Open Access Article
Open Access ArticleAdvances in porous organic polymers: syntheses, structures, and diverse applications
Mohamed Gamal
Mohamed
 *ab,
Ahmed. F. M.
EL-Mahdy
*ab,
Ahmed. F. M.
EL-Mahdy
 a,
Mohammed G.
Kotp
a and
Shiao-Wei
Kuo
a,
Mohammed G.
Kotp
a and
Shiao-Wei
Kuo
 *ac
*ac
aDepartment of Materials and Optoelectronic Science, Center for Functional Polymers and Supramoleuclar Materials and Center of Crystal Research, National Sun Yat-Sen University, Kaohsiung 804, Taiwan. E-mail: mgamal.eldin34@gmail.com; kuosw@faculty.nsysu.edu.tw
bChemistry Department, Faculty of Science, Assiut University, Assiut, 71516, Egypt
cDepartment of Medicinal and Applied Chemistry, Kaohsiung Medical University, Kaohsiung 807, Taiwan
First published on 23rd November 2021
Abstract
Porous organic polymers (POPs) are organic macromolecules that are considered emerging materials because of their high specific surface areas, tunable porosities, low densities, high chemical and thermal stabilities, variable compositions, convenient post-functionalization, extended π-conjugations, and their high contents of carbon, nitrogen, oxygen, and other non-metallic atoms. POPs have been classified into four types: covalent triazine frameworks (CTFs), hypercrosslinked polymers (HCPs), covalent organic frameworks (COFs), and conjugated microporous polymers (CMPs). These materials have potential applications in, for example, gas capture/separation, energy storage, H2 production from water, photocatalysis, chemical sensing, perovskite solar cells, water treatment, optical devices, and biomedicine. In this review, we provide an overview of recent reports describing the preparation and various applications of POPs.
1. Introduction
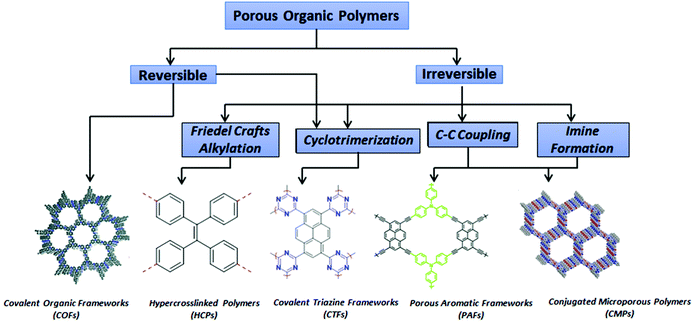
Porous structures have existed in nature for a very long time in, for example, charcoal, biological tissues, and rocks.1 The word “porous” is usually used to describe a material featuring interconnected permanent voids that allow the passage of gases or liquids.1 Porous materials are applied in many technological and scientific fields, with a huge number of new advanced porous materials having been developed during the last two decades.2–14 Based on the IUPAC classification, porous materials can be divided, based on their pore diameters, into microporous (<2 nm), mesoporous (>2 nm and <50 nm), and macroporous (>50 nm) materials.15–22 Various porous materials have been constructed from metal–organic frameworks (MOFs) and porous organic polymers (POPs).1,23–28 MOFs are typically crystalline porous materials or porous coordination polymers obtained through the formation of coordinate bonds between organic ligands and clusters or metal ions to form voids within the framework structure, thereby resulting in porosity.23–25 In the past two decades, many groups have prepared various subclasses of POP frameworks, including porous aromatic frameworks (PAFs), covalent triazine frameworks (CTFs), covalent organic frameworks (COFs), polymers of intrinsic microporosity (PIMs), hypercrosslinked polymers (HCPs), and conjugated microporous polymers (CMPs) (Fig. 1).1,29–33 The preparation of each kind of POP generally occurs through network reactions.1 All POPs are amorphous materials—except for a small number of CTFs and COFs that are crystalline materials with ordered structures prepared under thermodynamic control.1 Like nanoporous materials, POPs have many potential applications because of their high surface areas and uniform pore sizes, with large numbers of channels and active sites available for chemical reactions.3,34–37 Accordingly, POPs have attracted attention for their use in, for example, the degradation of organic pollutants, energy storage, photocatalysis, H2 evolution, light harvesting, photovoltaics, luminescence, gas adsorption and separation, chemical sensing, and drug loading and delivery.38–54 Various morphologies of POPs, including nanotubes, nanospheres, and nanosheets, can be controlled by varying the synthetic method, the monomer structure, or the reaction conditions.45,48,55 Furthermore, the surface areas of POPs, typically in the range 100–1000 m2 g−1, are higher than those of MOFs and porous carbons.3 In 2007, Yaghi et al. prepared a 3D-COF-103 possessing a high Brunauer–Emmett–Teller (BET) surface area of 4210 m2 g−1.56 Ben et al. used the Yamamoto reaction to prepare a PAF (PAF-1) exhibiting a high specific area (4210 m2 g−1).57 The insolubility of POPs in organic solvents, due to their rigid chemical bonds and high degrees of polymerization, can simplify the separation and recycling of POPs.3 In this review, we provide an overview of recent reports describing the preparation of POPs (including CTFs, COFs, HCPs, and CMPs) and their applications in photocatalytic water splitting for H2 evolution, gas capture and separation, chemosensing, the removal of dyes and metals, organic synthesis, and energy storage.2. Preparation of POPs
In this section, we discuss progress in the synthetic methodologies that are widely used for the preparation four kinds of POPs: HCPs, CTFs, CMPs, and COFs.2.1. Preparation of HCPs
HCPs are porous polymers that have attracted much attention for their extended conjugation; tunable porosities; high surface areas; high mechanical, chemical, and hydrothermal stabilities; variety of potential synthetic methods; and facile post-functionalization after HCP formation.58–63 Many methods have been used to determine the chemical structures, thermal stabilities, morphologies, and textural characteristics (e.g., pore size diameters and surface areas) of HCPs, including Fourier transform infrared (FTIR) spectroscopy, elemental analysis, solid state nuclear magnetic resonance (NMR) spectroscopy, transmission electron microscopy (TEM), optical microscopy, field emission scanning electron microscopy (SEM), N2 adsorption isotherms, and thermogravimetric analysis (TGA).58–63 Davankov and Tsyurupa were the first to generate three-dimensional (3D) network structures of linear polystyrene (PS) with high surface areas (up to 2000 m2 g−1) as the first HCP material, through post-crosslinking of a PS precursor in the swollen state.64 The formation HCPs can be achieved through acid-catalyzed Friedel–Crafts reactions involving a Lewis acid catalyst, a reaction solvent, and arene-based nucleophilic and electrophilic species.65 To avoid products forming through side reactions during the knitting step, the reactions are performed under Ar and N2 atmospheres to afford pure HCPs as solid materials.65 The incorporation of metal species into HCPs can be performed through post-modification—by introducing a metal salt or complex—or by using a metal complex as a co-monomer or monomer during the knitting reaction (Fig. 2).65 HCP materials have been employed in many applications, including energy storage; (supercapacitor devices, and lithium, sodium, and potassium batteries); chemical sensing; heterogeneous catalysis; optoelectronic devices; drug delivery; gas capture and separation; and chromatographic separation.58–65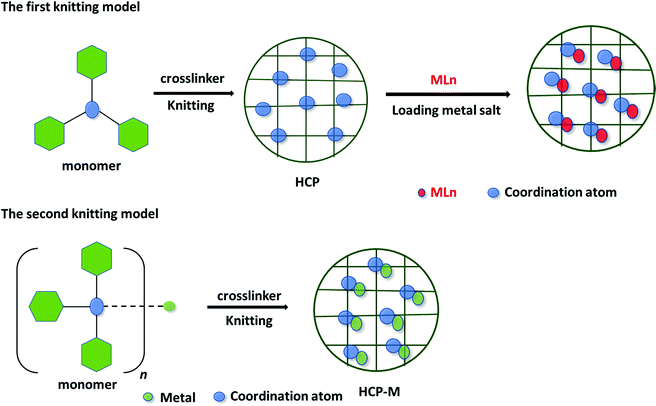 | ||
| Fig. 2 Schematic cartoon for incorporating metal species into HCP frameworks via two different knitting models. Reproduced from ref. 65 with permission from Wiley-VCH. | ||
2.2. Preparation of CTFs
CTFs are partially crystalline, amorphous porous materials possessing conjugated and layered structures.66 Thomas and co-workers were the first to report examples of CTF materials containing aromatic rings and triazine moieties in the core.6 CTFs have attracted much attention for their potential applications in, for example, CO2 reduction, pollutant degradation, organosynthesis, water splitting, H2O2 and H2 production, and organic semiconductor devices.67–72 Several approaches have been used for the preparation of CTFs, including cyclotrimerization of nitrile groups through direct or indirect methods in the presence of molten ZnCl2 at 400 °C; superacid-catalyzed trimerization; Suzuki cross-coupling; Ni-catalyzed Yamamoto coupling; and P2O5 catalysis at 400 °C (Fig. 3).66 The preparation of CTFs with highly crystalline structures requires the selection of monomers with planar characteristics; the use of nonplanar monomers leads to the formation amorphous or semicrystalline CTFs.73–76 To date, only a few crystalline CTFs have been reported. For example, Tang et al. found that the degree of crystallinity of CTFs and the formation of uniform layered structures increased upon increasing the microwave power from 20 to 100 W.77 Interestingly, Xu et al. prepared a two-dimensional (2D) CTF with a well stacked structure within 10 min at 25 °C through a TfOH-catalyzed interfacial reaction, with simple purification procedures applied after the trimerization reactions (Fig. 4).78 They found that the crystalline CTFs formed using this approach were AB-staggered; in contrast, mostly crystalline CTFs exhibiting the AA stacking mode were obtained when using the ionothermal method.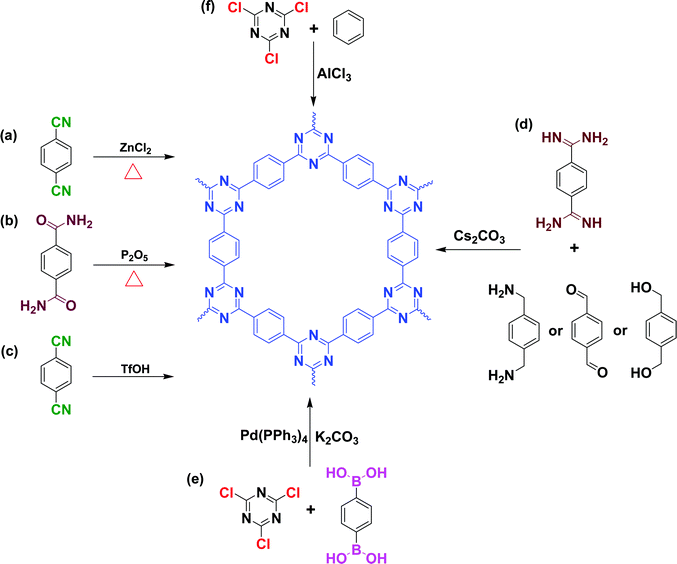 | ||
| Fig. 3 Formation of CTFs via direct or indirect approaches. Reproduced from ref. 66 with permission from American Chemical Society. | ||
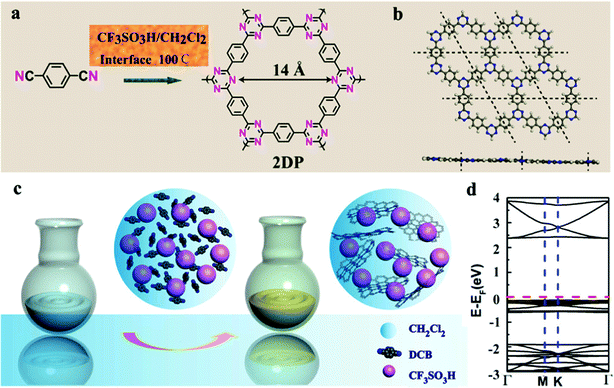 | ||
| Fig. 4 (a) and (b) Synthesis of 2D-CTFs through polymerization at CH2Cl2/CF3SO3H interfaces, (b) and (d) represent the atomic structure and DFT band structure of the 2D-CTF. Reproduced from ref. 78 with permission from American Chemical Society. | ||
2.3. Preparation of CMPs
CMPs are microporous organic polymers that are emerging materials because of their permanent building blocks; huge surface areas; structural adjustability, diversity, and modularity; expanded degrees of conjugation; and excellent physiochemical stabilities.79–82 Accordingly, CMPs have been used widely as materials for photovoltaic devices, light-harvesting, photocatalysis, adsorbents, luminescent materials, and clean energy applications (e.g., supercapacitors, H2 storage, photocatalytic cells, metal–ion rechargeable batteries, CO2 capture and conversion, and fuel cells).79–82 The interesting properties of CMPs arise from (i) their expanded conjugated structures along the polymer chains (significantly enhancing their chemical and physiochemical stabilities) and (ii) their high surface areas and highly crosslinked polymeric network structures (improving their electrochemical activities, cycle stabilities, and kinetics and preventing the active material from dissolution into organic electrolytes).79–84 The approaches used most widely for the preparation of CMPs include phenazine ring fusion, electropolymerization, alkyne metathesis, Schiff base formation, and Yamamoto, Heck, Sonogashira–Hagihara, Buchwald–Hartwig, and Suzuki–Miyaura cross-couplings. These methods allow good selectivities, a range of different of substrates, suitable reaction conditions, simple post-synthetic modifications, and the high-yield production of CMPs with high specific surface areas.2.4. Preparation of COFs
COFs are important emerging materials with potential applications in a wide range of fields, including optics, gas capture, lithium–sulfur batteries, electrocatalysis, metal adsorption, light harvesting, and sensing; they are easy to prepare with high surface areas and total pore volumes, moderate physical and chemical properties, and homogeneous micropores.85–92 COFs have been formed with 2D and 3D network structures comprising backbones containing carbon (C) and hydrogen (H) atoms as well as other elements, including oxygen (O), boron (B), sulfur (S), and nitrogen (N) atoms. Various topologies of 2D and 3D COFs can be induced by varying the geometries and dimensions of their building blocks (Fig. 5).93 Compared with other porous polymeric materials, the ability to predict the skeleton structures of COFs is unique. The many synthetic techniques available for the preparation of COFs include sonochemical methods, interfacial synthesis, ionothermal synthesis, mechanochemical methods, microwave-assisted synthesis, and solvothermal synthesis.93 The fabrication of COF skeletons with particular pore sizes and symmetries depends on the choice of suitable linkers and organic building blocks. The types of linkers that have been used for the preparation of COFs include hydrazone, phenazine, imine, azine, boroxine, imide, and triazine units (Fig. 6).93 2D COF frameworks can have nine different topologies: hexagonal layer (hxl), kgd, tth, mtf, bex, kagome (kgm), hexagonal tungsten bronze (htb), square lattice (sql), and hcb (Fig. 7).94 Feng and co-workers prepared a 3D anionic CDCOF (rra net) containing trinodal secondary building units through the condensation reaction of γ-CD with B(OMe)3 in the presence of LiOH under microwave conditions.95 In addition, Yaghi et al. obtained a COF-based tth topology when combining square-planar, hexagonal, and triangular building blocks and observed tth topology tiling.96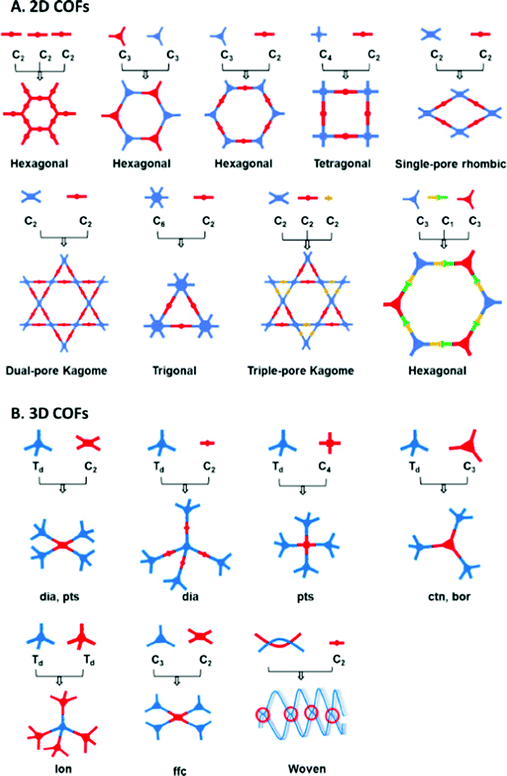 | ||
| Fig. 5 Topology diagrams representing a general basis for COF design and the construction of (A) 2D COFs and (B) 3D COFs.93 Reproduced from ref. 93 with permission from Elsevier. | ||
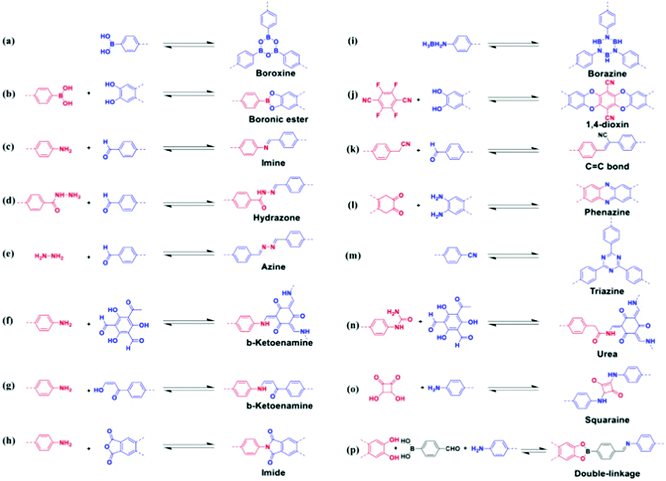 | ||
| Fig. 6 Various linkages for COF formation. Reproduced from ref. 93 with permission from Elsevier. | ||
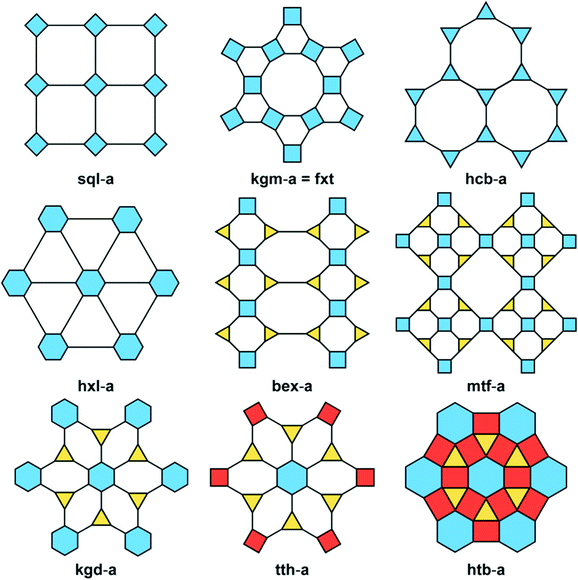 | ||
| Fig. 7 Various topologies in 2D COFs. Reproduced from ref. 94 with permission from American Chemical Society. | ||
3. Potential applications of POPs
3.1. CO2 capture
POPs are ideal candidates for capturing CO2 gas in a green and environmentally friendly manner. Very recently, the Tan group prepared two novel inorganic–organic crosslinked polymers (HCP-PN-1, and HCP-PN-2) based on phosphazene moieties through Friedel–Crafts alkylations for the nucleophilic substitution of hexachlorotriphosphazene with 2-naphthol (PN-Nap-1 and PN-Nap-2) to give nitrogen- and phosphorus-enriched HCPs (Fig. 8).97 Interestingly, these HCPs had hierarchical pore structures and high surface areas (based on BET measurements) that led to high degrees of CO2 adsorption (HCP-PN-1: 72 mg g−1; HCP-PN-2: 57 mg g−1; at 273 K). Li et al. prepared MOP-PZ containing N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and NH groups through a Scholl reaction of 3,5-diphenyl-1H-pyrazole in the presence of AlCl3 in CHCl3 as the solvent. They prepared the hybrid material MOP-PZ-Ag by embedding silver nanoparticles (NPs) into MOP-PZ, and found that MOP-PZ-Ag functioned as a heterogeneous catalyst for the production of propiolic acid through the carboxylation of some terminal alkynes; this stable hybrid material also displayed an excellent degree of CO2 adsorption (183.7 mg g−1).98 Wang and co-workers prepared nitrogen-enriched microporous polymers containing various contents of amino groups through condensation reactions of melamine with formohydrazide, formamide, N,N-dimethylformamide (DMF), and N-methylformamide. These four polymers possessed pore diameters of 0.52–1.10 nm and specific surface areas of 612–748 m2 g−1, with the BET method, and SEM, and TEM analysis revealing that they were agglomerates of tiny particles with an irregular shape and porous structures; the four polymers provided CO2 uptakes of up to 103 mg g−1 at 273 K.99 Liang et al. prepared four kinds of CMPs and used a post-knitting approach to improve their porosities, affording eight CMP-based HCPs (KCMPs) (Fig. 9).100 These KCMPs had high specific surface areas (up to 2267 m2 g−1), high pore volumes (up to 3.27 cm3 g−1), and excellent degrees of CO2 uptake (up to 175.12 mg g−1 at 273 K). Das et al. prepared two kinds of CTFs based on 1,2,3-triazolo units; their Tz-CTF polymeric frameworks exhibited excellent CO2 uptake capacities, with a high surface area, chemical and thermal stabilities, agglomerated tiny particles, and regular porous structures, based on SEM and TEM imaging.101 Our group also prepared two bicarbazole-based COFs (Cz-BD, and Cz-DHBD) through Schiff-base condensations of Cz-4CHO (as the C2-symmetric knot) with BD and DHBD.102 These Cz-BD and Cz-DHBD COFs possessed high surface areas (up to 2111 and 992.2 m2 g−1, respectively) as well as thermal stability up to 507 °C. The Cz-DHBD COF featured a kagome structure, due to intramolecular OH⋯N hydrogen bonding, that increased the steric bulk hinderance and decreased the nucleophilicity of the imino nitrogen atoms, thereby decreasing its ability to capture CO2 (110.59 mg g−1) relative to that of the Cz-BD COF (125.95 mg g−1). In addition, we have prepared hollow microspherical and microtubular carbazole-based COFs through condensations of Car-3NH2 and the triformyl linkers TPA-3CHO, TPP-3CHO, and TPT-3CHO with various degrees of planarity, obtaining high surface areas of 1334, 743, and 721 m2 g−1, respectively. Due to their high surface areas, these Car-TPA, Car-TPT, and Car-TPP COFs provided high degrees of CO2 capture, with recorded uptake rates of up to 61, 42, and 34 mg g−1, respectively, at 298 K. Although Car-TPT and Car-TPP had similar surface areas, the former provided a higher CO2 capture rate because of the higher nitrogen content of the triazine units in the Car-TPT COF.103 The effect of the nitrogen content on the CO2 capture ability of COFs was also observed in six 2D COFs displaying various planarities, symmetries, and nitrogen contents, synthesized through the condensation reaction of TPA-3NH2 and TPT-3NH2 (as triarylamine monomers) with the monomers TPA-3CHO, TPP-3CHO, and 2TPT-3CHO (with various degrees of planarity) (Fig. 10).104 Here, TPT-3NH2 is more planar than TPA-3NH2, and TPT-3CHO is more planar than TPP-3CHO and TPA-3CHO. The COFs with lower planarity possessed lower surface areas; those with higher nitrogen contents and higher nucleophilicities displayed higher degrees of CO2 capture. As expected, the higher nitrogen contents in the TPT-based COFs provided higher rates of CO2 capture (up to 92.38 mg g−1 at 273 K for TPT-COF-6), with the greater planarity of their building blocks also enhancing the morphology to favor CO2 capture.104 We have also prepared β-ketoenamine-linked COFs (TFP-TPA, TFP-Car, and TFP-TPP) through solvothermal Schiff-base [3+3] polycondensations of TFP-3OHCHO with three tris(aminophenyl)-presenting derivatives (possessing amino, carbazole, and pyridine units, respectively).105 The TFP-TPA, TFP-Car, and TFP-TPP COFs possessed different degrees of planarity and surface areas of 457, 362, and 686 m2 g−1, respectively. The higher degrees of planarity of the TFP-Car and TFP-TPP COFs resulted in higher thermal stabilities and stronger interactions with CO2 molecules at 273 K (up to 190 and 200 mg g−1, respectively) relative to those exhibited by the lower-planarity TPA-Car COF (183 mg g−1).105 Nagai et al. reported four types of PI-COF prepared through solvothermal reactions of TAPA and TAPB with PMDA and NTCDA (Fig. 11).106 These PI-COF materials had specific surface areas exceeding 500 m2 g−1 and were applied for CO2 uptake. We have used Schiff base formation, reduction, and Mannich and Sonogashira–Hagihara couplings to prepare two new CMPs (TPE-TPE-BZ, and Py-TPE-BZ) featuring benzoxazine-linked tetraphenylethylene and pyrene units (Fig. 12)107 Upon thermal treatment, the benzoxazine units in the backbones of the TPE-TPE-BZ and Py-TPE-BZ CMPs underwent ring opening polymerizations to form new materials displaying high performance for CO2 uptake, due to the presence of phenolic OH and Mannich bridges capable of hydrogen bonding with CO2 molecules.
C and NH groups through a Scholl reaction of 3,5-diphenyl-1H-pyrazole in the presence of AlCl3 in CHCl3 as the solvent. They prepared the hybrid material MOP-PZ-Ag by embedding silver nanoparticles (NPs) into MOP-PZ, and found that MOP-PZ-Ag functioned as a heterogeneous catalyst for the production of propiolic acid through the carboxylation of some terminal alkynes; this stable hybrid material also displayed an excellent degree of CO2 adsorption (183.7 mg g−1).98 Wang and co-workers prepared nitrogen-enriched microporous polymers containing various contents of amino groups through condensation reactions of melamine with formohydrazide, formamide, N,N-dimethylformamide (DMF), and N-methylformamide. These four polymers possessed pore diameters of 0.52–1.10 nm and specific surface areas of 612–748 m2 g−1, with the BET method, and SEM, and TEM analysis revealing that they were agglomerates of tiny particles with an irregular shape and porous structures; the four polymers provided CO2 uptakes of up to 103 mg g−1 at 273 K.99 Liang et al. prepared four kinds of CMPs and used a post-knitting approach to improve their porosities, affording eight CMP-based HCPs (KCMPs) (Fig. 9).100 These KCMPs had high specific surface areas (up to 2267 m2 g−1), high pore volumes (up to 3.27 cm3 g−1), and excellent degrees of CO2 uptake (up to 175.12 mg g−1 at 273 K). Das et al. prepared two kinds of CTFs based on 1,2,3-triazolo units; their Tz-CTF polymeric frameworks exhibited excellent CO2 uptake capacities, with a high surface area, chemical and thermal stabilities, agglomerated tiny particles, and regular porous structures, based on SEM and TEM imaging.101 Our group also prepared two bicarbazole-based COFs (Cz-BD, and Cz-DHBD) through Schiff-base condensations of Cz-4CHO (as the C2-symmetric knot) with BD and DHBD.102 These Cz-BD and Cz-DHBD COFs possessed high surface areas (up to 2111 and 992.2 m2 g−1, respectively) as well as thermal stability up to 507 °C. The Cz-DHBD COF featured a kagome structure, due to intramolecular OH⋯N hydrogen bonding, that increased the steric bulk hinderance and decreased the nucleophilicity of the imino nitrogen atoms, thereby decreasing its ability to capture CO2 (110.59 mg g−1) relative to that of the Cz-BD COF (125.95 mg g−1). In addition, we have prepared hollow microspherical and microtubular carbazole-based COFs through condensations of Car-3NH2 and the triformyl linkers TPA-3CHO, TPP-3CHO, and TPT-3CHO with various degrees of planarity, obtaining high surface areas of 1334, 743, and 721 m2 g−1, respectively. Due to their high surface areas, these Car-TPA, Car-TPT, and Car-TPP COFs provided high degrees of CO2 capture, with recorded uptake rates of up to 61, 42, and 34 mg g−1, respectively, at 298 K. Although Car-TPT and Car-TPP had similar surface areas, the former provided a higher CO2 capture rate because of the higher nitrogen content of the triazine units in the Car-TPT COF.103 The effect of the nitrogen content on the CO2 capture ability of COFs was also observed in six 2D COFs displaying various planarities, symmetries, and nitrogen contents, synthesized through the condensation reaction of TPA-3NH2 and TPT-3NH2 (as triarylamine monomers) with the monomers TPA-3CHO, TPP-3CHO, and 2TPT-3CHO (with various degrees of planarity) (Fig. 10).104 Here, TPT-3NH2 is more planar than TPA-3NH2, and TPT-3CHO is more planar than TPP-3CHO and TPA-3CHO. The COFs with lower planarity possessed lower surface areas; those with higher nitrogen contents and higher nucleophilicities displayed higher degrees of CO2 capture. As expected, the higher nitrogen contents in the TPT-based COFs provided higher rates of CO2 capture (up to 92.38 mg g−1 at 273 K for TPT-COF-6), with the greater planarity of their building blocks also enhancing the morphology to favor CO2 capture.104 We have also prepared β-ketoenamine-linked COFs (TFP-TPA, TFP-Car, and TFP-TPP) through solvothermal Schiff-base [3+3] polycondensations of TFP-3OHCHO with three tris(aminophenyl)-presenting derivatives (possessing amino, carbazole, and pyridine units, respectively).105 The TFP-TPA, TFP-Car, and TFP-TPP COFs possessed different degrees of planarity and surface areas of 457, 362, and 686 m2 g−1, respectively. The higher degrees of planarity of the TFP-Car and TFP-TPP COFs resulted in higher thermal stabilities and stronger interactions with CO2 molecules at 273 K (up to 190 and 200 mg g−1, respectively) relative to those exhibited by the lower-planarity TPA-Car COF (183 mg g−1).105 Nagai et al. reported four types of PI-COF prepared through solvothermal reactions of TAPA and TAPB with PMDA and NTCDA (Fig. 11).106 These PI-COF materials had specific surface areas exceeding 500 m2 g−1 and were applied for CO2 uptake. We have used Schiff base formation, reduction, and Mannich and Sonogashira–Hagihara couplings to prepare two new CMPs (TPE-TPE-BZ, and Py-TPE-BZ) featuring benzoxazine-linked tetraphenylethylene and pyrene units (Fig. 12)107 Upon thermal treatment, the benzoxazine units in the backbones of the TPE-TPE-BZ and Py-TPE-BZ CMPs underwent ring opening polymerizations to form new materials displaying high performance for CO2 uptake, due to the presence of phenolic OH and Mannich bridges capable of hydrogen bonding with CO2 molecules.
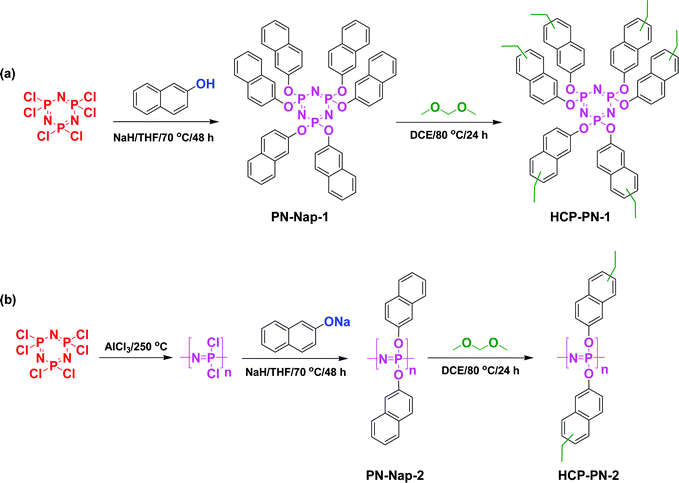 | ||
| Fig. 8 Synthesis of (a) PN-Nap-2 and HCP-PN-2, (b) and (c) CO2 adsorption of PN-Nap-2 and HCP-PN-2 at 0 and 25 °C. Reproduced from ref. 97 with permission from American Chemical Society. | ||
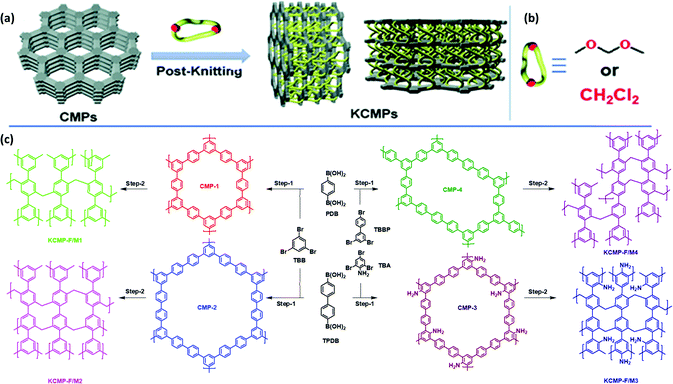 | ||
| Fig. 9 Schematic cartoon for the synthesis of CMPs via the post-knitting method. (b) The cross-linker structure. (c) The synthetic routes for the KCMP via palladium-catalyzed Suzuki coupling and a Lewis acid catalyzed Friedel–Crafts reaction. Reproduced from ref. 100 with permission from the Royal Society of Chemistry. | ||
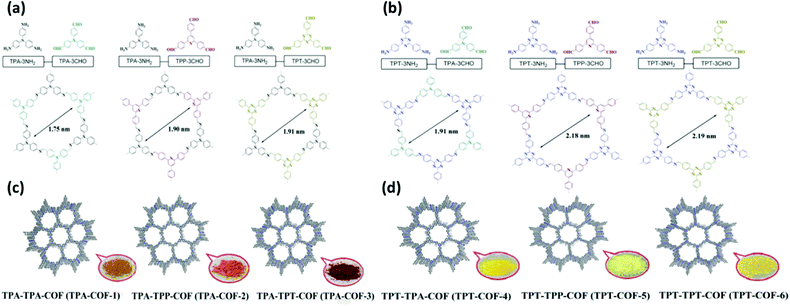 | ||
| Fig. 10 (a and b) Synthesis of TPA-COF-1, TPA-COF-2, TPA-COF-3, TPT-COF-1, TPT-COF-2, and TPT-COF-3 through Schiff base reactions. (c and d) Their color photos. Reproduced from ref. 104 with permission from the Royal Society of Chemistry. | ||
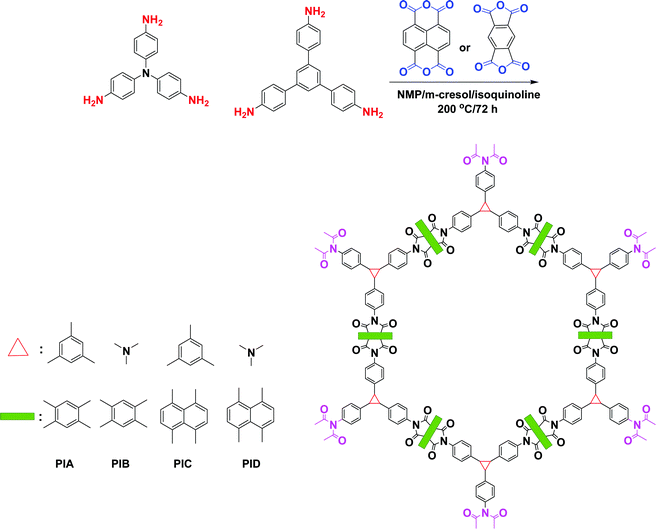 | ||
| Fig. 11 Synthesis of the four polyimide COFs. Reproduced from ref. 106 with permission from American Chemical Society. | ||
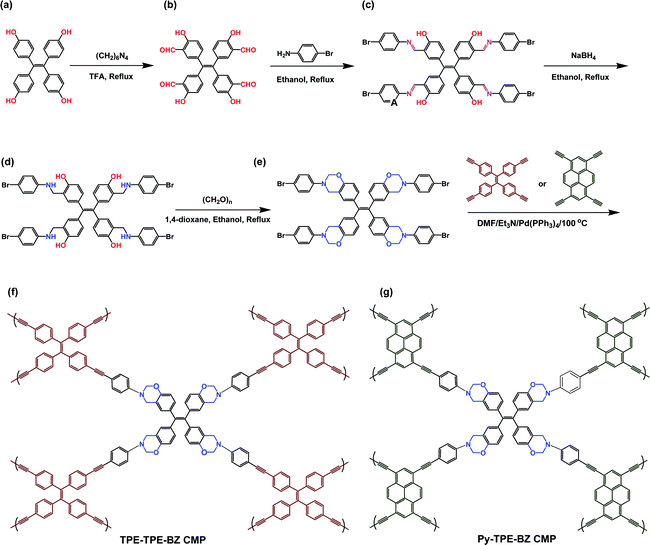 | ||
| Fig. 12 Synthesis of the TPE-TPE-BZ CMP, and TPE-TPE-BZ CMPs through a multistep reaction. Reproduced from ref. 107 with permission from American Chemical Society. | ||
3.2. Dyes and iodine adsorption
Yu et al. prepared four HCP materials (BCB, Py, CCBCB, and CCPy-CMP) based on perylene, bicarbazole, and triazine units (Fig. 13).108 According to BET analyses, the surface areas of BCB, Py, CCBCB and CCPy-CMP were 545, 624, 220, and 20 m2 g−1, respectively; their surface morphologies featured nanotubes, graphitic flakes, aggregated particles, and nanosheet stacks, respectively. The I2 uptake of the CCBCP-CMP sample [11.56 wt (m−2 g−1)] was higher than those of the other precursors because of its high surface area and high content of N-heteroatoms. Recently, we used Friedel–Crafts polymerization to prepare the hybrid inorganic/organic porous materials POSS-TPE and POSS-TPP based on cubic octavinylsilsesquioxane (OVS) and tetraphenylethene and tetraphenylpyrazine units, respectively. The as-prepared POSS-TPE had a high surface area (741 m2 g−1) and good thermal stability, as determined using N2 adsorption/desorption and TGA, respectively.109 The Alameddine group prepared five CMP materials through Cu-catalyzed [4+2] benzannulations of 2,5-bis(phenylethynyl)tetraphthaldehyde with a series of 1,4-diarylethynyltriptycene derivatives.110 The resultant CMPs 1–5 were microporous, with high BET surface areas (794 m2 g−1) and pore volumes (0.63 cm−3 g−1), and displayed good I2 uptake (166 wt%). Lin et al. constructed CTT-POP1, a POP containing triaryl triazine and featuring intramolecular hydrogen bonds, that functioned as an adsorbent for the dye methylene blue because of its surface wettability and capability for π–π interactions.111 The Das group prepared a series of covalent organic polymers (COPs) through the reactions of triptycene with derivatives of benzene-1,3,5-tricarboxaldehyde.112 Interestingly, the surface areas of these resulting polymers were strongly affected by the number of OH groups in the I-COPs, as predicted from the BET profiles. Due to their high surface areas and uniform spherical shapes, determined from SEM images, the I2 capacities of these I-COPs were outstanding (ca. 4860 mg g−1) when compared with those of other COP materials. The ability to tune the charge density of COFs, when combined with their high crystallinities and high surface areas, makes them good candidates for capturing various chemicals, including dyes. Our group employed the previously mentioned BFTB-PyTA, BFTB-BFTB, and BFTB-BCTA COFs for dye adsorption; the adsorption efficacies of the hollow-structured BFTB-PyTA and BFTB-BFTB COFs were higher than that of the non-hollow-structured BFTB-BCTA COF. These COFs displayed excellent adsorption performance (up to 99.2%) and confirmed that the controlling parameters of dye adsorption in the COFs were the surface area, the morphology, and the capability for π-stacking with the dye.113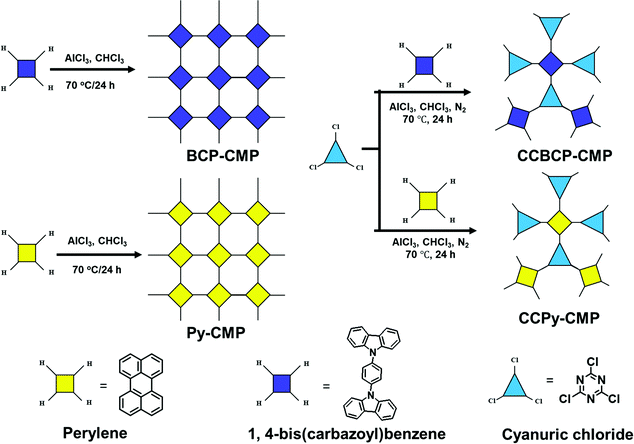 | ||
| Fig. 13 Synthesis routes for BCB-CMP, Py-CMP, CCBCB-CMP and CCPy-CMP. Reproduced from ref. 108 with permission from Elsevier. | ||
3.3. Energy storage
There are many reports of the use of POPs as excellent materials for energy storage. For example, we have prepared microporous CTFs based on pyrene units (Pyrene-CTF-10, and Pyrene-CTF-20) through ionothermal reactions of 1,3,6,8-cyanopyrene (TCNPy) with different mole ratios of ZnCl2 at 500 °C.114 Pyrene-CTF-10 and Pyrene-CTF-20 possessed high surface areas (819 and 1019 m2 g−1, respectively) and capacitances (380 and 500 F g−1, respectively). We have also prepared bicarbazole-based CTFs (Car-CTFs) based on Car-4CN. According to TGA and N2 adsorption/desorption analyses, our Car-CTFs had high specific surface areas (up to 1400 m2 g−1), and excellent coulombic efficiencies.115 Mohamed et al. synthesized two HCPs (TPE-CPOP1, and TPE-CPOP2) based on tetraphenylethene as the main building unit and these obtained materials had high BET surface areas, amorphous structures, and high specific capacitances.116 Furthermore, we prepared An-CPOP1 and An-CPOP2 through simple Friedel–Crafts alkylations of An-4Ph with 2,4,6-trichloro-1,3,5-triazine and formaldehyde dimethyl acetal as crosslinker reagents.117 An-CPOP1 possessed a tubular nanotube structure, based on SEM imaging, while An-CPOP2 displayed a capacitance of 98.10 F g−1 at 0.5 A g−1. Mohamed et al. prepared DPT-HPP through a Friedel–Crafts reaction of (4-(5,6-diphenyl-1H-benzimidazol-2-yl)triphenylamine in the presence of FeCl3; it exhibited a specific capacitance of 110 F g−1 and excellent cycling stability over 2000 cycles.118 We reported the TPPDATPPyr and TPPDA-TPTPE COFs prepared through Schiff-base condensations of TPPDA(NH2)4 with TPPyr(CHO)4 and TPTPE(CHO)4, respectively.119 The TPPDATPPyr and TPPDA-TPTPE COFs were highly thermally resistant, with decomposition temperatures (Td10) of up to 543 and 551 °C, respectively; in addition, they had high surface areas (up to 1020 and 1067 m2 g−1, respectively). Both the TPPDATPPyr and TPPDA-TPTPE COFs underwent reversible redox processes at a low sweep rate (5 mV s−1) over the potential range from +0.18 to −0.92 V, because of their redox-active triphenylamino groups. At a current density of 2 A g−1, the specific capacitance of the TPPDA-TPTPE COF (237.1 F g−1) was higher than that of the TPPDATPPyr COF (188.7 F g−1), due to the higher surface area of the former, as well as the easy access for electrolytes to its electrode surface, thanks to the presence of the heteroatoms. Furthermore, the TPPDATPPyr and TPPDA-TPTPE COFs displayed excellent cycling capacities, with retentions of up to 86.2 and 85.6%, respectively, after 5000 cycles. We have examined the effect of the COF surface area on capacitance through the formation of a sponge-like shell TPT-DAHQ COF through the template-free condensation of TPT-3CHO and DAHQ-2HCl.120 The TPT-DAHQ COF possessed an extraordinary surface area (up to 1855 m2 g−1) in addition to high thermal resistance (up to 452 °C). The high surface area of the TPT-DAHQ COF was the main factor behind its excellent capacitance (up to 256 F g−1 at current density of 0.5 A g−1); it displayed high durability, with up to 98.8% of its original capacitance retained without any obvious degradation after 1850 cycles at 10 A g−1. We also elucidated the effect of the active redox sites in COFs structures through one-pot syntheses of three biflurorenylidene-based COFs BFTB-PyTA, BFTB-BFTB, and BFTB-BCTA through reactions of BFTB-4CHO with PyTA-4NH2, BFTB-4NH2, and BCTA-4NH2, respectively.113 In addition to the BFTB-PyTA, BFTB-BFTB, and BFTB-BCTA COFs displaying record-high thermal resistances of up to 433, 416, and 449 °C, respectively, they also exhibited high BET surface areas (up to 1133, 1040, and 834 m2 g−1, respectively), determined through N2 isothermal analyses at 77 K. The specific capacitances of the BFTB-PyTA and BFTB-BFTB COFs were 68.0 and 84.5 F g−1, respectively, at a scan rate of 5 mV s−1; the specific capacitance of the BFTB-BCTA COF at the same scan rate was higher (89.9 F g−1), although it had the lowest surface area, revealing that the redox-active sites were the carbazole subunits. Furthermore, the smooth surface morphology of the BFTB-BFTB COF played an important role in its specific capacitance being higher than that of the BFTB-PyTA COF, which had a hollow-microtubule structure. The capacitive retentions of the BFTB-PyTA, BFTB-BFTB, and BFTB-BCTA COFs were 97.27, 85.23, and 91.21%, respectively. Using our previously mentioned TFP-COFs, we investigated the effects of redox-active amino, pyridine, and carbazole groups on the reversible redox processes occurring at a low sweep rate of 5 mV s−1. The specific capacitance of the TFP-TPA COF (up to 291.1 F g−1) was higher than those of the TFP-TPP and TFP-Car COFs, due to its higher nitrogen-atom percentage; therefore, it had a greater number of available redox active sites, even though it had a lower surface area. The TFP-TPA COF, TFP-TPP and TFP-Car COFs exhibited high durability after 5000 cycles at 10 A g−1, with retention of their original values of up to 91, 88.2, and 90.4%, respectively.105 Triphenylamine has been a highly studied redox-active unit because of its ability to store energy. Our group investigated the previously mentioned COFs 1–6 to test their validity as supercapacitors. Indeed, the TPA-COFs 1–4 exhibited supercapacitance, whereas the TPT-COFs 5 and 6 did not provide any redox curves during cyclic voltammetry, consistent with their absence of triphenylamine moieties. Notably, the unsaturated N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C units do not display any reactivity in acidic media, and increasing the number of triphenylamine groups enhanced the specific capacitance; as a result, the capacitance of TPA-TPA-COF-1, which featured six triphenylamine groups, was higher than those of the other COFs 2–4. Morphologies and chemical structures can also play a role in affecting the specific capacitances of supercapacitors. We confirmed these phenomena in a recent study of the previously mentioned Car-TPA, Car-TPP, and Car-TPT COFs, which possessed carbazole, pyridine, and triphenylamine units, respectively, making them ideal candidates for storing energy; at 0.2 A g−1, they displayed specific capacitances of 13.6, 14.5, and 17.4 F g−1, respectively.103 The Car-TPA and Car-TPP COFs had high surface areas and two redox groups, but provided lower capacitances; in contrast, the Car-TPT COF had a low surface area and only one redox group, but its microtubular structure and molecular design led to enhanced capacitance and retention during the charging/discharging process. Recently, Maha et al. used Sonogashira–Hagihara reactions to prepare a series of CMPs based on tetrabenzonaphthalene (TBN) moieties (TBN-TPE-CMP, TBN-Py-CMP, and TBN-Car-CMP) [Fig. 14(a)].121 Subsequently, we improved the conductivity of these materials by mixing them with single-walled carbon nanotubes (SWCNTs) [Fig. 14(b)]. Interestingly, the capacitance of the TBN-Py-CMP/SWCNT nanocomposite (430 F g−1 at 0.5 A g−1) was higher than those of the other samples, presumably because of strong π–π interactions between the pyrene units in the TBN-Py-CMP framework and the SWCNTs.122–125 Eddaoudi et al. prepared Hex-Aza-COF-2 and Hex-Aza-COF-3 through solvothermal condensations of benzoquinone with redox-functionalized aromatic tetramines and phenazine, respectively [Fig. 15(a)].126 They used powder X-ray diffraction (PXRD), solid state NMR spectroscopy, BET analysis, SEM, and TEM imaging to investigate the chemical structures, crystallinities, porosities, and morphologies of these materials [Fig. 15(b)–(i)]. The PXRD data and SEM images revealed that both materials had moderate crystallinities and aggregated spherical morphologies. Furthermore, based on electrochemical measurements, the specific capacitances of Hex-Aza-COF-2 (585 F g−1) and Hex-Aza-COF-3 (663 F g−1) were higher than those reported previously for porous polymeric and COF materials. Yang et al. prepared a new porous organic HCP through the reaction of pyrene with CHCl3 as the crosslinker and AlCl3 as the catalyst, with the subsequent hydrolysis affording HcPPy that possessed a high surface area (723 m2 g−1) and micropores (1 nm) and mesopores (2–3 and 3.5–4.5 nm).127 This material functioned as an anode in Li batteries and provided excellent current densities (up to 683 MA h g−1 at 1000 mA g−1). Wong et al. prepared materials named SP and HP through Sonogashira couplings of tri(4-ethynylphenylamine) with 9-ferrocenylidene-2,7-diiodo-9H-fluorene in the presence and absence of ZIF-67, respectively.128 They then prepared Fe-embedded magnetic carbon materials (SP and HP-MCMs) through the carbonization of SP and HP at 500 °C for 1 h. The obtained spherical SP-MCP had a microporous architecture, good redox performance, and good cycling stability when applied in lithium-ion batteries (LIBs) as an anode; its performance was superior to that of the HP-MCP. Zhao et al. prepared the 2D-COF-ETTA-ETTCA and COF-ETTA-ETTCA-S materials by loading 88.4 wt% of sulfur and applied these materials in lithium–sulfur batteries.129 The devices displayed high capacity (up to 1617 mA h g−1 at 0.1C), low capacitance decay after 528 cycles, and high coulombic performance (ca. 98%).
C units do not display any reactivity in acidic media, and increasing the number of triphenylamine groups enhanced the specific capacitance; as a result, the capacitance of TPA-TPA-COF-1, which featured six triphenylamine groups, was higher than those of the other COFs 2–4. Morphologies and chemical structures can also play a role in affecting the specific capacitances of supercapacitors. We confirmed these phenomena in a recent study of the previously mentioned Car-TPA, Car-TPP, and Car-TPT COFs, which possessed carbazole, pyridine, and triphenylamine units, respectively, making them ideal candidates for storing energy; at 0.2 A g−1, they displayed specific capacitances of 13.6, 14.5, and 17.4 F g−1, respectively.103 The Car-TPA and Car-TPP COFs had high surface areas and two redox groups, but provided lower capacitances; in contrast, the Car-TPT COF had a low surface area and only one redox group, but its microtubular structure and molecular design led to enhanced capacitance and retention during the charging/discharging process. Recently, Maha et al. used Sonogashira–Hagihara reactions to prepare a series of CMPs based on tetrabenzonaphthalene (TBN) moieties (TBN-TPE-CMP, TBN-Py-CMP, and TBN-Car-CMP) [Fig. 14(a)].121 Subsequently, we improved the conductivity of these materials by mixing them with single-walled carbon nanotubes (SWCNTs) [Fig. 14(b)]. Interestingly, the capacitance of the TBN-Py-CMP/SWCNT nanocomposite (430 F g−1 at 0.5 A g−1) was higher than those of the other samples, presumably because of strong π–π interactions between the pyrene units in the TBN-Py-CMP framework and the SWCNTs.122–125 Eddaoudi et al. prepared Hex-Aza-COF-2 and Hex-Aza-COF-3 through solvothermal condensations of benzoquinone with redox-functionalized aromatic tetramines and phenazine, respectively [Fig. 15(a)].126 They used powder X-ray diffraction (PXRD), solid state NMR spectroscopy, BET analysis, SEM, and TEM imaging to investigate the chemical structures, crystallinities, porosities, and morphologies of these materials [Fig. 15(b)–(i)]. The PXRD data and SEM images revealed that both materials had moderate crystallinities and aggregated spherical morphologies. Furthermore, based on electrochemical measurements, the specific capacitances of Hex-Aza-COF-2 (585 F g−1) and Hex-Aza-COF-3 (663 F g−1) were higher than those reported previously for porous polymeric and COF materials. Yang et al. prepared a new porous organic HCP through the reaction of pyrene with CHCl3 as the crosslinker and AlCl3 as the catalyst, with the subsequent hydrolysis affording HcPPy that possessed a high surface area (723 m2 g−1) and micropores (1 nm) and mesopores (2–3 and 3.5–4.5 nm).127 This material functioned as an anode in Li batteries and provided excellent current densities (up to 683 MA h g−1 at 1000 mA g−1). Wong et al. prepared materials named SP and HP through Sonogashira couplings of tri(4-ethynylphenylamine) with 9-ferrocenylidene-2,7-diiodo-9H-fluorene in the presence and absence of ZIF-67, respectively.128 They then prepared Fe-embedded magnetic carbon materials (SP and HP-MCMs) through the carbonization of SP and HP at 500 °C for 1 h. The obtained spherical SP-MCP had a microporous architecture, good redox performance, and good cycling stability when applied in lithium-ion batteries (LIBs) as an anode; its performance was superior to that of the HP-MCP. Zhao et al. prepared the 2D-COF-ETTA-ETTCA and COF-ETTA-ETTCA-S materials by loading 88.4 wt% of sulfur and applied these materials in lithium–sulfur batteries.129 The devices displayed high capacity (up to 1617 mA h g−1 at 0.1C), low capacitance decay after 528 cycles, and high coulombic performance (ca. 98%).
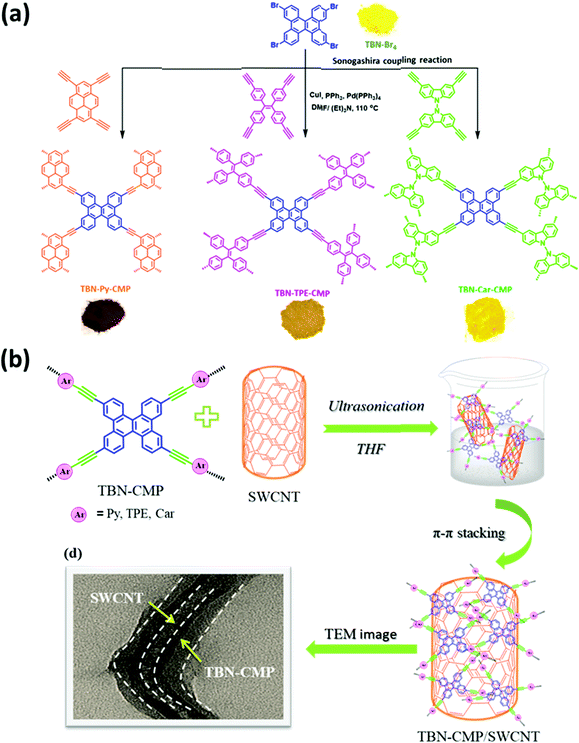 | ||
| Fig. 14 (a) Synthesis of TBN-Py-CMP, TBN-TPE-CMP, and TBN-Car-CMP and (b) formation of TBN-CMP/SWCNTs. Reproduced from ref. 121 with permission from American Chemical Society. | ||
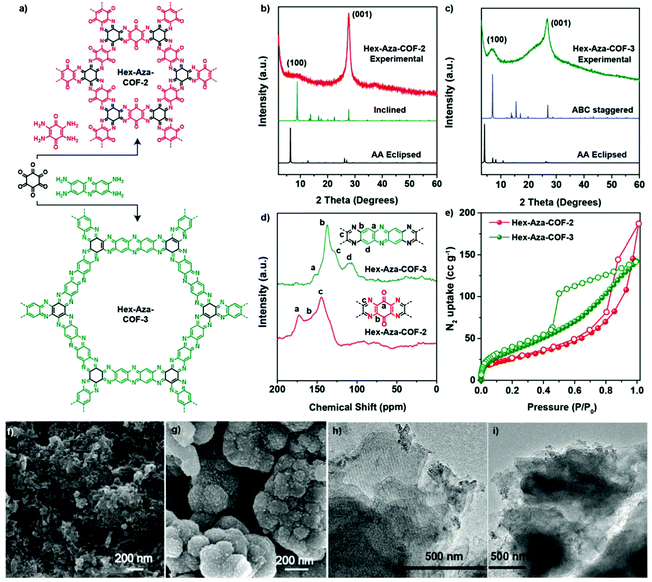 | ||
| Fig. 15 (a) Synthesis of Hex-Aza-2 and Hex-Aza-COF-3, (b) and (c) PXRD pattern for Hex-Aza-COF-2 and Hex-Aza-COF-3. (d) Solid-state 13C NMR for Hex-Aza-COF-2 and Hex- Aza-COF-3. (e) BET isotherms for Hex-Aza-COF-2 and Hex-Aza-COF-3. (f and g) SEM images for Hex-Aza-COF-2 and Hex-Aza-COF-3. (h and i) TEM images for Hex-Aza-COF-2 and Hex-Aza-COF-3. Reproduced from ref. 126 with permission from Wiley-VCH. | ||
3.4. Hydrogen evolution
Chi et al. synthesized a new electrochemical material (PMO10V2@CTF) through the fabrication of PMO10V2 with a cationic CTF as a support, itself prepared through trimerization of 1,3-bis(4-cyanophenyl)imidazolium chloride. PMO10V2@CTF functioned as the catalyst for the production of benzaldehyde and H2 through the oxidation of benzyl alcohol.130 Recently, der Voort et al. prepared metal-free electrocatalysts based on porous BINOL-CTF materials featuring various nitrogen-containing groups (pyridine-N-oxide, pyrrolic-N, quaternary-N, and pyridinic-N/triazine-N units) through cyclization reactions of 2,2′-dihydroxy[1,1′-binaphthalene]-6,6′-dicarbonitrile as the C and N atom sources in molten ZnCl2 at 400 and 500 °C, respectively.131 BINOL-10–500 displayed outstanding performance in the hydrogen evolution reaction (HER) and oxygen reduction reaction (ORR), relative to the other CTF samples, as a result of its large pores, high specific surface area, and high quaternary-N content. Recently, COFs have garnered an exciting position in the field of H2 production because of their ability to facilitate charge transfer and light harvesting. Accordingly, our group applied two of the previously mentioned COFs (PyTA-BC, and PyTA-BC-Ph), based on their excellent photophysical properties, as photocatalysts for H2 evolution from water in the presence of a sacrificial electron donor. The PyTA-BC and PyTA-BC-Ph COFs provided photocatalytic activities of up to 1183 and 417 µmol g−1 h−1, respectively, in the presence of ascorbic acid as the electron donor.132 Jiang et al. prepared, through the in situ growth of TFPT-DETH on the octahedral NH2-UiO-66 surface, an NH2-UiO-66@TFPT-DETH core–shell hetero-framework possessing an octahedral morphology, a smooth surface, a high surface area, light absorption properties, and both meso- and microporous structures; it mediated a high degree of H2 production from water (up to 7178 mmol g−1 h−1) (Fig. 16).133 Interestingly, Guo et al. prepared multivariate Tp(BTxTP1−x)-COFs for photocatalytic H2 evolution from water through condensation of the monomer 1,3,5-triformylphloroglucinol (Tp) with tertiary phenyl (TP) and benzothiadiazole-derived (BT) units [Fig. 17(a)].134 FTIR and solid state NMR spectroscopy confirmed the chemical structures of the resulting COFs, which possessed highly crystalline structures and high surface areas (>700 m2 g−1) [Fig. 17(b)–(e)]. The H2 evolution rate increased to 9839 µmol g−1 h−1 when the COF material contained 5 mol% of BT.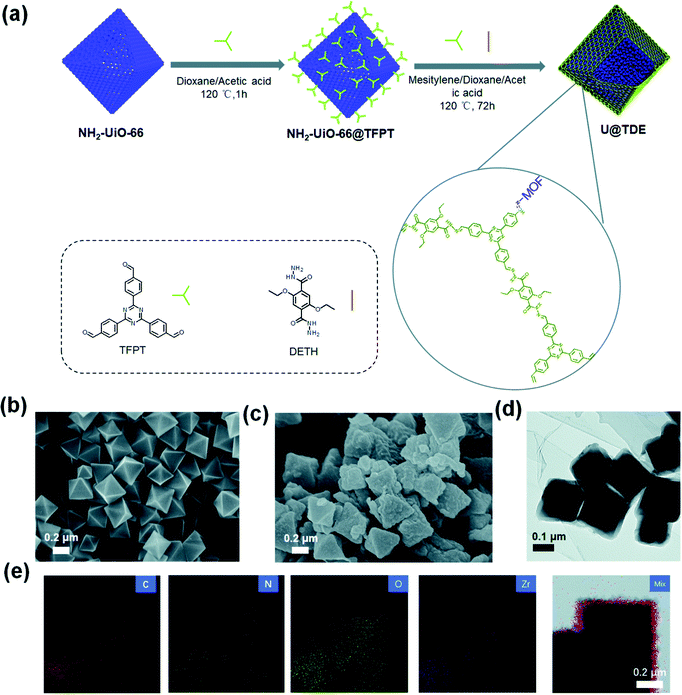 | ||
| Fig. 16 (a) Schematic for the preparation of U@TDEn core–shell hetero frameworks. (b) SEM image of NH2-UiO-66; (c) SEM, (d) TEM image and (e) EDX mapping of U@TDE4. Reproduced from ref. 133 with permission from the Royal Society of Chemistry. | ||
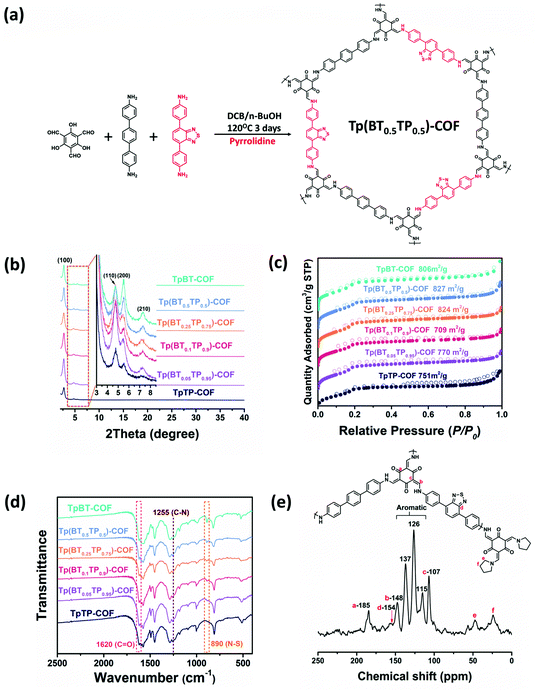 | ||
| Fig. 17 Synthesis of Tp(BTxTP1−x)-COFs. (b)–(d) PXRD, N2 adsorption–desorption and FT-IR profiles of Tp(BTxTP1−x)-COFs. (e) Solid-state 13C CP/MAS NMR spectrum of Tp(BT0.05TP0.95)-COF. Reproduced from ref. 134 with permission from the Royal Society of Chemistry. | ||
3.5. Chemical sensing
Laskar et al. prepared two branched conjugated mesoporous polymers (CMO1 and CMO2) displaying aggregation-induced emission and high quantum yields.135 CMO1 was more sensitive than CMO2 towards trinitrotoluene (TNT) and picric acid, due to the higher pore density and size in CMO1. The Zhong group prepared a highly fluorescent F-CTF-3 porous framework through the condensation of phenamidine hydrochloride with 4,4′-(benzo[c][1,2,5]thiadiazole-4,7-diyl)dibenzaldehyde.136 This fluorescent material functioned as a chemical sensor for phenylamine (PA) and phenylenediamine (PDA), with detection at concentrations of 11.7 and 1.47 nM, respectively. The excellent luminescence features of COFs can arise in addition to their low densities, high chemical stabilities, and high thermal stabilities, making them promising candidates for use as fluorescent sensors. Often, π-conjugation of the building blocks (e.g., triazine, carbazole, pyrene, and triphenylene) or the inherently rigid structure of the COFs leads to this fluorescence behavior. We reported the first applicable COF for the detection of amino acids. We used solvothermal conditions to prepare a Cu@TFPB-DHTH COF (Fig. 18) through polycondensation of TFPB-3CHO and DHTH and subsequent modification with Cu2+ ions.137 The BET surface area of the TFPB-DHTH COF was 360 m2 g−1; it displayed high thermal stability up to 322 °C. The TFPB-DHTH COF exhibited highly fluorescent yellow emission in addition to high chemical stability; the addition of Cu2+ ions quenched this fluorescence, due to aggregation. Anions had only marginal effects on this quenching. The fluorescence intensity increased upon the addition of cysteine and L-histidine. The limits of detection (LODs) for cysteine and L-histidine in the presence of the Cu@TFPB-DHTH COF were 340 and 520 nM, respectively, with high selectivity. Fluorescent COFs have also been used for the spectroscopic determination of very low concentrations (nanomolar) of HCl. We have fabricated the three luminescent COFs BCTB-PD, BCTA-TP, and BCTB-BCTA through Schiff base condensations of BCTB-4CHO with PD, BCTA-4NH2 with TP, and BCTB-4CHO with BCTA-4NH2, respectively.138 The thermal stabilities of the BCTB-PD and BCTB-BCTA COFs were higher than that of the BCTA-TP COF, revealing the positive effect of BCTB-4CHO as the aldehydic building block. The BET surface areas of the BCTB-PD, BCTB-BCTA, and BCTA-TP COFs were 2212, 1098, and 645 m2 g−1, respectively. Noncovalent interactions (hydrogen bonding, dipole effects) between these COFs and polar solvents led to red-shifting upon increasing the solvent polarity, in addition to enhancing the stability of the excited states of the COFs and, hence, their corresponding intramolecular charge transfer (ICT). The BCTB-4CHO-based COFs (BCTB-PD and BCTB-BCTA) displayed fluorescence emissions because their BCTB-4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N units functioned as electron donating groups that facilitated ICT between the carbazole BCTB-4CH
N units functioned as electron donating groups that facilitated ICT between the carbazole BCTB-4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N units and the acceptors; in contrast, the weakly electron donating BCTA-4N
N units and the acceptors; in contrast, the weakly electron donating BCTA-4N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH groups resulted in weak fluorescence for the BCTA-TP COF. The BCTB-BCTA COF displayed high fluorescence sensitivity for the detection of HCl, due to the increasing planarity of its constituent units upon protonation of the imino nitrogen atoms in the BCTB-BCTA COF architecture, resulting in red-shifting of the signal. The fluorescence lifetime of the BCTB-BCTA COF increased from 4.13 to 4.89 ns upon exposure to 1 mmol L−1 HCl, in addition to displaying an LOD of 10 nmol L−1. Increasing the planarity of the units in the COFs and converting them into quinoid structures were exploited by our group for HCl sensing using 2D PyTA-BC and PyTA-BC-Ph COFs, prepared through Schiff base condensations of PyTA-4NH2 with BC-4CHO and BC-Ph-4CHO, respectively, and separately under solvothermal conditions.125 The PyTA-BC and PyTA-BC-Ph COFs had thermal stabilities of up to 403 and 421 °C, respectively, and high surface areas (520 and 1445 m2 g−1, respectively); we attribute the superior thermal stability and surface area of the PyTA-BC-Ph COFs to their longer building blocks. Both the PyTA-BC and PyTA-BC-Ph COFs exhibited solvatochromism phenomena and red-shifting of their signals occurred, due to strong hydrogen bonding between the amino groups on the COF surfaces and the C
CH groups resulted in weak fluorescence for the BCTA-TP COF. The BCTB-BCTA COF displayed high fluorescence sensitivity for the detection of HCl, due to the increasing planarity of its constituent units upon protonation of the imino nitrogen atoms in the BCTB-BCTA COF architecture, resulting in red-shifting of the signal. The fluorescence lifetime of the BCTB-BCTA COF increased from 4.13 to 4.89 ns upon exposure to 1 mmol L−1 HCl, in addition to displaying an LOD of 10 nmol L−1. Increasing the planarity of the units in the COFs and converting them into quinoid structures were exploited by our group for HCl sensing using 2D PyTA-BC and PyTA-BC-Ph COFs, prepared through Schiff base condensations of PyTA-4NH2 with BC-4CHO and BC-Ph-4CHO, respectively, and separately under solvothermal conditions.125 The PyTA-BC and PyTA-BC-Ph COFs had thermal stabilities of up to 403 and 421 °C, respectively, and high surface areas (520 and 1445 m2 g−1, respectively); we attribute the superior thermal stability and surface area of the PyTA-BC-Ph COFs to their longer building blocks. Both the PyTA-BC and PyTA-BC-Ph COFs exhibited solvatochromism phenomena and red-shifting of their signals occurred, due to strong hydrogen bonding between the amino groups on the COF surfaces and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of the polar solvents, which facilitated ICT. Interactions of the PyTA-BC and PyTA-BC-Ph COFs with HCl also induced red-shifting from their original yellow color; the signals reverted back after exposure to NH3 vapor. This process, which could be repeated without considerable performance loss, resulted from the increase in planarity upon protonation and the formation of quinoid structures. The PyTA-BC and PyTA-BC-Ph COFs had very low LODs for HCl of 24 and 20 nmol L−1, respectively.132 Guo et al. obtained a new 1D-COF with a specific surface area of 426 m2 g−1 and thermal stability up to 360 °C through the reaction of TFPPy and DABP; they used this COF as a chemical H+ sensor in acidic solutions.139 Zhu et al. prepared a dual-luminescent COF (DL-COF) through Schiff base formation between ETTA and 9,10-anthracenedicarboxaldehyde and used it for the chemical sensing of explosive nitro compounds with high selectivity and sensitivity.140 Mohamed et al. used Heck reactions to prepare the four ultrastable microporous polymers An-HPP, TPT-HPP, Car-HPP, and TPE-HPP from octavinylsilsesquioxane (OVS) and brominated anthracene triphenyltriazine, bicarbazole, and tetraphenylethene, respectively; we revealed that these materials displayed good thermal stabilities because of the presence of the inorganic POSS units and the high crosslinking densities.141–144 Furthermore, these four fluorescent materials could be used for the detection of Fe3+ ions.
O groups of the polar solvents, which facilitated ICT. Interactions of the PyTA-BC and PyTA-BC-Ph COFs with HCl also induced red-shifting from their original yellow color; the signals reverted back after exposure to NH3 vapor. This process, which could be repeated without considerable performance loss, resulted from the increase in planarity upon protonation and the formation of quinoid structures. The PyTA-BC and PyTA-BC-Ph COFs had very low LODs for HCl of 24 and 20 nmol L−1, respectively.132 Guo et al. obtained a new 1D-COF with a specific surface area of 426 m2 g−1 and thermal stability up to 360 °C through the reaction of TFPPy and DABP; they used this COF as a chemical H+ sensor in acidic solutions.139 Zhu et al. prepared a dual-luminescent COF (DL-COF) through Schiff base formation between ETTA and 9,10-anthracenedicarboxaldehyde and used it for the chemical sensing of explosive nitro compounds with high selectivity and sensitivity.140 Mohamed et al. used Heck reactions to prepare the four ultrastable microporous polymers An-HPP, TPT-HPP, Car-HPP, and TPE-HPP from octavinylsilsesquioxane (OVS) and brominated anthracene triphenyltriazine, bicarbazole, and tetraphenylethene, respectively; we revealed that these materials displayed good thermal stabilities because of the presence of the inorganic POSS units and the high crosslinking densities.141–144 Furthermore, these four fluorescent materials could be used for the detection of Fe3+ ions.
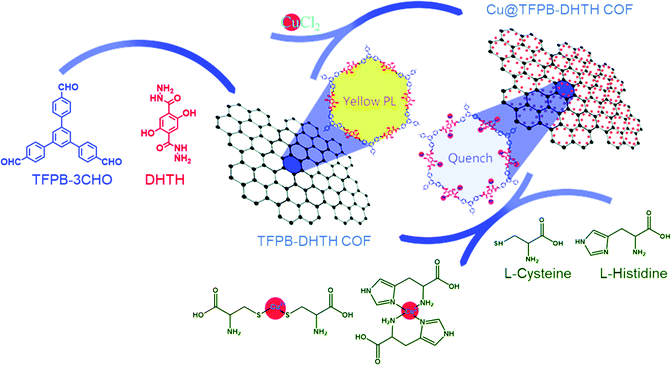 | ||
| Fig. 18 Schematic representation of Cu@TFPB-DHTH COF as a chemical sensor for Cys and L-His. Reproduced from ref. 137 with permission from the Royal Society of Chemistry. | ||
3.6. Other applications
Ning et al. prepared two CMPs containing organic dyes in their backbones through Sonogashira couplings of TDFB and TCT with TEB (Fig. 19).145 The TDFB-TEB CMP possessed a narrower band gap and a more planar π-conjugated structure and displayed higher photocatalytic performance towards the aerobic oxidants sulfur and amines with excellent recycling, compared with the behavior of TCT-TEF. Ma et al. reported CMP-S1, a CMP containing aromatic naphthalene moieties and a conjugated structure, that exhibited higher performance for aromatic capture than that for alkanes, based on organic vapor adsorption analysis.146 Xia et al. synthesized pTTT-BTD, pTTT-Ben, and pTTT-DMOB CMPs from 2,4,6-(tri-2-thienyl)-1,3,5-triazine as the knot block and the linkers BTD, Ben, and BMOB, respectively, each with different morphologies and electronic and optical properties. Interestingly, pTTT-Ben exhibited high photocatalytic performance, providing high production rates of succinic acid (4.66 mmol g−1 h−1) and 2,5-diformylfuran (0.53 mmol g−1 h−1) from biomass materials.147 Zhang et al. incorporated phosphoric acid into porous solids through Sonogashira coupling of 1,3,5-tris(4-ethynylphenyl)benzene with various aliphatic perfluoro monomers (Fig. 20).148 The obtained electrolytes of the CMP polymers had a low activation energy (0.4 eV) and high proton conductivity (4.39 × 10−3 S cm−1), due to their hydrophobic pores and hydrogen bonding between phosphoric acid and the perfluoroalkyl chains of the CMPs. Zhao et al. obtained the thiazolo[5,4-d]thiazole-linked CMPs TZTZ-TA and TZTZ-TP through condensations of 1,3,5-tri(4-formylphenyl)benzene with 2,4,6-tri(4-formylphenyl)-1,3,5-triamine (as the C3 symmetrical anode block) and with dithiooxamide (as the C2 monomer), respectively.149 The obtained CMPs had amorphous characteristics, different colors, good thermal stabilities, and BET surface areas in the range from 314.8 to 439.5 m2 g−1. Furthermore, the TZTZ-TA CMP containing the triazine units mediated the production of NADH (82.0 wt% within 5 min) to an outstanding degree when compared with that of TZTZ-TP (6.9 wt% during 10 min). Lang et al. prepared a different series of CMPs, based on fluorene and carbazole units as electron donating groups, and observed that MFC-CMP displayed a high BET specific surface area, more sites for oxidation, and good performance in the production of imines and oxygen through amine oxidation under visible light photocatalysis.150 Hua and co-workers used Sonogashira coupling to prepare a series of fluorescent CMPH, CMPNH2, and CMPN materials containing amino groups. CMPN, which featured N,N-diethylpropylamine units as side chains in the framework, exhibited good I2 capacity and excellent stability towards β- and γ-ray irradiation, which was the result of the formation of charge-transfer complexes between I2 and the amino, phenyl, and triazine groups.151 Maji et al. constructed a CMP containing dithienyl units as photochromic groups through Schiff-base condensation of dithienylethene aldehyde and benzene-1,3,5-tricarboxyhydrazide [Fig. 21(a)].152 They investigated its porosity, chemical structure, morphology, and optical and crystallinity properties [Fig. 21(e)–(t)]. The pcCMP-O structure had a yellow color that, after UV irradiation, changed to give the deep-green pcCMP-C; in addition, pcCMP-O functioned as an NH function logic gate. Li and co-workers prepared a metal-free heterogeneous catalyst (2,5-DCP-CTF) through the cyclotrimerization of 2,5-dicyanopyridine in the presence of molten ZnCl2.153 2,5-DCP-CTF possessed a high nitrogen atom content and a hierarchical porous structure; they used it for the preparation of cyclic carbonates with high selectivity and for the conversion of CO2. Hu et al. used silicon-promoted cationic polymerization to synthesize a series of HCPs with outstanding chemical stability, surface area, tunable porosity, and extended conjugation, which were capable of capturing dibenzothiophene (1335 mg g−1).154 Hou et al. prepared TFPPy-Td-COFs with a high surface area (1094 m2 g−1) and uniform pore size (2.87 nm) through the condensation of TFPPy with thiadiazole-2HN2.155 Interestingly, they prepared polymethylacrylates with a narrow polydispersity index when using TFPPy-Td-COFs as a heterogeneous photocatalyst for atom transfer radical polymerization (ATRP) photo-induced polymerization under irradiation with white light in the presence of Cu(I). In 2019, Chen et al. prepared two kinds of donor–acceptor 2D-COFs (TPB-BT-COF, and TAPT-BT-COF), featuring benzothiadiazole (BT) units as electron acceptors, through condensation reactions (Fig. 22).156 These materials possessed outstanding porosities, were chemically stable under strongly acidic and basic conditions, and had high crystallinities. The rate of photoreduction of an aqueous solution of hexavalent chromium [Cr(VI)] in the presence of the TPB-BT-COF was faster than that in the presence of the TAPT-BT-COF, due to the former having a narrower band gap and an abundant negative conduction band, allowing facile migration and separation of hole pairs/photogenerated electrons. In 2020, Jiang et al. synthesized the sp2-N enriched TM-TPT-COF through the reaction of TM with TPT in the presence of a basic catalyst.157 They then prepared Pt@COF through the reaction of the TM-TPT-COF with K2PtCl4; the presence of Pt NPs in the COF layers was confirmed using high-resolution TEM (HR-TEM), X-ray photoelectron spectroscopy (XPS), X-ray absorption spectroscopy, and WT-extended XAFS (EXAFS). The ORR mediated by the fabricated Pt@COF electrocatalyst in an acidic electrolyte was superior to that of a commercial Pt/C catalyst. In 2020, Huang et al. constructed a 2D-COF based on porphyrin units (TAPP-TFPP-COF) from the reaction of TAPP with TFPP; it exhibited excellent crystallinity, stability, tetragonal micropores, a high BET surface area of 962 m2 g−1 and a pore size of 1.8 nm (Fig. 23).158 The conductivity of the TAPP-TFPP-COF after doping with I2 increased from 1.12 × 10−10 to 1.46 × 10−7 S cm−1; furthermore, TAPP-TFPP-COF films displayed high sensitivity and selectivity at 700 nm, based on spectroscopic detection. Our group contributed to the development of perovskite solar cells through the preparation of the 2D-COFs Car-ETTA and TFPPy-ETTA through [4+4] solvothermal condensations of ETTA with Car-4CHO and TFPPy, respectively. The Car-ETTA and TFPPy-ETTA COFs possessed high thermal stabilities (up to 467 and 580 °C, respectively) and high surface areas (829 and 1156 m2 g−1, respectively). Perovskite solar cells modified with the Car-ETTA and TFPPy-ETTA COFs displayed high power conversion efficiencies (PCEs), up to 19.79 and 19.72%, respectively, resulting from minimized charge recombination at the perovskite-PTAA-COF interfaces, due to low energy levels of the highest occupied molecular orbitals of the COFs.159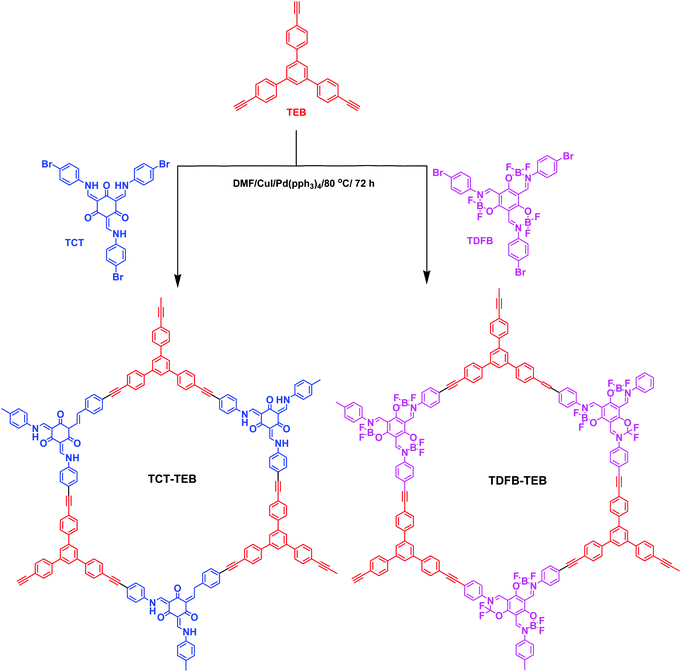 | ||
| Fig. 19 Preparation of TDFB-TEB and TCT-TEB. Reproduced from ref. 145 with permission the Royal Society of Chemistry. | ||
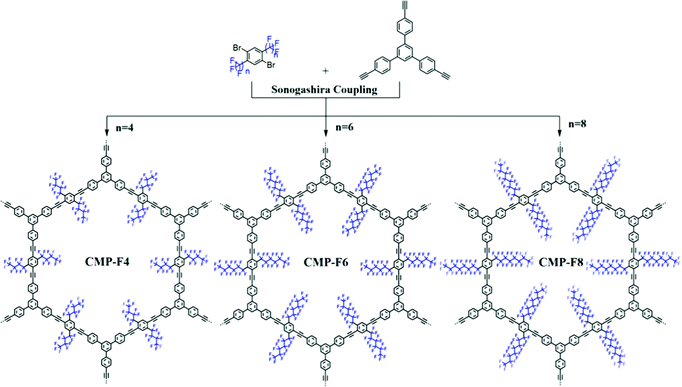 | ||
| Fig. 20 Synthesis of perfluoroalkyl-functionalized CMPs. Reproduced from ref. 148 with permission from American Chemical Society. | ||
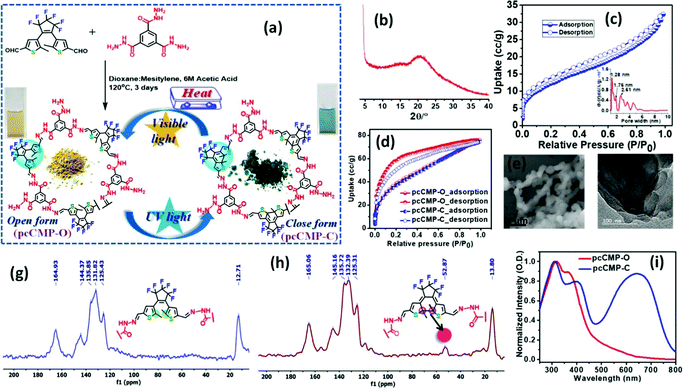 | ||
| Fig. 21 (a) Preparation of the pcCMP (b) PXRD for pcCMP-O; (c) N2 adsorption (77 K) for pcCMP-O; (d) CO2 adsorption for pcCMP-O and pcCMP-C; (e) FESEM and (f) TEM images for pcCMPO; (g and h) solid-state 13C NMR for pcCMP-O and pcCMP-C, respectively; and (i) UV-vis absorption spectra in the solid state for pcCMP-O and pcCMP-C. Reproduced from ref. 152 with permission from American Chemical Society. | ||
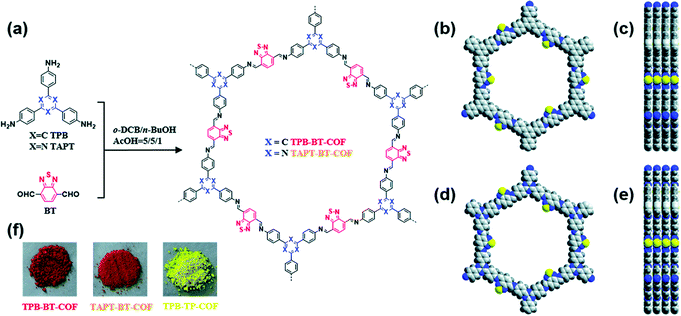 | ||
| Fig. 22 (a) Synthesis of BT-COFs; (b) top and (c) side view of TPB-BT-COF; (d) top and (e) side view of TAPT-BT-COF; (f) photographs of TPB-BT-COF, TAPT-BT-COF and TPB-TP-COF. Reproduced from ref. 156 with permission from the Royal Society of Chemistry. | ||
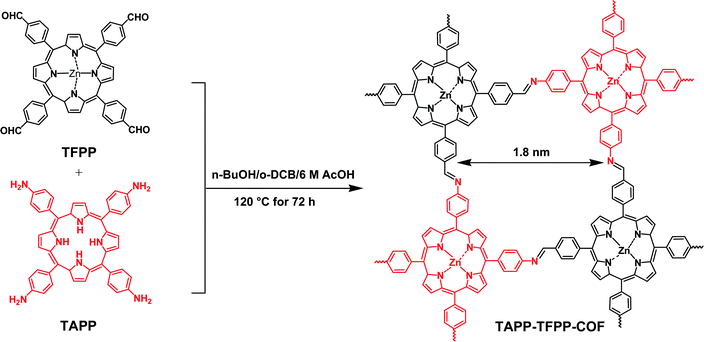 | ||
| Fig. 23 Synthesis of microporous TAPP-TFPP-COF. Reproduced from ref. 158 with permission from American Chemical Society. | ||
4. Conclusion and Outlook
POPs, including CTFs, COFs, HCPs, and CMPs, are attractive porous materials, and are comparable with metal–organic framework inorganic zeolites, because they possess excellent chemical and thermal stabilities, low densities, and tunable porosities, while being easy to prepare with flexible designs and structural diversity. These porous materials have been applied as useful platforms in many fields, including energy storage and conversion, gas separation, chemical sensing, luminescence, electronic devices, drug delivery, and H2 evolution from water. In this review, we discuss many examples of recent progress in the preparation of these materials, as well as the excellent performance of some POPs applied for CO2 uptake, energy storage, H2 evolution, photocatalysis, and photovoltaics. In the past few years, many research groups have prepared such materials with excellent properties; nevertheless, the use of novel synthetic methods for the preparation of new CMPs, CTFs, and COF remains worthwhile. For example, even COF materials with Frank–Kasper phases or Archimedean tiling patterns could have specific applications, and are interesting to consider. Finally, further exploration of the properties and applications of new POP materials will continue to open doors in both academia and industry.Author contributions
The manuscript was written through contributions from all authors.Abbreviation
| An | Anthracene |
| BTD | Benzothiadiazole |
| Ben | Benzene |
| BD | Benzidine |
| BT | Benzothiadiazole |
| Cz-4CHO | Bi-carbazole-4CHO |
| Car-4CN | [9,9′-Bicarbazole]-3,3′,6,6′-tetracarbonitrile |
| An-4Ph | 9,10-Bis(diphenylmethylene)-9,10-dihydroanthracene |
| BFTB-4CHO | 4,4′,4″,4‴-([9,9′-Bifluorenylidene]-3,3′,6,6′-tetrayl) tetrabenzaldehyde |
| BFTB-4NH2 | 4,4′,4″,4‴-([9,9′-Bifluorenylidene]-3,3′,6,6′-tetrayl)tetraaniline |
| BCTA-4NH2 | 4,4′,4″,4‴-([9,9′-Bicarbazole]-3,3′,6,6′-tetrayl)tetraaniline |
| BCTB-4CHO | 4,4′,4″,4‴-([9,9′-Bicarbazole]-3,3′,6,6′-tetrayl)tetrabenzaldehyde |
| BC-Ph-4CHO | 4,4′,4″,4‴-([9,9′-Bicarbazole]-3,3″,6,6″-tetrayl)tetrabenzaldehyde |
| TCNPy | 1,3,6,8-Cyanopyrene |
| CO2 | Carbon dioxide |
| OVS | Cubic octavinylsilsesquioxane |
| CTFs | Covalent triazine frameworks |
| COFs | Covalent organic frameworks |
| CMPs | Conjugated microporous polymers |
| DHBD | 3,3′-Dihydroxybenzidine |
| DAHQ-2HCl | 2,5-Diaminohydroquinone dihydrochloride |
| DHTH | 2,5-Dihydroxyterephthalohydrazide |
| BMOB | Dimethoxybenzene |
| DABP | 4,4′-Diaminobenzophenone |
| γ-CD | γ-Cyclodextrin |
| ETTA | 4,4′,4″,4‴-(Ethane-1,1,2,2-tetrayl)tetranilino |
| H2 | Hydrogen |
| H2O2 | Hydrogen peroxide |
| HCPs | Hypercrosslinked polymers |
| htb | Hexagonal tungsten bronze |
| hxl | Hexagonal layer |
| kgm | Kagome |
| IUPAC | International Union of Pure and Applied Chemistry |
| ICT | Intramolecular charge transfer |
| Li–S | Lithium–sulfur batteries |
| LiOH | Lithium hydroxide |
| MOFs | Metal–organic frameworks |
| NTCDA | 1,4,5,8-Naphthalenetetracarboxylic dianhydride |
| PS | Polystyrene |
| Py | Pyrene |
| POPs | Porous organic polymers |
| PAFs | Porous aromatic frameworks |
| PIMs | Polymers of intrinsic microporosity |
| PMDA | Pyromellitic dianhydride |
| PA | Phenylamine |
| PDA | Phenylenediamine |
| PD | p-Phenylenediamine |
| PyTA-4NH2 | 4,4′,4″,4‴-(Pyrene-1,3,6,8-tetrayl)tetraaniline |
| PyTA-4NH2 | 4,4′,4″,4‴-Pyrene-1,3,6,8-tetrayl)tetraaniline |
| SEM | Scanning electron microscope |
| TEM | Transmission electron microscope |
| TGA | Thermogravimetry analyses |
| TfOH | Trifluoromethanesulfonic acid |
| Car-3NH2 | Triamine 9-(4-aminophenyl)-carbazole-3,6-diamine |
| TPA-3CHO | Tris(4-formylphenyl)amine |
| TPP-3CHO | 2,4,6-Tris(4-formylphenyl)pyridine |
| TPT-3CHO | 2,4,6-Tris(4-formylphenyl)triazine |
| TPA-3NH2 | Tris(4-aminophenyl)amine |
| TPT-3NH2 | 2,4,6-Tris(4-aminophenyl)triazine |
| TFP-3OHCHO | 1,3,5-Triformylphloroglucinol |
| TAPA | Tris(4-aminophenyl)amine |
| TAPB | 1,3,5-Tris(4-aminophenyl)benzene |
| TPPDA(NH2)4 | Tetraphenyl-p-phenylenediamine |
| TPPyr(CHO)4 | 1,3,6,8-Tetrakis(4-formylphenyl)pyrene |
| TPTPE(CHO)4 | 1,1,2,2-Tetrakis[4-formyl-(1,1′-biphenyl)]ethane) |
| TBN | Tetrabenzonaphthalene |
| Tp | 1,3,5-Triformylphloroglucinol |
| TPE | Tetraphenylethene |
| Pyr-4Ph | Tetraphenylpyrazine |
| TNT | Trinitrotoluene |
| TFPB-3CHO | 1,3,5-Tris(4-formylphenyl)benzene |
| BC-4CHO | 3,3′,6,6″-Tetraformyl-9,9″-bicarbazole |
| TFPPy | 1,3,6,8-Tetrakis(p-formylphenyl)pyrene |
| TP | Terephthalaldehyde |
| TPT | Triphenyltriazine |
| Ben-T | 1,3,5-Tris(4-ethynylphenyl)benzene |
| TM | 2,4,6-Trimethyl-1,3,5-trizaine |
| Car-4CHO | 3,3′,6,6′-Tetraformyl-9,9′-bicarbazole |
| B(OMe)3 | Trimethyl borate |
| sql | Square lattice |
| SBUs | Secondary building units |
| SWCNTs | Single walled carbon nanotubes |
| TAPP | Zinc 5,10,15,20-tetra(4-aminophenyl)porphyrin |
| TFPP | Zinc 5,10,15,20-tetra(4-formylphenyl)porphyrin |
Conflicts of interest
The authors declare that they have no conflicts of interest.Acknowledgements
This study was supported financially by the Ministry of Science and Technology, Taiwan, under contracts MOST 106-2221-E-110-067-MY3, 108-2638-E-002-003-MY2, 108-2218-E-110-013-MY3, and 108-2221-E-110-014-MY3.References
- J. S. M. Lee and A. I. Cooper, Advances in Conjugated Microporous Polymers, Chem. Rev., 2020, 120, 2171–2214 CrossRef CAS PubMed.
- Y. Z. Cheng, X. Ding and B. H. Han, Porous Organic Polymers for Photocatalytic Carbon Dioxide Reduction, ChemPhotoChem, 2021, 5, 406–417 CrossRef CAS.
- T. X. Wang, H. P. Liang, D. A. Anito, X. Ding and B. H. Han, Emerging applications of porous organic polymers in visible-light photocatalysis, J. Mater. Chem. A, 2020, 8, 7003 RSC.
- K. Amin, N. Ashraf, L. Mao, C. F. J. Faul and Z. Wei, Conjugated microporous polymers for energy storage: Recent progress and challenges, Nano Energy, 2021, 85, 105958 CrossRef CAS.
- M. G. Mohamed, M. Y. Tsai, W. C. Su, A. F. M. EL-Mahdy, C. F. Wang, C. F. Huang, L. Dai, T. Chen and S. W. Kuo, Nitrogen-Doped microporous carbons derived from azobenzene and nitrile-functionalized polybenzoxazines for CO2 uptake, Mater. Today Commun., 2020, 24, 101111 CrossRef CAS.
- M. G. Mohamed, S. M. Ebrahium, A. S. Hammam, S. W. Kuo and K. I. Aly, Enhanced CO2 capture in nitrogen-enriched microporous carbons derived from Polybenzoxazines containing azobenzene and carboxylic acid units, J. Polym. Res., 2020, 27, 197 CrossRef CAS.
- S. Wang, C. Zhang, Y. Shu, S. Jiang, Q. Xia, L. Chen, S. Jin, I. Hussain, A. I. Cooper and B. Tan, Layered Microporous Polymers by Solvent Knitting Method, Sci. Adv., 2017, 3, e1602610 CrossRef PubMed.
- M. M. Samy, G. Mohamed, T. H. Mansoure, T. S. Meng, M. A. R. Khan, C. C. Liaw and S. W. Kuo, Solid state chemical transformations through ring-opening polymerization of ferrocene-based conjugated microporous polymers in host–guest complexes with benzoxazine-linked cyclodextrin, J. Taiwan Inst. Chem. Eng., 2021 DOI:10.1016/j.jtice.2021.10.010.
- M. G. Mohamed, M. H. Elsayed, A. M. Elewa, A. F. M. EL-Mahdy, C. H. Yang, A. A. K. Mohammed, H. H. Chou and S. W. Kuo, Pyrene-containing conjugated organic microporous polymers for photocatalytic hydrogen evolution from water, Catal. Sci. Technol., 2021, 11, 2229–2241 RSC.
- X. Li, C. Zhang, S. Cai, X. Lei, V. Altoe, F. Hong, J. J. Urban, J. Ciston, E. M. Chan and Y. Liu, Facile transformation of imine covalent organic frameworks into ultrastable crystalline porous aromatic frameworks, Nat. Commun., 2018, 9, 2998 CrossRef PubMed.
- M. G. Mohamed, W. C. Chen, A. F. M. El-Mahdy and S. W. Kuo, Porous organic/inorganic polymers based on double-decker silsesquioxane for high-performance energy storage, J. Polym. Res., 2021, 28, 219 CrossRef CAS.
- M. M. Samy, M. G. Mohamed and S. W. Kuo, Directly synthesized nitrogen-and-oxygen–doped microporous carbons derived from a bio-derived polybenzoxazine exhibiting high-performance supercapacitance and CO2 uptake., Eur. Polym. J., 2020, 138, 109954 CrossRef CAS.
- A. F. M. EL-Mahdy, T. C. Yu, M. G. Mohamed and S. W. Kuo, Secondary Structures of Polypeptide-Based Diblock Copolymers Influence the Microphase Separation of Templates for the Fabrication of Microporous Carbons, Macromolecules, 2021, 54, 1030–1042 CrossRef CAS.
- J. Y. Wu, M. G. Mohamed and S. W. Kuo, Directly synthesized nitrogen-doped microporous carbons from polybenzoxazine resins for carbon dioxide capture, Polym. Chem., 2017, 8, 5481–5489 RSC.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report), Pure Appl. Chem., 2015, 87, 1051 CAS.
- J. Wei, Z. Sun, W. Luo, Y. Li, A. A. Elzatahry, A. M. Al-Enizi, Y. Deng and D. Zhao, New Insight into the Synthesis of Large-Pore Ordered Mesoporous Materials, J. Am. Chem. Soc., 2017, 139, 1706–1713 CrossRef CAS PubMed.
- X. Y. Yang, L. H. Chen, J. C. Rooke and C. Sanchez, Hierarchically porous materials: synthesis strategies and structure design, Chem. Soc. Rev., 2017, 46, 481–558 RSC.
- K. I. Aly, M. M. Sayed, M. G. Mohamed, S. W. Kuo and O. Younis, A facile synthetic route and dual function of network luminescent porous polyester and copolyester containing porphyrin moiety for metal ions sensor and dyes adsorption, Microporous Mesoporous Mater., 2020, 298, 110063 CrossRef.
- M. G. Mohamed, E. C. Atayde Jr, B. M. Matsagard, J. Nag, Y. Yamauchi, K. C. W. Wu and S. W. Kuo, Construction Hierarchically Mesoporous/Microporous Materials Based on Block Copolymer and Covalent Organic Framework, J. Taiwan Inst. Chem. Eng., 2020, 112, 180–192 CrossRef CAS.
- W. S. Hung, M. M. M. Ahmed, M. G. Mohamed and S. W. Kuo, Competing hydrogen bonding produces mesoporous/macroporous carbons templated by a high-molecular-weight poly(caprolactone-b-ethylene oxide-b-caprolactone) triblock copolymer, J. Polym. Res., 2020, 27, 173 CrossRef CAS.
- J. G. Li, P. Y. Lee, M. M. M. Ahmed, M. G. Mohamed and S. W. Kuo, Varying the Hydrogen Bonding Strength in Phenolic/PEO-b-PLA Blends Provides Mesoporous Carbons Having Large Accessible Pores Suitable for Energy Storage, Macromol. Chem. Phys., 2020, 221, 2000040 CrossRef CAS.
- W. Li, Q. Yue, Y. Deng and D. Zhao, Ordered Mesoporous Materials Based on Interfacial Assembly and Engineering, Adv. Mater., 2013, 25, 5129–5152 CrossRef CAS PubMed.
- M. O’Keeffe, Design of MOFs and Intellectual Content in Reticular Chemistry: A Personal View, Chem. Soc. Rev., 2009, 38, 1215–1217 RSC.
- H. Furukawa, K. E. Cordova, M. O’Keeffe and O. M. Yaghi, The Chemistry and Applications of Metal-Organic Frameworks, Science, 2013, 341, 1230444 CrossRef PubMed.
- L. Li, M. Zhang, R. Li, H. Jiang and Z. Liu, Facile synthesis of highly luminescent rod-like terbium-based metal–organic frameworks for sensitive detection of olaquindox, Anal. Methods, 2021, 13, 3785–3791 RSC.
- T. Ben and S. Qiu, Porous Aromatic Frameworks: Synthesis, Structure and Functions, CrystEngComm, 2013, 15, 17–26 RSC.
- Y. Xu, S. Jin, H. Xu, A. Nagai and D. Jiang, Conjugated Microporous Polymers: Design, Synthesis and Application, Chem. Soc. Rev., 2013, 42, 8012–8031 RSC.
- T. Hasell and A. I. Cooper, Porous Organic Cages: Soluble, Modular and Molecular Pores, Nat. Rev. Mater., 2016, 1, 16053 CrossRef CAS.
- A. P. Côté, A. I. Benin, N. W. Ockwig, M. Keeffe, A. J. Matzger and O. M. Yaghi, Porous, Crystalline, Covalent Organic Frameworks, Science, 2005, 310, 1166–1170 CrossRef PubMed.
- P. Katekomol, J. Roeser, M. Bojdys, J. Weber and A. Thomas, Covalent Triazine Frameworks Prepared from 1,3,5-Tricyanobenzene, Chem. Mater., 2013, 25, 1542–1548 CrossRef CAS.
- J.-X. Jiang, F. Su, A. Trewin, C. D. Wood, N. L. Campbell, H. Niu, C. Dickinson, A. Y. Ganin, M. J. Rosseinsky, Y. Z. Khimyak and A. I. Cooper, Angew. Chem., Int. Ed., 2007, 46, 8574–8578 CrossRef CAS PubMed.
- S. Xu, Y. Luo and B. Tan, Recent Development of Hypercrosslinked Microporous Organic Polymers, Macromol. Rapid Commun., 2013, 34, 471–484 CrossRef CAS PubMed.
- K. Konstas, J. W. Taylor, A. W. Thornton, C. M. Doherty, W. X. Lim, T. J. Bastow, D. F. Kennedy, C. D. Wood, B. J. Cox, J. M. Hill, A. J. Hill and M. R. Hill, Lithiated Porous Aromatic Frameworks with Exceptional Gas Storage Capacity, Angew. Chem., Int. Ed., 2012, 51, 6639–6642 CrossRef CAS PubMed.
- A. M. Elewa, A. F. M. EL-Mahdy, M. H. Elsayed, M. G. Mohamed, S. W. Kuo and H. H. Chou, Sulfur-doped triazine-conjugated microporous polymers for achieving the robust visible-light-driven hydrogen evolution, Chem. Eng. J., 2021, 421, 129825 CrossRef CAS.
- X. Gao, C. Shu, C. Zhang, W. Ma, S. B. Ren, F. Wang, Y. Chen, J. H. Zeng and J. X. Jiang, Substituent effect of conjugated microporous polymers on the photocatalytic hydrogen evolution activity, J. Mater. Chem. A, 2020, 8, 2404–2411 RSC.
- Y. Xu, N. Mao, S. Feng, C. Zhang, F. Wang, Y. Chen, J. Zeng and J. X. Jiang, Perylene-containing conjugated microporous polymers for photocatalytic hydrogen evolution, Macromol. Chem. Phys., 2017, 218, 1700049 CrossRef.
- G. Gatti, M. Errahali, L. Tei, M. Cossi and L. Marchese, On the Gas Storage Properties of 3D Porous Carbons Derived from Hyper-Crosslinked Polymers, Polymers, 2019, 11, 588 CrossRef PubMed.
- M. Liu, L. Guo, S. Jin and B. Tan, Covalent triazine frameworks: synthesis and applications, J. Mater. Chem. A, 2019, 7, 5153–5172 RSC.
- S. Das, P. Heasman, T. Ben and S. Qiu, Porous organic materials: strategic design and structure–function correlation, Chem. Rev., 2017, 117, 1515–1563 CrossRef CAS PubMed.
- N. B. McKeown, The synthesis of polymers of intrinsic microporosity (PIMs), Sci. China: Chem., 2017, 60, 1023–1032 CrossRef CAS.
- S. J. Yang, X. Ding and B. H. Han, Conjugated microporous polymers with extended p-structures for organic vapor adsorption, Macromolecules, 2018, 51, 947–953 CrossRef CAS.
- C. Dai and B. Liu, Conjugated polymers for visible-light driven photocatalysis, Energy Environ. Sci., 2020, 13, 24–52 RSC.
- J. Byun and K. A. I. Zhang, Designing conjugated porous polymers for visible light-driven photocatalytic chemical transformations, Mater. Horiz., 2020, 7, 15–31 RSC.
- T. Zhang, G. Xing, W. Chen and L. Chen, Porous organic polymers: a promising platform for efficient photocatalysis, Mater. Chem. Front., 2020, 4, 332–353 RSC.
- W. Liu, X. Li, C. Wang, H. Pan, W. Liu, K. Wang, Q. Zeng, R. Wang and J. Jiang, A scalable general synthetic approach toward ultrathin imine-linked two-dimensional covalent organic framework nanosheets for photocatalytic CO2 reduction, J. Am. Chem. Soc., 2019, 141, 17431–17440 CrossRef CAS PubMed.
- Y. Yuan and G. Zhu, Porous aromatic frameworks as a platform for multifunctional applications, ACS Cent. Sci., 2019, 5, 409–418 CrossRef CAS PubMed.
- H. Urakami, K. Zhang and F. Vilela, Modification of conjugated microporous poly-benzothiadiazole for photosensitized singlet oxygen generation in water, Chem. Commun., 2013, 49, 2353–2355 RSC.
- W. Ma, Q. Zheng, Y. He, G. Li, W. Guo, Z. Lin and L. Zhang, Size-controllable synthesis of uniform spherical covalent organic frameworks at room temperature for highly efficient and selective enrichment of hydrophobic peptides, J. Am. Chem. Soc., 2019, 141, 18271–18277 CrossRef CAS PubMed.
- R. Xu, X. S. Wang, H. Zhao, H. Lin, Y. B. Huang and R. Cao, Rhenium-modified porous covalent triazine framework for highly efficient photocatalytic carbon dioxide reduction in a solid–gas system, Catal. Sci. Technol., 2018, 8, 2224–2230 RSC.
- L. Li, Z. Cai, Q. Wu, W.-Y. Lo, N. Zhang, L. X. Chen and L. Yu, Rational design of porous conjugated polymers and roles of residual palladium for photocatalytic hydrogen production, J. Am. Chem. Soc., 2016, 138, 7681–7686 CrossRef CAS PubMed.
- H. P. Liang, A. Acharjya, D. A. Anito, S. Vogl, T.-X. Wang, A. Thomas and B.-H. Han, Rhenium-metalated polypyridine-based porous polycarbazoles for visible-light CO2 photoreduction, ACS Catal., 2019, 9, 3959–3968 CrossRef CAS.
- B. C. Ma, S. Ghasimi, K. Landfester, F. Vilela and K. A. I. Zhang, Conjugated microporous polymer nanoparticles with enhanced dispersibility and water compatibility for photocatalytic applications, J. Mater. Chem. A, 2015, 3, 16064–16071 RSC.
- R. S. Sprick, B. Bonillo, M. Sachs, R. Clowes, J. R. Durrant, D. J. Adamsa and A. I. Cooper, Extended conjugated microporous polymers for photocatalytic hydrogen evolution from water, Chem. Commun., 2016, 52, 10008–10011 RSC.
- P. F. Wei, M. Z. Qi, Z. P. Wang, S. Y. Ding, W. Yu, Q. Liu, L. K. Wang, H. Z. Wang, W. K. An and W. Wang, Benzoxazole-linked ultrastable covalent organic frameworks for photocatalysis, J. Am. Chem. Soc., 2018, 140, 4623–4631 CrossRef CAS PubMed.
- R. R. Liang, R. Han A, S. Q. Xu, Q. Y. Qi and X. Zhao, Fabricating organic nanotubes through selective disassembly of two-dimensional covalent organic frameworks, J. Am. Chem. Soc., 2020, 142, 70–74 CrossRef CAS PubMed.
- H. M. El-Kaderi, J. R. Hunt, J. L. Mendoza-Cortés, A. P. Côté, R. E. Taylor, M. O'Keeffe and O. M. Yaghi, Designed synthesis of 3D covalent organic frameworks, Science, 2007, 316, 268–272 CrossRef CAS PubMed.
- T. Ben, H. Ren, S. Ma, D. Cao, J. Lan, X. Jing, W. Wang, J. Xu, F. Deng, J. M. Simmons, S. Qiu and G. Zhu, Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area, Angew. Chem., Int. Ed., 2009, 48, 9457–9460 CrossRef CAS PubMed.
- J. Huang and S. Richard Turner, Hypercrosslinked Polymers: A Review, Polym. Rev., 2018, 58, 1–41 CrossRef CAS.
- Z. Wei, Q. Chen and H. Liu, Hydroxyl modified hypercrosslinked polymers: targeting high efficient adsorption separation towards aniline, New J. Chem., 2021, 45, 11607–11617 RSC.
- M. Errahali, G. Gatti, L. Tei, G. Paul, G. Rolla, L. Canti, A. Fraccarollo, M. Cossi, A. Comotti, P. Sozzani and L. Marchese, Microporous Hyper-crosslinked Aromatic Polymers Designed for Methane and Carbon Dioxide Adsorption, J. Phys. Chem. C, 2014, 118, 28699–28710 CrossRef CAS.
- Y. Zhang, L. Zhang, X. Zhang, D. Yang, C. Du, L. Wan, C. Au, J. Chen and M. Xie, Pyridine-based hypercrosslinked polymers as support materials for palladium photocatalysts and their application in Suzuki–Miyaura coupling reactions, New J. Chem., 2020, 44, 15202–15208 RSC.
- L. Tan and B. Tan, Hypercrosslinked porous polymer materials: Design, synthesis, and applications, Chem. Soc. Rev., 2017, 46, 3322–3356 RSC.
- N. Fontanals, R. M. Marce, F. Borrull and P. A. G. Cormack, Hypercrosslinked materials: preparation, characterisation and applications, Polym. Chem., 2015, 6, 7231–7244 RSC.
- V. Davankov, V. Rogozhin and M. Tsjurupa, US Pat., 3729457, 1973 Search PubMed.
- Y. Gu, S. U. Son, T. Li and B. Tan, Low-Cost Hypercrosslinked Polymers by Direct Knitting Strategy for Catalytic Applications, Adv. Funct. Mater., 2021, 31, 2008265 CrossRef CAS.
- Z. Qian, Z. J. Wang and K. A. I. Zhang, Covalent Triazine Frameworks as Emerging Heterogeneous Photocatalysts, Chem. Mater., 2021, 33, 1909–1926 CrossRef CAS.
- P. Puthiaraj, Y. R. Lee, S. Zhang and W. S. Ahn, Triazine-Based Covalent Organic Polymers: Design, Synthesis and Applications in Heterogeneous Catalysis, J. Mater. Chem. A, 2016, 4, 16288–16311 RSC.
- M. Liu, K. Jiang, X. Ding, S. Wang, C. Zhang, J. Liu, Z. Zhan, G. Cheng, B. Li, H. Chen, S. Jin and B. Tan, Controlling Monomer Feeding Rate to Achieve Highly Crystalline Covalent Triazine Frameworks, Adv. Mater., 2019, 31, 1807865 CrossRef PubMed.
- M. Liu, Q. Huang, S. Wang, Z. Li, B. Li, S. Jin and B. Tan, Crystalline Covalent Triazine Frameworks by In Situ Oxidation of Alcohols to Aldehyde Monomers, Angew. Chem., Int. Ed., 2018, 57, 11968–11972 CrossRef CAS PubMed.
- X. Jiang, P. Wang and J. Zhao, 2D Covalent Triazine Framework: A New Class of Organic Photocatalyst for Water Splitting, J. Mater. Chem. A, 2015, 3, 7750–7758 RSC.
- J. Li, P. Liu, H. Huang, Y. Li, Y. Tang, D. Mei and C. Zhong, Metal-Free 2D/2D Black Phosphorus and Covalent Triazine Framework Heterostructure for CO2 Photoreduction, ACS Sustainable Chem. Eng., 2020, 8, 5175–5183 CrossRef CAS.
- C. Xu, Q. Xie, W. Zhang, S. Xiong, C. Pan, J. Tang and G. Yu, A Vinylene-Bridged Conjugated Covalent Triazine Polymer as a Visible-Light-Active Photocatalyst for Degradation of Methylene Blue, Macromol. Rapid Commun., 2020, 41, 2000006 CrossRef CAS PubMed.
- P. Katekomol, J. Roeser, M. Bojdys, J. Weber and A. Thomas, Covalent Triazine Frameworks Prepared from 1,3,5-Tricyanobenzene, Chem. Mater., 2013, 25, 1542–1548 CrossRef CAS.
- M. J. Bojdys, J. Jeromenok, A. Thomas and M. Antonietti, Rational Extension of the Family of Layered, Covalent, Triazine-Based Frameworks with Regular Porosity, Adv. Mater., 2010, 22, 2202–2205 CrossRef CAS PubMed.
- P. Kuhn, A. Thomas and M. Antonietti, Toward Tailorable Porous Organic Polymer Networks: A High-Temperature Dynamic Polymerization Scheme Based on Aromatic Nitriles, Macromolecules, 2009, 42, 319–326 CrossRef CAS.
- W. Zhang, C. Li, Y. P. Yuan, L. G. Qiu, A. J. Xie, Y. H. Shen and J. F. Zhu, Highly Energy- and Time-Efficient Synthesis of Porous Triazine-Based Framework: Microwave-Enhanced Ionothermal Polymerization and Hydrogen Uptake, J. Mater. Chem., 2010, 20, 6413–6415 RSC.
- J. Xie, S. A. Shevlin, Q. Ruan, S. J. A. Moniz, Y. Liu, X. Liu, Y. Li, C. C. Lau, Z. X. Guo and J. Tang, J. Efficient Visible Light-Driven Water Oxidation and Proton Reduction by an Ordered Covalent Triazine-Based Framework, Energy Environ. Sci., 2018, 11, 1617–1624 RSC.
- J. Liu, W. Zan, K. Li, Y. Yang, F. Bu and Y. Xu, Solution Synthesis of Semiconducting Two-Dimensional Polymer via Trimerization of Carbonitrile, J. Am. Chem. Soc., 2017, 139, 11666–11669 CrossRef CAS PubMed.
- S. Luo, Z. Zeng, H. Wang, W. Xiong, B. Song, C. Zhou, A. Duan, X. Tan, Q. He, G. Zeng, Z. Liu and R. Xiao, Recent progress in conjugated microporous polymers for clean energy: Synthesis, modification, computer simulations, and applications, Prog. Polym. Sci., 2021, 115, 101374 CrossRef CAS.
- X. Sheng, H. Shi, L. Yanga, P. Shao, K. Yu and X. Luo, Rationally designed conjugated microporous polymers for contaminants adsorption, Sci. Total Environ., 2021, 750, 141683 CrossRef CAS PubMed.
- Y. He, Z. Cheng, H. Zuo, C. Yan and Y. Liao, Green synthesis of pyridyl conjugated microporous polymers as precursors for porous carbon microspheres for efficient electrochemical energy storage, ChemElectroChem, 2020, 7, 959–966 CrossRef CAS.
- N. Chaoui, M. Trunk, R. Dawson, J. Schmidt and A. Thomas, Trends and challenges for microporous polymers, Chem. Soc. Rev., 2017, 46, 3302–3321 RSC.
- J. Chen, W. Yan, E. J. Townsend, J. Feng, L. Pan, V. D. A. Hernandez and C. F. J. Faul, Tunable surface area, porosity, and function in conjugated microporous polymers, Angew. Chem., Int. Ed., 2019, 58, 11715–11719 CrossRef CAS PubMed.
- S. Luo, Z. Zeng, G. Zeng, Z. Liu, R. Xiao, P. Xu, H. Wang, D. Huang, Y. Liu, B. Shao, Q. Liang, D. Wang, Q. He, L. Qin and Y. Fu, Recent advances in conjugated microporous polymers for photocatalysis: designs, applications, and prospects, J. Mater. Chem. A, 2020, 8, 6434–6470 RSC.
- A. Schneemann, R. Dong, F. Schwotzer, H. Zhong, I. Senkovska, X. Feng and S. Kaskel, 2D framework materials for energy applications, Chem. Sci., 2021, 12, 1600–1619 RSC.
- V. Singh and H. R. Byon, Advances in electrochemical energy storage with covalent organic frameworks, Mater. Adv., 2021, 2, 3188–3212 RSC.
- H. Vardhan, A. Nafady, A. M. Al-Enizi and S. Ma, Pore surface engineering of covalent organic frameworks: structural diversity and applications, Nanoscale, 2019, 11, 21679–21708 RSC.
- X. Zhao, P. Pachfule and A. Thomas, Covalent organic frameworks (COFs) for electrochemical applications, Chem. Soc. Rev., 2021, 50, 6871–6913 RSC.
- M. S. Lohse and T. Bein, Covalent Organic Frameworks: Structures, Synthesis, and Applications, Adv. Funct. Mater., 2018, 28, 1705553 CrossRef.
- C. Wu, Y. Liu, H. Liu, C. Duan, Q. Pan, J. Zhu, F. Hu, X. Ma, T. Jiu, Z. Li and Y. Zhao, Highly Conjugated Three-Dimensional Covalent Organic Frameworks Based on Spirobifluorene for Perovskite Solar Cell Enhancement, J. Am. Chem. Soc., 2018, 140, 10016–10024 CrossRef CAS PubMed.
- X. J. Zhao, P. Pachfule, S. Li, T. Langenhahn, M. Y. Ye, C. Schlesiger, S. Praetz, J. Schmidt and A. Thomas, Macro/Microporous Covalent Organic Frameworks for Efficient Electrocatalysis, J. Am. Chem. Soc., 2019, 141, 6623–6630 CrossRef CAS PubMed.
- X. Wang, M. Bahri, Z. Fu, M. A. Little, L. Liu, H. Niu, N. D. Browning, S. Y. Chong, L. Chen, J. W. Ward and A. I. Cooper, A Cubic 3D Covalent Organic Framework with nbo Topology, J. Am. Chem. Soc., 2021, 143, 15011–15016 CrossRef CAS PubMed.
- H. R. Abuzeid, A. F. M. EL-Mahdy and S. W. Kuo, Covalent organic frameworks: Design principles, synthetic strategies, and diverse applications, Giant, 2021, 6, 100054 CrossRef CAS.
- H. L. Nguyen and A. Alzamly, Covalent Organic Frameworks as Emerging Platforms for CO2 Photoreduction, ACS Catal., 2021, 11, 9809–9824 CrossRef CAS.
- Y. Zhang, J. Duan, D. Ma, P. Li, S. Li, H. Li, J. Zhou, X. Ma, X. Feng and B. Wang, Three-Dimensional Anionic Cyclodextrin- Based Covalent Organic Frameworks, Angew. Chem., Int. Ed., 2017, 56, 16313–16317 CrossRef CAS PubMed.
- B. Zhang, H. Mao, R. Matheu, J. A. Reimer, S. A. Alshmimri, S. Alshihri and O. M. Yaghi, Reticular Synthesis of Multinary Covalent Organic Frameworks, J. Am. Chem. Soc., 2019, 141, 11420–11424 CrossRef CAS PubMed.
- A. Abid, S. Razzaque, I. Hussain and B. Tan, Eco-Friendly Phosphorus and Nitrogen-Rich Inorganic–Organic Hybrid Hypercross-linked Porous Polymers via a Low-Cost Strategy, Macromolecules, 2021, 54(12), 5848–5855 CrossRef CAS.
- Y. Cui, Z. Xu, H. Y. Li, D. J. Young, Z. G. Ren and H. X. Li, Synthesis of a Pyrazole-Based Microporous Organic Polymer for High-Performance CO2 Capture and Alkyne Carboxylation, ACS Appl. Polym. Mater., 2020, 2, 4512–4520 CrossRef CAS.
- B. Zhang, J. Yan, G. Lia and Z. Wang, Cost-effective preparation of microporous polymers from formamide derivatives and adsorption of CO2 under dry and humid conditions, Polym. Chem., 2019, 10, 3371–3379 RSC.
- Y. Liu, S. Wang, X. Meng, Y. Ye, X. Song and Z. Liang, Increasing the surface area and CO2 uptake of conjugated microporous polymers via a post-knitting method, Mater. Chem. Front., 2021, 5, 5319–5327 RSC.
- S. Mukherjee, M. Das, A. Manna, R. Krishna and S. Das, Newly designed 1,2,3-triazole functionalized covalent triazine frameworks with exceptionally high uptake capacity for both CO2 and H2, J. Mater. Chem. A, 2019, 7, 1055–1068 RSC.
- H. R. Abuzeid, A. F. M. EL-Mahdy and S. W. Kuo, Hydrogen bonding induces dual porous types with microporous and mesoporous covalent organic frameworks based on bicarbazole units, Microporous Mesoporous Mater., 2020, 300, 110151 CrossRef CAS.
- A. F. M. El-Mahdy, C. Young, J. Kim, J. You, Y. Yamauchi and S. W. Kuo, Hollow Microspherical and Microtubular [3+3] Carbazole-Based Covalent Organic Frameworks and Their Gas and Energy Storage Applications, ACS Appl. Mater. Interfaces, 2019, 11, 9343–9354 CrossRef CAS PubMed.
- A. F. M. EL-Mahdy, C. H. Kuo, A. Alshehri, C. Young, Y. Yamauchi, J. Kim and S. W. Kuo, Strategic design of triphenylamine- and triphenyltriazine-based two-dimensional covalent organic frameworks for CO2 uptake and energy storage, J. Mater. Chem. A, 2018, 6, 19532–19541 RSC.
- A. F. M. EL-Mahdy, Y. H. Hung, T. H. Mansoure, H. H. Yu, Y. S. Hsu, K. C. W. Wu and S. W. Kuo, Synthesis of [3+3] β-ketoenamine-tethered covalent organic frameworks (COFs) for high-performance supercapacitance and CO2 storage, J. Taiwan Inst. Chem. Eng., 2019, 103, 199–208 CrossRef CAS.
- R. Jagt, A. Vasileiadis, H. Veldhuizen, P. Shao, X. Feng, S. Ganapathy, N. C. Habisreutinger, M. A. van der Veen, C. Wang, M. Wagemaker, S. van der Zwaag and A. Nagai, Synthesis and Structure–Property Relationships of Polyimide Covalent Organic Frameworks for Carbon Dioxide Capture and (Aqueous) Sodium-Ion Batteries, Chem. Mater., 2021, 33, 818–833 CrossRef PubMed.
- M. G. Mohamed, T. C. Chen and S. W. Kuo, Solid-State Chemical Transformations to Enhance Gas Capture in Benzoxazine-Linked Conjugated Microporous Polymers, Macromolecules, 2021, 54, 5866–5877 CrossRef CAS.
- Y. Zhang, D. Yi, P. Tu, S. Yang, Q. Xie, Z. Gao, S. Wu and G. Yu, Boosting radioactive iodine capture of microporous polymers through strengthened host–guest interaction, Microporous Mesoporous Mater., 2021, 321, 111148 CrossRef CAS.
- M. G. Mohamed, M. Y. Tsai, C. F. Wang, C. F. Huang, M. Danko, L. Dai, T. Chen and S. W. Kuo, Multifunctional Polyhedral Oligomeric Silsesquioxane (POSS) Based Hybrid Porous Materials for CO2 Uptake and Iodine Adsorption, Polymers, 2021, 13, 221 CrossRef CAS PubMed.
- N. Baig, S. Shetty, S. Al-Mousawi and B. Alameddine, Conjugated microporous polymers using a copper-catalyzed [4+2] cyclobenzannulation reaction: promising materials for iodine and dye adsorption, Polym. Chem., 2021, 12, 2282–2292 RSC.
- Y. Zhang, X. Hong, X. M. Cao, X. Q. Huang, B. Hu, S. Y. Ding and H. Lin, Functional Porous Organic Polymers with Conjugated Triaryl Triazine as the Core for Superfast Adsorption Removal of Organic Dyes, ACS Appl. Mater. Interfaces, 2021, 13, 6359–6366 CrossRef CAS PubMed.
- A. Hassan, A. Alam, M. Ansari and N. Das, Hydroxy functionalized triptycene based covalent organic polymers for ultra-high radioactive iodine uptake, Chem. Eng. Sci., 2022, 427, 130950 CrossRef CAS.
- A. F. M. EL-Mahdy, M. B. Zakaria, H. X. Wang, T. Chen, Y. Yamauchi and S. W. Kuo, Heteroporous bifluorenylidene-based covalent organic frameworks displaying exceptional dye adsorption behavior and high energy storage, J. Mater. Chem. A, 2020, 8, 25148–25155 RSC.
- M. G. Mohamed, A. F. M. EL-Mahdy, Y. Takashi and S. W. Kuo, Ultrastable conductive microporous covalent triazine frameworks based on pyrene moieties provide high-performance CO2 uptake and supercapacitance, New J. Chem., 2020, 44, 8241–8253 RSC.
- M. G. Mohamed, A. F. M. EL-Mahdy, M. M. M. Ahmed and S. W. Kuo, Directly Synthesized Microporous Bicarbazole-Based Covalent Triazine Frameworks for High-Performance Energy Storage and Carbon Dioxide Uptake, ChemPlusChem, 2019, 84, 1767–1774 CrossRef CAS PubMed.
- M. G. Mohamed, M. M. M. Ahmed, W. T. Du and S. W. Kuo, Meso/Microporous Carbons from Conjugated Hyper-Crosslinked Polymers Based on Tetraphenylethene for High-Performance CO2 Capture and Supercapacitor, Molecules, 2021, 26, 738 CrossRef CAS PubMed.
- M. G. Mohamed, X. Zhang, T. H. Mansoure, A. F. M. El-Mahdy, C. F. Huang, M. Danko, Z. Xin and S. W. Kuo, Hypercrosslinked porous organic polymers based on tetraphenylanthraquinone for CO2 uptake and high-performance supercapacitor, Polymer, 2020, 205, 122857 CrossRef CAS.
- M. G. Mohamed, A. F. M. EL-Mahdy, T. S. Meng, M. M. Samy and S. W. Kuo, Multifunctional Hypercrosslinked Porous Organic Polymers Based on Tetraphenylethene and Triphenylamine Derivatives for High-Performance Dye Adsorption and Supercapacitor, Polymers, 2020, 12, 2426 CrossRef CAS PubMed.
- A. F. M. EL-Mahdy, M. G. Mohamed, T. H. Mansoure, H. H. Yu, T. Chen and S. W. Kuo, Ultrastable tetraphenyl-p-phenylenediamine-based covalent organic frameworks as platforms for high-performance electrochemical supercapacitors, Chem. Commun., 2019, 55, 14890–14893 RSC.
- A. F. M. EL-Mahdy, Y. H. Hung, T. H. Mansoure, H. H. Yu, T. Chen and S. W. Kuo, A Hollow Microtubular Triazine-and Benzobisoxazole-Based Covalent Organic Framework Presenting Sponge-Like Shells That Functions as a High-Performance Supercapacitor, Chem. – Asian J., 2019, 14, 1429–1435 CrossRef CAS PubMed.
- M. M. Samy, M. G. Mohamed, A. F. M. El-Mahdy, T. H. Mansoure, K. C. W. Wu and S. W. Kuo, High-Performance Supercapacitor Electrodes Prepared From Dispersions of Tetrabenzonaphthalene-Based Conjugated Microporous Polymers and Carbon Nanotubes, ACS Appl. Mater. Interfaces, 2021, 13, 51906–51916 CrossRef CAS PubMed.
- M. M. Samy, M. G. Mohamed and S. W. Kuo, Pyrene-functionalized tetraphenylethylene polybenzoxazine for dispersing single-walled carbon nanotubes and energy storage, Compos. Sci. Technol., 2020, 119, 108360 CrossRef.
- M. G. Mohamed and S. W. Kuo, Functional Silica and Carbon Nanocomposites Based on Polybenzoxazines, Macromol. Chem. Phys., 2019, 220, 1800306 CrossRef.
- M. G. Mohamed, K. C. Hsu and S. W. Kuo, Bifunctional polybenzoxazine nanocomposites containing photo-crosslinkable coumarin units and pyrene units capable of dispersing single-walled carbon nanotubes, Polym. Chem., 2015, 6, 2423–2433 RSC.
- C. W. Huang, M. G. Mohamed, C. Y. Zhu and S. W. Kuo, Functional Supramolecular Polypeptides Involving π–π Stacking and Strong Hydrogen-Bonding Interactions: A Conformation Study toward Carbon Nanotubes (CNTs) Dispersion, Macromolecules, 2016, 49(15), 5374–5385 CrossRef CAS.
- S. Kandambeth, J. Jia, H. Wu, V. S. Kale, P. T. Parvatkar, J. C. Jóźwiak, S. Zhou, X. Xu, Z. O. Ameur, E. A. Hamad, A. H. Emwas, O. Shekhah, H. N. Alshareef and M. Eddaoudi, Covalent Organic Frameworks as Negative Electrodes for High-Performance Asymmetric Supercapacitors, Adv. Energy Mater., 2020, 10, 2001673 CrossRef CAS.
- H. Wang, Z. Li, Z. Meng, X. Guo, Y. Du and H. Yang, An easily obtained hypercrosslinked pyrene-based porous organic polymer as a high-performance electrode material for lithium-ion batteries, New J. Chem., 2021, 45, 7060–7064 RSC.
- Z. Wei, D. Wang, Y. Liu, X. Guo, Y. Zhu, Z. Meng, Z. Q. Yu and W. Y. Wong, Ferrocene-based hyperbranched polymers: a synthetic strategy for shape control and applications as electroactive materials and precursor-derived magnetic ceramics, J. Mater. Chem. C, 2020, 8, 10774–10780 RSC.
- B. Y. Lu, Z. Q. Wang, F. Z. Cui, J. Y. Li, X. H. Han, Q. Y. Qi, D. L. Ma, G. F. Jiang, X. X. Zeng and X. Zhao, A Covalent Organic Framework with Extended π-Conjugated Building Units as a Highly Efficient Recipient for Lithium–Sulfur Batteries, ACS Appl. Mater. Interfaces, 2020, 12, 34990–34998 CrossRef CAS PubMed.
- Z. Li, J. Zhang, X. Jing, J. Dong, H. Liu, H. Lv, Y. Chi and C. Hu, A polyoxometalate@covalent triazine framework as a robust electrocatalyst for selective benzyl alcohol oxidation coupled with hydrogen production, J. Mater. Chem. A, 2021, 9, 6152–6159 RSC.
- H. S. Jena, C. Krishnaraj, S. Parwaiz, F. Lecoeuvre, J. Schmidt, D. Pradhan and P. V. D. Voort, Illustrating the Role of Quaternary-N of BINOL Covalent Triazine-Based Frameworks in Oxygen Reduction and Hydrogen Evolution Reactions, ACS Appl. Mater. Interfaces, 2020, 12, 44689–44699 CrossRef CAS.
- A. F. M. EL-Mahdy, A. M. Elewa, S. W. Huang, H. H. Chou and S. W. Kuo, Dual-function fluorescent covalent organic frameworks: HCl sensing and photocatalytic H2 evolution from water, Adv. Opt. Mater., 2020, 8, 2000641 CrossRef CAS.
- Y. Chen, D. Yang, B. Shi, W. Dai, H. Ren, K. An, Z. Zhou, Z. Zhao, W. Wang and Z. Jiang, In situ construction of hydrazone-linked COF-based core–shell hetero-frameworks for enhanced photocatalytic hydrogen evolution, J. Mater. Chem. A, 2020, 8, 7724–7732 RSC.
- T. Zhou, X. Huang, Z. Mi, Y. Zhu, R. Wang, C. Wanga and J. Guo, Multivariate covalent organic frameworks boosting photocatalytic hydrogen evolution, Polym. Chem., 2021, 12, 3250–3256 RSC.
- D. Sengottuvelu, V. Kachwal, P. Raichure, T. Raghav and I. R. Laska, Aggregation-Induced Enhanced Emission (AIEE)-Active Conjugated Mesoporous Oligomers (CMOs) with Improved Quantum Yield and Low-Cost Detection of a Trace Amount of Nitroaromatic Explosives, ACS Appl. Mater. Interfaces, 2020, 12, 31875–31886 CrossRef CAS PubMed.
- Y. Tang, H. Huang, B. Peng, Y. Chang, Y. Li and C. Zhong, A thiadiazole-based covalent triazine framework nanosheet for highly selective and sensitive primary aromatic amine detection among various amines, J. Mater. Chem. A, 2020, 8, 16542–16550 RSC.
- L. R. Ahmed, A. F. M. EL-Mahdy, C. T. Pan and S. W. Kuo, A water-soluble copper-immobilized covalent organic framework functioning as an “OFF–ON” fluorescent sensor for amino acids, Mater. Adv., 2021, 2, 4617–4629 RSC.
- A. F. M. El-Mahdy, M. Y. Lai and S. W. Kuo, A highly fluorescent covalent organic framework as a hydrogen chloride sensor: roles of Schiff base bonding and π-stacking, J. Mater. Chem. C, 2020, 8, 9520–9528 RSC.
- Z. Chen, K. Wang, X. Hu, P. Shi, Z. Guo and H. Zhan, Novel One-Dimensional Covalent Organic Framework as a H+ Fluorescent Sensor in Acidic Aqueous Solution, ACS Appl. Mater. Interfaces, 2021, 13, 1145–1151 CrossRef CAS PubMed.
- M. Faheem, S. Aziz, X. Jing, T. Ma, J. Du, F. Sun, Y. Tian and G. Zhu, Dual luminescent covalent organic frameworks for nitro-explosive detection, J. Mater. Chem. A, 2019, 7, 27148–27155 RSC.
- M. G. Mohamed, N. Y. Liu, A. F. M. EL-Mahdy and S. W. Kuo, Ultrastable luminescent hybrid microporous polymers based on polyhedral oligomeric silsesquioxane for CO2 uptake and metal ion sensing, Microporous Mesoporous Mater., 2021, 311, 110695 CrossRef CAS.
- M. G. Mohamed and S. W. Kuo, Functional Polyimide/Polyhedral Oligomeric Silsesquioxane Nanocomposites, Polymers, 2019, 11, 26 CrossRef PubMed.
- M. G. Mohamed and S. W. Kuo, Polybenzoxazine/Polyhedral Oligomeric Silsesquioxane (POSS) Nanocomposites, Polymers, 2016, 8, 225 CrossRef PubMed.
- M. G. Mohamed, T. H. Mansoure, Y. Takashi, M. M. Samy, T. Chen and S. W. Kuo, Ultrastable porous organic/inorganic polymers based on polyhedral oligomeric silsesquioxane (POSS) hybrids exhibiting high performance for thermal property and energy storage, Microporous Mesoporous Mater., 2021, 328, 111505 CrossRef CAS.
- W. Gong, X. Deng, K. Dong, L. Liu and G. Ning, A boranil-based conjugated microporous polymer for efficient visible-light-driven heterogeneous photocatalysis, Polym. Chem., 2021, 12, 3153–3159 RSC.
- T. Chen, W. Zhang, B. Li, W. Huang, C. Lin, Y. Wu, S. Chen and H. Ma, Adsorptive Separation of Aromatic Compounds from Alkanes by π−π Interactions in a Carbazole-Based Conjugated Microporous Polymer, ACS Appl. Mater. Interfaces, 2020, 12, 56385–56392 CrossRef CAS PubMed.
- B. Chen, L. Chen, Z. Yan, J. Kang, S. Chen, Y. Jin, L. Ma, H. Yan and C. Xia, Conjugated microporous polymers as a visible light driven platform for photo-redox conversion of biomass derived chemicals, Green Chem., 2021, 23, 3607–3611 RSC.
- X. Jiang, K. Zhang, Y. Huang, B. Xu, X. Xu, J. Zhang, Z. Liu, Y. Wang, Y. Pan, S. Bian, Q. Chen, X. Wu and G. Zhang, Conjugated Microporous Polymer with C
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C and C–F Bonds: Achieving Remarkable Stability and Super Anhydrous Proton Conductivity, ACS Appl. Mater. Interfaces, 2021, 13, 15536–15541 CrossRef CAS PubMed.
C and C–F Bonds: Achieving Remarkable Stability and Super Anhydrous Proton Conductivity, ACS Appl. Mater. Interfaces, 2021, 13, 15536–15541 CrossRef CAS PubMed. - Y. Wang, H. Liu, Q. Pan, N. Ding, C. Yang, Z. Zhang, C. Jia, Z. Li, J. Liu and Y. Zhao, Construction of Thiazolo[5,4-d]thiazole-based Two-Dimensional Network for Efficient Photocatalytic CO2 Reduction, ACS Appl. Mater. Interfaces, 2020, 12, 46483–46489 CrossRef CAS PubMed.
- H. Xu, X. Li, H. Hao, X. Dong, W. Sheng and X. Lang, designing fluorene-based conjugated microporous polymers for blue light-driven photocatalytic selective oxidation of amines with oxygen, Appl. Catal., B, 2021, 285, 119796 CrossRef CAS.
- M. Xu, T. Wang, L. Zhou and D. Hua, Fluorescent conjugated mesoporous polymers with N,N-diethylpropylamine for the efficient capture and real-time detection of volatile iodine, J. Mater. Chem. A, 2020, 8, 1966–1974 RSC.
- A. Singh, P. Verma, S. Laha, D. Samanta, S. Roy and T. K. Maji, Photochromic Conjugated Microporous Polymer Manifesting Bio-Inspired pcFRET and Logic Gate Functioning, ACS Appl. Mater. Interfaces, 2020, 12, 20991–20997 CrossRef CAS PubMed.
- Y. M. Li, L. Yang, L. Sun, L. Ma, W. Q. Deng and Z. Li, Chemical fixation of carbon dioxide catalyzed via covalent triazine frameworks as metal free heterogeneous catalysts without a cocatalyst, J. Mater. Chem. A, 2019, 7, 26071–26076 RSC.
- Z. Shang, B. Zhao, Z. Wu, Y. Ding and A. Hu, Synthesis of Conjugated Mesoporous Hyper-cross-linked Polymers for Efficient Capture of Dibenzothiophene and Iodine, ACS Appl. Mater. Interfaces, 2020, 12, 56454–56461 CrossRef CAS PubMed.
- Z. Lu, X. Fu, H. Yang, Y. Zhao, L. Xiao and L. Hou, A covalent organic framework as a photocatalyst for atom transfer radical polymerization under white light irradiation, Polym. Chem., 2021, 12, 183–188 RSC.
- W. Chen, Z. Yang, Z. Xie, Y. Li, X. Yu, F. Lu and L. Chen, Benzothiadiazole functionalized D–A type covalent organic frameworks for effective photocatalytic reduction of aqueous chromium(VI), J. Mater. Chem. A, 2019, 7, 998–1004 RSC.
- L. Zhai, S. Yang, X. Yang, W. Ye, J. Wang, W. Chen, Y. Guo, L. Mi and Z. Wu, Constantinos Soutis, Qing Xu and Zheng Jiang, Conjugated Covalent Organic Frameworks as Platinum Nanoparticle Supports for Catalyzing the Oxygen Reduction Reaction, Chem. Mater., 2020, 32, 9747–9752 CrossRef CAS.
- X. Xu, S. Wang, Y. Yue and N. Huang, Semiconductive Porphyrin-Based Covalent Organic Frameworks for Sensitive Near-Infrared Detection, ACS Appl. Mater. Interfaces, 2020, 12, 37427–37434 CrossRef CAS PubMed.
- M. G. Mohamed, C. C. Lee, A. F. M. EL-Mahdy, J. Lüder, M. H. Yu, Z. Li, Z. Zhu, C. C. Chueh and S. W. Kuo, Exploitation of two-dimensional conjugated covalent organic frameworks based on tetraphenylethylene with bicarbazole and pyrene units and applications in perovskite solar cells, J. Mater. Chem. A, 2020, 8, 11448–11459 RSC.
| This journal is © The Royal Society of Chemistry 2022 |