Open Access Article
Open Access ArticleSustainable multicomponent indole synthesis with broad scope†
Xiaofang
Lei
ab,
Giasemi K.
Angeli
 a,
Constantinos G.
Neochoritis
a,
Constantinos G.
Neochoritis
 a and
Alexander
Dömling
a and
Alexander
Dömling
 *b
*b
aUniversity of Crete, Department of Chemistry, Heraklion, Greece
bUniversity of Groningen, Department of Pharmacy, Drug Design group, Groningen, The Netherlands. E-mail: a.s.s.domling@rug.nl
First published on 21st July 2022
Abstract
The most preferred heterocyclic indole core was de novo assembled by an innovative 2-step reaction from inexpensive and broadly available anilines, glyoxal dimethyl acetal, formic acid and isocyanides involving an Ugi multicomponent reaction followed by an acid induced cyclization. As opposed to many other indoles syntheses, our method delivers under mild and benign conditions using ethanol as solvent and no metal catalyst. The scope of the reactions was scouted and 20 derivatives are described.
Introduction
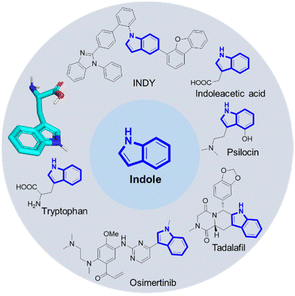
Arguably, indole belongs to the top 10 of the most important organic chemistry scaffolds, included in biochemicals, dyes, drugs, natural products, materials and agrochemicals (Fig. 1).1 Amongst many others, examples include the essential proteinogenic amino acid tryptophan also involved in many biochemical pathways, the cancer drug Osimertinib,2 the hallucinogenic Psilocin isolated from ‘magic mushrooms’ and investigated as the o-phosphorylated prodrug for the potential treatment of a range of mental illnesses,3 the highly conjugated INDY dye with superior phosphorescence properties used in OLEDs,4 the plant hormone indoleacetic acid (IAA) and the male impotency, extended duration drug Tadalafil.Not surprisingly myriads of synthetic methods were described to access the indole core. However, many of the well-established indole syntheses suffer from harsh reaction conditions, limited substrate scope, use of (halogenated) hydrocarbons as solvents or other sustainability issues. For example, the classical Fischer indole synthesis is often performed under highly acidic refluxing conditions of the precondensed phenylhydrazone.5 The synthesis of the hazardous phenylhydrazine precursor involves the diazotization of aniline, the reduction of the resulting diazonium salt with an excess amount of sodium sulfite, and the hydrolysis of phenylhydrazine sulfonic acid salt with hydrochloric acid, also known as the Fischer phenylhydrazine synthesis.6 Alternative access to phenylhydrazines involves metal catalyzed cross coupling of phenyl halides.7,8 Surprisingly, there are not many multicomponent reaction (MCR) approaches towards the assembly of the indole scaffold (Fig. 2). Takahashi synthesized 2,3-disubstituted indoles via palladium-catalyzed three-component coupling of aryl iodide, o-alkenylphenyl isocyanide and amine.9 Zhang and Fan described the elective formation of 3-cyano-1H-indoles from 1-bromo-2-(2,2-dibromovinyl)-benzenes with aldehydes and aqueous ammonia.10 3-Aminoindoles were accessed by Sorensen by a Brønsted acid mediated imine formation of anilines and aldehydes followed by cyclization with tert-butyl isocyanide.11 Kalinski performed an Ugi reaction of o-bromoanilines, cinnamaldehydes and isocyanides in the presence of formic acid, followed by an intramolecular Heck reaction to produce the desired indoles.12 Chen recently described a camphor sulfonic acid-mediated one-pot tandem consecutive approach to synthesize functionalized indole derivatives from the Ugi four-component reaction intermediate via a 5-exo-trig cyclization.13 We recently reacted anilines, TMS-azide, glyoxal dimethyl acetal and isocyanides to yield 2-tetrazolo substituted indoles (Fig. 2).14 MCR fundamentally complies with several aspects of the twelve principles of green chemistry, including high atom economy, convergent synthesis approach as opposed to sequential multistep synthesis, and often mild reaction conditions including environmentally benign solvents, avoidance of metal catalysts and waste production, while having a great scope and substrate compatibility.15
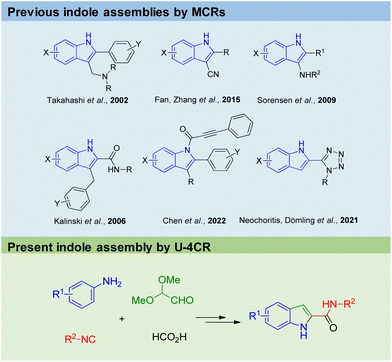 | ||
| Fig. 2 Previous and the present innovative MCR methods to assemble the indole core (highlighted in blue). | ||
Herein, we want to communicate our innovative just 2-step indole-2-carboxamide synthesis involving the Ugi-4CR of anilines, glyoxal dimethyl acetal, formic acid and isocyanides, followed by an acid-catalysed cyclization. Our protocol gives access on C2 functionalized indoles, is highly sustainable and sticks out by mild reaction conditions, low toxicity of the building blocks and a high safety standard, however not jeopardizing substrate scope and yields.
Results and discussion
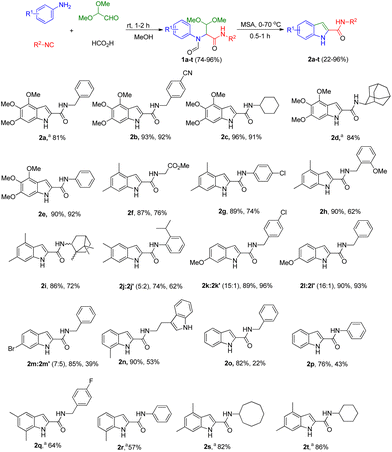
Explicitly, we reacted substituted anilines, glyoxal dimethyl acetal, formic acid and a multitude of isocyanides at room temperature, obtaining the desired Ugi four-components (U-4CR) adducts 1a–t in good to almost quantitative yields (74–96%) (Scheme 1). Satisfyingly, the acidic closure using methanesulfonic acid at 70 °C (MSA, ≥99% purity, freshly opened bottle), afforded a facile way to target multi-substituted indole-2-carboxamide derivatives 2a–t in 22–96% yield. Water free MSA is key for the successful cyclisation and often MSA from different suppliers contain variable amounts of water, as can be shown by 1H NMR. In several cases, both the U-4CRs 1 and the final adducts 2 precipitated during the reaction mixture greatly facilitating purification by simple filtration (see ESI†). Alternatively, both acetic acid and benzoic acid can be employed in the U-4CR instead of formic acid as both are removed under the second's step reaction conditions.The scope of the isocyanides is quite broad, as all the isocyanides that were employed, aryl (2e, 2g, 2j, 2p and 2r), benzylic (2a–b, 2h, 2k–m, 2o and 2q) and aliphatic (2c–d, 2f, 2i and 2s–t) with different substituents, afforded the desired Ugi product, and without interfering with the next step, the ring closure reaction. We preferentially employed anilines with electron donating groups (EDGs), mostly in meta position due to the presumed electrophilic ring closure mechanism. Substituted anilines with electron withdrawing groups (EWGs) or para substituted anilines with EDGs, i.e.2m and 2o–p, respectively reacted more sluggish in the cyclization reaction or not at all; for those substrates, we found that degradation of the U-4CR adducts was occurring, recovering the corresponding anilines. The indoles derived from meta-substituted anilines, such as 2j–m, were formed as two different regio isomers as expected.14 However, by selective precipitation (in case of 2j–l), we were able to separate the major regio isomers that are shown in Scheme 1 in isolated yields of 42%, 84% and 81%, respectively. Compound 2m was obtained as a mixture of regioisomers (see ESI†).
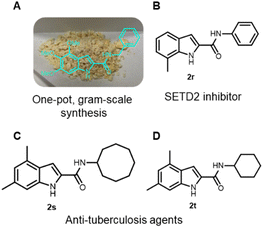
We have synthesized 20 multi-substituted indole-2-carboxamides in a 2-step procedure. Noteworthy, our method can access “double” free NH indole compound 2n, a strategy that can be extended to numerous other heterocycles. In order to render our synthetic methodology even more efficient, we explored the feasibility of the one-pot two-step approach synthesis. Thus, we were able to access 2a, 2d and 2q–t in a one-pot fashion in 81%, 84%, 64%, 57%, 82% and 86% overall yield, respectively (Scheme 1). Furthermore, our approach is easily scalable. We have synthesized 1a on a 10 mmol scale with 94% yield (4.06 g) or, 2a on a 5 mmol scale with 91% yield (1.55 g), after purification by column chromatography (Fig. 3, A).
To further show the usefulness of our protocol, we synthesized rapidly (0.5–1 h) in a mild and straightforward manner, a SETD2 inhibitor (2r) and two anti-tuberculosis agents (2s and 2t)16,17 in 57%, 82% and 86% overall yields, respectively (Fig. 2, B–D). In literature, the synthesis of the aforementioned compounds proceeds with the generation of 2-indole acetic acid, starting mostly from phenyl hydrazines and a subsequent amide formation. The conditions involve expensive and often difficult to access starting materials, harsh, strongly basic conditions (>140 °C heating),16 and less sustainable, costly catalysts and dry conditions.18 Overall, those compounds were all previously synthesized in >4 synthetic steps.
The single-crystal X-ray structures of 2f and 2g were obtained, showing the solid-state conformation of the scaffold involving intermolecular bifurcated hydrogen bonds involving the indole-NH and the 2-carbonyl of two adjacent molecules forming non covalent dimers (Fig. 4).
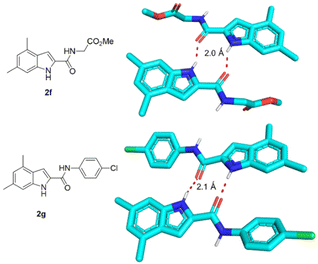 | ||
| Fig. 4 Hydrogen bonding-induced dimer formation of 2f (CCDC 2174979) and 2g (CCDC 2174980)† in solid state. | ||
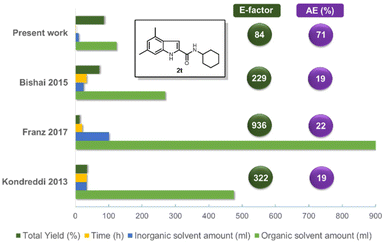
In order to demonstrate the green chemistry merits of our synthetic approach compared to the existing ones, we quantified various reaction parameters such as total yield, time (h), the amounts of inorganic and organic solvents (ml), process mass intensity (PMI), the E-factor and atom economy (AE) (Fig. 5 and ESI†). We chose as a representative example comparing the different procedures the indole amide 2t (Fig. 5). The substantial difference is that our methodology consists only of 2 steps and it can even be performed as one pot (Scheme 1). Besides, the employed starting materials are derived from inexpensive and abundant precursors such as anilines or primary amines or oxo components (isocyanide). Our procedure outperforms the others in every category, such as total yield, reaction time, amount of solvents (organic and inorganic), the E-factor and AE. This is also true if we take into account the additional isocyanide synthesis (see ESI†), although cyclohexyl isocyanide is cheaply commercially available. Noteworthy, there is no need of inert gas atmosphere, as is in all other compared procedures.
Conclusions
In summary, we have developed a short, mild, economic and scalable procedure towards C2 functionalized indole amides. The ecological footprint of our indole synthesis outperforms competing procedures by a factor of 2.5 to >10, as calculated by the E-factor and PMI. In detail, our innovative indole synthesis nicely addresses many of the twelve principles of green chemistry: prevention of waste by avoiding multistep procedures (1st principle); superior atom economy, as by definition ‘in MCRs most of the atoms of the starting materials are found in the product’ (2nd principle);19 also isocyanides are sometimes marked as toxic, it has to be mentioned that there was one isocyanide drug marketed, there are numerous isocyanide natural products, and there is more and more interest in the medicinal chemistry of isocyanides (3rd principle);20 our synthesis isn't using any auxiliaries in the first step, however, uses MSA in the second step (5th principle); the new indole synthesis is energy efficient, as the first step runs at room temperature and the second step is performed in a temperature range from 0 to 70 °C, depending on the substrate nature (6th principle); formic acid, a bio-renewable feedstock, is used twice in the synthesis, contributing the isocyanide-C and used as the acid component in the Ugi reaction (7th principle).21 Due to the multicomponent reaction nature numerous bioactive compounds can be easily and rapidly synthesized in just two steps or telescoped from inexpensive and readily available starting materials. Thus, we foresee a general acceptance of our novel indole synthesis, adding to the ever-growing tool box of synthetic indole chemistry.Author contributions
A. D. and C. G. N. conceptualized and directed the project. X. L. performed the syntheses and collected the analytical data. G. A. determined the single crystal X-ray structure. A. D., C. G. N. and X. L. contributed to the manuscript writing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research project (to C. G. N) was supported by the Hellenic Foundation for Research and Innovation (H.F.R.I.) under the “2nd Call for H.F.R.I. Research Projects to support Post-Doctoral Researchers” (Project Number: 0911). Xiaofang Lei acknowledges the China Scholarship Council for support.Notes and references
- E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed.
- S. S. Ramalingam, J. Vansteenkiste, D. Planchard, B. C. Cho, J. E. Gray, Y. Ohe, C. Zhou, T. Reungwetwattana, Y. Cheng, B. Chewaskulyong, R. Shah, M. Cobo, K. H. Lee, P. Cheema, M. Tiseo, T. John, M. C. Lin, F. Imamura, T. Kurata, A. Todd, R. Hodge, M. Saggese, Y. Rukazenkov and J. C. Soria, N. Engl. J. Med., 2020, 382, 41–50 CrossRef CAS PubMed.
- H. A. Geiger, M. G. Wurst and R. N. Daniels, ACS Chem. Neurosci., 2018, 9, 2438–2447 CrossRef CAS PubMed.
- Y. Chen, X. Wei, J. Cao, J. Huang, L. Gao, J. Zhang, J. Su and H. Tian, ACS Appl. Mater. Interfaces, 2017, 9, 14112–14119 CrossRef CAS PubMed.
- B. Robinson, Chem. Rev., 1963, 63, 373–401 CrossRef.
- E. Fischer, Ber. Dtsch. Chem. Ges., 1875, 8, 589–594 CrossRef.
- S. V. Kumar and D. Ma, Chin. J. Chem., 2018, 36, 1003–1006 CrossRef CAS.
- J. Y. Wang, K. Choi, S. J. Zuend, K. Borate, H. Shinde, R. Goetz and J. F. Hartwig, Angew. Chem., Int. Ed., 2021, 60, 399–408 CrossRef CAS PubMed.
- K. Onitsuka, S. Suzuki and S. Takahashi, Tetrahedron Lett., 2002, 43, 6197–6199 CrossRef CAS.
- B. Li, S. Guo, J. Zhang, X. Zhang and X. Fan, J. Org. Chem., 2015, 80, 5444–5456 CrossRef CAS PubMed.
- J. S. Schneekloth, J. J. Kim and E. J. Sorensen, Tetrahedron, 2009, 65, 3096–3101 CrossRef CAS PubMed.
- C. Kalinski, M. Umkehrer, J. Schmidt, G. Ross, J. Kolb, C. Burdack, W. Hiller and S. D. Hoffmann, Tetrahedron Lett., 2006, 47, 4683–4686 CrossRef CAS.
- S. P. Murugan, H.-J. Zhong, C.-Y. Wu, H.-W. Pan, C. Chen and G.-H. Lee, ACS Omega, 2022, 7, 5713–5729 CrossRef CAS PubMed.
- X. Lei, P. Lampiri, P. Patil, G. Angeli, C. G. Neochoritis and A. Dömling, Chem. Commun., 2021, 57, 6652–6655 RSC.
- R. C. Cioc, E. Ruijter and R. V. A. Orru, Green Chem., 2014, 16, 2958–2975 RSC.
- R. R. Kondreddi, J. Jiricek, S. P. S. Rao, S. B. Lakshminarayana, L. R. Camacho, R. Rao, M. Herve, P. Bifani, N. L. Ma, K. Kuhen, A. Goh, A. K. Chatterjee, T. Dick, T. T. Diagana, U. H. Manjunatha and P. W. Smith, J. Med. Chem., 2013, 56, 8849–8859 CrossRef CAS PubMed.
- O. K. Onajole, M. Pieroni, S. K. Tipparaju, S. Lun, J. Stec, G. Chen, H. Gunosewoyo, H. Guo, N. C. Ammerman, W. R. Bishai and A. P. Kozikowski, J. Med. Chem., 2013, 56, 4093–4103 CrossRef CAS PubMed.
- N. D. Franz, J. M. Belardinelli, M. A. Kaminski, L. C. Dunn, V. Calado Nogueira de Moura, M. A. Blaha, D. D. Truong, W. Li, M. Jackson and E. J. North, Bioorg. Med. Chem., 2017, 25, 3746–3755 CrossRef CAS PubMed.
- A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168–3210 CrossRef.
- A. Massarotti, F. Brunelli, S. Aprile, M. Giustiniano and G. C. Tron, Chem. Rev., 2021, 121, 10742–10788 CrossRef CAS PubMed.
- M. G. Mura, L. D. Luca, G. Giacomelli and A. Porcheddu, Adv. Synth. Catal., 2012, 354, 3180–3186 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, analytical data, NMR spectra, single-crystal data, e-factor calculations. CCDC 2174979 and 2174980. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2gc02060b |
| This journal is © The Royal Society of Chemistry 2022 |