Open Access Article
Open Access ArticleMaking more from bio-based platforms: life cycle assessment and techno-economic analysis of N-vinyl-2-pyrrolidone from succinic acid†
Moritz O.
Haus‡
 a,
Benedikt
Winter‡
a,
Benedikt
Winter‡
 bc,
Lorenz
Fleitmann
bc,
Lorenz
Fleitmann
 bc,
Regina
Palkovits
bc,
Regina
Palkovits
 *a and
André
Bardow
*a and
André
Bardow
 *bcd
*bcd
aChair of Heterogeneous Catalysis and Technical Chemistry, RWTH Aachen University, Worringerweg 2, DE-52074 Aachen, Germany. E-mail: palkovits@itmc.rwth-aachen.de
bEnergy and Process Systems Engineering, ETH Zurich, Tannenstraße 3, CH-8092 Zurich, Switzerland. E-mail: abardow@ethz.ch
cInstitute of Technical Thermodynamics, RWTH Aachen University, Schinkelstraße 8, DE-52062 Aachen, Germany
dInstitute of Energy and Climate Research (IEK 10), Research Center Jülich GmbH, Germany
First published on 18th August 2022
Abstract
The prospective assessment of the environmental and economic costs of emergent technologies based on biomass feedstocks has a vital role in guiding academic research and corporate investments. This study evaluates a new pathway for the synthesis of the established monomer N-vinyl-2-pyrrolidone (NVP) from succinic acid, a suggested biorefinery platform chemical. For this purpose, conceptual process design is combined with recent progress in catalysis research to deliver the life cycle inventories of the novel process route. LCA projects that bio-based NVP production reduces global warming impacts by 25–53% compared to the fossil alternative practiced today. Within this range, the magnitude of reductions depends on the biomass feedstock, the chosen hydrogenation catalyst, and separation technology. However, these global warming impact reductions have to be weighed against increasing other environmental impacts, mainly eutrophication and acidification, which are associated with farming and the expenditure of noble metal catalysts. The operational cost analysis suggests that succinic acid-based NVP production is competitive if the substrate cost ratio (succinic acid/γ-butyrolactone) is lower than 0.7. The insights gained in this study provide goals for catalyst research and process development.
1. Introduction
The integration of biomass feedstocks into traditionally fossil-based production sectors may be key to enabling human prosperity within the resource and climate constraints of planet earth.1–4 The biomass-based (short: bio-based) synthesis of chemicals and fuels has therefore been an important subject of political efforts,5–8 societal discussions, and academic research.9–14 In this context, polylactic acid,15,16 bio-ethanol17 and bio-diesel18 have emerged as encouraging examples, showing that bio-based production can be brought to the commercial scale.Yet, a broader transition to biomass feedstocks requires biorefineries that integrate a large network of processes converting the highly functionalized substrates into energy carriers and chemical building blocks.19 Regarding chemical building blocks, several studies20–23 have evaluated potential bio-economy platform chemicals based on criteria such as application versatility, market potential, and technology readiness. Often-reported key candidates include chemicals of carbohydrate origin, such as hydroxymethylfurfural (or derived furandicarboxylic acid), sorbitol (or derived glycols), and succinic acid.20,21,24 Consequently, value chains starting from these compounds should be of special interest to the chemical research community.
In the specific case of succinic acid, a major research focus has been on liquid-phase hydrogenations yielding γ-butyrolactone (GBL), 1,4-butanediol, and tetrahydrofuran (THF).25–29 While each of these products can be preferentially formed with the correct choice of catalyst system and reaction conditions, some selectivity issues remain due to the sequential nature of the ongoing hydrogenation steps.30 More importantly, however, current succinic acid price estimates (∼2.5 $ per kg)31 will challenge the prospects for economical production of comparably inexpensive lactones, alcohols and ethers (e.g. 1,4-butanediol price of 2.3 $ per kg).32
In contrast, the synthesis of heteroatom-containing chemicals from biomass may allow for easier market access due to higher prices and long synthesis pathways starting from fossil resources.1,33 Concerning the conversion of succinic acid into such chemicals, pyrrolidones could offer an attractive option with applications e.g. in pharmaceuticals and specialty polymers. As a potential pathway, White et al. discussed producing N-methyl-2-pyrrolidone (NMP) and 2-pyrrolidone (2PYD) from succinate-containing fermentation broth.34 The respective process comprises thermal imide formation, distillation, and reduction over a rhodium catalyst (220 °C, 100 bar H2). While the catalytic conversion of succinic anhydride into N-phenyl-2-pyrrolidone was also reported,35 the total number of studies on value chains from succinic acid to pyrrolidones has remained comparably limited, with patents comprising the bulk of available literature.36,37
In this context, we recently proposed a sequence of liquid phase amidation–hydrogenation and gas-phase dehydration processes to synthesize N-vinyl-2-pyrrolidone (NVP) from aqueous succinic acid solutions (Fig. 1).39,40 Yields of 70 mol% (succininc acid to NVP) were achieved with commercial hydrogenation catalysts and improved by further material development.41 Consequently, this reaction sequence seems to be a promising way of obtaining a high-value monomer from a potential biomass platform using two conversion steps. While this value proposition appears intriguing, a detailed evaluation of process inputs and outputs is required to determine the expected merit of the proposed transformation.
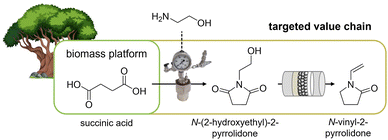 | ||
| Fig. 1 Targeted value chain from succinic acid, as a bio-based platform chemical, to N-vinyl-2-pyrrolidone.38 | ||
Here, life cycle assessment (LCA)42 offers a tool for the holistic analysis of value chains, as already demonstrated for biomass utilization scenarios.11,43 Previous early-stage LCA studies have resolved the influence of design choices, including catalyst materials and biomass feedstocks, thus helping to guide future research priorities.44,45 Consequently, to quantify the environmental and economic impact of bio-based NVP production, an LCA study on the current state of the succinic acid-to-NVP conversion technology is presented herein.
The study starts with defining the underlying LCA methodology (section 2), including the goal and scope definition (section 2.1), the system boundaries for bio- and fossil-based NVP production (section 2.2), the methodology for compilation and evaluation of process inventories (section 2.3), process simulations for bio-based NVP production (section 2.4) and economic evaluation (section 2.5). Life-cycle inventories of different process options are presented and discussed next (section 3.1). The following life cycle impact assessment (section 3.2) focuses first on global warming impact and is extended to other non-tox impacts of the CML2001/2016 method.46 Toxicology related impacts are available in the ESI.† Next to these environmental metrics, operational cost estimates are presented as an economic indicator (section 3.3). The comparative analysis highlights the environmental and economic potential of replacing fossil NVP production, considering different biomass feedstocks, separation technologies, and succinic acid market scenarios. After discussing uncertainties in the early stage evaluation (section 3.4), directions for future catalyst and process development are formulated (section 3.5). To the best of our knowledge, we present the first LCA study of bio-based pyrrolidone production.
2. Methodology
2.1. Goal and scope definition
The presented LCA aims to compare the environmental impacts of 1 kg of NVP produced through conventional and succinic acid-based processes. Thus, the functional unit of the evaluation is 1 kgNVP (molar purity >99%). Moreover, cradle-to-gate system boundaries are applied – including all processes from feedstock extraction to the factory gate. Since conventional and bio-based NVP are chemically identical at the factory gate, the use-phase of NVP can be assumed to be independent of the chemical's origin. Consequently, gate-to-grave impacts on LCA results cancel in the comparative approach and are therefore not considered.47,48Moreover, the evaluation is prospective and relates to a potential implementation of NVP production from succinic acid, obtained by sugar fermentation. To assess the impact of technological choices and developments that cannot be fully foreseen at this stage, several catalyst and separation scenarios are included. Furthermore, the source and type of biomass feedstock has been shown to have a vast effect on the environmental impact of bio-based chemicals.45,49 Thus, we include a set of typical first and second-generation feedstocks for sugar production in this study: sugar beet (base case), sugar cane, corn, and corn stover. While these feedstocks are often associated with distinct production regions,50 other regional differences are not considered for the early-stage assessment, and production of succinic acid and pyrrolidone is assumed to occur in Europe. Finally, the purified C5/C6-sugars derived from the named biomass feedstocks are assumed to yield identical results in succinic-acid production, though further research on feedstock variability may be required.51–53
2.2. Process description and system boundaries
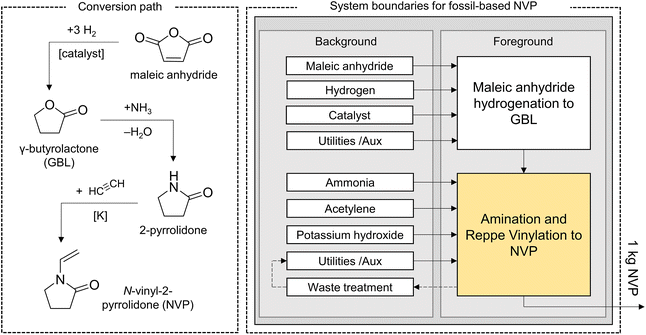 | ||
| Fig. 2 Conversion path and system boundaries for the evaluation of NVP synthesis from the maleic anhydride platform. Yellow color highlights process simulations conducted in this work. | ||
Several process options are disclosed in the patent literature, especially for realizing the last two stages (from GBL to NVP).56–60 A comparison of these options suggests that high-pressure, liquid-phase amination in a concentrated, aqueous solution without catalyst is state-of-the-art in 2-pyrrolidone synthesis.56 Subsequently, vinylation is performed with pressurized acetylene using potassium pyrrolidate, which forms from 2-pyrrolidone and KOH in water-free conditions, as a homogeneous catalyst.57,58
While this option is not implemented herein, an extensive array of patents has targeted the avoidance of acetylene in generating the vinyl-group of NVP.61,62 Most notably, the condensation of GBL and monoethanolamine yields N-(2-hydroxyethyl)-2-pyrrolidone (HEP), which can be dehydrated to NVP.63 Despite its dependence on fossil carbon due to the commercial sources of GBL, this alternative has inspired part of the succinic acid-based NVP production chain. This option also opens a path to bio-based NVP from bio-based GBL, which is not within the scope of the current article.64
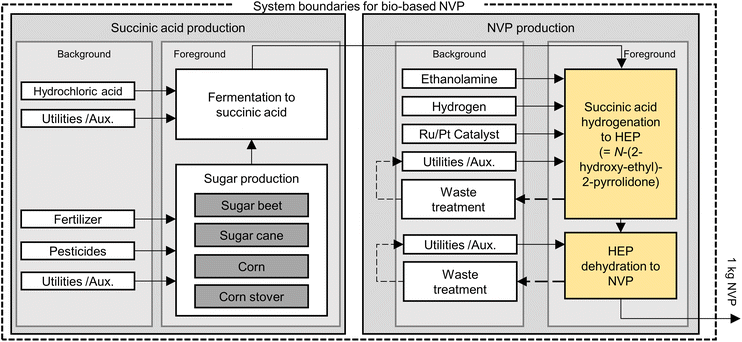 | ||
| Fig. 3 System boundaries for the evaluation of NVP synthesis from biomass via succinic acid. Yellow color highlights process simulations conducted in this work. Refer to Fig. 1 for a chemical representation of conversion steps. | ||
2.3. LCI compilation and LCIA
Since carbohydrates are frequently used as starting point of biomass-based chemical production, inventory data for the required agricultural and processing steps is present in scientific literature. The evaluation at hand specifically uses data compiled by Winter et al. for bio-based aniline production (ESI, section 3†).50 Moreover, information on fermentation to yield succinic acid was taken from Cok et al.65 Lastly, commercial inventory data on ethanolamine produced from ethylene oxide was obtained from IHS Markit.
In the case of fossil production, maleic anhydride reduction to GBL is the first process stage absent from the LCA database. Thus, commercial inventory data was obtained from IHS Markit. Subsequent steps in both value chains – starting from GBL and succinic acid, respectively – had to be analyzed by process flowsheet simulations due to a lack of published inventory data. These flowsheet simulations yielded a variety of output streams, some of which require further treatment. The output streams were categorized as (i) product, (ii) inert gas purge, (iii) wastewater, (iv) organic residue, and (v) combustible gas purge. While inert gases (ii) are released to the environment without further treatment, wastewater (iii) is discarded through a sequence of wet air oxidation and mechanical–biological treatment, and organic residue and combustible gases (iv–v) are jointly incinerated. The required inventory data for the treatment of (iii–v) is based on the literature analysis of waste treatment facilities in commercial plants.68,69
For the treatment of biogenic carbon, we followed Pawelzik et al. in accounting for all its uptake from and release into the atmosphere.70 Consequently, the GWI of bio-based NVP explicitly accounts for the amount of carbon uptake in plant cultivation but also the direct release during processing (e.g. fermentation) and NVP production. This treatment reflects the carbon flows from cradle-to-gate in line with the goal and scope of this study. Assuming combustion as an exemplary end-of-life scenario, the expansion towards cradle-to-grave boundaries closes the circle of carbon capture and release in the case of bio-based NVP, whereas conventional NVP would be burdened with additional carbon emissions, ultimately stemming from a fossil reservoir. However, our approach of explicitly accounting for biogenic carbon leads to very small – and sometimes negative – GWI attributed to succinic acid from cradle-to-gate. This negative carbon footprint does not imply that succinic acid production is a carbon-negative technology since the comparative assessment does not include end-of-life emissions.71
Moreover, the impacts of indirect land-use change (iLUC) have been discussed as a major factor in assessing chemicals and fuels from biomass.72 To incorporate this aspect despite the remaining large uncertainty in this field,73 iLUC effects on global warming impacts are estimated explicitly by exploiting knowledge on bio-ethanol which shares the sugar basis with bio-based NVP.50 The variability of results related to iLUC is shown by adapting values from several studies for each of the discussed biomass feedstocks.74–81 Lastly, it is noted that the models applied in the original iLUC research papers often do not exclude effects from direct land-use change. Keeping with the authors of those studies, the adaptation of their results to the bio-based NVP scenario is still referred to as iLUC. The reader is referred to the supplementary (section 4) for additional details on iLUC and other LCA aspects.
2.4. Process simulations
As discussed in section 2.3, part of the LCI data is obtained by rigorous process simulations using the flowsheeting software Aspen Plus® V11. Required process information is derived from scientific and patent literature as well as additional experiments (ESI, section 1 and 2†). In particular, amidation–hydrogenation of succinic acid with ethanolamine (Fig. 4, namine/nacid = 1, mwater/macid = 1) is implemented as a batch operation at 200 °C and 200 bar H2, with yield and by-product formation depending on catalyst choice. Three alternative simulations are implemented: (1) a commercial benchmark catalyst (Ru/C, 74% yield),39 (2) a recently described optimized system (Pt–Re/TiO2, 83% yield)41 and (3) a hypothetical future case with much reduced by-product formation (98% yield). To account for uncertainty in the handling of oligomeric hydrogenation by-products in the bio-based process, an alternative HEP separation is also considered supported by experiments (ESI, section 2b + c†): a sequence of (vacuum) distillations is implemented with or without prior removal of oligomers by extraction, resulting in conservative and optimistic process scenarios, respectively. Temperatures above the product's normal boiling point (>280 °C) are assumed to cause thermal stability issues. This limit imposes the need for vacuum separation, especially when high-boiling oligomers are removed by distillation.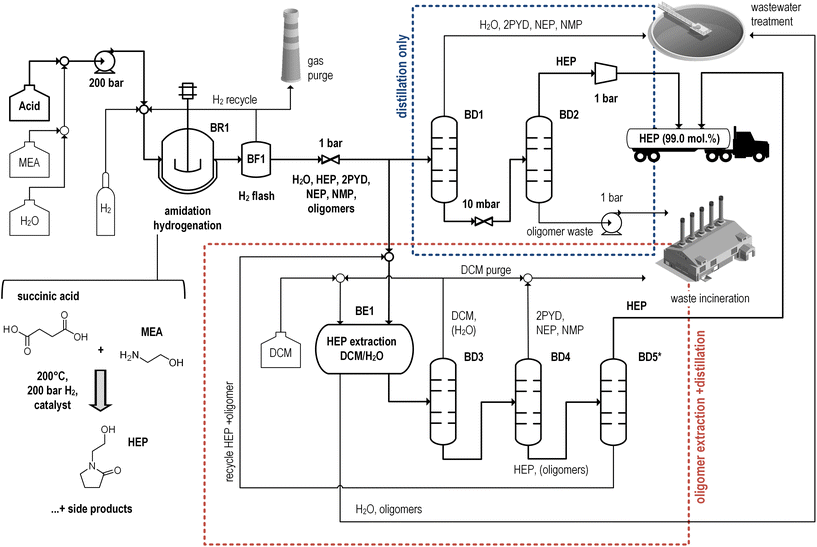 | ||
| Fig. 4 Flowsheet of HEP production from succinic acid and monoethanolamine as a first stage of the Bio-process simulation. Downstream purification alternatives (Bio-x-D and Bio-x-Ex) are shown in the blue and red boxes. “x” serves as indicator of the three hydrogenation catalyst options discussed in the text. *separation of oligomer traces leftover from extraction;82 BR labels reactors, BF flashes and BD distillation columns. | ||
Subsequent dehydration of purified HEP (Fig. 5) is based on results from continuous experimentation with a cost-effective Na2O/SiO2 catalyst.39,40 Conversion of 10 vol% HEP in nitrogen carrier gas at 385 °C yields 95% NVP and 3% of 2-pyrrolidone by-product. Pressure levels (199–66 mbar) in the ensuing distillation train are chosen in accordance with patent-reported product stability.83 The distillation train also removes co-formed water and acetaldehyde. Due to spatial limitations, flowsheets and detailed descriptions of the fossil-based process simulation are presented in the ESI (section 5†). All compared process scenarios are summarized in Table 1.
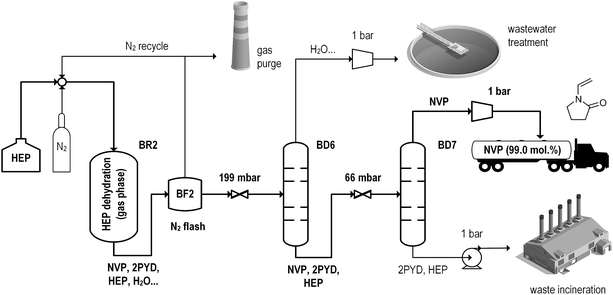 | ||
| Fig. 5 Flowsheet of NVP production from HEP as a second stage of the Bio-process simulations.82 | ||
| Process description | Acronym |
|---|---|
| Fossil NVP production from GBL (reference) | Fos |
| Succinic acid-based NVP production using Ru/C catalyst, distillation only | Bio-Ru-D |
| Succinic acid-based NVP production using Ru/C catalyst, extraction–distillation | Bio-Ru-Ex |
| Succinic acid-based NVP production using Pt–Re/TiO2 catalyst, extraction–distillation | Bio-Pt-Ex |
| Succinic acid-based NVP production using ideally optimized catalyst, extraction–distillation | Bio-Id-Ex |
Notably, the simulated processes contain a mixture of batch and continuous operations, with flowsheets omitting intermediate storage containers and heat exchangers for the sake of clarity. Reactors, distillation columns, and continuous extractions are implemented as RStoic, RadFrac, and Extract Aspen® models. All simulations are based on Henry (permanent gases) and NRTL (other components) thermodynamic models. Most single component parameters, especially those related to thermal separation (cpig, pv, ΔHv), are sourced from databases available in Aspen Plus®. For a few cases, including some properties of HEP, NVP, and oligomers, property data is estimated by COSMO-RS and the Benson group contribution method.84,85 Details are given in the ESI (section 6†). NRTL parameters for key component binary interactions unavailable in Aspen® databases are also estimated by COSMO-RS.
2.5. Economic evaluation
The life-cycle inventories for the process scenarios from the environmental impact assessment allow estimating operational cost estimates as an early-stage economic assessment. Prices for chemicals and utilities are given in the ESI (section 7†). All prices are given in US dollars in the year 2020. The cost of catalysts is estimated through heuristics given in the CatCost™ calculation tool (V1.0.4).86Given the scope of the evaluation and the low technology readiness level of bio-based NVP, capital investment is not estimated. However, the ESI (section 8†) also contains comparative lists of the main process equipment needed for conventional and bio-based NVP production. The units are characterized by their operating conditions and product-normalized flowrates from the equilibrium-based simulations. Both the bio-based route and the fossil-based route require a similar number and type of vessels.
3. Results and discussion
3.1. Life cycle inventory data
LCI data associated with GBL and succininc acid synthesis was compiled from commercial and open sources. The inventories for converting these intermediates to NVP were obtained through process simulations (section 2.4). In the following, these inventories are discussed to improve our understanding of the process scenarios. Initially, results are contrasted for the fossil (Fos) and an exemplary bio-based (Bio-Ru-Ex) NVP production scenario (Table 2).| Fos scenario | Bio-Ru-D scenario | Units | ||
|---|---|---|---|---|
| Stream | Quantity | Stream | Quantity | |
| a At T = −25 °C. b Calculated via the Carnot efficiency (Tref = 25 °C) of heat at 400 °C (natural gas), 200 °C (steam), −25 °C (cryogen). c Exemplary stream compositions and inventories of waste treatment in the ESI (section 10†); | ||||
| Substrates | Substrates | |||
| γ-Butyrolactone | 1.23 | Succinic acid | 1.71 | kg kgNVP−1 |
| Ammonia | 0.26 | Monoethanolamine | 0.89 | kg kgNVP−1 |
| Acetylene | 0.28 | Hydrogen | 0.088 | kg kgNVP−1 |
| Auxiliaries | Auxiliaries | |||
| Process water | 0.50 | Process water | 1.71 | kg kgNVP−1 |
| KOH | 0.016 | Nitrogen | 0.12 | kg kgNVP−1 |
| Ru/C | 8.6 × 104 | kg kgNVP−1 | ||
| Na2O/SiO2 | 2.5 × 103 | kg kgNVP−1 | ||
| Utilities | Utilities | |||
| Natural gas | 1.67 | Natural gas | 1.74 | MJ kgNVP−1 |
| Steam | 2.23 | Steam | 9.49 | MJ kgNVP−1 |
| Cryogena | 1.10 | Cryogena | 0.37 | MJ kgNVP−1 |
| Water cooling | 1.40 | Water cooling | 1.51 | MJ kgNVP−1 |
| Air cooling | 5.28 | Air cooling | 11.3 | MJ kgNVP−1 |
| Electricity | 0.79 | Electricity | 0.15 | kWh kgNVP−1 |
| (Total exergy)b | (4.82) | (Total exergy)b | (5.21) | MJ kgNVP−1 |
| Waste | Waste | |||
| Inert gas purge | 0.13 | kg kgNVP−1 | ||
| Flam. gas purge | 0.05 | Flam. gas purge | 0.044 | kg kgNVP−1 |
| Organic residue | 0.46 | Organic residue | 0.41 | kg kgNVP−1 |
| Wastewater | 0.78 | Wastewater | 2.94 | kg kgNVP−1 |
While molar NVP yields are similar for the GBL- (0.65 molGBL per molNVP) and succinic acid-based (0.67 molacid per molNVP) scenario, a notable difference can be observed in substrate consumption (fossil: 1.8 kgsubstrates per kgNVPvs. bio: 2.7 kgsubstrates per kgNVP). However, the higher substrate demand in the bio-based scenario is related to four equivalents of water, formed through condensation, hydrogenation, and dehydration reactions when succinic acid is converted to NVP. On the other hand, GBL is characterized by a lower C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio (higher degree of reduction), thus expelling only one equivalent of water in this value chain. While the high C
O ratio (higher degree of reduction), thus expelling only one equivalent of water in this value chain. While the high C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio of succinic acid relates to a common challenge in making chemicals from biomass,1 GBL itself is generated by hydrogenation of an acid anhydride and subsequent removal of co-produced water. However, the choice of our system boundaries for process simulations was not optimized towards the comparability of the utilized intermediates. These upstream steps are accounted for in the LCA.
O ratio of succinic acid relates to a common challenge in making chemicals from biomass,1 GBL itself is generated by hydrogenation of an acid anhydride and subsequent removal of co-produced water. However, the choice of our system boundaries for process simulations was not optimized towards the comparability of the utilized intermediates. These upstream steps are accounted for in the LCA.
The low amount of solvent (process water) and therefore high concentration of reactant/product streams is a clear benefit of the Fos scenario. Most importantly, the load on distillation columns is reduced and thus the need for steam and natural gas for heating. Yet, the conventional process scenario (Fos) is burdened with the expenditure of more costly utilities (electricity and sub-ambient cooling) due to the extensive use of vacuum distillations. Overall, exergy demand is thus comparable between the discussed alternatives, with 7.5% lower exergy demand for the fossil alternative.
Lastly, Fos and Bio-Ru-D differ in the amounts of generated wastewater, which are higher in the succinic acid-based production scenario. While the latter also comprises an additional inert gas purge (N2 from dehydration), quantities of the remaining waste streams (organics and flammable gas) are comparable between Fos and Bio-Ru-D, not least due to similar overall conversion selectivity.
The scenarios for NVP production from succinic acid consider two separation strategies (distillation (-D) and extraction (-Ex)) and three catalyst systems (ruthenium-based (-Ru-), platinum-based (-Pt-) and ideal, (-Id-)) (Table 3). Regarding separation, oligomer removal by extraction (Bio-Ru-Ex vs. Bio-Ru-D) comes at the cost of an organic solvent (dichloromethane, DCM) and higher cooling demand due its low boiling point (∼40 °C). Yet, this cost is balanced by lower steam and electricity requirements in Bio-Ru-Ex, which trace back to the heat of evaporating DCM as opposed to water and a reduced vacuum distillation need. Finally, waste quantities in the listed scenarios are affected by the purge of organic solvent and the difference in allocation of organic by-products to waste streams (see Fig. 4). For example, oligomeric by-products are allocated to organic residue and wastewater streams in distillation and extraction scenarios, respectively.
| Stream | Bio-Ru-D | Bio-Ru-Ex | Bio-Pt-Ex | Bio-Id-Ex | Units |
|---|---|---|---|---|---|
| Quantity | |||||
| a Ru/C or Pt–Re/TiO2 depending on scenario. b At T = −25 °C. c Calculated via the Carnot efficiency (Tref = 25 °C) of heat at 400 °C (natural gas), 200 °C (steam), −25 °C (cryogen). d Exemplary stream compositions and inventories of waste treatment in the ESI (section 10†). | |||||
| Substrates | |||||
| Succinic acid | 1.71 | 1.68 | 1.44 | 1.24 | kg kgNVP−1 |
| Monoethanolamine | 0.89 | 0.87 | 0.74 | 0.64 | kg kgNVP−1 |
| Hydrogen | 0.088 | 0.086 | 0.057 | 0.047 | kg kgNVP−1 |
| Auxiliaries | |||||
| Process water | 1.71 | 1.68 | 1.44 | 1.24 | kg kgNVP−1 |
| Dichloromethane | — | 0.11 | 0.084 | 0.068 | kg kgNVP−1 |
| Nitrogen | 0.12 | 0.12 | 0.12 | 0.12 | kg kgNVP−1 |
| Hydrogenation catalysta | 8.6 × 104 | 8.4 × 104 | 7.2 × 104 | 6.2 × 104 | kg kgNVP−1 |
| Na2O/SiO2 | 2.5 × 103 | 2.5 × 103 | 2.5 × 103 | 2.5 × 103 | kg kgNVP−1 |
| Utilities | |||||
| Natural gas | 1.74 | 1.58 | 1.70 | 3.19 | MJ kgNVP−1 |
| Steam | 9.49 | 6.93 | 5.68 | 3.25 | MJ kgNVP−1 |
| Cryogenb | 0.37 | 1.48 | 1.32 | 1.13 | MJ kgNVP−1 |
| Water cooling | 1.51 | 4.62 | 4.01 | 3.55 | MJ kgNVP−1 |
| Air cooling | 11.3 | 4.86 | 4.11 | 3.54 | MJ kgNVP−1 |
| Electricity | 0.15 | 0.087 | 0.076 | 0.065 | kWh kgNVP−1 |
| (Total exergy)c | (5.21) | (4.11) | (3.63) | (3.48) | MJ kgNVP−1 |
| Waste | |||||
| Inert gas purge | 0.13 | 0.13 | 0.13 | 0.13 | kg kgNVP−1 |
| Flam. gas purge | 0.044 | 0.043 | 0.011 | 7.2 × 103 | kg kgNVP−1 |
| Organic residue | 0.41 | 0.22 | 0.18 | 0.11 | kg kgNVP−1 |
| Wastewater | 2.94 | 3.14 | 2.57 | 2.11 | kg kgNVP−1 |
Lastly, further distinctions between Bio-x-Ex scenarios are caused by the choice of a hydrogenation catalyst, which determines overall NVP yields from succinic acid. While a recent improvement of the commercially available Ru/C catalyst, namely a bimetallic Pt–Re/TiO2 material,41 reduces normalized process inputs by about 14% relative, further optimization (Bio-Id-Ex) would allow for 26%. For reasons of mass balancing, waste quantities are inversely affected by the implemented hydrogenation catalyst. Still, the total exergy demand of the Bio-Pt-Ex scenario is less than 5% higher than for the ideal Bio-Id-Ex scenario, indicating small room for increasing energy efficiency through catalyst development.
3.2. Life cycle assessment
The environmental impacts of succinic acid-based NVP production were determined by evaluating the LCIs described in section 3.1 with the CML2001/2016 method. Results are summarized in Fig. 6 for the global warming impact (GWI); other categories are discussed subsequently.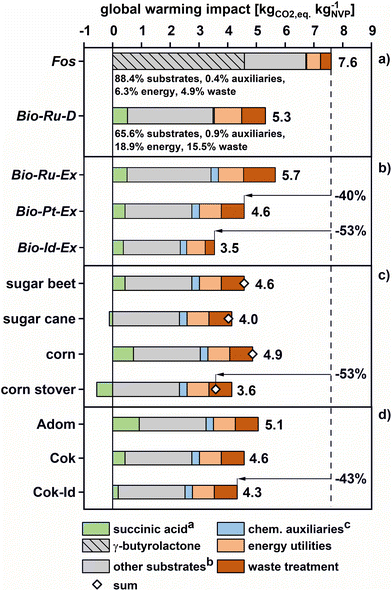 | ||
Fig. 6 Cradle-to-gate global warming impact of NVP production as a function of (a) value chain, (b) catalyst technology, (c) fermentation feedstock and (d) succinic acid production process. The process scenario is Bio-Pt-Ex, unless specified otherwise, a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Succinic acid is derived from sugar beet using a process by Cok et al.65 unless specified otherwise, b Succinic acid is derived from sugar beet using a process by Cok et al.65 unless specified otherwise, b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) other substrates include H2 and monoethanolamine (Bio) as well as ammonia and acetylene (Fos), c other substrates include H2 and monoethanolamine (Bio) as well as ammonia and acetylene (Fos), c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalysts (heterogeneous and KOH), solvents (H2O, DCM) and inerts (N2) are summarized as auxiliaries. catalysts (heterogeneous and KOH), solvents (H2O, DCM) and inerts (N2) are summarized as auxiliaries. | ||
The global warming impact of fossil NVP production is estimated at 7.6 kgCO2 eq. per kgNVP. Thus, even the commercial Ru/C catalyst in the Bio-Ru-D scenario (5.3 kgCO2 eq. per kgNVP) reduces GWI. Emission savings are mostly due to a large contribution of the fossil-derived GBL intermediate (∼60%) to the global warming impact in the Fos scenario. Bio-Ru-D, on the other hand, has a larger global warming impact due to energy use (0.95 kgCO2 eq. per kgNVPvs. 0.48 kgCO2 eq. per kgNVP) and waste disposal (0.83 kgCO2 eq. per kgNVPvs. 0.37 kgCO2 eq. per kgNVP). However, increases in utility impacts are more than compensated for by the low contribution of the succinic acid feedstock due to its biogenic origin. Avoiding four equivalents of fossil carbon per molecule of NVP lowers the global warming impact of the Bio-Ru-D scenario substantially.
Even utilizing bio-based feedstocks, the production of NVP is not carbon neutral. Remaining global warming impacts of bio-based NVP production are mainly driven by the utilization of fossil-based resources for energy provision or in the educts monoethanolamine and hydrogen. Global warming impacts could thus be decreased by utilizing renewable energy for electricity and heat generation and supplying monoethanolamine and hydrogen from renewable sources. However, such improvements would also lower the carbon intensity of the fossil-based supply chain.
Due to our cradle-to-gate system boundaries, the use phase and end-of-life of NVP is not considered. Assuming combustion with no heat recovery as the end of life of NVP would add additional 2.3 kgCO2 eq. per kgNVP to the global warming impacts of fossil and bio-based NVP.
The overall GWI of the extractive alternative (Bio-Ru-Ex) is in-between the Bio-Ru-D case and the Fos case, with a global warming impact of 5.7 kgCO2 eq. per kgNVP. Compared to Bio-Ru-D, the GWI primarily increases due to the production and disposal of the extraction solvent, which is partially purged to avoid the accumulation of impurities. Yet, Bio-Ru-Ex should be considered a base case of Bio scenarios due to its conservative approach to removing oligomeric residue. Thus, changes in the catalyst system are only discussed in detail for the extraction case (Bio-x-Ex).
Here, using the more efficient Pt–Re/TiO2 catalyst as opposed to Ru/C lowers substrate consumption and waste treatment demand, thereby reducing the global warming impacts of NVP production (Bio-Pt-Ex: 4.6 kgCO2 eq. per kgNVP). Notably, the large specific global warming impact of platinum (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 kgCO2 eq. per kgPt) as compared to ruthenium (3570 kgCO2 eq. per kgRu) has a negligible impact due to catalyst stability and metal recycling (ESI, section 3b + 4b†). In extrapolation of this case study, a potential improvement of the Pt-catalyst (Y(HEP) = 98 mol%) would bring the global warming impact of bio-based NVP production down to 3.5 kgCO2 eq. per kgNVP - 53% less than in the Fos scenario. This change is primarily due to an efficient succinic acid conversion to NVP, which reduces normalized process input and waste output quantities. Due to these benefits, an ideal catalyst would also reduce the carbon footprint by an additional 24% compared to the best current scenario Bio-Pt-Ex, much more than the small improvement potential found for energy in section 3.1.
600 kgCO2 eq. per kgPt) as compared to ruthenium (3570 kgCO2 eq. per kgRu) has a negligible impact due to catalyst stability and metal recycling (ESI, section 3b + 4b†). In extrapolation of this case study, a potential improvement of the Pt-catalyst (Y(HEP) = 98 mol%) would bring the global warming impact of bio-based NVP production down to 3.5 kgCO2 eq. per kgNVP - 53% less than in the Fos scenario. This change is primarily due to an efficient succinic acid conversion to NVP, which reduces normalized process input and waste output quantities. Due to these benefits, an ideal catalyst would also reduce the carbon footprint by an additional 24% compared to the best current scenario Bio-Pt-Ex, much more than the small improvement potential found for energy in section 3.1.
Also, fermentative succinic acid production is a rapidly developing field, with current efforts focusing, among others, on the use of alternative feedstocks.65,87 Thus, a sensitivity analysis considers four feedstocks for sugar production (Fig. 6c). While variations between cases (3.6–4.9 kgCO2 eq. per kgNVP) are smaller than those observed for different catalyst systems, the implementation of corn stover leads to the highest GWI savings (53% relative reduction vs. Fos). This finding is expected as corn stover represents a lignocellulosic side-product of food and feed production, with limited alternative use.
Further variability in the total GWI of Bio-Pt-Ex is connected to sugar fermentation and succinic acid separation. Three scenarios for succinic acid production are compared in this regard (Fig. 6d): (i) the base case of Cok et al.65 with glucose and energy demands of 1.35 kg and 9.9 MJ per kg of succinic acid, (ii) an alternative evaluation by Adom at al.94 demanding 1.90 kg and 17 MJ per kg of acid and (iii) a best case, where the theoretical minimum glucose demand of 1.04 kg per kg of acid95 is assumed within the process of Cok et al. (Cok-Id). Comparing the scenarios based on Cok et al. and Adom et al. shows a mild impact of assumptions for succinic acid production on the general advantage of Bio-Pt-Ex. Global warming impacts increase from 4.5 kgCO2 eq. per kgNVP to 5.1 kgCO2 eq. per kgNVP between these cases. On the other hand, pushing succinic acid fermentation to the theoretical limit could reduce the global warming impacts of the Bio-Pt-Ex scenario to 4.3 kgCO2 eq. per kgNVP.
Finally, impacts from indirect land use change (iLUC) have recently attained increasing interest in assessing biomass-based production, especially for first-generation feedstocks.88 Thus, several iLUC estimates for the proposed feedstocks were derived for the succinic acid-based production scenario Bio-Pt-Ex considered most realistic (Fig. 7). The analysis shows that iLUC (−0.1–0.7 kgCO2 eq. per kgNVP) has a mild impact on the environmental merits of Bio scenarios, but should always be analyzed in real-life implementation.
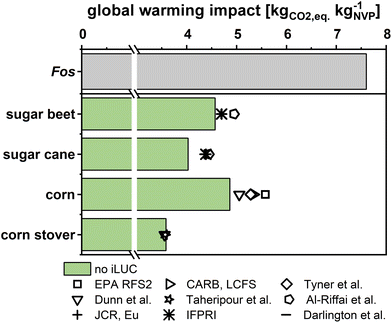 | ||
| Fig. 7 Impact of different iLUC estimates on total GWI for the Bio-Pt-Ex scenario.74–81 Further information are given in the ESI section 4c.† | ||
As far as other categories are concerned, environmental impacts are estimated to decrease by up to 65% relative (Bio-Id-Ex vs. Fos) in the categories of photochemical ozone creation, ozone layer depletion, and abiotic depletion of fossil energy (Fig. 8). In contrast, Bio scenarios increase impacts in the categories of eutrophication potential, acidification potential, and abiotic resource depletion. This increase is due to biomass feedstock production in the case of eutrophication and due to the supply of noble metal catalyst components in the case of acidification and resource depletion.
 | ||
| Fig. 8 Relative non-tox environmental impacts of bio-based NVP production compared to fossil-based production. Tabulated absolute values are given in the ESI section 11.† | ||
Moreover, heightened eutrophication presents a commonly observed drawback in bio-based chemical production and is primarily related to direct fertilizer emissions from agriculture. Thus, surges in this category vs. Fos vary between 70 and 560% relative, depending on biomass feedstock. The minimum of this range corresponds to corn stover (US) and the maximum to sugar cane (Thailand), due to high fertilizer use in the available inventories.
In the category of acidification, increases of up to 300% for routes using ruthenium catalysts and up to 690% for routes using platinum catalysts are found. Mining and refining activities of the noble metals are the root cause and contribute between 80–90% to the total impact estimate. These contributions, in turn, mostly stem from direct sulfur dioxide emissions in the refining process, which may be avoidable through the implementation of suitable gas treatment standards. Finally, the category of abiotic resource depletion is also dominated by the catalytic material, contributing 50–80% of the total impact estimate.
This analysis highlights the need to establish a sustainable catalyst supply chain to avoid excessive acidification and to utilize waste feedstocks such as corn stover to reduce eutrophication. Yet, it must be noted that current estimates on catalyst stability are conservative due to a lack of long-term stability data. Thus, catalyst-related impacts could be lowered in the course of future technology development.
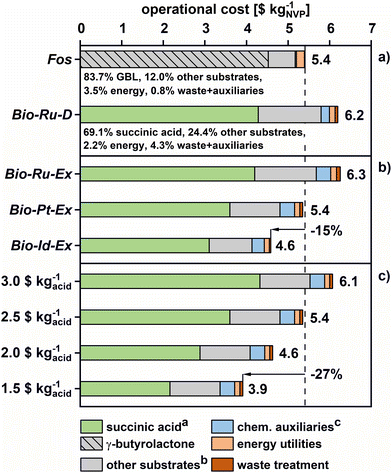
3.3. Operational cost analysis
Economic viability is another important factor in the implementation of bio-based processes. In contrast to the GWI estimates, an initial comparison of Bio-Ru-D (6.2 $ per kgNVP) and Fos (5.4 $ per kgNVP) scenarios shows higher operational costs associated with NVP from succinic acid (Fig. 9). The root cause of this difference between GWI and cost evaluations is the CO2 benefit of biomass feedstocks, which does not directly affect their economics.However, cost and GWI trends within the set of Bio scenarios are identical. Thus, Bio-Ru-Ex yields slightly higher costs (6.3 $ per kgNVP), mostly due to the expenditure of extraction agent and waste treatment. The cost of Bio-x-Ex can be reduced by up to 27% relative by new catalyst technologies in amidation–hydrogenation (Bio-Ru-Ex vs. Bio-Id-Ex). NVP from Bio-Id-Ex would then be produced at 15% lower cost than Fos.
The pronounced impact of catalyst selectivity on overall cost is rooted in the large contribution of substrate costs in Fos as well as Bio scenarios. For example, substrates account for 5.8 $ per kgNVP (93.5%) in Bio-Ru-D. This dominance of substrate cost is due to low solvent loads (see section 3.1) and straightforward separation schemes, which lead to modest energy demands. Also, waste treatment contributes more to emissions than to overall costs.
Within the substrate cost segment, succinic acid and GBL are the largest contributors, implying a strong dependence on the price of biogenic succinic acid set by a rapidly developing market. Thus, a sensitivity analysis for succinic acid price is presented in Fig. 9c (base case = 2.5 $ per kgacid).31 The analysis shows that bio-based NVP production through the improved Pt–Re/TiO2 catalyst (Bio-Pt-Ex) is likely to remain cost-competitive for succinic acid prices below 2.5 $ per kg. This break even point equates to a price ratio of 0.69 for the utilized C4 intermediates (succinic acid/GBL, mass-based), which could be used as a rough indicator of feasibility in different GBL market conditions.
Lastly, further improvements in fermentation and separation technology have been suggested to reduce the cost of succinc acid production to 1.5 $ per kgacid.89 Therefore, the operational cost for Bio-Pt-Ex may become as low as 3.9 $ per kgNVP – almost 30% less than in the Fos scenario. In addition, the ESI† explores the dependence of economic viability on patent-based assumptions for fossil NVP production, showing that a fossil-based production nearing stoichiometric yield would still have higher operating costs of 4.1 $ per kgNVP (ESI, section 9†).
3.4. Uncertainty
Previous sections have discussed the environmental and economic impacts of bio-based pyrrolidone production. Therein multiple process and market scenarios, covering a broad range of potential futures were considered. Thus, the ultimate feedstock choice and availability, the separation and catalyst technology are key variables in determining the environmental and economic performances of bio-based N-vinyl-2-pyrrolidone.It is clear that the presented, scenario-based sensitivity analysis does not include all potential uncertainties for a nascent process technology. For example, the energy system in general is in a state of transition and might change drastically till an eventual implementation of bio-based pyrrolidone production. Furthermore, bio-based succinic acid production is not yet established and thus production is simulated on literature, not industrial data. On the economic side, the market development for succinic acid provides the largest uncertainty.
Despite these unkowns, the presented spread of scenarios provides a suitable error margin for the given assessment. Accordingly, there is clear potential for improved environmental impacts through the production of NVP from succinic acid, whereas the economic success is very dependent on market developments.
3.5. Relevance and future research
To the best of our knowledge, the environmental impacts of bio-based pyrrolidone production have previously remained unreported. Lammens et al. have investigated the economics of NVP made from glutamic acid but did not address environmental impacts.90 Based on simplifying assumptions, these authors found economic potential for the production of NVP and the substrate (glutamic acid) price was identified as the dominant operational cost driver. Notably, the value chain studied by Lammens and coworkers was based on decarboxylation and vinylation instead of amidation-hydrogenation and dehydration reactions. Thus, our analysis not only adds environmental aspects to the evaluation of bio-based NVP, but also expands the scope of available synthesis pathways.The evaluation at hand can also direct future research towards an economically feasible and environmentally friendly process. In this regard, it is noted that the succinic acid conversion to NVP is a nascent technology with knowledge gaps to be addressed prior to industrial implementation. For example, the detailed properties and continuous separation behavior of oligomeric residue from amidation–hydrogenation must be explored to validate the proposed purification technologies. Moreover, DCM represents a suboptimal extraction agent in terms of performance and green chemistry,91 which leaves potential for more benign alternatives. However, our analysis followed a conservative approach by only considering DCM due to its demonstration in the laboratory.
Our results also indicate the large potential for further catalyst research, optimizing the selectivity and long-term stability of materials for amidation–hydrogenation. Promising catalyst candidates are other Pt–MeOx combinations (e.g. Pt–MoOx and Pt–VOx) already explored in the general context of amide reduction.92,93 Due to their impact on investment cost, factors such as operation pressure and space–time-yield will also become more important as the process moves to a pilot stage.
Finally, succinic acid from fermentation must be available at a stable, competitive price to generate investment interest in succinic acid conversion technologies to bio-products. It is therefore paramount to continue research on efficient succinic acid production and separation. Methods that allow for an integration of succinic acid production and downstream conversion are also promising to lower the effective cost contribution of substrates to NVP production.
4. Conclusions
In this work, we proposed and analyzed processes for the bio-based production of N-vinyl-2-pyrrolidone. Currently practiced fossil alternatives served as benchmark. The analysis is based on laboratory data, scientific and patent literature. Where necessary, life cycle inventories of process steps were derived from Aspen® flowsheet simulations. Finally, process scenarios were evaluated in terms of environmental impacts, such as global warming impact, and operational costs. Within these metrics, the succinic acid conversion scenario could reduce the impact/cost of NVP by up to 53% and 15%, respectively. Notably, the best current laboratory technology (Bio-Pt-Ex) still delivers substantial GWI reductions of 40% and reaches parity for operational cost.Yet, economic and environmental benefits of bio-based production via succinic acid largely depend on the availability of an efficient catalyst for amidation–hydrogenation. The catalyst must convert succinic acid and ethanolamine into N-(2-hydroxyethyl)-2-pyrrolidone, while limiting the formation of side-products, such as oligomers. This challenge is currently best addressed by a tailored Pt–Re/TiO2 material.41
On the supply side, the bio-based processes for NVP production would benefit significantly from further developments in succinic acid production from sugars and the emergence of a well-developed market for this platform chemical. Especially, lignocellulosic feedstocks would be desirable, resulting in lower succinic acid prices and reduced environmental impacts.
The study has also highlighted the potential of combining laboratory research on nascent processes with rigorous process modeling and assessment. In this regard, it should be rewarding to enter upscaling experiments for the chemical conversions and separations described herein. Respective results could be fed back, thus improving the quality of the given estimates and generating new research priorities in an iterative approach.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. A detailed CRediT statement is provided with the submission.Abbreviations
| NVP | N-Vinyl-2-pyrrolidone |
| GBL | γ-Butyrolactone |
| THF | Tetrahydrofurane |
| NMP | N-Methyl-2-pyrrolidone |
| 2PYD | 2-Pyrrolidone |
| LCA | Life cycle assessment |
| GWI | Global warming impact |
| HEP | N-(2-Hydroxyethyl)-2-pyrrolidone |
| LCI | Life cycle inventory |
| LCIA | Life cycle impact assessment |
| EP | Eutrophication potential |
| AP | Acidification potential |
| iLUC | Indirect land-use change |
| NRTL | Non-random two liquids |
| DCM | Dichloromethane. |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the German Federal Ministry of Education and Research (BMBF) through the projects BioPyrr and BioPVP [FKZ IBÖ-03 031B0249 and 031B0487 A] in the framework of “New Products for the Bioeconomy” (Neue Produkte für die Bioökonomie) and by funding from the European Union's Horizon 2020 research and innovation program under grant agreement no. 720695 (GreenSolRes). The authors wish to acknowledge the help of Stephanie Föller in conducting the presented experiments. Furthermore, Mr. Haus expresses his thanks towards the German Chemical Industry Fund (Fonds der chemischen Industrie, FCI), which generously supported his PhD project.References
- P. N. R. Vennestrøm, C. M. Osmundsen, C. H. Christensen and E. Taarning, Angew. Chem., Int. Ed., 2011, 50, 10502–10509 CrossRef PubMed.
- A. Pfennig , CBEN, 2019, 6, 90–104.
- Á. Galán-Martín, V. Tulus, I. Díaz, C. Pozo, J. Pérez-Ramírez and G. Guillén-Gosálbez, One Earth, 2021, 4, 565–583.
- R. Meys, A. Kätelhön, M. Bachmann, B. Winter, C. Zibunas, S. Suh and A. Bardow, Science, 2021, 374, 71–76 CrossRef CAS PubMed.
- European Comission, Bioeconomy, available at: https://ec.europa.eu/info/research-and-innovation/research-area/environment/bioeconomy_en, accessed 27 September 2021.
- RoadToBio Consortium, Welcome to RoadToBio, available at: https://www.roadtobio.eu/, accessed 27 September 2021.
- United States Department of Agriculture, BioPreferred, available at: https://www.biopreferred.gov/BioPreferred/, accessed 27 September 2021.
- T. W. House, Ind. Biotechnol., 2012, 8, 97–102 CrossRef.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed.
- A. D. Patel, K. Meesters, H. den Uil, E. de Jong, E. Worrell and M. K. Patel, ChemSusChem, 2013, 6, 1724–1736 CrossRef CAS PubMed.
- D. Kralisch, D. Ott and D. Gericke, Green Chem., 2015, 17, 123–145 RSC.
- J. Becker, A. Lange, J. Fabarius and C. Wittmann, Curr. Opin. Biotechnol., 2015, 36, 168–175 CrossRef CAS PubMed.
- M. Montazeri, G. G. Zaimes, V. Khanna and M. J. Eckelman, ACS Sustainable Chem. Eng., 2016, 4, 6443–6454 CrossRef CAS.
- (a) S. Spierling, E. Knüpffer, H. Behnsen, M. Mudersbach, H. Krieg, S. Springer, S. Albrecht, C. Herrmann and H.-J. Endres, J. Cleaner Prod., 2018, 185, 476–491 CrossRef; (b) S. Y. Lee, H. U. Kim, T. U. Chae, J. S. Cho, J. W. Kim, J. H. Shin, D. I. Kim, Y.-S. Ko, W. D. Jang and Y.-S. Jang, Nat. Catal., 2019, 2, 18–33 CrossRef CAS; (c) J. He, L. Chen, S. Liu, K. Song, S. Yang and A. Riisager, Green Chem., 2020, 22, 6714–6747 RSC.
- E. Castro-Aguirre, F. Iñiguez-Franco, H. Samsudin, X. Fang and R. Auras, Adv. Drug Delivery Rev., 2016, 107, 333–366 CrossRef CAS PubMed.
- M. L. Di Lorenzo and R. Androsch, Industrial Applications of Poly(lactic acid), Springer International Publishing, Cham, 1st edn., 2018, vol. 282 Search PubMed.
- S. Niphadkar, P. Bagade and S. Ahmed, Biofuels, 2018, 9, 229–238 CrossRef CAS.
- M. Rouhany and H. Montgomery, in Biodiesel: From Production to Combustion, ed. M. Tabatabaei and M. Aghbashlo, Springer International Publishing, Cham, 2019, pp. 1–14 Search PubMed.
- J. J. Bozell and M. K. Patel, Feedstocks for the Future, American Chemical Society, Washington, DC, 2006, vol. 921 Search PubMed.
- T. Werpy and G. Petersen, Top value added chemicals from biomass: volume I—results of screening for potential candidates from sugars and synthesis gas, National Renewable Energy Lab., Golden, CO (US), 2004 Search PubMed.
- M. J. Biddy, C. Scarlata and C. Kinchin, Chemicals from biomass: a market assessment of bioproducts with near-term potential, National Renewable Energy Lab.(NREL), Golden, CO (United States), 2016 Search PubMed.
- J.-V. Bomtempo, F. Chaves Alves and F. de Almeida Oroski, Faraday Discuss., 2017, 202, 213–225 RSC.
- S. Takkellapati, T. Li and M. A. Gonzalez, Clean Technol. Environ. Policy, 2018, 20, 1615–1630 CrossRef CAS PubMed.
- M. Dusselier, M. Mascal and B. F. Sels, in Selective Catalysis for Renewable Feedstocks and Chemicals, ed. K. M. Nicholas, Springer, Heidelberg, 2014, pp. 1–40 Search PubMed.
- L. Corbel-Demailly, B.-K. Ly, D.-P. Minh, B. Tapin, C. Especel, F. Epron, A. Cabiac, E. Guillon, M. Besson and C. Pinel, ChemSusChem, 2013, 6, 2388–2395 CrossRef CAS PubMed.
- K. H. Kang, U. G. Hong, Y. Bang, J. H. Choi, J. K. Kim, J. K. Lee, S. J. Han and I. K. Song, Appl. Catal., A, 2015, 490, 153–162 CrossRef CAS.
- C. S. Spanjers, D. K. Schneiderman, J. Z. Wang, J. Wang, M. A. Hillmyer, K. Zhang and P. J. Dauenhauer, ChemCatChem, 2016, 8, 3031–3035 CrossRef CAS.
- X. Di, C. Li, B. Zhang, J. Qi, W. Li, D. Su and C. Liang, Ind. Eng. Chem. Res., 2017, 56, 4672–4683 CrossRef CAS.
- X. Di, C. Li, G. Lafaye, C. Especel, F. Epron and C. Liang, Catal. Sci. Technol., 2017, 7, 5212–5223 RSC.
- A. Cukalovic and C. V. Stevens, Biofuels, Bioprod. Biorefin., 2008, 2, 505–529 CrossRef CAS.
- weastra s.r.o. WP 8.1. Determination of market potential for selected platform chemicals, available at: https://www.cbp.fraunhofer.de/content/dam/cbp/en/documents/project-reports/BioConSepT_Market-potential-for-selected-platform-chemicals_report1.pdf, accessed 13 October 2021.
- J.-P. Lange and S. H. Wadman, ChemSusChem, 2020, 13, 5329–5337 CrossRef CAS PubMed.
- M. J. Hülsey, H. Yang and N. Yan, ACS Sustainable Chem. Eng., 2018, 6, 5694–5707 CrossRef.
- J. F. White, J. E. Holladay, A. A. Zacher, J. G. Frye and T. A. Werpy, Top. Catal., 2014, 57, 1325–1334 CrossRef CAS.
- G. Budroni and A. Corma, J. Catal., 2008, 257, 403–408 CrossRef CAS.
- W. Fischer, D. Klein, A. Künkel, R. Pinkos and E. Scholten, US Patent, 8017790B2, 2011 Search PubMed.
- T. Thosukhowong, K. Roffi, S. R. More, R. L. Gustine and S. Tanielyan, WO Patent, 2013033649A1, 2013 Search PubMed.
- brgfx, Vector Image - Tree. <a href= https://de.freepik.com/vektoren/hintergrund>Hintergrund Vektor erstellt von brgfx - de.freepik.com </a>, available at: https://de.freepik.com/brgfx, accessed 17 October 2021.
- M. O. Haus, Y. Louven and R. Palkovits, Green Chem., 2019, 21, 6268–6276 RSC.
- H. Krishna, M. O. Haus and R. Palkovits, Appl. Catal., B, 2021, 286, 119933 CrossRef CAS.
- M. O. Haus, A. Meledin, S. Leiting, Y. Louven, N. C. Roubicek, S. Moos, C. Weidenthaler, T. E. Weirich and R. Palkovits, ACS Catal., 2021, 11, 5119–5134 CrossRef CAS.
-
(a)
International Organization for Standardization, ISO 14040
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2006, available at: https://www.iso.org/standard/37456.html, accessed 29 September 2021;
(b)
L. Ismail, J. Alcorta and N. Perou, The computational structure of life cycle assessmnet. Dordrecht, Kluwer (Eco-efficiency in industry and science, v. 11), London, 2002 Search PubMed.
2006, available at: https://www.iso.org/standard/37456.html, accessed 29 September 2021;
(b)
L. Ismail, J. Alcorta and N. Perou, The computational structure of life cycle assessmnet. Dordrecht, Kluwer (Eco-efficiency in industry and science, v. 11), London, 2002 Search PubMed. - M. Weiss, J. Haufe, M. Carus, M. Brandão, S. Bringezu, B. Hermann and M. K. Patel, J. Ind. Ecol., 2012, 16, 169–181 CrossRef.
- O. G. Griffiths, R. E. Owen, J. P. O'Byrne, D. Mattia, M. D. Jones and M. C. McManus, RSC Adv., 2013, 3, 12244–12254 RSC.
- I. Muñoz, K. Flury, N. Jungbluth, G. Rigarlsford, L. M. i Canals and H. King, Int. J. Life Cycle Assess., 2014, 19, 109–119 CrossRef.
- H. de Bruijn, R. van Duin, M. A. J. Huijbregts, J. B. Guinee, M. Gorree, R. Heijungs, G. Huppes, R. Kleijn, A. de Koning, L. van Oers, A. Wegener Sleeswijk, S. Suh and H. A. Udo de Haes, Handbook on Life Cycle Assessment, Springer Netherlands, Dordrecht, 2002, vol. 7 Search PubMed.
- N. von der Assen, J. von Jung and A. Bardow, Energy Environ. Sci., 2013, 6, 2721 RSC.
- N. von der Assen, P. Voll, M. Peters and A. Bardow, Chem. Soc. Rev., 2014, 43, 7982–7994 RSC.
- R. Pelton, Int. J. Life Cycle Assess., 2019, 24, 12–25 CrossRef CAS.
- B. Winter, R. Meys and A. Bardow, J. Cleaner Prod., 2021, 290, 125818 CrossRef CAS.
- C. Andersson, D. Hodge, K. A. Berglund and U. Rova, Biotechnol. Prog., 2007, 23, 381–388 CrossRef CAS PubMed.
- S. Chan, S. Kanchanatawee and K. Jantama, Bioresour. Technol., 2012, 103, 329–336 CrossRef CAS PubMed.
- C. Li, K. L. Ong, Z. Cui, Z. Sang, X. Li, R. D. Patria, Q. Qi, P. Fickers, J. Yan and C. S. K. Lin, J. Hazard. Mater., 2021, 401, 123414 CrossRef CAS PubMed.
- W. Schwarz, J. Schossig, R. Rossbacher and H. Höke, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2000 Search PubMed.
- A. L. Harreus, R. Backes, J.-O. Eichler, R. Feuerhake, C. Jäkel, U. Mahn, R. Pinkos and R. Vogelsang, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2000 Search PubMed.
- M. Rudloff, P. Stops, E. Henkes, H. Schmidtke, R.-H. Fischer, M. Julius, R. Lebkücher and K.-H. Ross, US Patent, 7164031B2, 2007 Search PubMed.
- W. Staffel, S. Käshammer, R. Kessinger, R. Vogelsang, A. Paul and L. Tuttelberg, WO Patent, 2009071479A1, 2009 Search PubMed.
- R. Vogelsang, S. Käshammer, W. Staffel, U. Eiden, A. Brand and L. Tuttelberg, WO Patent, 2009074534A1, 2009 Search PubMed.
- K.-C. Liu and P. D. Taylor, US Patent, 4824967A, 1989 Search PubMed.
- K.-C. Liu and P. D. Taylor, US Patent, 4873336A, 1989 Search PubMed.
- B. Puetzer, L. Katz and L. Horwitz, US Patent, 2775599A, 1956 Search PubMed.
- K. Ebel, M. Eiermann, T. Narbeshuber and E. Gehrer, US Patent, 5994562A, 1999 Search PubMed.
- Y. Shimasaki, H. Yano, H. Sugiura and H. Kambe, BCSJ, 2008, 81, 449–459.
- X. Li, W. Wan, J. G. Chen and T. Wang, ACS Sustainable Chem. Eng., 2018, 6, 16039–16046 CrossRef CAS.
- B. Cok, I. Tsiropoulos, A. L. Roes and M. K. Patel, Biofuels, Bioprod. Biorefin., 2014, 8, 16–29 CrossRef CAS.
- Sphera, GaBi Software, available at: https://gabi.sphera.com/deutsch/software/gabi-software/, accessed 10.16.2021.
- IHS Markit, Chemical Economics Handbook, 2017 Search PubMed.
- C. Seyler, T. B. Hofstetter and K. Hungerbühler, J. Cleaner Prod., 2005, 13, 1211–1224 CrossRef.
- A. Köhler, PhD Thesis, ETH Zurich, 2006. Available from DOI:10.3929/ethz-a-005206273, last visited 11.05.2022.
- P. Pawelzik, M. Carus, J. Hotchkiss, R. Narayan, S. Selke, M. Wellisch, M. Weiss, B. Wicke and M. K. Patel, Resour., Conserv. Recycl., 2013, 73, 211–228 CrossRef.
- S. E. Tanzer and A. Ramírez, Energy Environ. Sci., 2019, 12, 1210–1218 RSC.
- T. Searchinger, R. Heimlich, R. A. Houghton, F. Dong, A. Elobeid, J. Fabiosa, S. Tokgoz, D. Hayes and T.-H. Yu, Science, 2008, 319, 1238–1240 CrossRef CAS PubMed.
- J. A. Mathews and H. Tan, Biofuels, Bioprod. Biorefin., 2009, 3, 305–317 CrossRef CAS.
- W. E. Tyner, F. Taheripour, Q. Zhuang, D. Birur and U. Baldos, Land Use Changes and Consequent CO2 Emissions due to US Corn Ethanol, Purdue University, 2010 Search PubMed.
- L. Marelli, F. Ramos, R. Hiederer and R. Koeble, Estimate of GHG emissions from global land use change scenarios, 2011.
- D. Laborde Debucquet, Assessing the land use change consequences of European biofuel policies, 2011.
- California Air Resources Board, LCFS Land Use Change Assessment, available at: https://ww2.arb.ca.gov/resources/documents/lcfs-land-use-change-assessment, accessed 17 October 2021.
- P. Al-Riffai, B. V. Dimaranan and D. Laborde Debucquet, Global trade and environmental impact study of the EU biofuels mandate, 2010.
- T. Darlington, D. Kahlbaum, D. O'Connor and S. Mueller, Land Use Change Greenhouse Gas Emissions of European Biofuel Policies Utilizing the Global Trade Analysis Project (GTAP) Model, 2013.
- F. Taheripour and W. E. Tyner, Induced Land Use Emissions due to First and Second Generation Biofuels and Uncertainty in Land Use Emission Factors, Economics Research International, 2013 Search PubMed.
- J. B. Dunn, S. Mueller, H.-Y. Kwon and M. Q. Wang, Biotechnol. Biofuels, 2013, 6, 51 CrossRef CAS PubMed.
- macrovector, Vector Image - Industrial Building Icons. <a href= https://de.freepik.com/vektoren/abstrakt>Abstrakt Vektor erstellt von macrovector - de.freepik.com </a>, available at: https://de.freepik.com/macrovector, accessed 17 October 2021.
- Y. Yamaguchi, H. Yano, K. Hitoshi, A. Kurusu and Y. Shimasaki, EP Patent, 1094061B2, 2001 Search PubMed.
- S. W. Benson, Thermochemical kinetics, Wiley, 1976 Search PubMed.
- A. Klamt, F. Eckert and W. Arlt, Annu. Rev. Chem. Biomol. Eng., 2010, 1, 101–122 CrossRef CAS PubMed.
- ChemCatBio, CatCost, available at: https://catcost.chemcatbio.org/, accessed 13 October 2021.
- E. Mancini, S. S. Mansouri, K. V. Gernaey, J. Luo and M. Pinelo, Crit. Rev. Environ. Sci. Technol., 2020, 50, 1829–1873 CrossRef CAS.
- A. Broch, S. K. Hoekman and S. Unnasch, Environ. Sci. Policy, 2013, 29, 147–157 CrossRef.
- M. Morales, M. Ataman, S. Badr, S. Linster, I. Kourlimpinis, S. Papadokonstantakis, V. Hatzimanikatis and K. Hungerbühler, Energy Environ. Sci., 2016, 9, 2794–2805 RSC.
- T. M. Lammens, S. Gangarapu, M. C. Franssen, E. L. Scott and J. P. Sanders, Biofuels, Bioprod. Biorefin., 2012, 6, 177–187 CrossRef CAS.
- F. P. Byrne, S. Jin, G. Paggiola, T. H. M. Petchey, J. H. Clark, T. J. Farmer, A. J. Hunt, C. Robert McElroy and J. Sherwood, SustainableChem.Processes, 2016, 4, 7 CrossRef.
- K. Shimizu, W. Onodera, A. S. Touchy, S. M. A. H. Siddiki, T. Toyao and K. Kon, ChemistrySelect, 2016, 1, 736–740 CrossRef CAS.
- T. Mitsudome, K. Miyagawa, Z. Maeno, T. Mizugaki, K. Jitsukawa, J. Yamasaki, Y. Kitagawa and K. Kaneda, Angew. Chem., Int. Ed., 2017, 56, 9381–9385 CrossRef CAS PubMed.
- F. Adom, J. B. Dunn, J. Han and N. Sather, Environ. Sci. Technol., 2014, 48, 14624–14631 CrossRef CAS PubMed.
- T. Tiso, T. Narancic, R. Wei, E. Pollet, N. Beagan, K. Schröder, A. Honak, M. Jiang, S. T. Kenny, N. Wierckx, R. Perrin, L. Avérous, W. Zimmermann, K. O‘Connor and L. M. Blank, Metab. Eng., 2021, 66, 167–178 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Contents: 1 Experimental laboratory procedures; 2. Experimental results on amidation–hydrogenation; 3. Life cycle inventories; 4. Life cycle impact assessment; 5. Process descriptions; 6. Estimation of thermodynamic properties; 7. Operational cost analysis; 8. Exemplary comparison of unit operations; 9. Analysis of alternative scenarios for fossil-based NVP production; 10. Detailed waste stream compositions and LCIs; 11. Tabulated LCA results. See DOI: https://doi.org/10.1039/d2gc01219g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |