferreira, S. Sousa, P. Cossart and D. Cabanes, In vivo transcriptional profiling of listeria monocytogenes and mutagenesis identify new virulence factors involved in infection, PLoS Pathog., 2009, 5, e1000449 CrossRef PubMed.
- W. F. Du, M. Zhou, Z. G. Liu, Y. Chen and R. Li, Inhibition effects of low concentrations of epigallocatechin gallate on the biofilm formation and hemolytic activity of Listeria monocytogenes, Food Control, 2018, 85, 119–126 CrossRef CAS.
- J. M. Huang, B. W. Chen, H. H. Li, Q. H. Zeng, J. J. Wang, H. Q. Liu, Y. J. Pan and Y. Zhao, Enhanced antibacterial and antibiofilm functions of the curcumin-mediated photodynamic inactivation against Listeria monocytogenes, Food Control, 2020, 108, 106886 CrossRef CAS.
- A. Upadhyay, I. Upadhyaya, A. Kollanoor-Johny and K. Venkitanarayanan, Antibiofilm effect of plant derived antimicrobials on Listeria monocytogenes, Food Microbiol., 2013, 36, 79–89 CrossRef CAS PubMed.
- S. Zheng, M. Bawazir, A. Dhall, H. E. Kim, L. He, J. Heo and G. Hwang, Implication of surface properties, bacterial motility, and hydrodynamic conditions on bacterial surface sensing and their initial adhesion, Front. Bioeng. Biotechnol., 2021, 9, 643722 CrossRef PubMed.
- E. K. Chu, O. Kilic, H. Cho, A. Groisman and A. Levchenko, Self-induced mechanical stress can trigger biofilm formation in uropathogenic Escherichia coli, Nat. Commun., 2018, 9, 4087 CrossRef PubMed.
- J. Palmer, S. Flint and J. Brooks, Bacterial cell attachment, the beginning of a biofilm, J. Ind. Microbiol. Biotechnol., 2007, 34, 577–588 CrossRef CAS PubMed.
- L. R. Hoffman, D. A. D'Argenio, M. J. MacCoss, Z. Y. Zhang, R. A. Jones and S. I. Miller, Aminoglycoside antibiotics induce bacterial biofilm formation, Nature, 2005, 436, 1171–1175 CrossRef CAS PubMed.
- D. Rodriguez Sartori, M. Bertuola, A. Minan, E. Gonik, M. C. Gonzalez and M. Fernandez Lorenzo de Mele, Environmentally induced changes of commercial carbon nanotubes in aqueous suspensions. adaptive behavior of bacteria in biofilms, ACS Omega, 2021, 6, 5197–5208 CrossRef CAS PubMed.
- J. M. Lunden, M. K. Miettinen, T. J. Autio and H. J. Korkeala, Persistent Listeria monocytogenes strains show enhanced adherence to food contact surface after short contact times, J. Food Prot., 2000, 63, 1204–1207 CrossRef CAS PubMed.
- A. Grundling, L. S. Burrack, H. G. A. Bouwer and D. E. Higgins, Listeria monocytogenes regulates flagellar motility gene expression through MogR, a transcriptional repressor required for virulence, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 12318–12323 CrossRef PubMed.
- S. Vatanyoopaisarn, A. Nazli, C. E. R. Dodd, C. E. D. Rees and W. M. Waites, Effect of flagella on initial attachment of Listeria monocytogenes to stainless steel, Appl. Environ. Microbiol., 2000, 66, 860–863 CrossRef CAS PubMed.
- E. C. R. Bonsaglia, N. C. C. Silva, A. Fernades, J. P. Araujo, M. H. Tsunemi and V. L. M. Rall, Production of biofilm by Listeria monocytogenes in different materials and temperatures, Food Control, 2014, 35, 386–391 CrossRef CAS.
- Y. H. Chang, W. M. Gu, N. Fischer and L. McLandsborough, Identification of genes involved in Listeria monocytogenes biofilm formation by mariner-based transposon mutagenesis, Appl. Microbiol. Biotechnol., 2012, 93, 2051–2062 CrossRef CAS PubMed.
- T. Williams, B. Joseph, D. Beier, W. Goebel and M. Kuhn, Response regulator DegU of Listeria monocytogenes regulates the expression of flagella-specific genes, FEMS Microbiol. Lett., 2005, 252, 287–298 CrossRef CAS PubMed.
- G. M. Knudsen, J. E. Olsen and L. Dons, Characterization of DegU, a response regulator in Listeria monocytogenes, involved in regulation of motility and contributes to virulence, FEMS Microbiol. Lett., 2004, 240, 171–179 CrossRef CAS PubMed.
- C. Solano, M. Echeverz and I. Lasa, Biofilm dispersion and quorum sensing, Curr. Opin. Microbiol., 2014, 18, 96–104 CrossRef CAS PubMed.
- K. P. Lemon, N. E. Freitag and R. Kolter, The Virulence Regulator PrfA Promotes Biofilm Formation by Listeria monocytogenes, J. Bacteriol., 2010, 192, 3969–3976 CrossRef CAS PubMed.
- F. J. Vazquez-Armenta, M. A. Hernandez-Onate, M. A. Martinez-Tellez, A. A. Lopez-Zavala, G. A. Gonzalez-Aguilar, M. M. Gutierrez-Pacheco and J. F. Ayala-Zavala, Quercetin repressed the stress response factor (sigB) and virulence genes (prfA, actA, inlA, and inlC), lower the adhesion, and biofilm development of L. monocytogenes, Food Microbiol., 2020, 87, 103377 CrossRef CAS PubMed.
- C. U. Riedel, I. R. Monk, P. G. Casey, M. S. Waidmann, C. G. M. Gahan and C. Hill, AgrD-dependent quorum sensing affects biofilm formation, invasion, virulence and global gene expression profiles in Listeria monocytogenes, Mol. Microbiol., 2009, 71, 1177–1189 CrossRef CAS PubMed.
- A. Rieu, S. Weidmann, D. Garmyn, P. Piveteau and J. Guzzo, agr system of Listeria monocytogenes EGD-e: Role in adherence and differential expression pattern, Appl. Environ. Microbiol., 2007, 73, 6125–6133 CrossRef CAS PubMed.
- D. Garmyn, L. Gal, J. P. Lemaitre, A. Hartmann and P. Piveteau, Communication and autoinduction in the species Listeria monocytogenes: A central role for the agr system, Commun. Integr. Biol., 2009, 2, 371–374 CrossRef PubMed.
- K. NicAogain and C. P. O’Byrne, The role of stress and stress adaptations in determining the fate of the bacterial pathogen listeria monocytogenes in the food chain, Front. Microbiol., 2016, 7, 1865 Search PubMed.
- A. M. Kocot and M. A. Olszewska, Biofilm formation and microscopic analysis of biofilms formed by Listeria monocytogenes in a food processing context, LWT – Food Sci. Technol., 2017, 84, 47–57 CrossRef CAS.
- C. A. Taylor, M. Beresford, H. A. S. Epton, D. C. Sigee, G. Shama, P. W. Andrew and I. S. Roberts, Listeria monocytogenes relA and hpt mutants are impaired in surface-attached growth and virulence, J. Bacteriol., 2002, 184, 621–628 CrossRef CAS PubMed.
- S. van der Veen and T. Abee, Importance of SigB for listeria monocytogenes static and continuous-flow biofilm formation and disinfectant resistance, Appl. Environ. Microbiol., 2010, 76, 7854–7860 CrossRef CAS PubMed.
- M. R. Garner, B. L. Njaa, M. Wiedmann and K. J. Boor, Sigma B contributes to Listeria monocytogenes gastrointestinal infection but not to systemic spread in the guinea pig infection model, Infect. Immun., 2020, 74, 876–886 CrossRef PubMed.
- M. Wiedmann, T. J. Arvik, R. J. Hurley and K. J. Boor, General stress transcription factor SigmaB and its role in acid tolerance and virulence of Listeria monocytogenes, J. Bacteriol., 1998, 180, 3650–3656 CrossRef CAS PubMed.
- L. A. Becker, M. S. Cetin, R. W. Hutkins and A. K. Benson, Identification of the gene encoding the alternative Sigma factor Sigma(B) from Listeria monocytogenes and its role in osmotolerance, J. Bacteriol., 1998, 180, 4547–4554 CrossRef CAS PubMed.
- A. Ferreira, C. P. O'Byrne and K. J. Boor, Role of Sigma(B) in heat, ethanol, acid, and oxidative stress resistance and during carbon starvation in Listeria monocytogenes, Appl. Environ. Microbiol., 2001, 67, 4454–4457 CrossRef CAS PubMed.
- F. Alonzo, P. D. McMullen and N. E. Freitag, Actin polymerization drives septation of listeria monocytogenes namA hydrolase mutants, demonstrating host correction of a bacterial defect, Infect. Immun., 2011, 79, 1458–1470 CrossRef CAS PubMed.
- M. A. Hamon, D. Ribet, F. Stavru and P. Cossart, Listeriolysin O: The Swiss army knife of listeria, Trends Microbiol., 2012, 20, 360–368 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ma00403h |
| This journal is © The Royal Society of Chemistry 2022 |