Open Access Article
Open Access ArticleFormulation of PLGA nano-carriers: specialized modification for cancer therapeutic applications
Manosree
Chatterjee
 *ab and
Nripen
Chanda
*ab and
Nripen
Chanda
 *a
*a
aMaterial Processing and Microsystem Laboratory, CSIR – Central Mechanical Engineering Research Institute, Durgapur-713209, India. E-mail: manosree87@gmail.com; n_chanda@cmeri.res.in; Fax: +91-343-2546745; Tel: +91-9474112053 Tel: +91-9933034370
bDepartment of Biotechnology, National Institute of Technology Durgapur, Durgapur-713209, India
First published on 2nd December 2021
Abstract
The eminence of nano-scale materials prevailed after the invention of high-resolution microscopes. Nowadays, nanoparticles are predominantly found in every application, including biomedical applications. In nanomedicine, the unique properties make nano-scale particles an efficient delivery vehicle to overcome the adverse effects of therapeutic molecules, which are directly administered. The polymeric nanoparticles have gradually gained interest as a nano-carrier over various non-polymeric types of nanoparticles due to their biocompatible nature. PLGA is the most frequently used polymer to synthesize polymeric nano-carriers as it is a clinically approved biodegradable polymer and has a broad scope of modification of its inherent properties. PLGA polymer, before or after nanoparticle formation, can be functionalized using various non-covalent and covalent modification techniques to suit desired applications. Since the beginning of PLGA nanoparticle usage, different synthesis methods have evolved progressively with various advantages and limitations. The present review also discusses the post-surface modification characterization of PLGA nanoparticles and their imaging and drug delivery applications.
1 Introduction
The concept of nanoparticles was theoretically introduced in the 19th century by Max Planck and Albert Einstein, which gradually became an imperative field after the invention of the transmission electron microscope.1 From the beginning of the 20th century, nano-materials gained more attention after flourishing sophisticated high-resolution characterization techniques and became a promising technology for a wide range of applications.1,2 In 1908, Paul Ehrlich, who won the Nobel Prize in Medicine, has given the concept of “magic bullet”, which was a carrier of drug molecules with another agent of selectivity that only destroys diseased cells without any harmful effect on healthy cells.1 Nowadays, nanoparticles’ unique physicochemical properties make them an indispensable aspect of biomedical research that has both monitoring and therapeutic applications in disease.3–5 Its nano-scale size provides (i) high surface area to volume ratio that can accommodate a massive amount of external agents, (ii) distinctive optical property, which can help in imaging, (iii) electrical property with specific zeta (ζ) potential significant for interaction with cells, (iv) chemical property that can deliver an opportunity for functionalization with small molecules, and (v) colloidal stability, which helps in the application by preventing irreversible aggregation.6–10 Different kinds of nanoparticles, such as polymeric, metallic, and liposome nanoparticles, were meticulously investigated to treat many lethal diseases, and many of them are in clinical trials.11–15 Detection and monitoring of pathologies are essential to identify the disease and its intensity.16 Iron oxide, gold, and gadolinium nanoparticle have proven their potentials in magnetic resonance imaging (MRI).17,18 Various metallic nanoparticles are used in computerized tomography (CT), ultrasound, photoacoustic imaging, positron emission tomography, and optical imaging.19–25 Though several drug delivery systems have been effectively used in clinical practices, a major constraint of such systems, specifically with metallic nanoparticles (e.g., silver nanoparticles) is bio-accumulation over the due course of treatment. Since the metal-based nanoparticles are not metabolized in physiological conditions, prolonged treatment with such nano-formulations leads to undesirable complications.26,27These limitations can be addressed by polymer-based biocompatible nano-materials that have emerged as an alternative platform for drug delivery and imaging applications. Among all types of US Food and Drug Administration (FDA) approved nanoparticles used as a drug delivery system, polymeric nanoparticles are the most widely used in clinical practices.15 In addition to the biocompatibility, the polymeric nanoparticles offer a few other advantages, e.g., (a) enhancing the encapsulation of small molecules, (b) preventing degradation or deactivation of the drug in the bloodstream before reaching the target, (c) prolonging blood circulation time, (d) controlling the release of drugs in target tissues or cells, (e) improving the drug loading capacity, (f) increasing the bioavailability of drugs, and finally (g) speeding the passive accumulation of drugs at tumor sites based on the enhanced permeability and retention (EPR) effect.28–32 Besides, there is a flexibility to modify its pristine polymer chain and surface characteristics to enhance drug delivery and imaging efficacy, making them a superior therapeutic nano-platform.
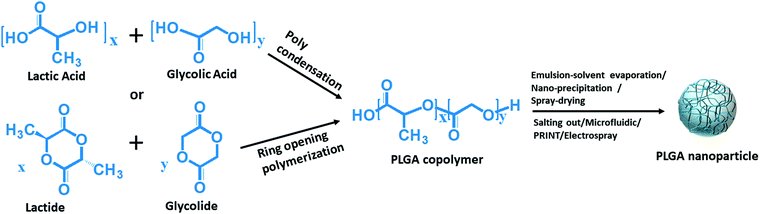
The majority of the polymeric nanoparticles are fabricated using numerous biocompatible polymers to reduce the undesirable systemic toxicity of the drug transporter.33 Among these, FDA and European Medicine Agency (EMA) approved biodegradable PLGA polymer is most widely used as a versatile and clinically proved elemental polymer for the synthesis of efficient nano-carriers.34–36 Simultaneous drug enrichment to the tumor site and minimizing toxicity to normal tissues are indispensable aspects of nanoparticle-mediated drug delivery purposes. The use of biocompatible polymers like PLGA to achieve these aspects through nanoparticle synthesis has been an ever-growing arena in the field of safe drug delivery. PLGA polymer is composed of varying ratios of lactic acid and glycolic acid monomer units that are ester bonded to form the polyester polymer. In an in vivo system, PLGA polymer decomposed upon hydrolysis at the ester bond and eventually metabolized through the kerbs cycle with the nontoxic end products (H2O and CO2), which are eliminated from the body.37–39 The globally accepted and clinically approved PLGA polymer chains randomly orient themselves to form PLGA nanoparticles (Fig. 2). PLGA copolymers with low molecular weights (MW < 10 kDa) are synthesized by the polycondensation reaction of lactic acid and glycolic acid in various ratios and higher molecular weight copolymers (generally used 10–100 kDa) are synthesized by ring-opening polymerization of cyclic dimers (Fig. 2).35,39 Biodegradation of PLGA nanoparticles depends on the integral properties of the PLGA copolymer chain, which include the ratio of lactic acid and glycolic acid monomers in its chain composition and molecular weight.36,40 The drug release from PLGA nanoparticles by the degradation of PLGA copolymer can be regulated by tuning the ratio of lactic acid and glycolic acid monomers in the PLGA chain. If the ratio of lactic acid is increased in the PLGA chain, the hydrophobicity also increases proportionally, resulting in a slow degradation of PLGA as it absorbs less water.35,36,40 On the contrary, faster hydrolysis is observed when the glycolic acid content in PLGA is higher, resulting in the rapid release of drugs from nanoparticles. An acid-terminated PLGA chain with lower molecular weight and equal ratio of lactic acid (LA) and glycolic acid (GA) (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 PLGA) is frequently used to prepare drug delivery vehicles due to its optimum degradation rate (less than two months at 37 °C in an aqueous medium).35,41,42 PLGA copolymer with higher molecular weight causing slower degradation rate of nanoparticles exhibits a slower drug release.40 Various clinically significant biomolecules are efficiently encapsulated inside the PLGA nanoparticle's core through weak covalent interactions for imaging and drug delivery. The physicochemical properties of nanoparticles predominantly depend on the composition and molecular weight of the PLGA polymer.39 The polymer can be dissolved in a wide range of organic solvents like acetone, dichloromethane, tetrahydrofuran, ethyl acetate, and chloroform, which is advantageous for nanoparticle synthesis.38 Moreover, PLGA nanoparticles prove its excellence as a nano-carrier system as it possesses a wide range of degradation rates that provide a desirable formulation opportunity, stability in long-term storage, and high encapsulation efficiency. The first targeting (prostate-specific membrane antigen (PSMA)–targeted) nanomedicine BIND-014 containing docetaxel, which was tested on humans, was prepared with PLGA polymer.43,44 Currently available PLGA polymer-based antitumor drugs approved for clinical practices to treat various types of cancer are listed in Table 1.45–51
50 PLGA) is frequently used to prepare drug delivery vehicles due to its optimum degradation rate (less than two months at 37 °C in an aqueous medium).35,41,42 PLGA copolymer with higher molecular weight causing slower degradation rate of nanoparticles exhibits a slower drug release.40 Various clinically significant biomolecules are efficiently encapsulated inside the PLGA nanoparticle's core through weak covalent interactions for imaging and drug delivery. The physicochemical properties of nanoparticles predominantly depend on the composition and molecular weight of the PLGA polymer.39 The polymer can be dissolved in a wide range of organic solvents like acetone, dichloromethane, tetrahydrofuran, ethyl acetate, and chloroform, which is advantageous for nanoparticle synthesis.38 Moreover, PLGA nanoparticles prove its excellence as a nano-carrier system as it possesses a wide range of degradation rates that provide a desirable formulation opportunity, stability in long-term storage, and high encapsulation efficiency. The first targeting (prostate-specific membrane antigen (PSMA)–targeted) nanomedicine BIND-014 containing docetaxel, which was tested on humans, was prepared with PLGA polymer.43,44 Currently available PLGA polymer-based antitumor drugs approved for clinical practices to treat various types of cancer are listed in Table 1.45–51
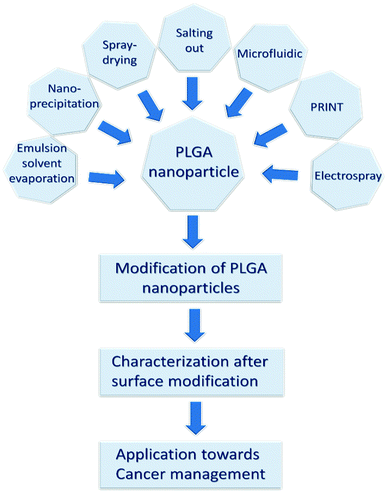 | ||
| Fig. 1 The review's structure based on PLGA nano-carrier formulation, surface modification, and cancer therapeutic applications. | ||
| Product (manufacturers’ name) | Drug name | Dosage type | PLGA content and LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GA GA |
Drug dosages | Targeted tumor | Approved year |
|---|---|---|---|---|---|---|
| Decapeptyl® (Ferring Pharmaceuticals Pvt. Ltd.) | Triptorelin | Microsphere | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
3.75 mg in every 28 days for 6 months | Prostate, breast | 1986 |
| Zoladex Depot® (AstraZeneca UK Limited) | Goserelin acetate | Implant | 13.3–14.3 mg per dose; 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
3.6 mg in every 28 days | Prostate | 1989 |
| Lupron Depot® (Abbvie Endocrine Inc) | Leuprolide | Microsphere | 33.1 mg per dose; 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
3.75 mg in every month | Prostate | 1989 |
| Sandostatin Lar® (Novartis Pharmaceuticals UK Ltd) | Octreotide acetate | Microsphere | 188.8,377.6 and 566.4 mg per dose; 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (glucose-initiated PLGA) 50 (glucose-initiated PLGA) |
10 mg, 20 mg or 30 mg in every 4 weeks | Acromegaly | 1998 |
| Trelstar® (Ferring Pharmaceuticals Pvt.) | Triptorelin pamoate | Microsphere | 136, 118, 182 mg per dose; 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
3.75 mg in every 4 weeks or 11.25 mg in every 12 weeks or 22.5 mg in every 24 weeks | Prostate | 2000 |
| Eligard® (Zydus Cadila Healthcare Ltd.) | Leuprolide acetate | In situ forming implant | 82.5 mg per dose of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (in 7.5 mg), 158.6 mg per dose of 75 50 (in 7.5 mg), 158.6 mg per dose of 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 (in 22.5 mg), 211.5 mg per dose of 75 25 (in 22.5 mg), 211.5 mg per dose of 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 (in 30 mg) and 165 mg per dose of 85 25 (in 30 mg) and 165 mg per dose of 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 (in 45 mg) 15 (in 45 mg) |
1 mg in every day 7.5 mg in a month 22.5 mg in every 3 months 30 mg in every 4 months 45 mg in every 6 months | Prostate | 2002 |
| Signifor Lar® (Novartis Pharmaceuticals Corporation) | Pasireotide pamoate | Microsphere | 26.29, 52.58, 78.87 mg per dose of PLGA-50-60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40–50 and PLGA-50 40–50 and PLGA-50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
20 mg, 40 mg, 60 mg in every 28 days | Acromegaly | 2014 |
In the present study, we review the synthesis, surface properties, and superiority of PLGA nano-carriers towards cancer therapeutic applications. It includes a comprehensive discussion on the existing PLGA nanoparticle synthesis methods, surface functionalization processes, and subsequent characterization techniques. It also covers the optimization and limitations of the existing PLGA nano-formulation procedures. Finally, we scrutinized the theranostic efficiency of the PLGA nano-carrier system, a promising alternative to conventional drugs. Then, we have summarized the reported research on the cancer therapeutic applications of the PLGA nano-carrier. The review's complete structure is depicted in Fig. 1. This review article provides an extensive understanding of all the milestones for making a PLGA nano-carrier, which is a potential cancer therapeutic system.
2 Synthesis techniques of PLGA nanoparticles
Synthesis techniques of PLGA nanoparticles depend on their application and the type of molecules they have to encapsulate. Therefore, it is required to select an appropriate synthesis method for the proper designing of nanoparticles. The size distribution and shape of PLGA nanoparticles are mainly determined by the PLGA chain orientation and parameters of the synthesis method. The synthesis method also has a strong influence on the colloidal stability, encapsulation efficiency of the external agents, and behavior of the nanoparticles in the cellular model. Moreover, the release rate of the encapsulated agent depends on the synthesis parameters of the particular synthesis procedure.39 Here, the existing synthesis procedures are discussed in two categories. First, the comparatively older methods are denoted as conventional methods, and second, the comparatively newer ones are denoted as non-conventional methods. The process parameters, critical characteristics of the synthesized particles, advantages, and limitations of the methods are tabulated in Table 2.| Name of the method | Parameters that affect nanoparticles size | LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GA and size of nanoparticles (nm) GA and size of nanoparticles (nm) |
Drug loading (%) and encapsulation efficiency (%) | Advantages | Limitations | Ref. |
|---|---|---|---|---|---|---|
| Conventional methods | ||||||
| Emulsion-solvent evaporation | Polymer concentration, speed of agitation, stabilizer concentration | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 75 50, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 85 25, 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15; and 100–500 15; and 100–500 |
0.022–8 and 20–80 | Spherical morphology, easy and rapid procedure of nanoparticle fabrication, colloidal stability | Heterogeneous in size, residual stabilizer remains, particle agglomeration, drugs may lose activity due to high shear stress | 52–57,59,60 |
| Nano-precipitation | Polymer concentration, speed of agitation, stabilizer concentration | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 75 50, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 85 25, 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15; and 50–300 15; and 50–300 |
1.7–10 and 40–90 | High yield, easy and reproducible | High polydispersity index, use of stabilizer, high speed agitation may degrade drug molecules | 61–66 |
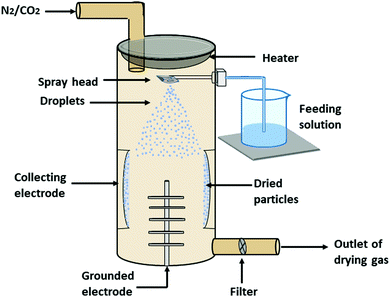
| Spray-drying | Spray mesh hole size, concentration of the polymer, density of the spray liquid, flow rate | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 75 50, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25; and > 300 25; and > 300 |
1.5–7.4 and 65–90 | It produced powder nanoparticles, stable in storage because free of moisture, nanoparticles produced free from contamination of other chemicals | Degradation of the temperature sensitive drug due to the high heat, high operating cost, and agglomeration of nanoparticles | 67–73 |
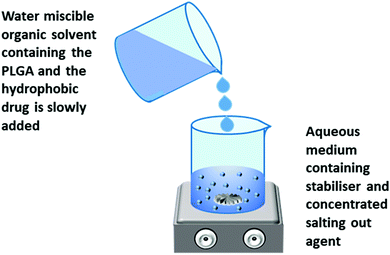
| Salting out | Polymer concentration, stirring speed, stabilizer concentration, concentration of salting out agent | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 75 50, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25; and 55–500 25; and 55–500 |
5 and 55–80 | Use of nontoxic oil phase, nanoparticles size can be controlled by adjusting different parameters | Purifying the nanoparticles is very hectic due to the presence of salting out agent, high speed agitation may result in loss of drug activity | 74–76 |
| Nonconventional methods | ||||||
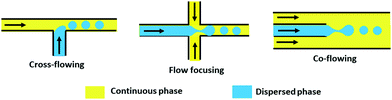
| Microfluidic | Channel geometry, flow rate ratio of the continuous phase and dispersed phase, interfacial tension between two phases, Mixing time | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 75 50, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25; and 40–200 25; and 40–200 |
10–18 and ∼90% | Narrow size distribution, reproducible | Swelling of PDMS polymer alters channel geometry, very low yield | 77–80 |
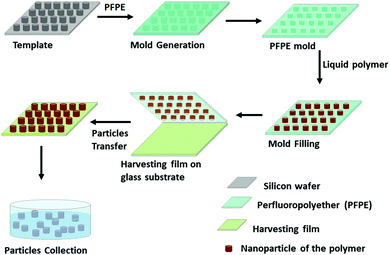
| Template patterns, mold preparation | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 85 50, 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15; and 10–300 15; and 10–300 |
1–40 and > 90% | Monodispersed particle, high encapsulation efficiency, reproducible | Low yield, degradation of clinically important fragile molecules during solidification | 81–86 | |
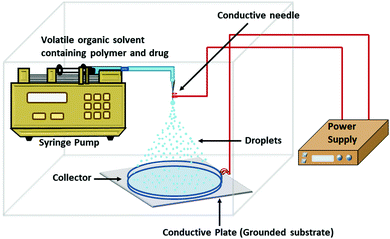
| Electrospray | Concentration of the polymer, nature of solvent, needle diameter flow rate, potential difference and distance between needle and grounded electrode | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 and 10–500 50 and 10–500 |
5–43 and > 90% | High yield in a short duration of time, single step method, surfactant and high speed agitation free process | 87–89 | |
2.1 Conventional methods
The most frequently applied earlier methods for the synthesis of PLGA nanoparticles in this category are discussed below.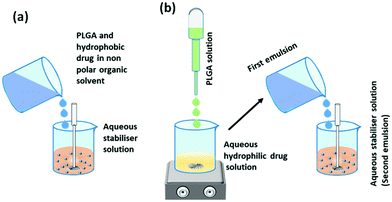 | ||
| Fig. 3 Synthesis of PLGA nanoparticles by (a) single emulsion-solvent evaporation method and (b) double emulsion solvent evaporation method. | ||
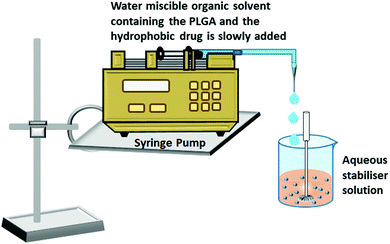
In the single emulsion solvent evaporation method, both PLGA and hydrophobic drugs are dissolved in a non-polar organic solvent, which is then added drop-wise to the aqueous solution of surfactants (stabilizers) with continuous agitation (ultra-sonication or homogenization). The high shear stress disperses the oil–water emulsion into nanoparticles, which hardens after evaporation of the organic solvent by continuous stirring (Fig. 3a).56,57 For the encapsulation of hydrophilic drugs, the PLGA solution is drop-wise added to the aqueous drug solution with continuous stirring that produces the first emulsion. Then, the first emulsion is transferred to the aqueous solution of surfactants (water in oil in water) under ultra-sonication or homogenization, which forms the second emulsion that finally transforms into the hydrophilic drug encapsulated PLGA nanoparticles (Fig. 3b).55,57 The nanoparticles are solidified after the evaporation step like the single emulsion method. The vigorous mechanical agitation step in the stabilizer containing aqueous medium disperses the emulsion into nano-droplets of varying sizes with a layer of the stabilizer surrounding it. Nanoparticle size increases with the increase in PLGA concentration. At higher PLGA concentrations, the viscosity of the organic phase obstructs the disruption of the emulsion into very small-sized nano-droplets; as a result, a high number of PLGA polymer resides in the droplet, which eventually produces nanoparticles with a larger size.58,59 Manchanda et al. reported that a higher concentration of the stabilizer in an aqueous medium increases the overall shear force on the emulsion droplets by reducing the organic/aqueous interfacial tension, which eventually helps the formation of nanoparticles with a smaller mean diameter. They also reported that the drug encapsulation efficiency increases with the increase in PLGA concentration as high viscosity resists the diffusion of the drug into the aqueous medium. A high polymer ratio also provides a dense network to trap the drug molecules.60 The agitation speed also has a remarkable effect on the size of the nanoparticles. The high agitation speed produces smaller nanoparticles by rupturing the emulsion droplets into smaller ones containing lesser PLGA polymer. Kadriye Kizilbey conducted a series of experiments to optimize the parameters to encapsulate hydrophobic drugs inside PLGA nanoparticles using the single emulsion solvent evaporation method. He reported that the diameter and encapsulation efficiency of nanoparticles increase with the increase in PLGA concentration. At the same time, the increasing concentration of PVA (stabilizer) has a similar effect on size but has a reverse effect on encapsulation efficiency.56
2.2 Nonconventional approaches
Nowadays, various interdisciplinary approaches have been introduced to overcome several limitations like multi-step synthesis process, reduced drug loading, low yield, polydispersity in size, and the involvement of biohazard materials. Conventional ways of PLGA nanoparticle synthesis are emulsion-solvent evaporation, nano-precipitation, salting out, and spray-drying methods. These methods usually employ more than one solvent phase with stabilizer/cross-linker and high shear stress for a prolonged time, resulting in polydispersity in the nanoparticle size. Nevertheless, after several washes of the synthesized nanoparticles, some residual solvent may remain, which may result in undesirable toxic effects in the application phase.Every type of polymer with a specific molecular weight has a particular number of chain entanglements in a specific type of solvent, which increases with the increasing polymer concentration. As the concentration of the polymer increases, the viscosity of the polymer solution gets enhanced, producing a higher chain entanglement density that is physical overlapping of the polymer chain. Above the critical chain overlap concentration, the electrospinning that is fiber formation begins by inhibiting Rayleigh disintegration of the droplets. Polymer concentration below the critical chain overlap concentration favors electrospraying. A lower PLGA concentration decreases the nanoparticle's size as there is no or low chain entanglement in the solution.88 The solvent should be highly volatile and conductive in electrospray-mediated polymer nanoparticle synthesis. During the flight of the nano-droplets, the whole solvent must completely evaporate before reaching the collector.88 A specific flow rate of the spray solution through the conductive needle is indispensable to get a stable spray for nanoparticle formation. For the synthesis of monodispersed nanoparticles, a steady flow with a sufficiently low rate is required. At a high flow rate, an intermittent jet is produced instead of an electrospray. The particle size also depends on the flow rate. Smaller particles are formed from the slower flow rate.88 Stable electrospraying depends on the needle diameter when the flow rate, applied voltage, and electrospray setup are stable. A smaller needle diameter produces more stable and smaller spherical nanoparticles.88 The stable coulomb fission can be obtained only above a certain applied voltage at a fixed flow rate and electrospray setup. As the voltage increases, the spray solution's dribbling from the conductive needle starts to be finer and eventually becomes nano-spray.88 The distance between the conductive needle and the grounded electrode decides the electric field strength, which influences the droplets’ rupturing. Shorter distance intensifies the electric field strength, which leads to smaller-sized particle formation when other parameters are fixed. A sufficient distance is also required for the complete evaporation of the solvent. If the distance becomes more, then a higher voltage is required to overcome the spray jet.88
3 Modification of PLGA nanoparticles
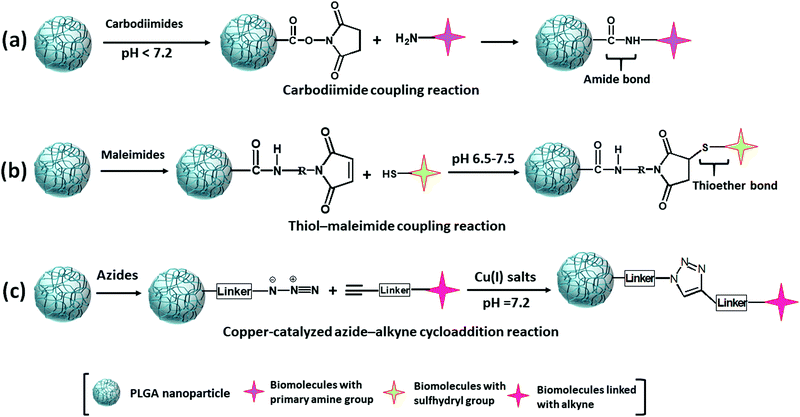
The clinically important molecules are less efficient to reach and accomplish the desired diagnostic or therapeutic aim solely due to their various unfavorable and adverse effects (hydrophobicity, toxicity, ionic form, and coagulation). Drug delivery systems overcome these limitations by providing a frontier solution to increase the drug molecules’ pharmacological efficacy and bioavailability by entrapping the drug molecules inside their core. As PLGA is deficient in functional groups, it is necessary to functionalize it with additional functional groups to convey the clinically important molecules at the site where it is required. Modification techniques of PLGA nanoparticles are summarised in Table 3. The modification also provides a high degree of flexibility by altering the polymer and particle surface properties to make it suitable for therapeutic and imaging applications.| Type of interaction | Conjugating molecule | Coupling agent | Reaction environment | Type of bond formation | Ref. |
|---|---|---|---|---|---|
| Non-covalent | |||||
| Hydrophobic | Hydrophobic biomolecules | — | Depends on the conjugating molecule | Hydrophobic | 92 |
| Electrostatic | Positively charged biomolecules | — | Depends on the conjugating molecule | Electrostatic | 92 |
| Hydrogen bond | Biomolecules with carboxyl, amine and hydroxyl groups | — | Depends on the conjugating molecule | Hydrogen bond | 92 |
| Avidin–Biotin | Any type of biomolecules | Avidin and Biotin | Wide range of pH | Protein and ligand | 93–95 |
| Covalent | |||||
| Carbodiimide coupling reaction | Biomolecules with primary amine group | Carbodiimides | pH < 7.2 | Amide bond | 91,98–101 |
| Thiol-maleimide coupling reaction | Biomolecules with sulfhydryl or thiol group | Maleimide | pH 6.5–7.5 | Thioether bond | 106–109 |
| Copper-catalyzed azide–alkyne cycloaddition (CuAAC) reaction | Biomolecules linked with alkyne group | Azide and alkyne | In the presence of copper(I) catalyst at pH 7.2 | 5-Membered heteroatom ring (1,2,3-triazole) | 117,119,121 |
3.1 Non-covalent
Non-covalent binding is a reversible interaction that provides easy attachment and detachment of the desired molecules at predefined conditions. This interaction is weak in nature; it includes hydrophobic, hydrogen bonds, and electrostatic interactions (Fig. 10a–c).92 As there is no stable bond formed between PLGA and ligand, the conjugation is very unstable. When a faster rate of release is needed, this type of conjugation provides an excellent option. This type of conjugation does not hamper the molecular properties of the ligand. Conjugation of biomolecules using this type of weak interaction is a faster one-step process, and additional use of chemical and purification steps can be avoided. However, unlike covalent conjugation, there is a risk of premature release from the nanoparticle's surface. Another frequently used and the most robust non-covalent interaction is the Avidin–Biotin interaction (Fig. 10d).92,93 In non-covalent interactions, small molecules are randomly conjugated, which sometimes block the group required for receptor-specific interaction. Avidin–Biotin interaction strategy helps to bind each other with high binding affinity.92,94 Avidin is a basic tetrameric glycoprotein that irreversibly binds with biotin (Vitamin B7) in a wide range of pH and temperature, facilitating the site-specific quantitative conjugation of clinically important small molecules with PLGA polymer as well as nanoparticles.95 Using avidin–biotin interaction, Sirianni et al. stably conjugated radioactive isotopes F-18 labelled PEGylated biotin ([18F]-fluorobenzylamide-poly(ethylene glycol)4-biotin) with avidin-modified PLGA nanoparticles for efficient delivery and positron emission tomography imaging in intact rat brain.96 Despite these advantages, some limitations are there, like non-specific binding at physiological pH (due to basic pI and presence of terminal glycoside moiety) and strong irreversible interaction causing difficulty in the release. To avoid these limitations, streptavidin and neutravidin, which are analogs of avidin can more specifically bind with biotin to make the reaction reversible.92,95 For this reason, biotin analogs like desthiobiotin are also in use.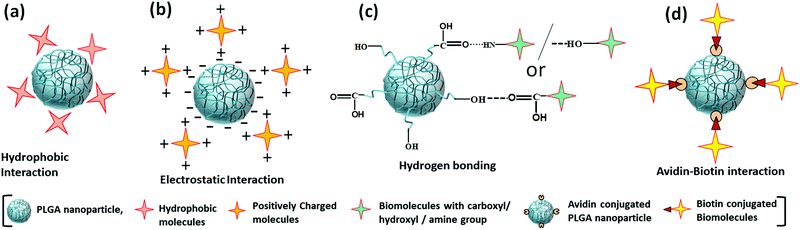 | ||
| Fig. 10 Non-covalent interactions for the modification of PLGA nanoparticles (a) hydrophobic interaction, (b) electrostatic interaction, (c) hydrogen bonding, (d) avidin–biotin interaction. | ||
3.2 Covalent
Covalent conjugation is a stable conjugation procedure that plays a vital role in the stable transportation of clinically important molecules at a predefined location and rate. It employs multiple steps to form a strong bond between PLGA polymer/nanoparticle surface and small ligands that facilitate long circulation and controlled release at the desired position. It lowers the chance to change the inherent molecular property of the conjugated molecules. For the functionalization of PLGA polymer/nanoparticle, three coupling reactions are generally used, such as carbodiimide coupling, thiol-maleimide coupling, and copper–catalyzed azide–alkyne cycloaddition.4 Characterizations of PLGA nano-carriers after surface modification
It is necessary to perceive the surface properties of the PLGA nano-carrier after functionalization with desired ligands to get a critical insight into the suitability of therapeutic application. Characteristics of nano-carriers depend on the modification of the PLGA polymer, nanoparticle surface, conjugated molecules, and the type of interaction at the time of conjugation. Intrinsic properties of the nano-carrier like size, charge, shape, excitation and emission wavelength, molecular composition, and type of bond involved in conjugation are analyzed using various sophisticated analytical methods.124,125 These physico-chemical properties of the nanoparticle decide the stability, drug encapsulation efficiency, and release kinetics of the nanoparticle, which reflects its applicability as a nano-carrier system. The tuning of the synthesis and functionalization process parameters leads to the modification of the properties of nanoparticles that regulate the behavior of the nanoparticles.126,127Hydrodynamic size and charge of the PLGA nano-carrier are analyzed using a particle size analyzer by the dynamic light scattering technique.128 The size and charge (Zeta (ζ) potential) are the determining factors to cross the cell-associated barrier for delivering the drug or tracking through an imaging agent.129 Nano-carrier with size less than 200 nm internalized efficiently by the cell through endocytosis and EPR (enhanced permeability and retention) effect of tumor vasculature. Nanoparticles with a size greater than 200 nm were prematurely eliminated from the body by the reticuloendothelial system (RES).130,131 A higher ζ potential of PLGA nanoparticles provides colloidal stability of the nanoparticles. The higher surface charge of the nanoparticles produces electrostatic repulsion; thus, they remain suspended in solution, which prevents agglomeration and maintains the size.132 Nanoparticles were analyzed using atomic force microscopy (AFM), field emission scanning electron microscopy (FESEM), and transmission electron microscopy (TEM) to study the nanoparticle's shape, increased diameter, and surface texture after surface modification (Fig. 12).90,91 The nanoparticle's shape and morphology depend on the synthesis procedure, which has an effect on the cellular uptake of the nanoparticles. A three-dimensional view and line roughness graph of nanoparticles with the sub-nanometer resolution is observed under AFM in atmospheric or submerged conditions.133 FESEM and TEM analyses also provide information about elemental composition by energy-dispersive X-ray spectroscopy (EDX).134
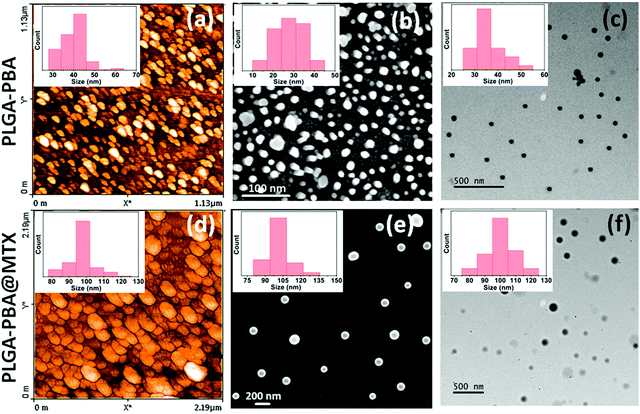 | ||
| Fig. 12 (a) AFM, (b) FESEM and (c) TEM images of 1-pyrenebutyric acid conjugated PLGA nanoparticles and the same after methotrexate conjugation on the nanoparticles surface through amide linkage shown in (d–f), respectively. The inset shows the corresponding particle size distribution histogram where the increase in particle size establishes the conjugation (Reproduced from ref. 91 with permission from the Royal Society of Chemistry). | ||
Ultraviolet-visible spectroscopy (UV-vis spectroscopy) is used to study the encapsulated and conjugated ligands/biomolecules in PLGA nano-carriers that absorb light in the UV or visible regions of the electromagnetic spectrum.135 In a complementary way of UV-vis spectroscopy, fluorescent molecule conjugated PLGA nanoparticles absorb a specific wavelength of light (usually ultraviolet light), then re-emit the light to return from electronically excited states to the ground state, which is detected by fluorescence spectroscopy (Fig. 13).136 These techniques are used to quantify the conjugates present in modified PLGA nanoparticles to measure the loading and encapsulation efficiency. The wavelength of maximum absorption (λmax) and maximum emission (λem) are utilized to study the release kinetics of the drug molecules carried by the PLGA nanoparticles. These optical characteristics help detect, quantify the concentration, and find the level of degradation of the nano-carrier in biological samples.137
 | ||
| Fig. 13 Fluorescence spectra of PLGA, 1-pyrenebutyric acid (PBA) conjugated PLGA nanoparticles (PLGA-PBA), and methotrexate (MTX) conjugated PLGA-PBA nanoparticles (PLGA-PBA@MTX). The images of nanoparticles suspensions under UV lamp (λmax = 265 nm) are represented in the inset (Reproduced from ref. 91 with permission from the Royal Society of Chemistry). | ||
Elemental characterization provides a deeper understanding of physical, chemical, and biological phenomena of the nano-carrier by the Fourier-transform infrared spectroscopy (FTIR), mass spectroscopy, nuclear magnetic resonance (NMR) spectroscopy, X-ray photoelectron spectroscopy (XPS), and Raman spectroscopy.90,91 The most common technique to determine the elemental composition and type of bonds between the molecules in the nano-carrier by their unique stretching frequencies is FTIR analysis (Fig. 14).138 The charge to mass ratio of fragmented ions of the nanoparticles during electrospray ionization in the mass spectroscopic analysis technique helps detect the presence of certain molecules and to predict the structure.139 Proton (1H) and carbon-13 (13C) NMR spectroscopy are generally used to establish the purity, detect the molecular structure, formation of bonds during conjugation, and analyze the molecules’ chemical environment in the nano-conjugate (Fig. 14).127,135 The chemical state and the electronic state of the elements within the nano-carrier are analyzed using the XPS technique.135 Raman spectroscopy is employed to analyze the chemical composition and structure of the nano-carrier through a non-contact and non-destructive way by the molecular fingerprint that is the signature vibrational, rotational, and other low-frequency modes of biomolecules.140
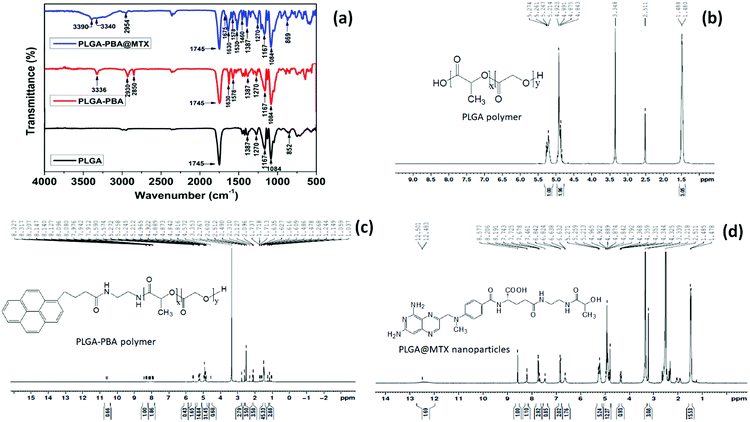 | ||
| Fig. 14 (a) FTIR spectrum of PLGA polymer, 1-pyrenebutyric acid (PBA) conjugated PLGA polymer (PLGA-PBA) and methotrexate (MTX) conjugated PLGA-PBA nanoparticles (PLGA-PBA@MTX) showing chemical conjugation of PBA and MTX with PLGA before and after nanoparticle formation. 1H NMR analysis of all the conjugation step i.e. (b) PLGA polymer (c) PLGA-PBA polymer and (d) PLGA@MTX nanoparticles (Reproduced from ref. 91 with permission from the Royal Society of Chemistry). | ||
Analysis of the nano-carrier through all these characterization procedures makes a complete vision about the complete characteristics, which helps determine their mode of effect in further application phases and their limitations.
5 Application of PLGA nanoparticles in cancer
Cancer is one of the fast-growing burdens and also the second leading cause of death around the world.141 The International Agency for Research on Cancer (IARC) is a part of the world health organization (WHO), which provides an estimation of the global pattern of cancer incidence and mortality in 185 countries for 36 types of cancer in the Globocan database.142 According to their report in 2018, new cancer cases were 18.1 million, and deaths were 9.6 million, which has risen to 19.3 million new cancer cases and 10 million cancer deaths in 2020 worldwide. They also predict that new cancer cases will be 29.4 million in 2040.143–145 According to Globocan 2020, one in five people develop cancer during their lifetime; among them, one in eight men and one in eleven women die due to cancer globally.144 From the mid of 20th century, typical therapeutic procedures for this lethal disease are radiotherapy, chemotherapy, and surgery.146,147 In spite of good response, these treatment strategies face diverse challenges such as (i) in radiation therapy, X-ray and γ-ray cause adverse side effects by damaging the nearby healthy tissues, (ii) inadequate drug concentration due to instability in systemic circulation and multi-drug resistance of conventional chemotherapeutic drugs force to increase the therapeutic dose to get an effective concentration of the drug at the disease site that results in adverse side effects, (iii) non-specific bio-distribution produce systemic toxicity, and (iv) limited scope to monitor the administrated drug and the treatment response.148–150 The recent nano-carrier approach of chemotherapy is a revolutionary step that overwhelms the limitations of the usual chemotherapeutic treatment strategy to encounter the increasing rate of cancer.151–153 Since the last three decades, the application of nanoparticles has been rapidly evolving as a compact platform for efficient drug delivery and monitoring system, along with an increasing understanding of the interaction with the complex biological organization and tumor microenvironment.146,1475.1 Cellular imaging capabilities
Accurate analysis of tumor margin is fundamental for effective therapeutic assessment in pre-treatment and intra-treatment to avoid treatment failure and recurrence of cancer. Xu et al. Developed PLGA nanobubble for intraoperative real-time cancer imaging by the fluorescence and ultrasound method simultaneously. They conjugated HuCC49DCH2 antibody with Texas Red fluorescence dye encapsulated PLGA nanobubble for one-step binding with TAG-72 antigen overexpressed on LS174T human colon cancer cell line. For three-step targeting, biotin conjugated HuCC49DCH2 antibody, streptavidin, and Texas Red encapsulated biotin conjugated PLGA nanobubble were successively applied to the LS174T cells for more accurate targeting.154 CT and MRI are the common imaging modalities clinically used for the diagnosis and evaluation of therapeutic efficacy. Nowadays, inorganic nanocrystals are frequently used for molecular imaging methods with more specific detection by a high payload of contrast agents. Mieszawska et al. used electron-dense gold nanocrystals (AuNCs), manifesting high X-ray attenuation coefficient and quantum dots (QDs) emitting light in the near infra-red region to integrate with lipid coated PLGA nanoparticles for tunable bio-imaging by computed tomography and fluorescence with high quantum yield.155 Likewise, for detecting malignancies and monitoring the effects of therapeutic agents using MRI, Mariano et al. synthesized a novel, highly sensitive MRI contrast agent by the entrapment of amphiphilic gadolinium(III) complex (Gd-DOTAMA) in PLGA nanoparticles. The hydrophobic steric chains are confined inside the PLGA core and the hydrophilic Gd coordination cage is partially exposed on the nanoparticle surface, which enhanced the longitudinal relaxation rate in contact with an aqueous solvent that facilitates higher sensitivity in MRI visualization in murine melanoma xenograft.156 The traveling path of the administrated nanoparticles by real-time fluorescence tracking provides excellent control over the therapeutic action and immediate response. To fulfill this purpose, Wang et al. prepared a multifunctional PLGA based nano-platform (PEI-PLGA-PTX-MNPs) by simultaneous encapsulation of supermagnetic γ-Fe2O3 nanoparticles (MNPs) and antitumor drug paclitaxel (PTX) inside its core. They also labeled that nanoplatform with polyethyleneimine (PEI)-conjugated fluorescein isothiocyanate (FITC), which is finally applied on human brain glioblastoma U251 cells to study cellular imaging and drug delivery efficacy.1575.2 Drug delivery abilities
PLGA nanoparticles are enormously investigated as a highly effective alternative for the efficient delivery of active therapeutic agents at cancerous tissues by minimizing systemic toxicity. The release rate of anti-proliferative drugs with a high therapeutic payload at the target site from PLGA nano-carriers can be controlled by various formulation processes. The hydrophobic core of PLGA nanoparticles may accommodate various types of drug molecules to transport in a protective manner by prolonging the systemic circulation, which increases the intratumoral accumulation of active therapeutics. One of the major focuses in developing nano-drug delivery systems is to make them target specific to avoid non-specific distribution by conjugating a specific targeting ligand that can strongly bind with the molecules expressed explicitly on the cancerous tissues. The ability of PLGA nano-carriers to cross the leaky tumor vasculature by the EPR effect facilitates passive accumulation at the tumor location. Many research groups are continuously trying to overcome the present treatment challenges by improving the drug delivery process for clinical translation from bench to bedside. Amoozgar et al. developed low molecular-weight chitosan (LMWC) coated PLGA nanoparticles from PLGA-LMWC conjugate, facilitating pH-sensitive cell interaction and encapsulated paclitaxel release inside the weak acidic tumor microenvironment by electrostatic interactions with glycocalyx of the cell membrane. The LMWC surface coating also protects them in systemic circulation from opsonization and phagocytic uptake at neutral pH.158 Devulapally et al. put their effort to conquer the limitations like tumor recurrence and metastasis of the existing treatment strategy of hepatocellular carcinoma (HCC). The reasons behind the above limitations are intrinsic and acquired drug resistance and low permeability of drugs. They formulated a new drug delivery carrier by the co-encapsulation of antisense-miRNA-21 and gemcitabine (GEM) inside PEGylated-PLGA nanoparticles enhancing the treatment efficacy. The antisense-miRNA-21 downregulates endogenous miRNA-21, which in turn increases the expression of tumor suppressor protein (PTEN) that significantly decreases cell proliferation and increases cytotoxicity in combination with chemotherapeutic drug GEM on the HCC cells.159 Multi-drug resistant cancer cells show their drug resistance by the efflux of the potent chemotherapeutic drugs like doxorubicin, docetaxel, and paclitaxel. Wu et al. synthesized a novel biocompatible functional surfactant based on amphiphilic vitamin E-oligo(methyl diglycol L-glutamate) (VEOEG) that can inhibit drug efflux and with prolonged blood circulation time in an in vivo system. VEOEG surfactant coated and paclitaxel (PTX) loaded PLGA nanoparticle was conjugated with hyaluronic acid (PTX-HA-PLGA NPs), specifically targeting CD44 receptor overexpressed on various cancer cells. These PTX-HA-PLGA NPs exhibited excellent stability without any burst release of PTX with increased plasma half-life in in vivo.160 To achieve the most critical aspects of the therapeutic delivery vehicle, such as colloidal stability, high therapeutic payload, and controlled drug release at the target site, Wang et al. synthesized doxorubicin (DOX) encapsulated bio-responsive multifunctional PLGA nanoparticles from reversible crosslinking of lipoic acid modified star PLGA polymer. Specific release of doxorubicin from those nanoparticles in the intracellular reductive environment of cancer cells improved accumulation inside the tumor and reduced systemic side effects, which significantly enhanced cancer chemotherapeutic efficacy.161The research works carried out in the last decade on PLGA nano-carriers and their applications in cancer therapy are discussed in Table 4.
| Nanoparticle formulation | LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GA and Type of imaging/drug molecules GA and Type of imaging/drug molecules |
Synthesis method | Size (nm) and encapsulation efficiency | Application on and administration route | Ref. |
|---|---|---|---|---|---|
| Poly(L-lysine)-poly(ethylene glycol)-folate (PLL-PEG-FOL) adsorbed Fe3O4 or CdSe/ZnS and DOXO encapsulated PLGA nanoparticle | Fe3O4 nanocrystals, CdSe/ZnS nanocrystals and Doxorubicin (DOXO) | Single-emulsion solvent evaporation | 100–200;— | KB cancer cells for MR and optical imaging and drug delivery | 162 |
| VCR and VRP encapsulated PLGA nanoparticle | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25; Vincristine (VCR), and verapamil (VRP) 25; Vincristine (VCR), and verapamil (VRP) |
Combining single emulsion solvent evaporation and salting-out method | 98.8 ± 8.4; 67.86 ± 5.10% for VCR and 80.29 ± 4.55% for VRP | Multidrug resistant breast cancer cells (MCF-7/ADR) for drug delivery | 163 |
| POSS-containing conjugated polymer (CP) loaded and Herceptin conjugated PLGA nanoparticle | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; Polyhedral oligomeric silsesquioxanes (POSS)-containing conjugated polymer (CP) 50; Polyhedral oligomeric silsesquioxanes (POSS)-containing conjugated polymer (CP) |
Single-emulsion solvent evaporation | 230 ± 3–243 ± 6; 44% | Breast cancer cells (SKBR-3, MCF-7) for optical imaging | 164 |
| Herceptin conjugated, Hydrophobic and hydrophilic drugs encapsulated, MNP embedded PLGA nanoparticle | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35; Magnetic nanoparticles (MNP), paclitaxel (Pac) + rapamycin (Rapa) and paclitaxel (Pac) + carboplatin (Carbo) 35; Magnetic nanoparticles (MNP), paclitaxel (Pac) + rapamycin (Rapa) and paclitaxel (Pac) + carboplatin (Carbo) |
Double emulsion solvent evaporation | 304 ± 4.1; Pac (80.6 ± 2.7%) + Rapa (86.6 ± 3.1%) and 310 ± 3.9; Pac (83.5 ± 3.0%) + Carbo (47.8 ± 1.5%) | Breast cancer cells (MCF-7), pancreatic cancer cells (PANC-1) and rat model for MR imaging and drug delivery; administered through saphenous vein | 165 |
| AS1411 aptamer conjugated and paclitaxel-loaded PLGA nanoparticle | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; Paclitaxel 50; Paclitaxel |
Single-emulsion solvent evaporation | 200; — | Human glial cancer cells (GI-1 cells) for drug delivery | 166 |
| Curcumin and bortezomib co-encapsulated and alendronate (Aln). conjugated PLGA nanoparticles | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; Curcumin and bortezomib 50; Curcumin and bortezomib |
Single-emulsion solvent evaporation | 235 ± 70.30; — | Intraosseous mice model of bone metastasis of breast cancer for drug delivery; administered through tail vein | 167 |
| Cyclic peptide (cRGD)-modified monomethoxy (polyethylene glycol)-PLGA-poly (L-lysine) nanoparticle encapsulated either DHAQ or Rb | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; Mitoxantrone (DHAQ) or rhodamine B (Rb) 50; Mitoxantrone (DHAQ) or rhodamine B (Rb) |
Double emulsion solvent evaporation | 180; 85.3% | Breast cancer cells (MDA-MB-231) for optical imaging or drug delivery | 168 |
| siRNA encapsulated and lipid coated PLGA nanoparticle | Small interfering RNAs (siRNA) | 207 ± 4.461; 46% | Human cervical (HeLa), prostate (PC3, DU145, LNCaP) and liver (HepG2) cancer cells for siRNA delivery | 85 | |
| Curcumin loaded PLGA nanoparticles | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; Curcumin 50; Curcumin |
Single-emulsion solvent evaporation | 120; 80% | Breast cancer cells (MCF7) for drug delivery | 169 |
| DOX and VER combined chitosan shell coated MNPs encapsulated and cRGD peptide functionalized PLGA nanoparticle. | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; Doxorubicin (DOX), verapamil (VER) and magnetic nanoparticles (MNPs) 50; Doxorubicin (DOX), verapamil (VER) and magnetic nanoparticles (MNPs) |
Double emulsion solvent evaporation | 144; 74.8% for DOX and 53.2% for VER | Male S-180 sarcoma-bearing mice for drug delivery; administered through tail vein | 170 |
| Holo-transferrin conjugated and bortezomib-loaded PLGA nanoparticle | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; Bortezomib (BTZ) 50; Bortezomib (BTZ) |
Single-emulsion solvent evaporation | 200; 53 ± 6% | Human pancreatic cancer cells (SUIT-2) for drug delivery | 171 |
| DOX encapsulated and low-molecular-weight protamine-surface modified PLGA nanoparticle | Doxorubicin (DOX) | Nanoprecipitation | 206.2; 83% | Mice harboring drug-resistant breast tumors for drug delivery; administered through tail vein | 172 |
| Tam encapsulated herceptin conjugated polyvinyl-pyrrolidone coated PLGA nanoparticle | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; Tamoxifen (Tam) 50; Tamoxifen (Tam) |
Double emulsion solvent evaporation | 93.44; 72.4 ± 2.3% | Human breast cancer cells (MCF-7) for drug delivery | 173 |
| BSA-Gd complexes and DOX encapsulated and poly(ethylene glycol) conjugated PLGA nanoparticle | Bovine serum albumin gadolinium (BSA-Gd) complexes and Doxorubicin (DOX) | Single-emulsion solvent evaporation | 280; 20.9% | Human cervical cancer cells (HeLa) and female nude mice bearing tumor for MR imaging and drug delivery; administered through tail vein | 174 |
| SPION, QDs and the anticancer drug busulfan encapsulated PLGA nanoparticle | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; Superparamagnetic iron oxide nanoparticles (SPION), manganese-doped zinc sulfide (Mn 50; Superparamagnetic iron oxide nanoparticles (SPION), manganese-doped zinc sulfide (Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnS) quantum dots (QDs) and busulfan ZnS) quantum dots (QDs) and busulfan |
Single-emulsion solvent evaporation | 93; 89 ± 2% | Murine macrophage cells (J774A) and rat model for MR and optical imaging; administered through intravenous mode | 175 |
| Antisense-miR-21 and antisense-miR-10b co-loaded urokinase plasminogen activator receptor (uPAR) conjugated PLGA nanoparticle | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; Antisense-miR-21 and antisense-miR-10b 50; Antisense-miR-21 and antisense-miR-10b |
Double emulsion solvent evaporation | 100 to 200; 72.4 ± 6.2% | Triple negative breast cancer (TNBC) cells and TNBC tumor xenografts in nude mice for antisense-miRNAs delivery; administered through intravenous mode | 176 |
| Nutlin-3a loaded EpCAM aptamer and quantum dots conjugated PLGA nanoparticle | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; Nutlin-3a 50; Nutlin-3a |
Single-emulsion solvent evaporation | 292 ± 10; 51.24 ± 6.7% | Human breast cancer cells (MCF-7 and ZR751) and ovarian cancer cells (SKOV3) for fluorescence imaging and drug delivery | 177 |
| T7-peptide conjugate, MNPs, PTX and CUR co-encapsulated PLGA nanoparticle | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; Iron oxide nanoparticles (MNPs), paclitaxel (PTX) and curcumin (CUR) 50; Iron oxide nanoparticles (MNPs), paclitaxel (PTX) and curcumin (CUR) |
Single-emulsion solvent evaporation | 130; 68% for PTX and 18% for CUR | Human malignant glioma (U87) and mice bearing orthotopic glioma (U87-Luc) for drug delivery and MRI; administered through tail vein | 178 |
| DOX-loaded lipid hybrid PLGA nanoparticles | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25; Doxorubicin (DOX) 25; Doxorubicin (DOX) |
Double emulsion solvent evaporation | 198 ± 12; 86.4 ± 8.5% | Human breast cancer cells (MDA-MB-231/ADR) and human squamous carcinoma cells (KB) for drug delivery | 179 |
| Superparamagnetic iron oxide (SPIO3 NPs4) loaded PLGA nanospheres | Oleic acid-coated superparamagnetic iron oxide (SPIO3 NPs4) | Multiple emulsion solvent evaporation method | 130; 90.2 ± 0.3% | T1-weighted MRI scans of C26 colon carcinoma xenograft model; administered through tail vein | 180 |
| Rhodamine B encapsulated PLGA nanoparticles | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; Rhodamine B 50; Rhodamine B |
Dewetting technique | 80; 93.26% | Human A549lung cancer cells for fluorescence imaging | 181 |
| AS1411 aptamer conjugated curcumin and SPIONs encapsulated PLGA nanocapsule | Curcumin and superparamagnetic iron oxide nanoparticles (SPIONs) | Nanoprecipitation | 150; — | Pancreatic cancer cells (PANC-1 and MIA PaCa-2) for optical, MRI, and photoacoustic imaging | 182 |
| CN-PPV and NIR dye encapsulate PLGA nanoparticle | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; Red-emitting conjugated polymer (CN-PPV) and near-infrared dye (NIR) 50; Red-emitting conjugated polymer (CN-PPV) and near-infrared dye (NIR) |
Single-emulsion solvent evaporation | 50; — | Cervical cancer cells (HeLa) for optical imaging | 183 |
| Doxorubicin encapsulated and Cy5.5 labeled PLGA nanoparticle | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; Doxorubicin, Cy5.5 50; Doxorubicin, Cy5.5 |
Double emulsion solvent evaporation | 114; ∼80% | U87 human glioma cell line for optical imaging and drug delivery | 184 |
| Polyethyleneimine-polyethylene glycol-folic acid functionalized quantum dots, Fe3O4 nanocrystals, and doxorubicin (DOX) encapsulated and (shRNA) adsorbed PLGA nanocomposites | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; CdSe/ZnS quantum dots, superparamagnetic Fe3O4 nanocrystals, Doxorubicin (DOX) and vascular endothelial growth factor (VEGF)-targeted small hairpin RNA (shRNA) 50; CdSe/ZnS quantum dots, superparamagnetic Fe3O4 nanocrystals, Doxorubicin (DOX) and vascular endothelial growth factor (VEGF)-targeted small hairpin RNA (shRNA) |
Double emulsion solvent evaporation | ∼300 nm; ∼62.97% for DOX | Cervical cancer cells (HeLa) and subcutaneous EMT-6 tumor xenograft mice model for MR and fluorescence imaging and drug delivery; Administered through intratumoral injection | 185 |
| Curcumin encapsulated PLGA nanoparticle | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; Curcumin 50; Curcumin |
Microfluidic | 30–70; 67% | Leukemia Jurkat cells for drug delivery | 186 |
| Chitosan and PEG-coated curcumin-loaded PLGA nanoparticles (CNPs) | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; Curcumin 50; Curcumin |
Single-emulsion solvent evaporation | 264 nm; 60% | Human pancreatic cancer cell lines PANC-1 and Mia Paca-2 for drug delivery | 187 |
| OX26 type monoclonal antibody functionalized TMZ encapsulated PLGA nanoparticle | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; Temozolomide (TMZ) 50; Temozolomide (TMZ) |
Single-emulsion solvent evaporation | 176; 48 ± 10% | Glioblastoma cells (U215 and U87) for drug delivery | 188 |
| DOX encapsulated and Au nanoparticle decorated PLGA nanoparticle | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; Doxorubicin (DOX) 50; Doxorubicin (DOX) |
Double emulsion solvent evaporation | ∼160; — | Mouse colon cancer cells (CT26), Murine breast cancer cells (4T1) and mice bearing 4T1 tumors for photoacoustic imaging and drug delivery; administered through intravenous mode | 189 |
| Transferrin decorated paclitaxel and elacridar co-encapsulated PLGA nanoparticle | Transferrin and elacridar | Nanoprecipitation | 226.9; 76% | Drug-resistant breast cancer cells (EMT6/AR1.0) for drug delivery | 190 |
| MTX and CUR co-encapsulated PLGA nanoparticle | Methotrexate (MTX) and curcumin (CUR) | Double emulsion solvent evaporation | 142.3 ± 4.07; MTX 71.32 ± 7.8% and CUR 85.64 ± 6.3% | Breast cancer cells SK-Br-3 cell line and chemically induced mammary tumors in female Sprague Dawley rats for drug delivery; administered through intravenous mode | 191 |
| iRGD conjugated and PTX-loaded PLGA nanoparticle | Paclitaxel (PTX) | Single-emulsion solvent evaporation | 147.5 ± 9.5; 88.2% | Human colorectal cancer cells (LS174T, COLO205, HCT116, and SW620) and LS174T tumor-bearing BALB/c (nu/nu) mice for drug delivery; Administered through intravenous mode | 192 |
| Curcumin and Niclosamide encapsulated PLGA nanoparticle | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; Curcumin and niclosamide 50; Curcumin and niclosamide |
Nanoprecipitation | 225.9; 58.09% for Curcumin and 85.36% for Niclosamide | Breast cancer cells (MDA-MB-231) for drug delivery | 193 |
| Epidermal growth factor functionalized 5Fu and perfluorocarbon (PFC) co-loaded PLGA nanoparticle | 5-Fluorouracil (5Fu) | Double emulsion solvent evaporation | 200; 81.6 ± 5.7% | Human colon cancer cells (SW620) for drug delivery | 194 |
| Cannabidiol (CBD) loaded PLGA nanoparticle | Cannabidiol (CBD) | Single-emulsion solvent evaporation | 240; 95% | Epithelial ovarian cancer cells (SKOV-3) for drug delivery | 195 |
| Sal and Tam encapsulated (PEG)–PLGA nanoparticle | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25; Salidroside (Sal) and Tamoxifen (Tam) 25; Salidroside (Sal) and Tamoxifen (Tam) |
Double emulsion solvent evaporation | 275.3 ± 44.0; Sal 32.63% ± 0.73% and Tam 49.18% ± 3.04% | Mouse breast cancer cell line (4T1) and female BALB/c mice for drug delivery; administered through intraperitoneal mode | 196 |
| Tg conjugated PLGA nanoparticle | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; 6-Thioguanine (Tg) 50; 6-Thioguanine (Tg) |
Electrospray | ∼60 nm; 97.22% | Cervical cancer cells (HeLa) for drug delivery and fluorescence imaging | 90 |
| PBA conjugated and MTX decorated PLGA nanoparticle | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; 1-pyrenebutyric acid (PBA) and methotrexate (MTX) 50; 1-pyrenebutyric acid (PBA) and methotrexate (MTX) |
Electrospray | ∼105 nm 91.4% | MTX resistant metastatic breast cancer cells (MCF-7 and MDA-MB-231) for fluorescence imaging and drug delivery | 91 |
| Molybdenum octahedral clusters encapsulated PLGA nanoparticle | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; Molybdenum octahedral clusters 50; Molybdenum octahedral clusters |
Single-emulsion solvent evaporation | 75.7–144.7; 29.2–73.9% | Ovarian cancer cell line (A2780) for photodynamic therapy (PDT) | 197 |
| PNAs encapsulated PLGA nanoparticle | Short cationic peptide nucleic acids (PNAs) | Double emulsion solvent evaporation | 145; — | HeLa, A549, HEK-293, SUDHL-5, U2932 cell line and xenograft mouse model for drug delivery; administered through tail vein | 198 |
6 Current limitations and future perspectives
After surveying the literature, it is found that there are some limitations in the reported PLGA nanoparticle synthesis. Most of the methods employ more than one solvent phase with stabilizer/cross-linker and high shear stress for a prolonged time, resulting in drug loss, agglomeration, and polydispersity in the nanoparticle size. Nevertheless, some residual solvents may remain after several washes, imparting undesirable toxic effects during the application phase. Moreover, during drug delivery, it is necessary to track the traveling pathway of nanoparticles. Therefore, stable imaging property with high intensity imaging is essential for monitoring the drug delivery vehicle after it is administered. Incorporating therapeutic and imaging molecules in a single nano-platform requires an environment in which both of them would not lose their molecular properties by interacting with each other. Thus, there are considerable challenges to encapsulate both molecules in a single particle such as (a) ionic property and hydrophobicity of both molecules may precipitate out one of them in a single solvent phase, (b) encapsulation efficiency of the drug may reduce, (c) imaging molecules may also release from the nanoparticles, which would result in misleading of the tracking of nanoparticles. To overcome these limitations, a fabrication process is required, which should be facile, efficient, and tuneable for achieving different integral properties like narrow size distribution, sustained-release kinetics, and capability to deliver different imaging/drug molecules by a single carrier. This requires developing an automated PLGA fabrication setup to synthesize nanoparticles with different molecules in a pre-programmed way that could save time and effort for large-scale synthesis.There is a vast research scope to continue this exploration with modern drug molecules that can be conveyed using PLGA nano-carriers and unveil their applications beyond the cellular model. Their behavior may be studied in in vitro 3D tumor spheroid, which mimics the complex in vivo tumor vasculature on a benchtop. It would be beneficial for the real-time study of drug delivery and imaging efficacy of nano-carriers. This type of study may also provide information regarding tumor vasculature penetration capability, size reducing efficiency of the tumor, cytotoxic efficiency, and monitoring ability of the therapeutic responses. Finally, the nano-carrier efficiency may be studied in clinical trials to understand better the effectiveness as a theranostic nano-system in cancer management.
7 Conclusion
The nanoparticle is becoming a promising alternative to conventional chemotherapeutic drugs as they cover-up all the limitations that reduce the success rate of the chemotherapeutic treatment. Nevertheless, it also appears to be an excellent tool for monitoring the diseased area and treatment responses. For this purpose, polymeric nanoparticles have gained huge interest due to their flexibility in synthesis, chemical modification, and drug release kinetics. The majority of the polymeric nanoparticles are fabricated using numerous biocompatible polymers to reduce the undesirable systemic toxicity of the drug transporter. Among various polymers, FDA and EMA approved biodegradable PLGA polymer is the most widely used as a versatile and clinically proven polymer for the synthesis of efficient nano-carrier for biomedical applications. An equal ratio of lactic acid and glycolic acid containing PLGA chain with low molecular weight is predominantly used to synthesize the nano-carrier system as it manifests optimum hydrolysis rate compared to other monomeric ratio compositions in PLGA chains. PLGA nano-carrier can be synthesized through a wide range of techniques. It has better stability, allows easy modification, and provides control over the drug release rate. The existing nanoparticle synthesis techniques face some challenges and that might compromise the therapeutic efficiency of the nano-carrier. Among all the techniques, the electrospray method is comparatively newer, convenient, and effective against most of the existing limitations. For active delivery of drug/imaging molecules, functionalization steps are used for covalent and noncovalent modifications of the polymer before or after synthesis of the nanoparticle. There is some lack of long term stability and constant intensity of the monitoring agents as they are simply encapsulated inside the particles. For stable monitoring, the inherent imaging quality of theranostic nanoparticles is necessary to study the real time effect of the delivery agents.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the director of CSIR-CMERI, Durgapur, and the director of NIT Durgapur for their support and encouragement. The authors are thankful to Dr Nibedita Mahata for her help and support. The authors also thank CSIR for its financial support from 12th FYP project no. ESC0112.References
- R. Krukemeyer, M. G. Krenn, V. Huebner, F. Wagner and W. Resch, J. Nanomed. Nanotechnol., 2015, 6(6), 336 Search PubMed.
- S. Bayda, M. Adeel, T. Tuccinardi, M. Cordani and F. Rizzolio, Molecules, 2020, 25, 1–15 Search PubMed.
- A. P. Ramos, M. A. E. Cruz, C. B. Tovani and P. Ciancaglini, Biophys. Rev., 2017, 9, 79–89 CrossRef CAS PubMed.
- H. A. Khan, M. K. Sakharkar, A. Nayak, U. Kishore and A. Khan, Nanoparticles for biomedical applications: An overview, Elsevier Ltd., 2018 Search PubMed.
- R. Shukla, N. Chanda, A. Zambre, A. Upendran, K. Katti, R. R. Kulkarni, S. K. Nune, S. W. Casteel, C. J. Smith, J. Vimal, E. Boote, J. D. Robertson, P. Kan, H. Engelbrecht, L. D. Watkinson, T. L. Carmack, J. R. Lever, C. S. Cutler, C. Caldwell, R. Kannan and K. V. Katti, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 12426–12431 CrossRef CAS PubMed.
- P. Christian, F. Von Der Kammer, M. Baalousha and T. Hofmann, Ecotoxicology, 2008, 17, 326–343 CrossRef CAS PubMed.
- M. A. Gatoo, S. Naseem, M. Y. Arfat, A. Mahmood Dar, K. Qasim and S. Zubair, Biomed Res. Int., 2014, 498420 Search PubMed.
- P. Kumbhakar, S. S. Ray and A. L. Stepanov, J. Nanomater., 2014, 2014, 2–4 Search PubMed.
- H. Zhu, E. Prince, P. Narayanan, K. Liu, Z. Nie and E. Kumacheva, Chem. Commun., 2020, 56, 8131–8134 RSC.
- D. Suresh, A. Zambre, N. Chanda, T. J. Hoffman, C. J. Smith, J. D. Robertson and R. Kannan, Bioconjugate Chem., 2014, 25, 1565–1579 CrossRef CAS PubMed.
- L. Zhang, F. X. Gu, J. M. Chan, A. Z. Wang, R. S. Langer and O. C. Farokhzad, Clin. Pharmacol. Ther., 2008, 83, 761–769 CrossRef CAS PubMed.
- R. Wang, P. S. Billone and W. M. Mullett, J. Coast. Life Med., 2013, 629681 Search PubMed.
- V. Weissig, T. K. Pettinger and N. Murdock, Int. J. Nanomed., 2014, 9, 4357–4373 CrossRef CAS PubMed.
- Y. H. Choi and H. K. Han, J. Pharm. Invest., 2018, 48, 43–60 CrossRef CAS PubMed.
- C. L. Ventola, Pharmacol. Ther., 2017, 42, 742–755 Search PubMed.
- A. Zambre, N. Chanda, S. Prayaga, R. Almudhafar, Z. Afrasiabi, A. Upendran and R. Kannan, Anal. Chem., 2012, 84, 9478–9484 CrossRef CAS PubMed.
- B. Blasiak, F. C. J. M. Van Veggel and B. Tomanek, J. Nanomater., 2013, 148578 Search PubMed.
- H. Bin Na, I. C. Song and T. Hyeon, Adv. Mater., 2009, 21, 2133–2148 CrossRef.
- Y. Liu, K. Ai, J. Liu, Q. Yuan, Y. He and L. Lu, Adv. Healthcare Mater., 2012, 1, 461–466 CrossRef CAS PubMed.
- E. Kang, H. S. Min, J. Lee, M. H. Han, H. J. Ahn, I. C. Yoon, K. Choi, K. Kim, K. Park and I. C. Kwon, Angew. Chem., Int. Ed., 2010, 49, 524–528 CrossRef CAS PubMed.
- X. Cai, W. Li, C. Kim, Y. Yuan, L. V. Wang and Y. Xia, ACS Nano, 2011, 5, 9658–9667 CrossRef CAS PubMed.
- H. Xie, Z. J. Wang, A. Bao, B. Goins and W. T. Phillips, Int. J. Pharm., 2010, 395, 324–330 CrossRef CAS PubMed.
- K. Kim, J. H. Kim, H. Park, Y. S. Kim, K. Park, H. Nam, S. Lee, J. H. Park, R. W. Park, I. S. Kim, K. Choi, S. Y. Kim, K. Park and I. C. Kwon, J. Controlled Release, 2010, 146, 219–227 CrossRef CAS PubMed.
- N. Chanda, A. Upendran, E. J. Boote, A. Zambre, S. Axiak, K. Selting, K. V. Katti, W. M. Leevy, Z. Afrasiabi, J. Vimal, J. Singh, J. C. Lattimer and R. Kannan, J. Biomed. Nanotechnol., 2014, 10, 383–392 CrossRef CAS PubMed.
- C. A. Wathen, C. Caldwell, N. Chanda, A. Upendran, A. Zambre, Z. Afrasiabi, S. E. Chapaman, N. Foje, W. M. Leevy and R. Kannan, Contrast Media Mol. Imaging, 2015, 10, 188–193 CrossRef CAS PubMed.
- Y. F. Li and C. Chen, Small, 2011, 7, 2965–2980 CrossRef CAS PubMed.
- X. D. Zhang, H. Y. Wu, D. Wu, Y. Y. Wang, J. H. Chang, Z. Bin Zhai, A. M. Meng, P. X. Liu, L. A. Zhang and F. Y. Fan, Int. J. Nanomed., 2010, 5, 771–781 CrossRef CAS PubMed.
- N. Larson and H. Ghandehari, Chem. Mater., 2012, 24, 840–853 CrossRef CAS PubMed.
- H. Maeda, T. Sawa and T. Konno, J. Controlled Release, 2001, 74, 47–61 CrossRef CAS PubMed.
- G. Pasut and F. M. Veronese, Prog. Polym. Sci., 2007, 32, 933–961 CrossRef CAS.
- Y. Shen, E. Jin, B. Zhang, C. J. Murphy, M. Sui, J. Zhao, J. Wang, J. Tang, M. Fan, E. Van Kirk and W. J. Murdoch, J. Am. Chem. Soc., 2010, 132, 4259–4265 CrossRef CAS PubMed.
- L. Dai, R. Liu, L. Q. Hu, Z. F. Zou and C. L. Si, ACS Sustainable Chem. Eng., 2017, 5, 8241–8249 CrossRef CAS.
- E. Calzoni, A. Cesaretti, A. Polchi, A. Di Michele, B. Tancini and C. Emiliani, J. Funct. Biomater., 2019, 10, 1–15 Search PubMed.
- M. Hirenkumar and S. Steven, Polymers, 2012, 3, 1–19 Search PubMed.
- P. Gentile, V. Chiono, I. Carmagnola and P. V. Hatton, Int. J. Mol. Sci., 2014, 15, 3640–3659 CrossRef CAS PubMed.
- F. Danhier, E. Ansorena, J. M. Silva, R. Coco, A. Le Breton and V. Préat, J. Controlled Release, 2012, 161, 505–522 CrossRef CAS PubMed.
- E. M. M. L. Houchin, TOPP, J. Pharm. Sci., 2007, 97, 2395–2404 CrossRef PubMed.
- A. Kumari, S. K. Yadav and S. C. Yadav, Colloids Surf., B, 2010, 75, 1–18 CrossRef CAS PubMed.
- E. Swider, O. Koshkina, J. Tel, L. J. Cruz, I. J. M. de Vries and M. Srinivas, Acta Biomater., 2018, 73, 38–51 CrossRef CAS PubMed.
- S. Rezvantalab, N. I. Drude, M. K. Moraveji, N. Güvener, E. K. Koons, Y. Shi, T. Lammers and F. Kiessling, Front. Pharmacol., 2018, 9, 1–19 CrossRef PubMed.
- M. Ayyoob and Y. J. Kim, Polymers, 2018, 10, 1–16 CrossRef PubMed.
- T. D. Farahani, A. A. Entezami, H. Mobedi and M. Abtahi, Iran. Polym. J., 2005, 14, 753–763 CAS.
- D. D. Von Hoff, M. M. Mita, R. K. Ramanathan, G. J. Weiss, A. C. Mita, P. M. Lorusso, H. A. Burris, L. L. Hart, S. C. Low, D. M. Parsons, S. E. Zale, J. M. Summa, H. Youssoufian and J. C. Sachdev, Clin. Cancer Res., 2016, 22, 3157–3163 CrossRef CAS PubMed.
- A. C. Anselmo and S. Mitragotri, Bioeng. Transl. Med., 2016, 1, 10–29 CrossRef PubMed.
- H. Zhong, G. Chan, Y. Hu, H. Hu and D. Ouyang, Pharmaceutics, 2018, 10, 1–19 CrossRef PubMed.
- K. Park, S. Skidmore, J. Hadar, J. Garner, H. Park, A. Otte, B. K. Soh, G. Yoon, D. Yu, Y. Yun, B. K. Lee, X. Jiang and Y. Wang, J. Controlled Release, 2019, 304, 125–134 CrossRef CAS PubMed.
- Y. Dang and J. Guan, Smart Mater. Med., 2020, 1, 10–19 CrossRef PubMed.
- J. Hadar, S. Skidmore, J. Garner, H. Park, K. Park, Y. Wang, B. Qin and X. Jiang, J. Controlled Release, 2019, 304, 75–89 CrossRef CAS PubMed.
- ELIGARD®, https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021731s005,021488s010,021379s010,021343s015lbl.pdf.
- SIGNIFOR LAR®, https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/203255s008lbl.pdf.
- J. Zhou, J. Walker, R. Ackermann, K. Olsen, J. K. Y. Hong, Y. Wang and S. P. Schwendeman, Mol. Pharm., 2020, 17, 1502–1515 CrossRef CAS PubMed.
- S. Pieper and K. Langer, Mater. Today Proc., 2017, 4, S188–S192 CrossRef.
- H. Y. Kwon, J. Y. Lee, S. W. Choi, Y. Jang and J. H. Kim, Colloids Surf., A, 2001, 182, 123–130 CrossRef CAS.
- C. E. Astete, C. S. S. R. Kumar and C. M. Sabliov, Colloids Surf., A, 2007, 299, 209–216 CrossRef CAS.
- D. Cun, D. K. Jensen, M. J. Maltesen, M. Bunker, P. Whiteside, D. Scurr, C. Foged and H. M. Nielsen, Eur. J. Pharm. Biopharm., 2011, 77, 26–35 CrossRef CAS PubMed.
- K. Kizilbey, ACS Omega, 2019, 4, 555–562 CrossRef CAS.
- M. J. Ramalho and M. C. Pereira, J. Chem. Educ., 2016, 93, 1446–1451 CrossRef CAS.
- K. Y. Hernández-Giottonini, R. J. Rodríguez-Córdova, C. A. Gutiérrez-Valenzuela, O. Peñuñuri-Miranda, P. Zavala-Rivera, P. Guerrero-Germán and A. Lucero-Acuña, RSC Adv., 2020, 10, 4218–4231 RSC.
- Y. Javadzadeh, F. Ahadi, S. Davaran, G. Mohammadi, A. Sabzevari and K. Adibkia, Colloids Surf., B, 2010, 81, 498–502 CrossRef CAS PubMed.
- R. Manchanda, A. Fernandez-Fernandez, A. Nagesetti and A. J. McGoron, Colloids Surf., B, 2010, 75, 260–267 CrossRef CAS PubMed.
- J. M. Barichello, M. Morishita, K. Takayama, T. Nagai, J. M. Barichello and M. Morishita, Drug Dev. Ind. Pharm., 1999, 25, 471–476 CrossRef CAS PubMed.
- G. Wang, B. Yu, Y. Wu, B. Huang, Y. Yuan and C. S. Liu, Int. J. Pharm., 2013, 446, 24–33 CrossRef CAS PubMed.
- W. S. Saad and R. K. Prud’Homme, Nano Today, 2016, 11, 212–227 CrossRef CAS.
- F. Lince, D. L. Marchisio and A. A. Barresi, J. Colloid Interface Sci., 2008, 322, 505–515 CrossRef CAS PubMed.
- C. G. Barreras-Urbina, B. Ramírez-Wong, G. A. López-Ahumada, S. E. Burruel-Ibarra, O. Martínez-Cruz, J. A. Tapia-Hernández and F. Rodríguez Félix, Int. J. Food Prop., 2016, 19, 1912–1923 CrossRef.
- M. S. Muthu, M. K. Rawat, A. Mishra and S. Singh, Nanomedicine, 2009, 5, 323–333 CrossRef CAS PubMed.
- C. Arpagaus, J. Pharm. Invest., 2019, 49, 405–426 CrossRef CAS.
- Y. Kohl, C. Kaiser, W. Bost, F. Stracke, M. Fournelle, C. Wischke, H. Thielecke, A. Lendlein, K. Kratz and R. Lemor, Nanomedicine, 2011, 7, 228–237 CrossRef CAS PubMed.
- D. M. K. Jensen, D. Cun, M. J. Maltesen, S. Frokjaer, H. M. Nielsen and C. Foged, J. Controlled Release, 2010, 142, 138–145 CrossRef PubMed.
- A. Panda, J. Meena, R. Katara and D. K. Majumdar, Pharm. Dev. Technol., 2016, 21, 43–53 CrossRef CAS PubMed.
- X. Li, N. Anton, C. Arpagaus, F. Belleteix and T. F. Vandamme, J. Controlled Release, 2010, 147, 304–310 CrossRef CAS PubMed.
- A. Sosnik and K. P. Seremeta, Adv. Colloid Interface Sci., 2015, 223, 40–54 CrossRef CAS PubMed.
- N. Mendoza-Munoz, D. Quintanar-Guerrero and E. Allemann, Recent Pat. Drug Delivery Formulation, 2012, 6, 236–249 CrossRef CAS PubMed.
- Y. N. Konan, R. Gurny and E. Allemann, Int. J. Pharm., 2002, 233, 239–252 CrossRef CAS PubMed.
- X. Song, Y. Zhao, W. Wu, Y. Bi, Z. Cai, Q. Chen, Y. Li and S. Hou, Int. J. Pharm., 2008, 350, 320–329 CrossRef CAS PubMed.
- M. L. T. Zweers, D. W. Grijpma, G. H. M. Engbers and J. Feijen, J. Biomed. Mater. Res., Part B, 2003, 66, 559–566 CrossRef PubMed.
- S. Rezvantalab and M. Keshavarz Moraveji, RSC Adv., 2019, 9, 2055–2072 RSC.
- L. L. Li, X. Li and H. Wang, Small Methods, 2017, 1, 1–9 CAS.
- J. Wang, W. Chen, J. Sun, C. Liu, Q. Yin, L. Zhang, Y. Xianyu, X. Shi, G. Hu and X. Jiang, Lab Chip, 2014, 14, 1673–1677 RSC.
- Z. Mahmoodi, J. Mohammadnejad, S. Razavi Bazaz, A. Abouei Mehrizi, M. A. Ghiass, M. Saidijam, R. Dinarvand, M. Ebrahimi Warkiani and M. Soleimani, Drug Delivery Transl. Res., 2019, 9, 707–720 CrossRef CAS PubMed.
- J. P. Rolland, B. W. Maynor, L. E. Euliss, A. E. Exner, G. M. Denison, J. M. Desimone, C. Hill, N. Carolina and C. Engineering, J. Am. Chem. Soc., 2005, 127, 10096–10100 CrossRef CAS PubMed.
- E. M. Enlow, J. C. Luft, M. E. Napier and J. M. Desimone, Nano Lett., 2011, 11, 808–813 CrossRef CAS PubMed.
- B. L. Banik, P. Fattahi and J. L. Brown, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2016, 8, 271–299 Search PubMed.
- D. Essa, P. P. D. Kondiah, Y. E. Choonara and V. Pillay, Front. Bioeng. Biotechnol., 2020, 8, 1–20 CrossRef PubMed.
- W. Hasan, K. Chu, A. Gullapalli, S. S. Dunn, M. Elizabeth, Φ. Enlow, J. C. Luft, S. Tian, M. E. Napier, P. D. Pohlhaus, J. P. Rolland, J. M. Desimone, U. States and N. York, Nano Lett., 2012, 12, 287–292 CrossRef CAS PubMed.
- K. S. Chu, W. Hasan, S. Rawal, M. D. Walsh, E. M. Enlow, J. C. Luft, A. S. Bridges, J. L. Kuijer, M. E. Napier, W. C. Zamboni and J. M. DeSimone, Nanomedicine, 2013, 9, 686–693 CrossRef CAS PubMed.
- J. A. Tapia-Hernández, P. I. Torres-Chávez, B. Ramírez-Wong, A. Rascón-Chu, M. Plascencia-Jatomea, C. G. Barreras-Urbina, N. A. Rangel-Vázquez and F. Rodríguez-Félix, J. Agric. Food Chem., 2015, 63, 4699–4707 CrossRef PubMed.
- D. N. Nguyen, C. Clasen and G. Van den Mooter, J. Pharm. Sci., 2016, 105, 2601–2620 CrossRef CAS PubMed.
- B. Almería, W. Deng, T. M. Fahmy and A. Gomez, J. Colloid Interface Sci., 2010, 343, 125–133 CrossRef PubMed.
- M. Chatterjee, N. Jaiswal, A. Hens, N. Mahata and N. Chanda, Mater. Sci. Eng., C, 2020, 114, 111029 CrossRef CAS PubMed.
- M. Chatterjee, R. Maity, S. Das, N. Mahata, B. Basu and N. Chanda, Mater. Adv., 2020, 1, 3033–3048 RSC.
- A. Juan, F. J. Cimas, I. Bravo, A. Pandiella, A. Ocaña and C. Alonso-Moreno, Pharmaceutics, 2020, 12, 1–20 CrossRef PubMed.
- J. Nicolas, S. Mura, D. Brambilla, N. Mackiewicz and P. Couvreur, Chem. Soc. Rev., 2013, 42, 1147–1235 RSC.
- W. X. Ren, J. Han, S. Uhm, Y. J. Jang, C. Kang, J. H. Kim and J. S. Kim, Chem. Commun., 2015, 51, 10403–10418 RSC.
- C. Akshay and J. Kun, J. Controlled Release, 2017, 245, 27–40 CrossRef PubMed.
- R. W. Sirianni, M. Q. Zheng, T. R. Patel, T. Shafbauer, J. Zhou, W. M. Saltzman, R. E. Carson and Y. Huang, Bioconjugate Chem., 2014, 25, 2157–2165 CrossRef CAS PubMed.
- N. Nakajima and Y. Ikada, Bioconjugate Chem., 1995, 6, 123–130 CrossRef CAS PubMed.
- Q. Yan, H. N. Zheng, C. Jiang, K. Li and S. J. Xiao, RSC Adv., 2015, 5, 69939–69947 RSC.
- J. V. Staros, R. W. Wright and D. M. Swingle, Anal. Biochem., 1986, 156, 220–222 CrossRef CAS PubMed.
- D. F. Detar and R. Silverstein, J. Am. Chem. Soc., 1966, 88, 1013–1019 CrossRef CAS.
- H. Shen, A. M. Jawaid and P. T. Snee, ACS Nano, 2009, 3, 915–923 CrossRef CAS PubMed.
- N. Jaiswal, A. Hens, M. Chatterjee, N. Mahata and N. Chanda, J. Colloid Interface Sci., 2019, 534, 122–130 CrossRef CAS PubMed.
- N. Zhang, C. Chittasupho, C. Duangrat, T. J. Siahaan and C. Berkland, Bioconjugate Chem., 2008, 19, 145–152 CrossRef CAS PubMed.
- N. Graf, D. R. Bielenberg, N. Kolishetti, C. Muus, J. Banyard, O. C. Farokhzad and S. J. Lippard, ACS Nano, 2012, 6, 4530–4539 CrossRef CAS PubMed.
- Y. Liu, K. Li, B. Liu and S. S. Feng, Biomaterials, 2010, 31, 9145–9155 CrossRef CAS PubMed.
- B. H. Northrop, S. H. Frayne and U. Choudhary, Polym. Chem., 2015, 6, 3415–3430 RSC.
- K. Renault, J. W. Fredy, P. Y. Renard and C. Sabot, Bioconjugate Chem., 2018, 29, 2497–2513 CrossRef CAS PubMed.
- L. Martínez-Jothar, S. Doulkeridou, R. M. Schiffelers, J. Sastre Torano, S. Oliveira, C. F. van Nostrum and W. E. Hennink, J. Controlled Release, 2018, 282, 101–109 CrossRef PubMed.
- Sulfhydryl-reactive Crosslinker Chemistry, https://www.thermofisher.com/in/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/sulfhydryl-reactive-crosslinker-chemistry.html.
- J. Su, Gels, 2018, 4, 72 CrossRef CAS PubMed.
- A. Vasconcelos, E. Vega, Y. Pérez, M. J. Gómara, M. L. García and I. Haro, Int. J. Nanomed., 2015, 10, 609–631 Search PubMed.
- P. Akkapeddi, S. A. Azizi, A. M. Freedy, P. M. S. D. Cal, P. M. P. Gois and G. J. L. Bernardes, Chem. Sci., 2016, 7, 2954–2963 RSC.
- G. D. Paka and C. Ramassamy, Mol. Pharm., 2017, 14, 93–106 CrossRef CAS PubMed.
- P. J. Kennedy, F. Sousa, D. Ferreira, C. Pereira, M. Nestor, C. Oliveira, P. L. Granja and B. Sarmento, Acta Biomater., 2018, 81, 208–218 CrossRef CAS PubMed.
- C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057–3064 CrossRef PubMed.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS PubMed.
- V. Hong, S. I. Presolski, C. Ma and M. G. Finn, Angew. Chem., Int. Ed., 2009, 48, 9879–9883 CrossRef CAS PubMed.
- J. Lu, M. Shi and M. S. Shoichet, Bioconjugate Chem., 2009, 20, 87–94 CrossRef CAS PubMed.
- E. Haldón, M. C. Nicasio and P. J. Pérez, Org. Biomol. Chem., 2015, 13, 9528–9550 RSC.
- C. Spiteri and J. E. Moses, Angew. Chem., Int. Ed., 2010, 49, 31–33 CrossRef CAS PubMed.
- F. Himo, T. Lovell, R. Hilgraf, V. V. Rostovtsev, L. Noodleman, K. B. Sharpless and V. V. Fokin, J. Am. Chem. Soc., 2005, 127, 210–216 CrossRef CAS PubMed.
- A. O. Saeed, J. P. Magnusson, E. Moradi, M. Soliman, W. Wang, S. Stolnik, K. J. Thurecht, S. M. Howdle and C. Alexander, Bioconjugate Chem., 2011, 22, 156–168 CrossRef CAS PubMed.
- Z. Zhou, A. Badkas, M. Stevenson, J. Y. Lee and Y. K. Leung, Int. J. Pharm., 2015, 487, 81–90 CrossRef CAS PubMed.
- C. Fonseca, S. Simões and R. Gaspar, J. Controlled Release, 2002, 83, 273–286 CrossRef CAS PubMed.
- C. E. Astete and C. M. Sabliov, J. Biomater. Sci., Polym. Ed., 2006, 17, 247–289 CrossRef CAS PubMed.
- W. Huang and C. Zhang, Biotechnol. J., 2018, 13, 1–19 Search PubMed.
- B. Zhang, P. Sai Lung, S. Zhao, Z. Chu, W. Chrzanowski and Q. Li, Sci. Rep., 2017, 7, 1–8 CrossRef PubMed.
- M. Chatterjee, A. Hens, K. Mahato, N. Jaiswal, N. Mahato, Nagahanumaiah and N. Chanda, J. Colloid Interface Sci., 2017, 506, 126–134 CrossRef CAS PubMed.
- D. H. Jo, J. H. Kim, T. G. Lee and J. H. Kim, Nanomedicine, 2015, 11, 1603–1611 CrossRef CAS PubMed.
- N. Ma, C. Ma, C. Li, T. Wang, Y. Tang, H. Wang, X. Mou, Z. Chen and N. He, J. Nanosci. Nanotechnol., 2013, 13, 6485–6498 CrossRef CAS PubMed.
- S. Prabha, G. Arya, R. Chandra, B. Ahmed and S. Nimesh, Artif. Cells, Nanomed., Biotechnol., 2016, 44, 83–91 CrossRef CAS PubMed.
- S. Honary and F. Zahir, Trop. J. Pharm. Res., 2013, 12, 265–273 Search PubMed.
- J. Sitterberg, A. Özcetin, C. Ehrhardt and U. Bakowsky, Eur. J. Pharm. Biopharm., 2010, 74, 2–13 CrossRef CAS PubMed.
- R. Carvalho Silva, L. Alexandre Muehlmann, J. Rodrigues Da Silva, R. de Bentes Azevedo and C. Madeira Lucci, Microsc. Res. Tech., 2014, 77, 691–696 CrossRef CAS PubMed.
- S. Spek, M. Haeuser, M. M. Schaefer and K. Langer, Appl. Surf. Sci., 2015, 347, 378–385 CrossRef CAS.
- A. Romani, C. Clementi, C. Miliani and G. Favaro, Acc. Chem. Res., 2010, 43, 837–846 CrossRef CAS PubMed.
- H. Freichels, F. Danhier, V. Préat, P. Lecomte and C. Jérôme, Int. J. Artif. Organs, 2011, 34, 152–160 CrossRef CAS PubMed.
- L. M. do, A. C. Gaspar, A. C. S. Dórea, D. Droppa-Almeida, I. S. de Mélo Silva, F. E. Montoro, L. L. Alves, M. L. H. Macedo and F. F. Padilha, J. Nanoparticle Res., 2018, 20, 289 CrossRef.
- S. F. Peng, C. Y. Lee, M. J. Hour, S. C. Tsai, D. H. Kuo, F. A. Chen, P. C. Shieh and J. S. Yang, Int. J. Oncol., 2014, 44, 238–246 CrossRef CAS PubMed.
- C. S. S. R. Kumar, Raman spectroscopy for nanomaterials characterization, 2012, vol. 9783642206 Search PubMed.
- Cancer, https://www.who.int/news-room/fact-sheets/detail/cancer.
- No Title, https://www.iarc.fr/featured-news/latest-global-cancer-data-cancer-burden-rises-to-19-3-million-new-cases-and-10-0-million-cancer-deaths-in-2020/.
- Global Cancer Data: GLOBOCAN 2018, https://www.uicc.org/news/new-global-cancer-data-globocan-2018.
- GLOBOCAN 2020: New Global Cancer Data, https://www.uicc.org/news/globocan-2020-new-cancer-data#.
- V. Hanf and R. Kreienberg, WHO report on cancer: setting priorities, investing wisely and providing care for all, 2003 Search PubMed.
- M. Arruebo, N. Vilaboa, B. Sáez-Gutierrez, J. Lambea, A. Tres, M. Valladares and Á. González-Fernández, Cancers, 2011, 3, 3279–3330 CrossRef CAS PubMed.
- L. Salvioni, M. A. Rizzuto, J. A. Bertolini, L. Pandolfi, M. Colombo and D. Prosperi, Cancers, 2019, 11(12), 1855 CrossRef CAS PubMed.
- G. Bor, I. D. M. Azmi and A. Yaghmur, Ther. Delivery, 2019, 10, 113–132 CrossRef CAS PubMed.
- K. H. Bae, H. J. Chung and T. G. Park, Mol. Cells, 2011, 31, 295–302 CrossRef CAS PubMed.
- X. Wang, L. Yang, Z. Chen and D. M. Shin, CA-Cancer J. Clin., 2008, 58, 97–110 CrossRef PubMed.
- L. Smith, Z. Kuncic, K. Ostrikov and S. Kumar, J. Nanomater., 2012, 2012, 891318 Search PubMed.
- S. Chapman, M. Dobrovolskaia, K. Farahani, A. Goodwin, A. Joshi, H. Lee, T. Meade, M. Pomper, K. Ptak, J. Rao, R. Singh, S. Sridhar, S. Stern, A. Wang, J. B. Weaver, G. Woloschak and L. Yang, Nano Today, 2013, 8, 454–460 CrossRef CAS PubMed.
- J. Zugazagoitia, C. Guedes, S. Ponce, I. Ferrer, S. Molina-Pinelo and L. Paz-Ares, Clin. Ther., 2016, 38, 1551–1566 CrossRef PubMed.
- J. S. Xu, J. Huang, R. Qin, G. H. Hinkle, S. P. Povoski, E. W. Martin and R. X. Xu, Biomaterials, 2010, 31, 1716–1722 CrossRef CAS PubMed.
- A. J. Mieszawska, A. Gianella, D. P. Cormode, Y. Zhao, A. Meijerink, R. Langer, O. C. Farokhzad, Z. A. Fayad and W. J. M. Mulder, Chem. Commun., 2012, 48, 5835–5837 RSC.
- R. N. Mariano, D. Alberti, J. C. Cutrin, S. G. Crich and S. Aime, Mol. Pharm., 2014, 11, 4100–4106 CrossRef CAS PubMed.
- X. Wang, L. Yang, H. Zhang, B. Tian, R. Li, X. Hou and F. Wei, Colloids Surf., B, 2018, 172, 708–717 CrossRef CAS PubMed.
- Z. Amoozgar, J. Park, Q. Lin and Y. Yeo, Mol. Pharm., 2012, 9, 1262–1270 CrossRef CAS PubMed.
- R. Devulapally, K. Foygel, T. V. Sekar, J. K. Willmann and R. Paulmurugan, ACS Appl. Mater. Interfaces, 2016, 8, 33412–33422 CrossRef CAS PubMed.
- J. Wu, J. Zhang, C. Deng, F. Meng and Z. Zhong, Biomacromolecules, 2016, 17, 2367–2374 CrossRef CAS PubMed.
- X. Wang, R. Cheng, L. Cheng and Z. Zhong, Biomacromolecules, 2018, 19, 1368–1373 CrossRef CAS PubMed.
- J. Kim, J. E. Lee, S. H. Lee, J. H. Yu, J. H. Lee, T. G. Park and T. Hyeon, Adv. Mater., 2008, 20, 478–483 CrossRef CAS.
- X. R. Song, Z. Cai, Y. Zheng, G. He, F. Y. Cui, D. Q. Gong, S. X. Hou, S. J. Xiong, X. J. Lei and Y. Q. Wei, Eur. J. Pharm. Sci., 2009, 37, 300–305 CrossRef CAS PubMed.
- K. Li, Y. Liu, K. Y. Pu, S. S. Feng, R. Zhan and B. Liu, Adv. Funct. Mater., 2011, 21, 287–294 CrossRef CAS.
- A. Singh, F. Dilnawaz, S. Mewar, U. Sharma, N. R. Jagannathan and S. K. Sahoo, ACS Appl. Mater. Interfaces, 2011, 3, 842–856 CrossRef CAS PubMed.
- A. Aravind, S. H. Varghese, S. Veeranarayanan, A. Mathew, Y. Nagaoka, S. Iwai, T. Fukuda, T. Hasumura, Y. Yoshida, T. Maekawa and D. S. Kumar, Cancer Nanotechnol., 2012, 3, 1–12 CrossRef CAS PubMed.
- S. I. Thamake, S. L. Raut, Z. Gryczynski, A. P. Ranjan and J. K. Vishwanatha, Biomaterials, 2012, 33, 7164–7173 CrossRef CAS PubMed.
- P. Liu, L. Qin, Q. Wang, Y. Sun, M. Zhu, M. Shen and Y. Duan, Biomaterials, 2012, 33, 6739–6747 CrossRef CAS PubMed.
- P. Verderio, P. Bonetti, M. Colombo, L. Pandolfi and D. Prosperi, Biomacromolecules, 2013, 14, 672–682 CrossRef CAS PubMed.
- J. M. Shen, F. Y. Gao, T. Yin, H. X. Zhang, M. Ma, Y. J. Yang and F. Yue, Pharmacol. Res., 2013, 70, 102–115 CrossRef CAS PubMed.
- M. F. Frasco, G. M. Almeida, F. Santos-Silva, M. Do Carmo Pereira and M. A. N. Coelho, J. Biomed. Mater. Res., Part A, 2015, 103, 1476–1484 CrossRef PubMed.
- H. Wang, Y. Zhao, H. Wang, J. Gong, H. He, M. C. Shin, V. C. Yang and Y. Huang, J. Controlled Release, 2014, 192, 47–56 CrossRef CAS PubMed.
- R. Vivek, R. Thangam, V. Nipunbabu, C. Rejeeth, S. Sivasubramanian, P. Gunasekaran, K. Muthuchelian and S. Kannan, ACS Appl. Mater. Interfaces, 2014, 6, 6469–6480 CrossRef CAS PubMed.
- Q. Liu, H. Zhu, J. Qin, H. Dong and J. Du, Biomacromolecules, 2014, 15, 1586–1592 CrossRef CAS PubMed.
- F. Ye, Å. Barrefelt, H. Asem, M. Abedi-Valugerdi, I. El-Serafi, M. Saghafian, K. Abu-Salah, S. Alrokayan, M. Muhammed and M. Hassan, Biomaterials, 2014, 35, 3885–3894 CrossRef CAS PubMed.
- R. Devulapally, N. M. Sekar, T. V. Sekar, K. Foygel, T. F. Massoud, J. K. Willmann and R. Paulmurugan, ACS Nano, 2015, 9, 2290–2302 CrossRef CAS PubMed.
- M. Das, W. Duan and S. K. Sahoo, Nanomedicine, 2015, 11, 379–389 CrossRef CAS PubMed.
- Y. Cui, M. Zhang, F. Zeng, H. Jin, Q. Xu and Y. Huang, ACS Appl. Mater. Interfaces, 2016, 8, 32159–32169 CrossRef CAS PubMed.
- J. B. Du, Y. Cheng, Z. H. Teng, M. L. Huan, M. Liu, H. Cui, B. Le Zhang and S. Y. Zhou, Mol. Pharm., 2016, 13, 1711–1722 CrossRef CAS PubMed.
- J. Mosafer, K. Abnous, M. Tafaghodi, H. Jafarzadeh and M. Ramezani, Colloids Surf., A, 2017, 514, 146–154 CrossRef CAS.
- M. Chatterjee, A. Hens, K. Mahato, N. Jaiswal, N. Mahato, Nagahanumaiah and N. Chanda, J. Colloid Interface Sci., 2017, 506, 126–134 CrossRef CAS PubMed.
- B. Sivakumar, R. G. Aswathy, R. Romero-Aburto, T. Mitcham, K. A. Mitchel, Y. Nagaoka, R. R. Bouchard, P. M. Ajayan, T. Maekawa and D. N. Sakthikumar, Biomater. Sci., 2017, 5, 432–443 RSC.
- E. Kemal, T. F. Abelha, L. Urbano, R. Peters, D. M. Owen, P. Howes, M. Green and L. A. Dailey, RSC Adv., 2017, 7, 15255–15264 RSC.
- Y. Malinovskaya, P. Melnikov, V. Baklaushev, A. Gabashvili, N. Osipova, S. Mantrov, Y. Ermolenko, O. Maksimenko, M. Gorshkova, V. Balabanyan, J. Kreuter and S. Gelperina, Int. J. Pharm., 2017, 524, 77–90 CrossRef CAS PubMed.
- X. Shen, T. Li, Z. Chen, Y. Geng, X. Xie, S. Li, H. Yang, C. Wu and Y. Liu, Int. J. Nanomed., 2017, 12, 4299–4322 CrossRef CAS PubMed.
- M. H. M. Leung and A. Q. Shen, Langmuir, 2018, 34, 3961–3970 CrossRef CAS PubMed.
- G. Arya, M. Das and S. K. Sahoo, Biomed. Pharmacother., 2018, 102, 555–566 CrossRef CAS PubMed.
- M. J. Ramalho, E. Sevin, F. Gosselet, J. Lima, M. A. N. Coelho, J. A. Loureiro and M. C. Pereira, Int. J. Pharm., 2018, 545, 84–92 CrossRef CAS PubMed.
- J. Xi, W. Wang, L. Da, J. Zhang, L. Fan and L. Gao, ACS Biomater. Sci. Eng., 2018, 4, 1083–1091 CrossRef CAS PubMed.
- H. Tonbul, A. Sahin, E. Tavukcuoglu, G. Esendagli and Y. Capan, J. Drug Delivery Sci. Technol., 2019, 54, 101380 CrossRef.
- M. A. Vakilinezhad, A. Amini, T. Dara and S. Alipour, Colloids Surf., B, 2019, 184, 110515 CrossRef CAS PubMed.
- Y. Zhong, T. Su, Q. Shi, Y. Feng, Z. Tao, Q. Huang, L. Li, L. Hu, S. Li, H. Tan, S. Liu and H. Yang, Int. J. Nanomed., 2019, 14, 8543–8560 CrossRef CAS PubMed.
- R. S. Prabhuraj, K. Bomb, R. Srivastava and R. Bandyopadhyaya, J. Polym. Res., 2020, 27, 133 CrossRef.
- P. Wu, Q. Zhou, H. Zhu, Y. Zhuang and J. Bao, BMC Cancer, 2020, 20, 1–10 CrossRef PubMed.
- A. I. Fraguas-Sánchez, A. I. Torres-Suárez, M. Cohen, F. Delie, D. Bastida-Ruiz, L. Yart, C. Martin-Sabroso and A. Fernández-Carballido, Pharmaceutics, 2020, 12, 1–19 CrossRef PubMed.
- X. Yu, L. Sun, L. Tan, M. Wang, X. Ren, J. Pi, M. Jiang and N. Li, AAPS PharmSciTech, 2020, 21, 85 CrossRef CAS PubMed.
- N. Brandhonneur, Y. Boucaud, A. Verger, N. Dumait, Y. Molard, S. Cordier and G. Dollo, Int. J. Pharm., 2021, 592, 120079 CrossRef CAS PubMed.
- S. Malik, J. Lim, F. J. Slack, D. T. Braddock and R. Bahal, J. Controlled Release, 2020, 327, 406–419 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |