Direct conversion of glyceric acid to succinic acid by reductive carbonylation†
Abstract
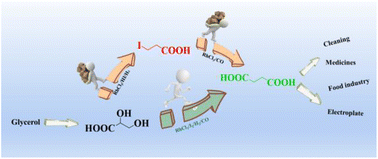
Glyceric acid (GA), a glycerol derivative, can be used as a versatile platform molecule to produce value-added chemicals. Succinic acid (SA) is a very important bio-based molecule that acts as a fine chemical and is used in biodegradable polymer synthesis. It has been shown earlier that GA can be effectively converted to the platform chemical 3-iodopropionic acid (3-IPA) with a RhCl3/HI catalyst system. In this work, we first explored the production of SA from 3-IPA by carbonylation. With RhCl3 as the catalyst and acetic acid (AA) and water as the solvent, as high as 78% yield of SA can be achieved. Considering the similarity of the catalysts used in two steps, we then further probed the one-step synthesis of SA directly from GA. Under optimized reaction conditions, 71% yield of SA can be obtained directly from GA by reductive carbonylation with RhCl3 and I2 as the catalyst under H2 and CO atmospheres. This is the first report of the direct conversion of GA to SA, which provides a novel method for glycerol valorization.


 Please wait while we load your content...
Please wait while we load your content...