Solvent-free aerobic photocatalytic oxidation of alcohols to aldehydes over ZnO/C3N4†
Abstract
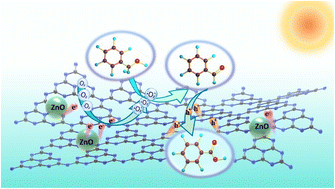
It is attractive but challenging to develop a non-noble metal-based photocatalytic system for the selective oxidation of benzyl alcohol to benzaldehyde under solvent-free conditions. Herein, a Z-scheme heterojunction photocatalyst of ZnO/C3N4 was designed and prepared. Under solvent-free irradiation conditions, benzyl alcohol can be exclusively oxidized to benzaldehyde with a yield of 99.8% and a formation rate of 16 633 μmol g−1 h−1 at room temperature. To the best of our understanding, the photocatalytic activity and selectivity of this oxidation process are much better than those reported for noble and non-noble metal-based photocatalyses. The water generated in the solvent-free oxidation process is revealed to disfavor the activity and selectivity. In addition, the mechanism of the title reaction is proposed. The present solvent-free photocatalytic system can be applied for the oxidation of benzylic, allylic, heterocyclic, and aliphatic alcohols to their corresponding aldehydes.


 Please wait while we load your content...
Please wait while we load your content...