Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Electro-responsive polymer-based platforms for electrostimulation of cells
Akel Ferreira
Kanaan
and
Ana Paula
Piedade
 *
*
CEMMPRE, Department of Mechanical Engineering, University of Coimbra, 3030-788 Coimbra, Portugal. E-mail: ana.piedade@dem.uc.pt
First published on 1st February 2022
Abstract
Tissue engineering is a fascinating branch of science that aims to develop strategies to respond to tissue/organ damage with the slightest biological rejection. It is currently known that electric cues can trigger several cell metabolic responses such as differentiation, proliferation, growth, attachment, alignment, and apoptosis. Electro-responsive polymer-based materials are considered one of the most appropriate classes of materials for developing advanced conducting systems that can fulfill this purpose. These materials act as conducting platforms that can properly transmit electrical signals to cells and work as a physical environment to ensure cell nutrition and development. The motivation of this review is to summarize the recent work in terms of polymer-based conducting scaffolds for electro-assisted cell culture. An overview of the most commonly studied polymers and scaffold design methodologies is presented. Moreover, the mechanisms behind the effect of electric cues on cell fate are briefly described. Finally, the conclusions and future perspectives regarding conducting polymer-based scaffolds for tissue engineering are also given.
1. Introduction
Stimuli-responsive materials, also known as “smart” materials, have been mainly utilized to design several advanced systems, namely sensors, actuators, robotics, electrochromic devices, and controlled/sustained drug delivery, which attempt to contemplate the specific and vast array of necessities of our current society. Among the family of “smart” materials, electro-responsive ones are currently being investigated to design advanced electro-conductive scaffolds for noble applications as cell culture/tissue engineering platforms.1–3These materials allow the communication of exogenous applied electrical stimulation (ES), a recent physical methodology to trigger/induce the complex cell process cascades, with living cells to enhance cell-to-cell and/or cell-to-scaffold interactions. Moreover, these electro-responsive scaffolds also provide a suitable and adequate environment for developing several tissues, including bone, muscle, and neural networks.4 The development of these tissues is pivotal in regenerative medicine approaches for tissue repair/replacement of damaged sites.
Several polymers have been utilized in the development of electro-responsive scaffolds. Careful selection of the chemical nature of the scaffold's constituent polymer and its physicochemical properties (e.g., porous volume, swelling water capacity, charge density, and topography) can significantly influence the overall characteristics of the final tissue. Additionally, other factors such as the intensity and magnitude of applied exogenous ES, type of cell line, and scaffold development strategy also play an important role in the cell's activities.4,5 The current challenge is to engineer a suitable scaffold that satisfies specific requirements that induce a desired cell response in complex environments (e.g., in vivo) at physiological applied electrical potentials and also present biodegradability and biocompatibility without compromising its performance.
This review focuses on the description of recent electro-responsive polymer-based scaffolds for tissue engineering applications. The objective is to demonstrate an array of electro-responsive polymer-based materials that have been employed in the development of advanced scaffolds for electro-assisted cell culture. Additionally, a brief description of the mechanisms behind the cell's responses to ES is presented. Finally, the future trends regarding electro-responsive polymer-based tissue engineering scaffolds and their limitations and current challenges are also highlighted.
2. Tissue engineering scaffolds: the influence of fabrication technologies and physicochemical cues on cell fate
Several types of technologies are explored to develop conductive tissue engineering scaffolds, which include (but are not restricted to) electrospinning, solvent casting, lyophilisation, self-assembly, and micropatterning.4,6,7 The former is undoubtedly the most studied approach to obtain scaffolds due to the possibility of producing materials with oriented/organized nanofibrous pattern pathways that resemble natural extracellular matrix (ECM) structures.Electrospinning is the most conventional fabrication method studied for obtaining tissue engineering scaffolds. This technology consists of forming polymer-based fibres in the sub-micron or microscale (fibre diameter <1 μm or >1 μm, respectively) through the application of strong electric potentials (∼20 kV). A classical electrospinning apparatus consists of a high voltage (DC or AC) power supply, a syringe pump, and a collector (Fig. 1).8 Briefly, the syringe is filled with a polymer solution or melt, and it is held at the capillary's tip due to surface tension forces. High electrical potentials are established between the syringe (usually positively charged) and the collector (either oppositely charged or grounded). Once the electrical input is given, electrostatic repulsions occur among the polymeric chains in the polymer solution. Thus, polymer solution drops assume a cone-shape format (so-called “Taylor cone”)9 due to the accumulation of charges in the polymer solution or melt at the syringe's tip.6,8 By augmenting the magnitude of applied electrical stimulus, electrostatic repulsion forces surpass the surface tension of the polymer solution resulting in the formation of a jet. The jet travels towards the oppositely charged collector at a controlled rate provided by the syringe pump. In this process, most of the solvent is evaporated before depositing onto the collector in a fibre-like matrix structure. Due to the spiral/whipping motion (also known as bending instability), the deposited fibre mats are randomly oriented.
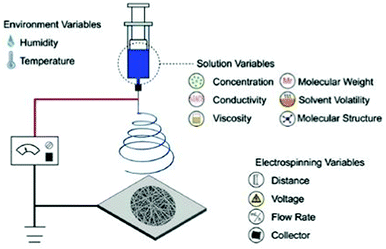 | ||
| Fig. 1 Illustration of a conventional electrospinning apparatus for scaffold design and general parameters that can tune the physicochemical properties of the final electrospun mat. Reprinted from ref. 10, Copyright 2019 Elsevier. | ||
The application of high electrical potentials leads to great plastic deformation of the polymer solution or melt. Consequently, the bending instability stretches the jet size (at least 1000×), resulting in very long thin fibers.8,11 Finally, several parameters such as viscosity, surface tension and chemical nature of the polymer, the magnitude of applied electrical stimulus, and distance between the syringe's tip and collector can be used to tune the physicochemical properties of the obtained fibres (Fig. 1).10
To shorten the production rate time of electrospun fibres, different electrospinning configurations are presented as an alternative to the classical needle-syringe setup, namely the rotating cylindrical electrode and collector, wire electrode, and multichannel needles with a rotating cylindrical collector.8
Additive manufacturing (AM) technologies such as fused filament fabrication (FFF), bioprinting, and inkjet printing have been regarded as emergent methods to obtain electro-responsive scaffolds with a defined intricate architecture and shape.4,12 AM technologies allow precise tuning of printing parameters and, thus, higher control over physical properties/characteristics of the final material. Therefore, the obtained scaffolds are expected to have enhanced reproducibility, which can be regarded as an advantage over conventional electrospinning technologies. Electro-responsive polymer-based materials developed by 3D printing technologies are still in their infancy.13 Consequently, electro-responsive scaffolds obtained by AM technologies can be considered a new and less explored research area regarding advanced platforms for tissue engineering applications.
As defined, a scaffold is a support material that can adequately host and accommodate living organisms (e.g. cells) and ensure transport of nutrients and metabolites to promote their natural behaviour. Therefore, it is crucial to pre-determine the physicochemical properties of the scaffold platform in order to control the cell fate during cell culture.
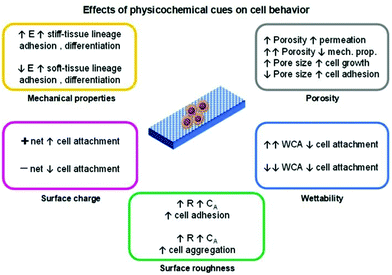
The technology employed for the development of a scaffold can determine its physicochemical properties and thus regulate the cell fate which is an essential factor when tissue engineering applications are envisaged. The physicochemical cues of a scaffold such as porosity, mechanical properties (stiffness), topography/roughness, wettability, and surface charge are variables that can influence the cell's biological responses (Fig. 2).4,14–16 The conjugation of these variables is mandatory to resemble the complex nature of the ECM.
Among the mentioned cues, the porosity (pore density, volume, size, and shape) is one of the most important and studied variables regarding cell behaviour influence for tissue engineering applications. In general, the porosity parameter plays a significant role in cell growth, adhesion and migration behaviour, nutrition exchange inside the scaffold, and the mechanical properties of the scaffold.4,14
Generally, the pore diameter of a scaffold (micrometric scale) is directly related to cell growth and adhesion. Larger pores induce cell migration from the outer layers towards the inner layer of the scaffold. With smaller pore diameters, enhanced cell adhesion is observed due to a higher specific area that prompts greater cell attachment. When the pore size is much higher than the size of the cells, their growth profile is similar to that observed in flat surfaces.
It is complicated to establish an optimal pore size for a widespread application because different cell behaviours are observed for distinct cell lines. Additionally, it is also essential to consider a suitable pore size according to the test environment (e.g., in vitro or in vivo).14 Nevertheless, most tissue engineering scaffolds present a broad pore size range of 100–500 μm.4
The pore connection and morphology also influence the cell behaviour. Highly porous platforms with interconnected cavities are interesting to promote and ensure the transport of nutrients and oxygen to cells and thus stimulate their growth.
A porosity of 96.7% is considered to be an optimal value to guarantee proper permeability. However, by augmenting the porosity, poorer mechanical properties of the scaffolds are attained.4 One strategy to overcome this drawback is by altering the pore geometry of the scaffold. The mechanical strength increases according to the following trend: diagonal > stagger > lattice.14 Nevertheless, the complexity of the pore geometry should not interfere with the metabolic activities of cells.
The mechanical properties of the scaffold (e.g., stiffness) are another important factor in regulating the cell fate. Living cells can respond to external mechanical stimulation and transduce this information into electrical and biochemical signals, which results in different cell differentiation, growth, migration, and adhesion. Cell lines that give origin to soft (<100 kPa; brain, and muscle) or rigid/stiff (>1 MPa; cartilage, and bone)17 tissue present enhanced adhesion on soft or stiff scaffolds, respectively. The differentiation behaviour of these cells follows the same trend, as cells seeded on soft/stiff scaffolds tend to differentiate into soft/stiff tissues, respectively.15,17
The topography and roughness of the scaffold also influence the behaviour of cells. Generally, by increasing the surface roughness, a higher contact area is obtained that promotes protein adsorption and, thus enhances the cell–protein–scaffold interactions resulting in greater adhesion/attachment.13 Moreover, cells tend to aggregate and form composite layers when seeded on rougher surfaces than in smoother ones.3 These composite layers prompt higher cell–cell signalling which ultimately enhances differentiation.
The wettability of a given scaffold for cell culture is a crucial and simple parameter to correlate the cell behaviour with scaffold characteristics. In general, superhydrophobic or superhydrophilic materials tend to present low/poor cell attachment/adhesion. This conduct is due to weak protein–scaffold forces, resulting in poor protein-meditated cell–scaffold interaction and, subsequently, a reduced cell attachment. Moderate water contact angle and surface energy values (for example, ∼85° and ∼ 70 mJ m−2, respectively) are considered to be the optimal conditions to induce cell attachment, growth, and proliferation.15,18
When considering electro-responsive polymers for the design of tissue engineering scaffolds, the effect of the surface charge of these materials is relevant in determining the cell fate. Besides the aforementioned interactions (such as hydrophobic/hydrophilic, and physisorption), the electrostatic ones are also responsible for inducing cell activities. Generally, cells bear a negative net charge that favours electrostatic interactions with positively charged scaffolds resulting in higher cell adhesion and growth. Conversely, a poor cell–scaffold interaction is observed when negatively charged surfaces are employed due to cell–scaffold electrostatic repulsion.15,19
3. Electrostimulation of cells: influence of electrical cues on cell functions
Among the types of physicochemical cues mentioned earlier, ES is another factor that can be used to manipulate cell behaviour (in vitro and in vivo) and also promote/enhance tissue repair.20–26 This process may be regarded as a potential alternative to commonly employed cell growth factors in the field of tissue engineering and regenerative medicine.5There are several methods to deliver ES onto cells, namely direct electrode contact, capacitive stimulation, inductive/electromagnetic stimulation (EMS), and conductive scaffold-mediated ES (Fig. 3).5,20,23,27
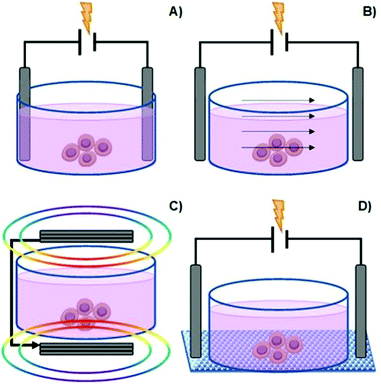 | ||
| Fig. 3 Common methods to manipulate culture cells in vitro: direct contact (A), capacitive stimulation (B), electromagnetic induction (C) and conductive substrate-mediated ES (D). | ||
The direct electrode contact method, also known as direct coupling, is the primarily employed method for cell ES due to its simplicity and convenience (Fig. 3A).
It consists of a pair of electrodes (e.g., Ag/AgCl, Pt, or Cu) submerged in a cell culture medium (highly conductive electrolyte solution) containing the cells and connected to a given power supply. Exogenous electrical fields utilized in this configuration are in the range of 101–102 mV mm−1.20 The advantages associated with this methodology include multi-well stimulation and excellent reproducibility.
Nevertheless, it presents some disadvantages that are intrinsic to electrode-based systems, such as alteration of pH at the vicinities of electrodes (pH waves), formation of cytotoxic faradaic by-products on the surface of the working electrodes, and electrophoresis (mass transport) of the components in the complex cell culture medium. Moreover, water electrolysis can also occur when the applied voltage between electrodes is higher than water's potential of 1.23 V. In this case, frequent culture medium replenishing is often performed to attenuate the potential change in medium composition.
One strategy to overcome this problem is by direct coupling through salt bridges. The salt bridge-mediated ES avoids the formation of cytotoxic components, which result from redox reactions at the surface of the electrode in contact with the cell culture medium. Consequently, minimal culture medium composition perturbation occurs. Salt-bridges for ES of cells are regularly composed of a polysaccharide (usually Agar) hydrogel loaded with electrolytes (e.g., KCl, KNO3, or NaClO4). This approach also has drawbacks, including a higher circuit resistance (compared to direct coupling), reduced working area, complex experimental setup, and concentration gradients between the bridge contents and the electrolyte media, resulting in limited ES exposure time.5,20
Capacitive stimulation (Fig. 3B) is a non-invasive ES method to induce cell metabolic activities. The experimental apparatus consists of placing electrodes outside the cell culture medium to guarantee indirect contact. Thus, a given insulator is mounted between the electrode and the culture medium. Finally, by applying ES, an electrical field is obtained, which further induces polarization of the components in the cell culture medium (such as the cell membrane, water molecules, and proteins). The advantage of this system is that it avoids the drawbacks associated with electrode-based ES. Moreover, it allows a homogenous particle redistribution to all cells (electrophoretic mass transfer) due to the perpendicular electrical field to the culture plate established between electrodes. The associated drawback with the capacitive ES approach consists of providing high-voltage power sources in order to overcome the natural high impedance of the insulator material required for this system.5,23,27
Another non-invasive strategy is the inductive EMS (Fig. 3C). An electric current passes through coiled electrodes (placed around the cell culture medium), where uniform electromagnetic fields are obtained. This uniform EMS is attained due to the specific spatial configuration of the electrodes that present an electrode–electrode distance equal to their radius (Helmholtz configuration).28 As an indirect method, EMS provides the stimulus potential near the target cell instead of direct ES, as mentioned for the electrode-based approach. The disadvantage of this method is great time and resource consumption.5,23,27
Finally, another type of non-invasive cell ES is given by the conductive scaffold-mediation (Fig. 3D). The cells “feel” the ES indirectly by its contact with the charged surface of the conductive scaffold. This approach comprises a conductive polymer-based scaffold sandwiched between the chamber (containing the cell culture medium) and an insulator material to avoid liquid leakage from the culture medium. Thus, the tip ends of the scaffold (that remained outside the culture medium) are connected to a power source to promote electrical stimulation to previously seeded cells. In this case, the electrical current is transmitted directly to the cells by the scaffold instead of to the culture medium. The advantage of this method is that the typical drawbacks associated with electrode-based systems are avoided by utilizing scaffolds with different physicochemical and mechanical properties and complex surface geometries.
Moreover, the ES-scaffold–cell synergy allows the evaluation of the effect of conductive scaffold properties in the cell's fate under electrical potential. Depending on the chemical nature of the polymer-based scaffold, several disadvantages are associated with this approach. The most common disadvantages are the instability of polymer-based scaffolds in aqueous culture medium, low biocompatibility and high cytotoxicity of the scaffold, limited attachment of the cells to the scaffold, significant impedance, and low surface wettability of the conductive substrate.5
Bioelectricity, also called endogenous ES, is defined as the electrical currents and potentials naturally produced by living organisms. Bioelectric currents are produced by the transport/flux of electrolytes across the cellular membrane and through tissues at a physiological magnitude range of ∼3–500 mV mm−2. It is present in both cytoplasmic environments and extracellular media. Therefore, endogenous ES plays an important role in several biological processes, namely tissue repair, wound healing, and embryogenesis.5,20,29–32 The application of exogenous ES in living cells is expected to influence their metabolic activities and thus, allow the development of functional therapeutic approaches.
The application of exogenous ES impacts cell processes such as migration, adhesion, proliferation, and differentiation (Fig. 4).5,20,22,23,33–35 The impact of the electrical cue on cell metabolic activities is still not completely clarified in terms of how cells interact with electrical signals. However, the literature attributes it to the cell membrane, the part responsible for most of its sensing capability towards electrical information.5
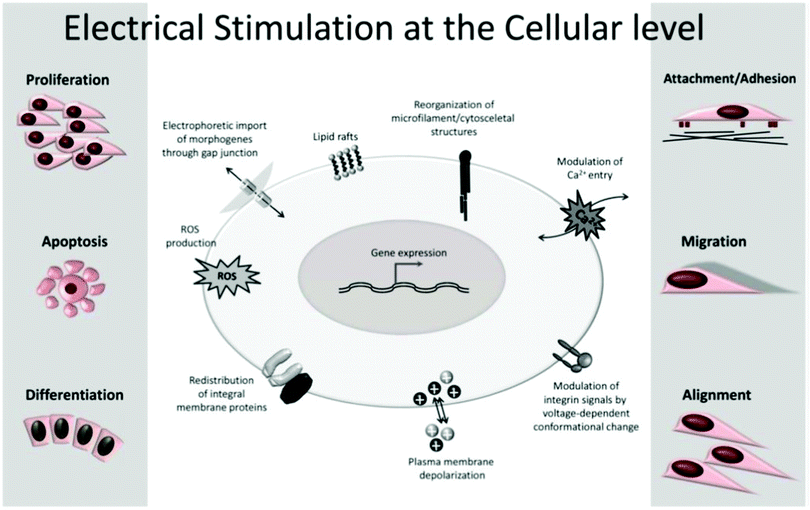 | ||
| Fig. 4 Common cell metabolic activities influenced by exogenous ES. Reprinted from ref. 20, Copyright 2020 Springer. | ||
All cells are embedded in a complex electrolyte aqueous medium. Ion pumps control the dynamic equilibrium among the ions within and outside the cell membrane through transmembrane ion channels. The natural redistribution of these ions results in an electrical potential difference that regulates the cell membrane potential by activating or inhibiting its ion channels.5,35 Upon exogenous ES, the ionic equilibrium outside the cell membrane, and consequently inside of it, is perturbed, resulting in membrane permeability alteration, which ultimately triggers several cellular processes (e.g., cytoskeletal arrangement, protein distribution, electrotaxis, and Ca2+ flux). These ES-induced alterations are the potential mechanisms behind cellular responses such as proliferation, migration, differentiation, alignment, adhesion, and growth.5,20,22,23,25,27
Signal transduction pathway
The major transduction pathway responsible for converting electrical information into biochemical cues is the activation of the mitogen-activated protein kinase (MAPK) cascades. These cascades regulate the messenger RNA (mRNA) transcriptions in response to exogenous ES. As a result, extracellular signal-regulated kinases (ERK 1/2 and ERK 5) and Jun amino-terminal kinases (JNK and p38MAPK) mediate several cell metabolic activities, including proliferation, differentiation, and apoptosis.20,22,36–38Ca2+ transients
The calcium ion signalling pathway is one of the most critical factors that regulate cell activities, especially in the case of migration and differentiation. Ca2+ ions play a pivotal role in several vital activities of cells. Exogenous ES affects the membrane potential by altering the concentration of intracellular and extracellular ions such as Na+, K+, and Ca2+. As a result, oscillations in intracellular Ca2+ concentration occur via two main routes: higher calcium ion influx from the extracellular medium through ion channels or release of calcium ions from the endoplasmic reticulum (ER) internal stores. This intracellular increase in calcium ion concentration triggers the cytoskeletal calmodulin, resulting in more significant proliferation and a higher growth factor expression, such as vascular endothelial growth factor (VEGF) and transforming growth factor-beta 1 (TGF-β1). Moreover, these alterations in the ion concentration inside and outside the cell lead to a difference in the cell membrane potential that alters its polarization and ultimately affects its migration under ES.5,22,27,39–45Cytoskeleton reorganization and actin distribution
Cells, in the presence of an exogenous electrical field, can also respond by presenting a mechanical activity. This process is known as the reverse mechanotransduction effect. Upon an electrical field, cells undergo mechanical deformation (generating a mechanical tension) due to the reorganization and redistribution of the cytoskeletal protein filaments of actin. Moreover, the dipoles of actin become aligned along with the electrical field orientation polarization in response to ES. This ES-induced cytoskeletal structure rearrangement prompts cell electrotaxis/migration.20,22,40,46 The cell migration orientation is commonly observed towards the cathode; however, it depends on the studied cell line.Surface receptor redistribution
Due to the high electrical resistivity of the cellular plasma membrane, electric-assisted cell processes occur in the outer environment of the cell rather than in its interior. The presence of applied ES can influence the redistribution of charged ligand-receptors (such as integrins) present in the cell membrane surface through electrophoresis. These electrical-induced spatial responses prompt the interaction of integrins (and also their linking proteins: actinin, talin, and vinculin) with actin microfilaments. Consequently, the integrin–actin interactions result in the formation of focal adhesion complexes, which ultimately influence the cell behavior in terms of adhesion and motility.20,22,47Adenosine triphosphate (ATP) synthesis
Cell membrane ATPases possess the capability to absorb electrical energy (at a given frequency and magnitude) to induce and regulate the activity of membrane proteins. The presence of an electrical field orients the migration of protons (from the cytosol) by electrophoresis towards the mitochondrial membrane via H+-ATPases proton pumps resulting in ATP generation. In general, this increase in ATP concentration enhances any cell activity that relies on energy consumption, such as regenerative processes.20,22,48 Moreover, ATP level oscillations might induce cytoskeleton reorganization40 (following the previously described cytoskeleton redistribution mechanism), affecting cell attachment and migration.Heat shock proteins (HSPs)
By definition, HSPs are proteins that cells produce when exposed to temperature stress (above their growth threshold) as a physiological stress response. It is hypothesized that the presence of an exogenous electrical input mimics this physiological stress acting as a stress inducer. Thus, it triggers a cellular response prompting the formation of stress proteins such as HSPs. These HSPs can interact with several transcription factors and extrinsic/intrinsic signalling pathways behind cell proliferation and differentiation.20,22,49Reactive oxygen species (ROS)
Electrical field-induced ROS generation is considered another important factor that influences the metabolic activity of cells. ROS are naturally produced by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase reactions. It is postulated that through ES, the membrane potential is affected (ion channels via electroporation), which influences the redox of NAPH that ultimately leads to ROS generation.50 It is commonly known that ROS leads to detrimental effects such as apoptosis, necrosis, DNA damage, and oxidation of proteins/lipids (oxidative stress). Nevertheless, moderate levels of intracellular ROS can induce cell differentiation and proliferation.20,22,51–53Lipid rafts
It is generally accepted that the electrical field induces the polarization of cell membrane proteins and receptors that can regulate cell migration. Therefore, discrete glycolipid domains in the plasma membrane redistribute and congregate (originating lipid rafts) in response to the exogenous electrical field. Thus, lipid raft microdomains act as initial sensors towards electrical signals. Lipid rafts congregate in response to the electrical field, increasing in size and reducing their motility. Finally, lipid rafts polarize, which consequently induces intracellular activities that modulate cell migration.20,22,54,55The briefly described cell mechanisms upon ES are interconnected in a complex cascade of events in cells and tissues. The success of exogenous ES on cells depends on several variables, including the chemical nature of the culture medium, experimental setup methodology of ES, and frequency, duration, and magnitude of the applied electrical stimulus. The latter is considered a crucial factor to modulate cell death upon ES.56
Considering cells seeded on a conductive scaffold (Fig. 3D), a general electrical stimulation dose threshold of ≤1 V cm−1 was reported.5,57 Conversely, the literature also reports greater vales of ES ∼ 500 V cm−1 to induce cell death if cells are seeded on non-conductive scaffolds.58 This indicates that the chemical nature of the polymer-based scaffold and its conductivity plays a significant role in establishing an electrical threshold. Moreover, another critical parameter to be considered when determining the successful outcome of ES on cells is the type of cell line. Each cell line presents some unique responses/behaviours and mechanisms under the applied electrical field, and detailed, thorough descriptions are beyond the scope of the present review.
4. Common electro-responsive polymers utilized in the design of electro conductive scaffolds regarding ES-assisted cell engineering
Conductive polymers can establish the required potential gradient by electrically stimulating cells and inducing several metabolic activities. Compared to non-conductive polymers, electro-responsive ones present some advantages, such as the development of ionic and/or electro-conductive scaffolds and the design of electro-responsive platforms with different sizes and shapes presenting intricate and complex geometries. Moreover, conductive scaffolds work as a bridge to ensure the communication between the precisely tuned exogenous electrical potential and cell lines. Thus, it properly delivers electrical signals to cells and provides a suitable environment to accommodate cells and support their metabolic activities promptly.1,5,59–62Several electro-responsive polymers are used in the development of advanced conductive cell culture/tissue engineering scaffolds, including those from the conjugated polymer family poly(pyrrole) (PPy),63–65 polyaniline (PANI),66–70 poly(3,4-ethylene dioxythiophene) (PEDOT)),71–74 and polysaccharides (chitosan (CS)),75–81 hyaluronic acid (HA),82 and alginate (ALG).83,84 Conductive scaffolds are also commonly obtained by combining highly conductive carbon-based materials (e.g., carbon nanotubes (CNTs),85–88 multiwalled carbon nanotubes (MWCNTs),78,89,90 graphene (GR),91–93 graphene oxide (GO)94 and reduced graphene oxide (rGO))95,96 with non-conductive polymers such as poly(lactic acid) (PLA), poly(ε-caprolactone) (PCL), poly(ethylene glycol) (PEG), collagen and its derivatives.
To investigate the possibility of employing electro-responsive polymers for developing conductive scaffolds for electro-assisted stimulation of cells, understanding the conducting mechanism of the constituent polymer is essential.
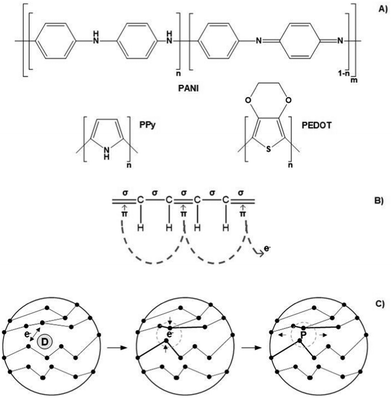
Conjugated polymers (CPs) are by far the most electro-responsive polymers commonly employed in the development of conductive scaffolds for tissue engineering applications (Fig. 5A). Conjugated polymers belong to the class of electro-conductive polymers, and their conducting mechanism is intrinsically related to their organic backbone structure and doping process (Fig. 5B and Fig. 5C, respectively).
Briefly, conducting polymers (such as PPy, PEDOT, and PANI) present a unique chemical structure configuration consisting of alternating single (σ) and double (π) bonds. Unlike saturated single bonds, unsaturated double bonds also present weakly localized π-bonds. Their p-orbitals are overlapped with each other creating a more easily delocalized pathway. This pathway prompts electrons to flow alongside its backbone, allowing electro-conductivity. To enhance or endow electrical conductivity, CPs undergo doping processes that involve adding (reduction) or removing (oxidizing) electrons to/from polymeric backbones through the utilization of dopants (e.g., hydrochloric acid or sulphonic acid). Oxidizing agents (p-dopants) remove electrons from the valence band (highest occupied orbital) and transfer them to the conduction band (lowest unoccupied orbital).
Conversely, reduction agents (n-dopants) add electrons to the conduction band where a radical anion is formed (polaron). A polaron consists of a loosely held electron surrounded by a crystal lattice distortion. Upon ES, the charge is carried in the form of these polarons that travels towards the working electrode, disrupting the stable conjugated backbone allowing the conduction of electrons. In general, the conductivity range of these classes of polymers is 10−2–105 S cm−1. However, several variables influence the conductivity amplitude, including the chemical nature of the CPs, polymer chain length, temperature, dopant concentration, and size and steric factors.5,97–99
Finally, these polymers are often combined with other materials (e.g., CS, collagen, PEG, and poly(vinyl alcohol) (PVA)) in order to compensate for their brittleness, low degree of swelling, and insolubility.7,99
Similarly, carbon-based materials such as GR, GO, rGO, CNTs, and MWCNTs (Fig. 6A and B) possess electro-conductivity based on their chemical structural organization. Like CPs, carbon-based materials present alternating σ–π bonds arranged in long sheets of hexagonal aromatic rings. Once again, this specific organization of single–double–single chemical bonds allows the formation of a “sea of delocalized electrons” (due to the overlap of p-orbitals from the π-bonds) that prompts the flow of electrons through this easily delocalized pathway, which, ultimately, results in a high electro-conductivity of up to 107 S m−1. The unique atomic organization of the aromatic rings that comprise these materials directly influences their electrical properties.
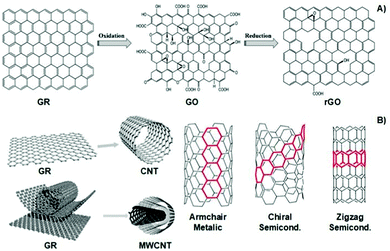 | ||
| Fig. 6 Structural illustration of graphene-based materials (A), carbon nanotubes and their atomic arrangement configuration (B). Reprinted from ref. 100, Copyright 2014; reprinted from ref. 101, Copyright 2019 John Wiley & Sons Ltd, reprinted from ref. 102, Copyright 2016 Royal Society of Chemistry. | ||
The classic examples are armchair, zigzag, and chiral conformations (Fig. 6B). The armchair conformation presents the highest electrical conductivity due to its lower bandgap.24,100–103 Carbon-based materials are often utilized as filler agents to endow high conductivity to polymeric matrixes regarding tissue engineering applications.
Polyelectrolytes are a unique class of materials that present ionisable acidic and/or basic groups alongside their polymer backbone. Different from the electron-conducting materials presented earlier, polyelectrolytes belong to the class of ion-conducing materials. Their conducting mechanism is based on the electrophoretic diffusion of their mobile counterion (an anion in the case of a polycation) in response to an applied electrical field (Fig. 7).104
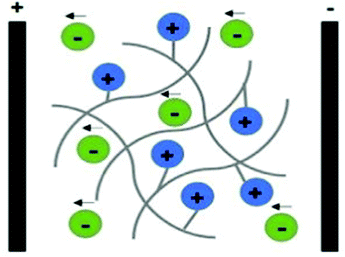 | ||
| Fig. 7 Schematic representation of the ion conducting mechanism of polyelectrolytes (considering a polycation as an example). | ||
These materials can be classified as single ion-conducting since the primary ion is chemically attached to the polymeric backbone, and its counterion is free to move. Upon ES, mobile counter ions diffuse towards oppositely charged electrodes (free anions electrophoretically diffuse towards the anode), acting as charge carriers, allowing the electric current to flow.
They are present in various configurations depending on the chemical nature of the polymer, namely polycations, polyanions, amphoteric polyelectrolytes, and polyzwitterions. Classic examples of polyelectrolytes include natural polymers (such as CS, alginate, and hyaluronic acid) and synthetic ones (poly(acrylic acid), Nafion®, and Flemion®).
Several variables can be used to tune the ionic conductivity of these materials, including the degree of crosslinking, chemical nature of the polyelectrolyte, the magnitude of the electrical field, temperature, swelling degree, size of counterions, and ionic strength of the aqueous environment.105–109 Polyelectrolytes are frequently used in the design of conducting scaffolds for cell culture due to their gel-forming capability and versatility.
5. Current electro-responsive polymer-based scaffolds for ES-assisted cell engineering
Several studies report the use of conductive polymer-based scaffolds for cell culture and tissue engineering applications without ES. These systems rely on the synergy between the endogenous ES of the cells and the conducting behaviour of the scaffold that could prompt cell metabolic activity. Nevertheless, these systems are not considered in the present review since they focus on electro-conductive platforms for electro-assisted cell culture.Table 1 summarizes the main information from several exogenous ES-assisted cell stimulation studies throughout the literature (considering the last 5 years) of conductive platforms based on different polymeric materials. A more detailed description of each system is given in the next section of this work.
| Conductive material | Cell line | Electrical stimulus | Main findings | Ref. |
|---|---|---|---|---|
| PPy σ = 57.8 ± 4.2 S m−1 | hNPCs | 0 to ∼40 V m−1 for 1 h in vitro | • Correlation between the scaffold's physical conformation and electrical stimulation on gene profile of hNPCS | |
| • Significant changes in neurotrophic gene expressions under ES | ||||
| • Physical conformation affects spatial electrical conductivity and nutrient availability | ||||
| PPy-coated cellulose σ = ∼2.4 × 10−5 S cm−1 | SH-SY5Y | 100 mV mm−1 at 1 Hz pulses in vitro | • Conductivity independent of fiber orientation of synthesized electrospun | 64 |
| • Longer and more branched neurites were observed upon ES onto conductive scaffolds | ||||
| • Aligned surface morphology combined with ES resulted in oriented neurite outgrowth | ||||
| PAN/PANI/Ni σ = 47.0 ± 1.0 mS cm−1 | SCs | 100 mV cm−1 for 1 h per day over 5 days in vitro | • Doping of Ni nanoparticles as an interesting approach to enhance electrical conductivity | 70 |
| • Higher electrical conductivity ensures proper electrostimulation of nerve cell growth | ||||
| • More rapid (2.1× faster) SC proliferation upon ES. | ||||
| PDA-functionalized PU/PANI σ = ∼9.0 × 10−4 S cm−1 | rBMSCs | ±500 mV cm−1 for 1 h per day in vitro | • Higher amount of PDA led to an increase in cell adhesion and spread under ES | 67 |
| • Greater (∼1.3× greater) ALP expression upon ES | ||||
| PEDOT-co-PDLLA CSC = 417 ± 62 μC cm−2 | NIH-3T3 | +1 mV over 1800 s in vitro | • The presence of exogenous ES enhanced (up to 2.4× greater) cell adhesion | 72 |
| • Higher cell proliferation was observed under ES | ||||
| PEDOT:PEG σ = 2.49 × 10−3 S cm−1 | C2C12 | 2 V, 10 ms at 1 Hz for 4 h per day over 7 days in vitro | • Both morphological and electrical cues had a synergistic influence on cell activity | 71 |
| • Upon ES, higher (∼2× greater) gene expression was observed | ||||
| PLA-CS doped with NPs σ = — | MG-63 | 5 V DC over 7 days in vitro | • Hydrogel's chemical nature dependence on cell response upon ES | 77 |
| • Metallic NPs granted higher conductivities that prompted greater biomineralization upon ES | ||||
| CS/PPy-PLA/PCL σ = 1.03 S m−1 | PC12 | 100 mV for 2 h per day over 5 days in vitro | • Synergy between scaffold morphology and ES in cell growth and differentiation | 65 |
| • Neurite length and cell differentiation were slightly superior under ES | ||||
| • Organized surface morphology and ES guided directional growth of neurites | ||||
| HA-CNTs CSC = ∼35 mF cm−2 | Lumbar dorsal root ganglia | 150, 200, and 250 mV mm−1 AC, 25 Hz, ±25 ms over 30 or 60 min in vitro | • An electrical threshold of 200 mV mm−1 (for 30 min) was suggested to significantly induce neurite outgrowth | 82 |
| • Longer neurites were observed under ES | ||||
| Collagen-GR σ = 3.93 mS cm−1 | BM-MSCs | 1 V for 5 min in vitro | • Conducting materials support cell growth and proliferation, and also an increase in gene expression under ES | 92 |
| • Exogenous ES led to an increase in several neuronal lineage markers during differentiation experiments | ||||
| Agarose-MWCNTs σ = ∼1.5 S m−1 | RSC96 | 3 – 4 V for 30 min per day, 10 Hz in vitro | • Modular cell adhesion (upon ES) according to MWCNT content was observed | 90 |
| • Better cell layer arrangement and adhesion under ES. | ||||
| PCL-rGO σ = 6.8 ± 0.36 × 10−5 S m−1 | hBM-SCs | 30 V for 3 h in vitro | • Cell viability dependent on rGO content under ES | 95 |
| • Higher cell adhesion observed upon exogenous ES | ||||
5.1 Polypyrrole (PPy)
PPy is one of the most explored conjugated polymers used in the design of conductive scaffolds for biomedical applications due to its high conductivity, and biocompatibility.An interesting study performed by Song et al. evaluated the effect of scaffold geometry on human neural progenitor cell (hNPC) metabolism under electrical fields of 0 and ∼40 V m−1. In their study, 2D (thin films) and 3D (tubes) PPy-based conductive scaffolds were developed, and hNPC–alginate solutions were dispensed to each scaffold for further electro-assisted cell culture. hNPC-seeded scaffolds were electrically stimulated for 1 h and incubated for 24 h prior to quantitative gene expression analysis. The results demonstrated that (for a fixed scaffold geometry), several gene expression factors such as heparin-binding EGF like growth factor (HBEGF), heat shock protein family member 1 (HSPB1), glial cell-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NTF3) and enolase 2 (ENO2) were up to 33-fold higher under ES when compared to those results without the application of an electrical field (Fig. 8).
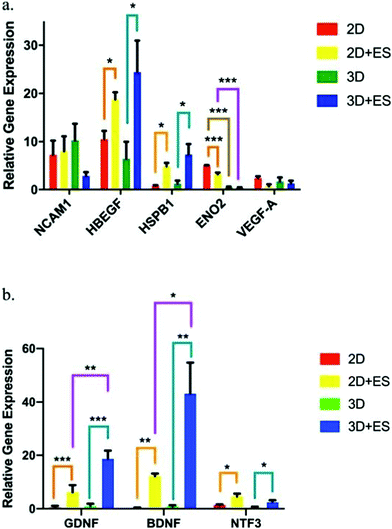 | ||
| Fig. 8 hNPC gene expression factor fluctuations using 2D (thin films) and 3D (tubes) PPy-based scaffolds under ES (ANOVA *p ≤ 0.05, **p ≤ 0.01 and ***p ≤ 0.001). (a) NCAM1, HBEGF, HSPB1, ENO2, and VEGF-A and (b) GDNF, BDNF, and NTF3. Reprinted from ref. 63, Copyright 2019 Nature. | ||
More interestingly, when comparing the results of gene expression values obtained for 2D and 3D scaffolds, it was observed that the scaffold shape presented an influence in determining the therapeutic potential of hNPCs. Under ES, 2D scaffolds enhanced the gene expression of ENO2 (Fig. 8a), whereas 3D scaffolds were more efficient in GDNF and BDNF neurotrophic factor expression (Fig. 8b). These gene expression factor variations are essential in several neurodegenerative diseases, namely Parkinson's, Alzheimer's, and Huntington's.110
Elashnikov conducted another interesting study regarding cellulose acetate butyrate (CAB)/PPy-based nanofiber scaffolds for the electrical stimulation of human neuroblastoma cells (SH-SY5Y).64 The focus was to observe the synergy interplay between the scaffold's physical cues and electrical signals on neuronal cell behaviour.
The results demonstrated that the surface topography of conductive scaffolds, as well as electrical signals (100 mV mm−1 at 1 Hz pulses for 1 h), presented a direct influence on cell growth, orientation, and alignment. Compared to control (TCP), the presence of conductive PPy on the scaffold induced significant changes in the morphology of SH-SY5Y cells, for both stimulated and non-stimulated samples.
Additionally, the surface topography of conductive scaffolds prompted oriented cell growth when comparing aligned and uniaxial aligned platforms.
The effect of electrical stimulation on cell metabolic activity was also observed for electro-conducting CAB/PPy-based scaffolds. In general, applying an electrical stimulus on SH-SY5Y cells increased the average neurite length and branching points. The cell viability assay was also performed to address the effect of ES upon cell metabolic activity.
The results demonstrated a linear relationship between long periods of ES time (up to 7 days) and a decrease in the cell metabolic activity (inhibited proliferation). The authors suggest that this reduction in SH-SY5Y proliferation is because these cells undergo differentiation after electrical stimulation.64
5.2 Polyaniline (PANI)
PANI is another classical CP widely utilized in developing conductive scaffolds due to its excellent conductivity, chemical stability, and processability. Polyacrylonitrile(PAN)/PANI electrospun nanofibers doped with Ni nanoparticles were synthesized to investigate the effect of scaffold impedance on Schwann cell (SC) metabolism after electrical stimulation.70The results demonstrated that the electro-conductivity of the scaffolds was enhanced by the presence of PANI (compared to control) and significantly higher (∼6.5× higher) when further doped with Ni nanoparticles, as expected. To evaluate the interplay between the scaffold's conductivity and SC fate under ES, a proliferation experiment was performed (Fig. 9). The results suggest that similar optical density (OD) values were observed for samples without ES, regardless of the intrinsic conductivity.
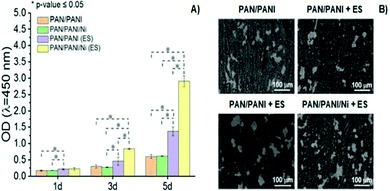 | ||
| Fig. 9 Proliferation behaviour (A) and SEM images (B) of SCs as a function of the chemical nature of conductive scaffolds and presence of ES (100 mV cm−1 for 1 h per day over 5 days) (ANOVA * significant difference p < 0.05). Reprinted from ref. 70, Copyright 2021 Elsevier. | ||
Conversely, ES samples doped with Ni nanoparticles (and thus those with higher electro-conductivity) presented the highest (4.7× greater) cell proliferation when compared to those samples measured without ES. Moreover, SEM micrographs revealed higher cell attachment onto scaffolds with higher electro-conductivity (PAN/PANI/Ni) when electro stimulated, which corroborates with previously reported cell proliferation results.70
Another interesting study regarding electro-responsive PANI-based scaffolds was conducted by Ghorbani et al. In this study, the influence of the chemical composition of prepared electrospun polyurethane (PU)/PANI scaffolds, modified with bioactive polydopamine (PDA), on metabolic responses of rat bone marrow mesenchymal stem cells (rBMSCs) in the presence/absence of ES was determined.67
Different electrospun formulations were prepared, including PU/PANI + PDA and PU/PANI/PVA + PDA. The presence of PVA in the chemical composition of electrospun scaffolds significantly influenced several physicochemical, electronic, and mechanical properties. Therefore, lower water contact angles (∼2× inferior), higher Young's modulus (∼1.3× greater), and lower conductivity (∼1.3× lower) were observed for PU/PANI/PVA + PDA samples when compared to PU/PANI + PDA. Moreover, higher osseointegration was observed for PU/PANI/PVA + PDA scaffolds immersed in simulated body fluid (SBF) due to the higher hydrophilic character of PVA that prompted the ionic interactions of SBF ions with the scaffold, which ultimately promoted hydroxyapatite formation.
The effect of ES (±500 mV cm−1 for 1 h per day) was evaluated on rBMSC adhesion and viability. In general, the applied electrical field enhanced cell spreading and filopodia formation onto conductive PANI-based scaffolds. Finally, the influence of exogenous electrical fields on cell differentiation capability (osseoblast generation) was addressed and evaluated in terms of alkaline phosphatase (ALP) expression. The results demonstrated that a higher concentration of ALP expression (up to ∼1.3× greater) under ES was observed for 14 days. The developed electro-conductive PANI-based scaffolds could be considered promising platforms for bone repair applications.67
5.3 Poly(3,4-ethylene dioxythiophene) (PEDOT)
The poly(thiophene) derivative, PEDOT, is another classic example of a conducting polymer commonly employed in the development of conductive scaffolds for tissue engineering applications. PEDOT presents high conductivity, biocompatibility, and hydrophobicity similar to other electron-conducting polymers from the CP family. Combining PEDOT with more biodegradable and hydrophilic polymers is a strategy used to overcome its low intrinsic biodegradability. Copolymers of PEDTO-co-poly(DL-lactide) (PDLLA) films were synthesized.The developed scaffolds worked as conducting platforms for fibroblast cell culture, focusing on exploring the effect of ES in cell adhesion and proliferation processes.72 Proliferation studies of NIH-3T3 cells seeded on conductive scaffolds showed a significant influence of the applied exogenous electrical field (Fig. 10A). An increase in fibroblast proliferation was observed for electro-stimulated conductive PEDOT-based samples, which prompted better development of NIH-3T3 cells for 11 days. Therefore, SEM micrographs also revealed that in the presence of electrical potential, higher cell adhesion and growth were obtained when compared to samples without ES (Fig. 10B).72
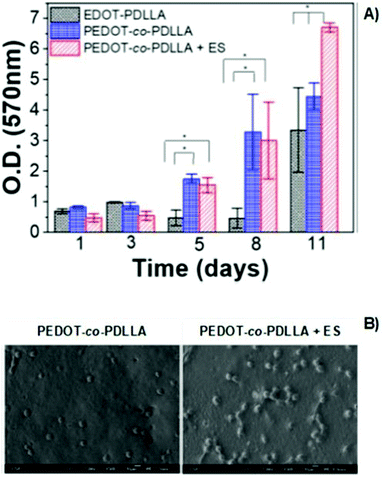 | ||
| Fig. 10 MTT assay of PEDOT-based films (A) and SEM micrographs (B) of NIH-3T3 fibroblasts in the presence or absence of electrical potential of 1 mV (ANOVA * significant difference p < 0.05). Reprinted from ref. 72, Copyright 2020 American Vacuum Society. | ||
The presence of conducting PEDOT on PEG-based hydrogels was demonstrated to enhance the differentiation of C2C12 myoblasts into myotubes.71 The results demonstrated an interesting interplay between scaffold surface pattern and conductivity in myoblast differentiation and migration (Fig. 11). The myotubes' spatial configuration was different according to the surface of each hydrogel (either flat (F) or patterned (P)). For example, thicker and shorter myotubes were observed in hydrogels presenting a flat surface compared to patterned ones. Moreover, concerning the influence of ES, myotubes were more connected to each other and highly oriented when compared to the same samples where no ES was applied. PEG-PEDOT hydrogels reveal the importance of conjugating surface properties (anisotropic architecture) with electro-conductivity resulting in the design of advanced scaffolds with promising tissue engineering applications.71
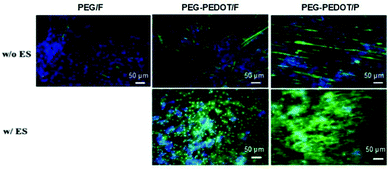 | ||
| Fig. 11 Influence of the physicochemical nature of hydrogels on myotube formation with (w/) and without (w/o) ES. Immunostaining images reveal the expression of myosin heavy chain (green) and nuclei (blue) on day 7 of culture. Reprinted from ref. 71, Copyright 2019 American Chemical Society. | ||
5.4 Chitosan (CS)
The deacetylate form of chitin, also known as chitosan (CS), is a natural polymer found in exoskeletons of arthropods and cell walls in fungi. It is a polycationic polysaccharide broadly explored for a diverse array of biomedical applications due to its intrinsic properties, namely biodegradability, biocompatibility, availability, bioadhesion, antimicrobial activity, pH-responsive capacity, processing versatility, and gel-forming capability.111,112 Taking advantage of the array of CS properties, this polysaccharide is often combined with conductive materials to obtain advanced biocompatible/biodegradable and conducting scaffolds for ES-assisted tissue engineering. PLA-CS hydrogels loaded with different nanoparticles (such as titanium dioxide (TiO2), gold (Au), and platinum (Pt)) were developed.77The effect of direct current ES (of 5V) was directly assessed by biomineralisation experiments with human osteosarcoma cell line (MG-63) in SBF medium. The results demonstrated that a difference in the DC-induced biomineralisation percentage was observed according to the type of metallic nanoparticles present in each hydrogel. Au-loaded samples present the highest (up to 1.25× higher) biomineralisation percentage compared to Pt-/TiO2-loaded samples and control (PLA-CS hydrogels without nanoparticle loading). This result is attributed to the highest electro-conductivity of gold nanoparticles that provoked the ionic interactions between the scaffold and the electrolytes (e.g., calcium and phosphorous) present in the SBF medium.77
Another interesting study regarding chitosan-based conductive platforms has been recently performed for nerve repair and regeneration applications. Therefore, CS/PPy-PLA/PCL fibre films were prepared for ES of PC12 cells.65 The study was focused on the influence of electrical cues, and the presence of chitosan on cell metabolic activities such as differentiation, neurite growth, and adhesion. In general, the results demonstrated a synergetic interplay between the chemical nature of chitosan and the electrical signals. For instance, the neurite growth and cell differentiation rate (Fig. 12A and B, respectively) were greater (at least 3× greater) for samples containing CS without applying an electrical stimulus. Moreover, the application of ES (100 mV, 2 h per day for 5 days) led to a discrete increase in the neurite length and cell differentiation ratio with the cultivation time.
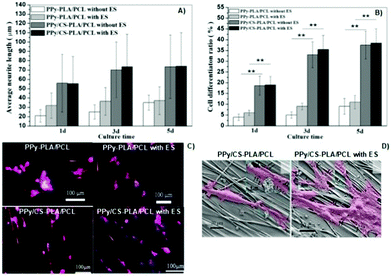 | ||
| Fig. 12 Effect of the chemical nature of the prepared films and also of the electrical cues on neurite length (A) and cell differentiation ratios (B); immunofluorescence (phalloidin/DAPI) micrographs of cells seeded on synthesized samples (orange arrows indicate the direction of fibre axis) (C); SEM micrographs of PC12 cells attached to the surface of CS-based samples with and without ES (cells are marked in pink and filopodia sites are highlighted by black arrows) (D). Reprinted from ref. 65, Copyright 2019 John Wiley & Sons Ltd. | ||
Concomitantly, immunofluorescence micrographs revealed that the presence of chitosan induced higher cell outgrowth. This experiment also demonstrated that upon ES, longer neurites were observed for samples containing CS compared to those without CS in its formulation (Fig. 12C). Finally, the impact of electrical cues on PC12 attachment onto CS-based films was evaluated (Fig. 12D).
The results demonstrated that neurites attached to the surface of samples and growth cones were more elongated, presenting more filopodia around them.65 Moreover, the fibre-patterned surfaces of the prepared films were able to guide and orient neurite growth along the fibre axis.
5.5 Other polymers conjugated with carbon-based materials
Carbon-based materials such as CNTs, MWCNTs, GR, GO, and rGO are widely utilized as fillers to induce or promote high electro-conductivity in several platforms regarding tissue engineering applications.103A recent work developed conductive platforms based on HA-CNTs nanofillers.82 The influence of exogenous ES magnitude (AC of 150, 200, and 250 mV mm−1, 25 Hz biphasic square waves, ± 25 ms pulse width and ± 50% duty cycle) on lumbar dorsal root ganglia cell metabolism was evaluated. In general, the results demonstrated that upon ES, longer neurite outgrowth was observed compared to samples without electrical stimulus. More interestingly, the results suggest an electrical field threshold of 200 mV mm−1 for 30 min to obtain significantly longer neurites. Another important observation is the ES application time regime. Independent of the chemical nature of the synthesized scaffold, the neurite length was similar at either 200 or 250 mV mm−1 applied for 30 min. Nevertheless, for 60 min of ES at 200 mV mm−1, higher neurite growth was observed for HA-CNT scaffolds when compared to HA or unstimulated samples.82
Conductive collagen–graphene cryogels with potential tissue engineering applications were also developed.92 The effect of the electrical stimulus on rat bone marrow-derived mesenchymal stem cells (BM-MSCs) and their differentiation into neuronal-like cells was demonstrated. Herein, amine- functionalized graphene was utilized as a crosslinker agent and as a filler to promote electro-conductivity in the designed cryogels. Electrical cues of 1 V for 5 min were utilized to promote electro-stimulation of cells previously seeded on scaffolds. The authors have discovered that different gene expressions (CD73 and CD90) could be obtained according to the chemical nature of the scaffolds under ES.
Moreover, these gene expression fluctuations were also time-dependent. For instance, a decrease in CD73 gene expression was observed as the graphene content of scaffolds increased for up to 3 days of measurements under ES.
Conversely, the increase in graphene content and the presence of an electrical field significantly augmented the CD73 gene expression levels at day 5. In the case of CD90 gene expressions, the increase in graphene content led to a decrease in CD90 levels after day 5 of ES.
Additionally, an inverse relationship was observed between the CD90 gene expression and the graphene content without ES. The differentiation capability of BM-MSCs under the influence of electrical fields was studied in vitro for cells seeded on conducting scaffolds. The immunocytochemistry results (Fig. 13) reveal that conducting scaffolds could promote cell differentiation in the presence and absence of ES. In the presence of ES, expressions of mature (Neu N) and immature neuron marker (β-tubulin III) were observed for BM-MSCs cultured on scaffolds. This result suggests that the developed scaffolds are promissing platforms to potentially induce cell differentiation upon ES.92
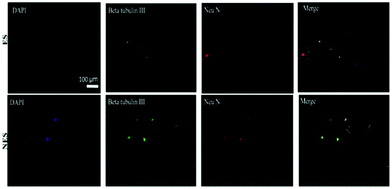 | ||
| Fig. 13 Immunochemistry images of BM-MSC differentiation behaviour on conducting scaffolds in the presence and absence of an electrical field. Codes are denoted as: electrically stimulated (ES), non-electrically stimulated (NES) and (4′,6-diamidino-2-phenylindole) (DAPI) blue-fluorescent DNA stain. Reprinted from ref. 92, Copyright 2021 Elsevier. | ||
Agarose hydrogels filled with MWCNTs were developed to evaluate the influence of ES on the Schwann cell line (RSC96).89 An electrical stimulus of 3–4 V for 30 min per day at a frequency of 10 Hz was applied to induce RSC96 cell growth onto the scaffolds. The results demonstrated highly conductive scaffolds (conductivity up to σ = ∼1.5 S m−1) and supported cell growth without ES. Nevertheless, a higher cell arrangement was attained along the longitudinal axis of scaffolds upon applying electrical signals.
Additionally, the presence of an electric field-induced higher cell adhesion on hydrogels with 0.025 and 0.05 wt% MWCNTs is observed compared to control (0% MWCNTs) and 0.1% MWCNTs scaffolds.90
Another attractive conducting scaffold was obtained by combining PCL with rGO particles.95 The metabolic activity of human bone marrow-derived mesenchymal stem cells (hBM-SCs) on 3D-printed scaffolds in the presence of electrical cues (30 V for 3 h) was evaluated. Briefly, the presence of rGO (10 wt%) particles in the 3D-printed scaffold increased the cell–scaffold interactions by reducing its hydrophobicity and, thus, prompted cell adhesion and increased cytocompatibility. Moreover, the presence of electrical cues led to an increase in cell adhesion (at least 1.5× higher) for all samples (independent of the presence of rGO particles).95
6. Conclusions
The electrical stimulation of cells can induce several metabolic pathways, namely modulation of Ca2+ concentration, cell membrane polarization, spatial redistribution of membrane proteins, reorganization of actin microfilaments/cytoskeletal structures, and alteration of the conformation of integrins. These alterations lead to different cell metabolic activities such as proliferation, differentiation, adhesion, alignment, migration, and apoptosis.Different methodology strategies were employed to develop conductive scaffolds for ES-assisted cell culture/tissue engineering, namely electrospinning, lyophilization, solvent casting, AM-related methodologies, and micropatterning. The former is the most commonly employed approach to designing conductive scaffolds with oriented surface patterns.
Several polymers were utilized in the design of advanced conductive scaffolds with tuned response upon ES, including conjugated polymers (PEDOT, PPy or PANI), natural polymers (CS and collagen), and carbon-based materials (e.g. CNTs, MWCNTs, GO, and rGO) combined with PEG, PCL, and PLA matrixes. Most of these systems are composed of two or more of these materials in attempting to obtain high conductivity–biocompatibility/low cytotoxicity–biodegradability synergy.
The physicochemical and electrical properties of the scaffolds play a significant role in determining cell fate. Moreover, cell metabolic activities triggered by ES cues are described by several complex mechanisms which are not fully understood. Obtaining advanced engineered scaffolds that present precise and finely reproducible results regarding cell culture systems are still a considerable challenge. With this in mind, the lack of information in the literature regarding the washing procedure of designed scaffolds is an important factor that must be considered. Residual components present in the produced scaffold can affect its physicochemical, mechanical, and electrical properties, impacting cell metabolism and, thus, cell fate.
Another key factor that is often disregarded is the biodegradability of the polymer-based conducting platforms. To the best of our knowledge, the majority of the studies developed so far are more focused on providing “proofs of concepts” with respect to ES-assisted cell culture and biocompatibility rather than its biodegradability. Indeed, there is a clear effort to utilize polymers that are potential candidates to induce biodegradability (such as chitosan, cellulose, alginate, collagen, PLA and PEG) by enzymatic and/or hydrolytic pathways. Nevertheless, few data are currently presented and should be addressed in the following studies regarding conducting platforms for ES-assisted cell culture.
Efforts are currently being made to develop conducting scaffolds that can precisely control/regulate cell metabolism under ES. Furthermore, the synergy between the physicochemical and mechanical properties of the scaffolds and cell metabolism is explored. Nevertheless, due to the complexity of each cell line and its specificities, the application of these advanced conducting scaffolds in complex environments (e.g., in vivo) is an important subject that requires more clarification and data.
The obtainment of highly engineered scaffolds that simultaneously present several properties including biocompatibility, antifouling, and antibacterial capacity, presence of interconnected porous cavities, and electro-responsiveness capable of being transversal to different cell lines is the huge challenge to be addressed in future investigations. Moreover, thoroughly evaluating the performance of in vitro ES-cultured tissues and in vivo systems could also be an important step in shaping and guiding further scaffold technologies for electro-assisted cell culture engineering.
Abbeviations
| ALG | Alginate |
| ALP | Alkaline phosphatase |
| AM | Additive manufacturing |
| ATP | Adenosine triphosphate |
| BDNF | Brain-derived neurotrophic factor |
| CA | Contact area |
| CAB | Cellulose acetate butyrate |
| CNTs | Carbon nanotubes |
| CPs | Conjugated polymers |
| CS | Chitosan |
| E | Elastic (Young) moduli |
| ECM | Extracellular matrix |
| EGF | Endothelial growth factor |
| EMS | Electromagnetic stimulation |
| ENO2 | Enolase 2 |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinases |
| ES | Electrical stimulation |
| FFF | Fused filament fabrication |
| GDNF | Glial cell-derived neurotrophic factor |
| GO | Graphene oxide |
| GR | Graphene |
| HA | Hyaluronic acid |
| HBEGF | Heparin-binding EGF |
| hNPCs | Human neural progenitor cells |
| HSPB1 | Heat shock protein family member 1 |
| HSPs | Heat shock proteins |
| JNK | Jun amino-terminal kinases |
| MAPK | Mitogen-activated protein kinase |
| mRNA | Messenger RNA |
| MWCNTs | Multiwalled carbon nanotubes |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NTF3 | Neurotrophin 3 |
| OD | Optical density |
| PAN | Polyacrylonitrile |
| PANI | Polyaniline |
| PCL | Poly(e-caprolactone) |
| PDA | Polydopamine |
| PDLLA | PEDTO-co-poly(DL-lactide) |
| PEDOT | Poly(3,4-ethylene dioxythiophene) |
| PEG | Poly(ethylene glycol) |
| PLA | Poly(lactic acid) |
| PPy | Polypyrrole |
| PU | Polyurethane |
| PVA | Poly(vinyl alcohol) |
| R | Surface roughness |
| rBMSCs | Rat bone marrow mesenchymal stem cells |
| rGO | Reduced graphene oxide |
| ROS | Reactive oxygen species |
| SBF | Simulated body fluid |
| SCs | Schwann cells |
| SH-SY5Y | Human neuroblastoma cells |
| TGF-β1 | Transforming growth factor-beta 1 |
| VEGF | Vascular endothelial growth factor |
| WCA | Water contact angle |
Author contributions
Conceptualization: Akel F. Kanaan and Ana P. Piedade. Writing—original draft preparation: Akel F. Kanaan. Writing—review and editing: Akel F. Kanaan and Ana P. Piedade. Project administration and funding acquisition: Ana P. Piedade. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was partially supported by FEDER funds through the programs COMPETE 2020 – Programa Operacional Fatores de Competitividade – Portugal 2020 and by national funds through FCT – Fundacão para a Ciência e a Tecnologia, I.P. under the projects POCI-01-0145-FEDER-030767, POCI-01-0247-FEDER-024533 and UIDB/00285/2020.References
- H. Palza, P. A. Zapata and C. Angulo-Pineda, Materials, 2019, 12, 277 CrossRef CAS PubMed.
- C. Ning, Z. Zhou, G. Tan, Y. Zhu and C. Mao, Prog. Polym. Sci., 2018, 81, 144–162 CrossRef CAS PubMed.
- R. Dong, P. X. Ma and B. Guo, Biomaterials, 2020, 229, 119584 CrossRef CAS PubMed.
- F. Xing, L. Li, C. Zhou, C. Long, L. Wu, H. Lei, Q. Kong, Y. Fan, Z. Xiang and X. Zhang, Stem Cells Int., 2019, 2180925 CAS.
- Z. Zhang, M. Rouabhia and S. E. Moulton, Conductive Polymers, CRC Press, Taylor & Francis Group, Boca Raton, FL, 1st edn, 2018 Search PubMed.
- T. Lu, Y. Li and T. Chen, Int. J. Nanomed., 2013, 8, 337–350 CrossRef PubMed.
- N. Alegret, A. Dominguez-Alfaro and D. Mecerreyes, Biomacromolecules, 2019, 20, 73–89 CrossRef CAS PubMed.
- A. Keirouz, M. Chung, J. Kwon, G. Fortunato and N. Radacsi, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2020, 12, 1–32 Search PubMed.
- T. Peijs, in Comprehensive Composite Materials II, ed. P. W. R. Beaumont and C. H. Zweben, Elsevier Inc., Amsterdam, Netherlands, 2018, vol. 6, pp. 162–200 Search PubMed.
- Y. Long, X. Yan, X. Wang, J. Zhang and M. Yu, in Electrospinning: Nanofabrication and Applications, ed. B. Ding, X. Wang and J. Yu, Elsevier Inc., Amsterdam, Netherlands, 2019, pp. 21–52 Search PubMed.
- R. Asmatulu and W. S. Khan, Synthesis and applications of electrospun nanofibers, Elsevier Inc., Amsterdam, Netherlands, 2019, pp. 17–39 Search PubMed.
- S. K. Sinha, in 3D and 4D Printing of Polymer Nanocomposite Materials, ed. E. Hayes, Elsevier Inc., Amsterdam, Netherlands, 2020, pp. 119–160 Search PubMed.
- A. F. Kanaan, A. C. Pinho and A. P. Piedade, Polymers, 2021, 13, 2713 CrossRef CAS PubMed.
- M. Ebrahimi, Front. Mater. Sci., 2021, 15, 352–373 CrossRef.
- N. Muzzio, S. Moya and G. Romero, Pharmaceutics, 2021, 13, 792 CrossRef CAS PubMed.
- X. Chen, H. Fan, X. Deng, L. Wu, T. Yi, L. Gu, C. Zhou, Y. Fan and X. Zhang, Nanomaterials, 2018, 8, 1–15 Search PubMed.
- C. F. Guimarães, L. Gasperini, A. P. Marques and R. L. Reis, Nat. Rev. Mater., 2020, 5, 351–370 CrossRef.
- B. Majhy, P. Priyadarshini and A. K. Sen, RSC Adv., 2021, 11, 15467–15476 RSC.
- M. Ferrari, F. Cirisano and M. Carmen Morán, Colloids Interfaces, 2019, 3, 48 CrossRef CAS.
- L. Leppik, K. M. C. Oliveira, M. B. Bhavsar and J. H. Barker, Eur. J. Trauma Emerg. Surg., 2020, 46, 231–244 CrossRef PubMed.
- Y. Wang, M. Rouabhia and Z. Zhang, Biochim. Biophys. Acta, Gen. Subj., 2016, 1860, 1551–1559 CrossRef CAS PubMed.
- G. Thrivikraman, S. K. Boda and B. Basu, Biomaterials, 2018, 23(150), 60–86 CrossRef PubMed.
- C. Chen, X. Bai, Y. Ding and I. S. Lee, Biomater. Res., 2019, 23, 1–12 CrossRef PubMed.
- L. P. da Silva, S. C. Kundu, R. L. Reis and V. M. Correlo, Trends Biotechnol., 2020, 38, 24–49 CrossRef CAS PubMed.
- R. Zhu, Z. Sun, C. Li, S. Ramakrishna, K. Chiu and L. He, Exp. Neurol., 2019, 319, 112963 CrossRef PubMed.
- H. Cheng, Y. Huang, H. Yue and Y. Fan, Stem Cells Int., 2021, 6697574 CAS.
- R. Balint, N. J. Cassidy and S. H. Cartmell, Tissue Eng., Part B, 2013, 19, 48–57 CrossRef CAS PubMed.
- M. Saqib, N. S. Francis and N. J. Francis, Int. Youth Conf. Radio Electron. Electr. Power Eng., 2020, 2020, 1–5 Search PubMed.
- M. Levin, Mol. Biol. Cell, 2014, 25, 3835–3850 CrossRef PubMed.
- A. Jahanshahi, L. M. Schönfeld, E. Lemmens, S. Hendrix and Y. Temel, Mol. Neurobiol., 2014, 49, 1005–1016 CrossRef CAS PubMed.
- E. Serena, E. Figallo, N. Tandon, C. Cannizzaro, S. Gerecht, N. Elvassore and G. Vunjak-Novakovic, Exp. Cell Res., 2009, 315, 3611–3619 CrossRef CAS PubMed.
- R. H. W. Funk, Front. Physiol., 2015, 6, 1–8 Search PubMed.
- S. Zhao, A. S. Mehta and M. Zhao, Cell. Mol. Life Sci., 2020, 77, 2681–2699 CrossRef CAS PubMed.
- M. R. Love, S. Palee, S. C. Chattipakorn and N. Chattipakorn, J. Cell. Physiol., 2018, 233, 1860–1876 CrossRef CAS PubMed.
- B. Cortese, I. E. Palamà, S. D’Amone and G. Gigli, Integr. Biol., 2014, 6, 817–830 CrossRef CAS PubMed.
- Y. D. Shaul and R. Seger, Biochim. Biophys. Acta, Mol. Cell Res., 2007, 1773, 1213–1226 CrossRef CAS PubMed.
- Y. Sun, W.-Z. Liu, T. Liu, X. Feng, N. Yang and H.-F. Zhou, J. Recept. Signal Transduction, 2015, 35, 600–604 CrossRef CAS PubMed.
- H. Rubinfeld and R. Seger, in MAP Kinase Signaling Protocols, ed. R. Seger, Humana Press, Totowa, NJ, 2004, pp. 1–28 Search PubMed.
- H. Zhuang, W. Wang, R. M. Seldes, A. D. Tahernia, H. Fan and C. T. Brighton, Biochem. Biophys. Res. Commun., 1997, 237, 225–229 CrossRef CAS PubMed.
- I. Titushkin and M. Cho, Biophys. J., 2009, 96, 717–728 CrossRef CAS PubMed.
- J. F. Perez-Zoghbi, C. Karner, S. Ito, M. Shepherd, Y. Alrashda and M. J. Sanderson, Pulm. Pharmacol. Ther., 2009, 22, 388–397 CrossRef CAS PubMed.
- F. M. P. Tonelli, A. K. Santos, D. A. Gomes, S. L. da Silva, K. N. Gomes, L. O. Ladeira and R. R. Resende, in Calcium Signaling, ed. M. S. Islam, Springer, Dordrecht, Netherlands, 2012, pp. 891–916 Search PubMed.
- R. E. Dolmetsch, K. Xu and R. S. Lewis, Nature, 1998, 392, 933–936 CrossRef CAS PubMed.
- T. Nakano, M. J. Moore, F. Wei, A. V. Vasilakos and J. Shuai, IEEE Trans Nanobioscience, 2012, 11, 135–148 Search PubMed.
- R. J. McMurray, M. J. Dalby and P. M. Tsimbouri, J. Tissue Eng. Regener. Med., 2015, 9, 528–539 CrossRef PubMed.
- J. T. Parsons, A. R. Horwitz and M. A. Schwartz, Nat. Rev. Mol. Cell Biol., 2010, 11, 633–643 CrossRef CAS PubMed.
- A. M. Das and D. A. Harris, Biochim. Biophys. Acta, Mol. Basis Dis., 1991, 1096, 284–290 CrossRef CAS.
- E. Esfandiari, S. Roshankhah, M. Mardani, B. Hashemibeni, E. Naghsh, M. Kazemi and M. Salahshoor, Iran. J. Basic Med. Sci., 2014, 17, 571–576 Search PubMed.
- T. E. DeCoursey, Immunol. Rev., 2016, 273, 194–218 CrossRef CAS PubMed.
- M. Wartenberg, N. Wirtz, A. Grob, W. Niedermeier, J. Hescheler, S. C. Peters and H. Sauer, Bioelectromagnetics, 2008, 29, 47–54 CrossRef CAS PubMed.
- K. Srirussamee, S. Mobini, N. J. Cassidy and S. H. Cartmell, Biotechnol. Bioeng., 2019, 116, 3421–3432 CrossRef CAS PubMed.
- E. Serena, E. Figallo, N. Tandon, C. Cannizzaro, N. Elvassore and G. Vunjak-novakovic, Cell, 2010, 315, 3611–3619 Search PubMed.
- M. M. Lozano, J. S. Hovis, F. R. Moss and S. G. Boxer, J. Am. Chem. Soc., 2016, 138, 9996–10001 CrossRef CAS PubMed.
- B. J. Lin, S. H. Tsao, A. Chen, S. K. Hu, L. Chao and P. H. G. Chao, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 8568–8573 CrossRef CAS PubMed.
- T. Batista Napotnik, T. Polajžer and D. Miklavčič, Bioelectrochemistry, 2021, 141, 107871 CrossRef CAS PubMed.
- G. Thrivikraman, G. Madras and B. Basu, Biomaterials, 2014, 35, 6219–6235 CrossRef CAS PubMed.
- B. Mercadal, N. Beitel-White, K. N. Aycock, Q. Castellví, R. V. Davalos and A. Ivorra, Ann. Biomed. Eng., 2020, 48, 1451–1462 CrossRef PubMed.
- A. Gelmi and C. E. Schutt, Adv. Healthcare Mater., 2021, 10, 1–30 Search PubMed.
- F. Alvarado-Hidalgo, K. Ramírez-Sánchez and R. Starbird-Perez, Molecules, 2020, 25, 5286 CrossRef CAS PubMed.
- H. Nekounam, S. Gholizadeh, Z. Allahyari, H. Samadian, N. Nazeri, M. A. Shokrgozar and R. Faridi-Majidi, Mater. Res. Bull., 2021, 134, 111083 CrossRef CAS.
- I. Rocha, G. Cerqueira, F. Varella Penteado and S. I. Córdoba de Torresi, Front. Med. Technol., 2021, 3, 1–18 Search PubMed.
- S. Vijayavenkataraman, S. Kannan, T. Cao, J. Y. H. Fuh, G. Sriram and W. F. Lu, Front. Bioeng. Biotechnol., 2019, 7, 1–14 CrossRef PubMed.
- S. Song, D. Amores, C. Chen, K. McConnell, B. Oh, A. Poon and P. M. George, Sci. Rep., 2019, 9, 19565 CrossRef CAS PubMed.
- R. Elashnikov, S. Rimpelová, L. Děkanovský, V. Švorčík and O. Lyutakov, J. Mater. Chem. B, 2019, 7, 6500–6507 RSC.
- Y. Xu, Z. Huang, X. Pu, G. Yin and J. Zhang, Cell Proliferation, 2019, 52, 1–11 CrossRef PubMed.
- Y. Li, X. Li, R. Zhao, C. Wang, F. Qiu, B. Sun, H. Ji, J. Qiu and C. Wang, Mater. Sci. Eng., C, 2017, 72, 106–112 CrossRef CAS PubMed.
- F. Ghorbani, B. Ghalandari, A. L. Khan, D. Li, A. Zamanian and B. Yu, Biotechnol. Prog., 2020, 36, 1–16 Search PubMed.
- Y. Arteshi, P. Mohammad Hoseinpour and S. Davaran, Mater. Today Proc., 2019, 42, 1579–1587 CrossRef.
- D. Shan, S. R. Kothapalli, D. J. Ravnic, E. Gerhard, J. P. Kim, J. Guo, C. Ma, J. Guo, L. Gui, L. Sun, D. Lu and J. Yang, Adv. Funct. Mater., 2018, 28, 1801787 CrossRef PubMed.
- M. Wang, P. L. Tremblay and T. Zhang, Bioelectrochemistry, 2021, 140, 107750 CrossRef CAS PubMed.
- H. Y. Gong, J. Park, W. Kim, J. Kim, J. Y. Lee and W. G. Koh, ACS Appl. Mater. Interfaces, 2019, 11, 47695–47706 CrossRef CAS PubMed.
- A. C. da Silva, R. A. da Silva, M. J. P. G. Souza, P. M. Montoya, R. Bentini, T. Augusto, R. M. Torresi, L. H. Catalani and S. I. Córdoba de Torresi, Biointerphases, 2020, 15, 021003 CrossRef CAS PubMed.
- S. Wang, S. Guan, W. Li, D. Ge, J. Xu, C. Sun, T. Liu and X. Ma, Mater. Sci. Eng., C, 2018, 93, 890–901 CrossRef CAS PubMed.
- S. Wang, S. Guan, Z. Zhu, W. Li, T. Liu and X. Ma, Mater. Sci. Eng., C, 2017, 71, 308–316 CrossRef CAS PubMed.
- J. Radwan-Pragłowska, Ł. Janus, M. Piątkowski, D. Bogdał and D. Matýsek, Polymers, 2020, 12, 159 CrossRef PubMed.
- M. O. Oftadeh, B. Bakhshandeh, M. M. Dehghan and A. Khojasteh, J. Biomed. Mater. Res., Part A, 2018, 106, 1200–1210 CrossRef CAS PubMed.
- J. Radwan-Pragłowska, Ł. Janus, M. Piatkowski, D. Bogdał and D. Matysek, Polymers, 2020, 12, 792 CrossRef PubMed.
- A. Abedi, B. Bakhshandeh, A. Babaie, J. Mohammadnejad, S. Vahdat, R. Mombeiny, S. R. Moosavi, J. Amini and L. Tayebi, Mater. Chem. Phys., 2021, 258, 123842 CrossRef CAS.
- R. Khalili, P. Zarrintaj, S. H. Jafari, H. Vahabi and M. R. Saeb, Int. J. Biol. Macromol., 2020, 154, 18–24 CrossRef CAS PubMed.
- S. Grossemy, P. P. Y. Chan and P. M. Doran, Integr. Biol., 2019, 11, 264–279 CrossRef PubMed.
- B. Bagheri, P. Zarrintaj, A. Samadi, R. Zarrintaj, M. R. Ganjali, M. R. Saeb, M. Mozafari, O. O. Park and Y. C. Kim, Int. J. Biol. Macromol., 2020, 147, 160–169 CrossRef CAS PubMed.
- E. M. Steel, J. Y. Azar and H. G. Sundararaghavan, Materialia, 2020, 9, 100581 CrossRef CAS.
- A. Serafin, C. Murphy, M. C. Rubio and M. N. Collins, Mater. Sci. Eng., C, 2021, 122, 111927 CrossRef CAS PubMed.
- S. Homaeigohar, T. Y. Tsai, T. H. Young, H. J. Yang and Y. R. Ji, Carbohydr. Polym., 2019, 224, 115112 CrossRef CAS PubMed.
- E. M. Steel, J. Y. Azar and H. G. Sundararaghavan, Materialia, 2020, 9, 100581 CrossRef CAS.
- Y. Sun, X. Liu, M. N. George, S. Park, B. Gaihre, A. Terzic and L. Lu, J. Biomed. Mater. Res., Part A, 2021, 109, 193–206 CrossRef CAS PubMed.
- L. He, Q. Xiao, Y. Zhao, J. Li, S. Reddy, X. Shi, X. Su, K. Chiu and S. Ramakrishna, ACS Appl. Mater. Interfaces, 2020, 12, 53150–53163 CrossRef CAS PubMed.
- S. Mombini, J. Mohammadnejad, B. Bakhshandeh, A. Narmani, J. Nourmohammadi, S. Vahdat and S. Zirak, Int. J. Biol. Macromol., 2019, 140, 278–287 CrossRef CAS PubMed.
- M. Imaninezhad, K. Pemberton, F. Xu, K. Kalinowski, R. Bera and S. P. Zustiak, J. Neural Eng., 2018, 15, 56034 CrossRef PubMed.
- Z. Liu, M. Yushan, Y. Alike, Y. Liu, S. Wu, C. Ma and A. Yusufu, Biomed. Res. Int., 2020, 4794982 CAS.
- P. Hitscherich, A. Aphale, R. Gordan, R. Whitaker, P. Singh, L. Hua Xie, P. Patra and E. J. Lee, J. Biomed. Mater. Res., Part A, 2018, 106, 2923–2933 CrossRef CAS PubMed.
- G. Agarwal, N. Kumar and A. Srivastava, Mater. Sci. Eng., C, 2021, 118, 111518 CrossRef CAS PubMed.
- C. Zhang, S. Fan, H. Shao, X. Hu, B. Zhu and Y. Zhang, Carbon, 2019, 148, 16–27 CrossRef CAS.
- J. Zhu, Z. Qi, C. Zheng, P. Xue, C. Fu, S. Pan and X. Yang, J. Nanomater., 2020, 5892506 CAS.
- C. Angulo-Pineda, K. Srirussamee, P. Palma, V. M. Fuenzalida, S. H. Cartmell and H. Palza, Nanomaterials, 2020, 10, 9–13 CrossRef PubMed.
- J. Wang, Y. Cheng, L. Chen, T. Zhu, K. Ye, C. Jia, H. Wang, M. Zhu, C. Fan and X. Mo, Acta Biomater., 2019, 84, 98–113 CrossRef CAS PubMed.
- P. Fomby, A. J. Cherlin, A. Hadjizadeh, C. J. Doillon, V. Sueblinvong, D. J. Weiss, J. H. T. Bates, T. Gilbert, W. C. Liles, C. Lutzko, J. Rajagopal, D. J. Prockop, D. Chambers, A. Giangreco, A. Keating, D. Kotton, P. I. Lelkes, D. E. Wagner and D. J. Prockop, Ann. Am. Thorac. Soc., 2010, 12, 181–204 Search PubMed.
- T. Nezakati, A. Seifalian, A. Tan and A. M. Seifalian, Chem. Rev., 2018, 118, 6766–6843 CrossRef CAS PubMed.
- R. Balint, N. J. Cassidy and S. H. Cartmell, Acta Biomater., 2014, 10, 2341–2353 CrossRef CAS PubMed.
- R. Vidu, M. Rahman, M. Mahmoudi, M. Enachescu, T. D. Poteca and I. Opris, Front. Syst. Neurosci., 2014, 8, 1–24 Search PubMed.
- S. Bhattacharjee, R. Joshi, A. A. Chughtai and C. R. Macintyre, Adv. Mater. Interfaces, 2019, 6, 1–27 Search PubMed.
- T. J. Sisto, L. N. Zakharov, B. M. White and R. Jasti, Chem. Sci., 2016, 7, 3681–3688 RSC.
- W. Wang, Y. Hou, D. Martinez, D. Kurniawan, W. H. Chiang and P. Bartolo, Polymers, 2020, 12, 1–38 Search PubMed.
- N. Wanasingha, P. Dorishetty, N. K. Dutta and N. R. Choudhury, Gels, 2021, 7, 148 CrossRef CAS PubMed.
- J. P. S. Sagou, S. Ahualli and F. Thomas, J. Colloid Interface Sci., 2015, 459, 212–217 CrossRef CAS PubMed.
- S. De, A. Ostendorf, M. Schönhoff and C. Cramer, Polymers, 2017, 9, 1–16 CrossRef PubMed.
- H. Li, A. Erbaş, J. Zwanikken and M. Olvera De La Cruz, Macromolecules, 2016, 49, 9239–9246 CrossRef CAS.
- C. J. Lee, H. Wu, Y. Hu, M. Young, H. Wang, D. Lynch, F. Xu, H. Cong and G. Cheng, ACS Appl. Mater. Interfaces, 2018, 10, 5845–5852 CrossRef CAS PubMed.
- A. Ostendorf, M. Schönhoff and C. Cramer, Phys. Chem. Chem. Phys., 2019, 21, 7321–7329 RSC.
- C. Zuccato and E. Cattaneo, Nat. Rev. Neurol., 2009, 5, 311–322 CrossRef CAS PubMed.
- Z. Shariatinia, Adv. Colloid Interface Sci., 2019, 263, 131–194 CrossRef CAS PubMed.
- D. Zhao, S. Yu, B. Sun, S. Gao, S. Guo and K. Zhao, Polymers, 2018, 10, 462 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2022 |